Could the Adoptive Transfer of Memory Lymphocytes be an Alternative Treatment for Acinetobacter baumannii Infections?
Abstract
1. Introduction
2. Results
2.1. Characterization of Test Strains
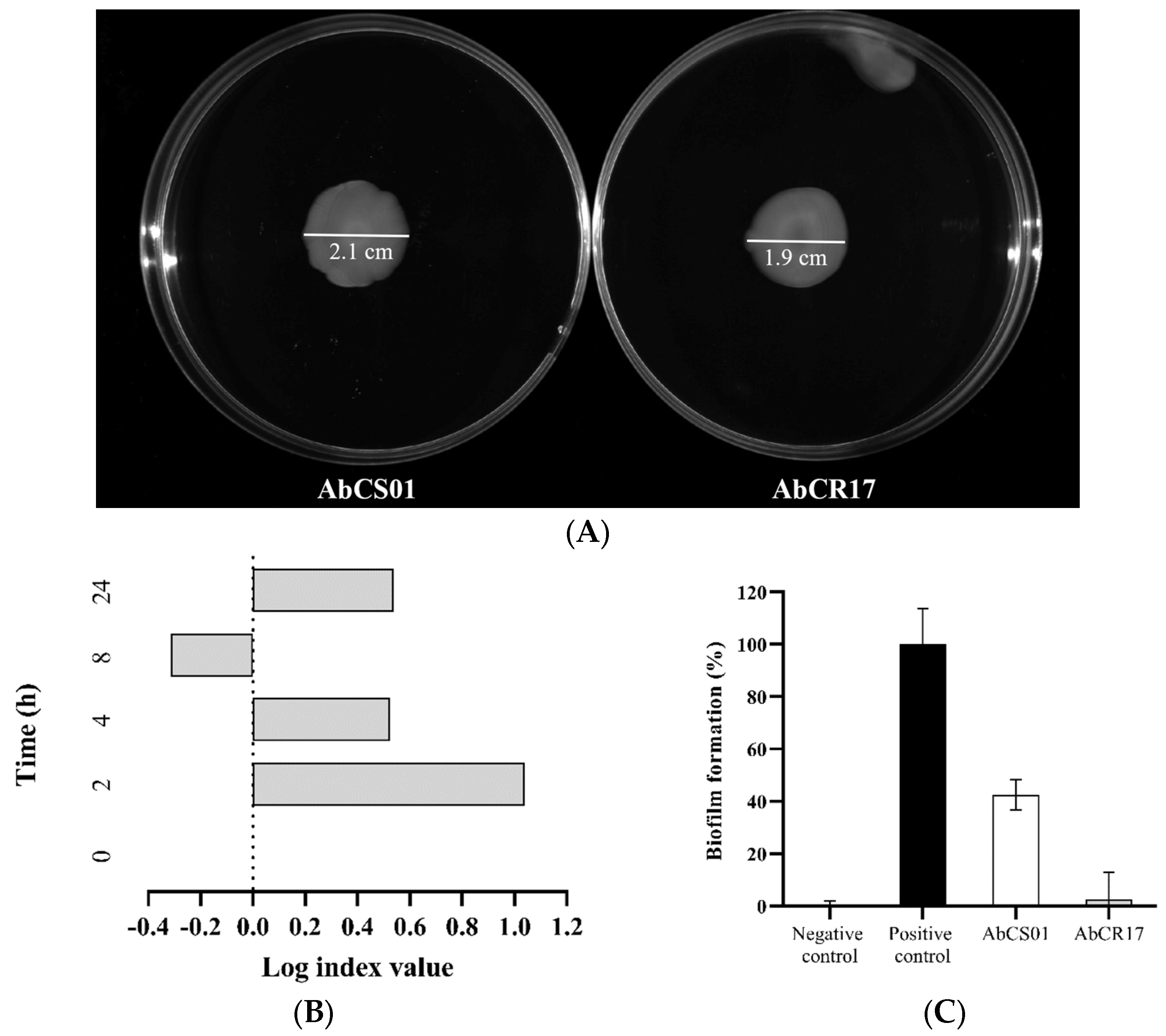
2.1.1. Surface Motility Assay
2.1.2. In Vitro Growth Curves and Competition Indices (CI)
2.1.3. Biofilm Assay
2.2. In Vivo Studies
Efficacy Studies in a Pneumonia Murine Model Infected with A. baumannii AbCS01 and A. baumannii AbCR17 Clinical Strains
3. Discussion
4. Materials and Methods
4.1. Antibiotics
4.2. Bacterial Strains
4.3. Characterization of Test Strains
4.3.1. Surface Motility Assay
4.3.2. In Vitro Growth Curves and CIs
4.3.3. Biofilm Assay
4.4. In Vivo Studies
4.4.1. Animals
4.4.2. Single-Cell Preparations of Splenocytes
4.4.3. Adoptive Transfer of Memory Lymphocytes
4.4.4. Efficacy Studies in a Pneumonia Murine Model Infected with A. baumannii AbCS01 and A. baumannii AbCR17 Clinical Strains
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amanati, A.; Sajedianfard, S.; Khajeh, S.; Ghasempour, S.; Mehrangiz, S.; Nematolahi, S.; Shahhosein, Z. Bloodstream infections in adult patients with malignancy, epidemiology, microbiology, and risk factors associated with mortality and multi-drug resistance. BMC Infect. Dis. 2021, 21, 636. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Resistance Collaborators. The burden of antimicrobial resistance in the Americas in 2019: A cross-country systematic analysis. Lancet Reg. Health Am. 2023, 25, 100561. [Google Scholar] [CrossRef]
- Hassoun-Kheir, N.; Harbarth, S. Estimating antimicrobial resistance burden in Europe-what are the next steps? Lancet Public Health 2022, 7, e886–e887. [Google Scholar] [CrossRef] [PubMed]
- WHO (World Health Organization). Bacterial Priority Pathogens List, 2024: Bacterial Pathogens of Public Health Importance to Guide Research, Development and Strategies to Prevent and Control Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- WHO (World Health Organization). 2020 Antibacterial Agents in Clinical and Preclinical Development: An Overview and Analysis; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Butler, M.S.; Gigante, V.; Sati, H.; Paulin, S.; Al-Sulaiman, L.; Rex, J.H.; Fernandes, P.; Arias, C.A.; Paul, M.; Thwaites, G.E.; et al. Analysis of the Clinical Pipeline of Treatments for Drug-Resistant Bacterial Infections: Despite Progress, More Action Is Needed. Antimicrob. Agents Chemother. 2022, 66, e0199121. [Google Scholar] [CrossRef] [PubMed]
- Restifo, N.P.; Dudley, M.E.; Rosenberg, S.A. Adoptive immunotherapy for cancer: Harnessing the T cell response. Nat. Rev. Immunol. 2012, 12, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, O.; Schroth, J.; Tree, T.; Turner, M.R.; Shaw, P.J.; Henson, S.M.; Malaspina, A. Senescent-like Blood Lymphocytes and Disease Progression in Amyotrophic Lateral Sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2023, 10, e200042. [Google Scholar] [CrossRef]
- Zander, R.; Kasmani, M.Y.; Chen, Y.; Topchyan, P.; Shen, J.; Zheng, S.; Burns, R.; Ingram, J.; Cui, C.; Joshi, N.; et al. Tfh-cell-derived interleukin 21 sustains effector CD8(+) T cell responses during chronic viral infection. Immunity 2022, 55, 475–493. [Google Scholar] [CrossRef] [PubMed]
- Maurice, N.M.; Bedi, B.; Sadikot, R.T. Pseudomonas aeruginosa Biofilms: Host Response and Clinical Implications in Lung Infections. Am. J. Respir. Cell Mol. Biol. 2018, 58, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Patino, M.G.; Garcia-Contreras, R.; Licona-Limon, P. The Immune Response against Acinetobacter baumannii, an Emerging Pathogen in Nosocomial Infections. Front. Immunol. 2017, 8, 441. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.M.; Soper, N.; Bennett, M.; Fallon, J.K.; Michell, A.R.; Alter, G.; Liu, G.Y.; Thomsen, I. Adoptive Transfer of Serum Samples from Children With Invasive Staphylococcal Infection and Protection Against Staphylococcus aureus Sepsis. J. Infect. Dis. 2021, 223, 1222–1231. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Ibrahim, A.S.; Baquir, B.; Palosaari, A.; Spellberg, B. Luminescent-activated transfected killer cells to monitor leukocyte trafficking during systemic bacterial and fungal infection. J. Infect. Dis. 2012, 205, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Tacke, R.; Sun, J.; Uchiyama, S.; Polovina, A.; Nguyen, D.G.; Nizet, V. Protection Against Lethal Multidrug-Resistant Bacterial Infections Using Macrophage Cell Therapy. Infect. Microbes Dis. 2019, 1, 61–69. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, A.; Cheng, W.; Li, Y.; Li, D.; Wang, L.; Zhang, X.; Xiao, Y. Adoptive macrophage directed photodynamic therapy of multidrug-resistant bacterial infection. Nat. Commun. 2023, 14, 7251. [Google Scholar] [CrossRef] [PubMed]
- Dunkley, M.L.; Clancy, R.L.; Cripps, A.W. A role for CD4+ T cells from orally immunized rats in enhanced clearance of Pseudomonas aeruginosa from the lung. Immunology 1994, 83, 362–369. [Google Scholar] [PubMed]
- Schreiber, S.; Dressler, L.S.; Loffredo-Verde, E.; Asen, T.; Färber, S.; Wang, W.; Groll, T.; Chakraborty, A.; Kolbe, F.; Kreer, C.; et al. CARs derived from broadly neutralizing, human monoclonal antibodies identified by single B cell sorting target hepatitis B virus-positive cells. Front. Immunol. 2024, 15, 1340619. [Google Scholar] [CrossRef] [PubMed]
- Wilk, M.M.; Misiak, A.; McManus, R.M.; Allen, A.C.; Lynch, M.A.; Mills, K.H.G. Lung CD4 Tissue-Resident Memory T Cells Mediate Adaptive Immunity Induced by Previous Infection of Mice with Bordetella pertussis. J. Immunol. 2017, 199, 233–243. [Google Scholar] [CrossRef]
- Iwanaga, N.; Chen, K.; Yang, H.; Lu, S.; Hoffmann, J.P.; Wanek, A.; McCombs, J.E.; Song, K.; Rangel-Moreno, J.; Norton, E.B.; et al. Vaccine-driven lung TRM cells provide immunity against Klebsiella via fibroblast IL-17R signaling. Sci. Immunol. 2021, 6, eabf1198. [Google Scholar] [CrossRef] [PubMed]
- Gachoud, D.; Bertelli, C.; Rufer, N. Understanding the parameters guiding the best practice for treating B-cell-depleted patients with COVID-19 convalescent plasma therapy. Br. J. Haematol. 2023, 200, e25–e27. [Google Scholar] [CrossRef] [PubMed]
- Specht, C.A.; Wang, R.; Oliveira, L.V.N.; Hester, M.M.; Gomez, C.; Mou, Z.; Carlson, D.; Lee, C.K.; Hole, C.R.; Lam, W.C.; et al. Immunological correlates of protection mediated by a whole organism, Cryptococcus neoformans, vaccine deficient in chitosan. mBio 2024, 15, e0174624. [Google Scholar] [CrossRef] [PubMed]
- Lombard-Vadnais, F.; Collin, R.; Daudelin, J.F.; Chabot-Roy, G.; Labrecque, N.; Lesage, S. The Idd2 Locus Confers Prominent Resistance to Autoimmune Diabetes. J. Immunol. 2022, 208, 898–909. [Google Scholar] [CrossRef] [PubMed]
- Roland, M.M.; Peacock, T.E.; Hall, N.; Mohammed, A.D.; Ball, R.; Jolly, A.; Alexeev, S.; Dopkins, N.; Nagarkatti, M.; Nagarkatti, P.; et al. B-cell-specific MhcII regulates microbiota composition in a primarily IgA-independent manner. Front. Immunol. 2023, 14, 1253674. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, M.; Kinoshita, M.; Nakashima, H.; Kato, A.; Mori, K.; Koiwai, K.; Shinomiya, N.; Seki, S. Mouse Liver B Cells Phagocytose Streptococcus pneumoniae and Initiate Immune Responses against Their Antigens. J. Immunol. 2022, 209, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Sigal, L.J. Activation of CD8 T Lymphocytes during Viral Infections. Encycl. Immunobiol. 2016, 4, 286–290. [Google Scholar] [CrossRef]
- Jiang, W.; He, Y.; He, W.; Wu, G.; Zhou, X.; Sheng, Q.; Zhong, W.; Lu, Y.; Ding, Y.; Lu, Q.; et al. Exhausted CD8+T Cells in the Tumor Immune Microenvironment: New Pathways to Therapy. Front. Immunol. 2020, 11, 622509. [Google Scholar] [CrossRef] [PubMed]
- Smani, Y.; Docobo-Pérez, F.; McConnell, M.J.; Pachón, J. Acinetobacter baumannii-induced lung cell death: Role of inflammation, oxidative stress and cytosolic calcium. Microb. Pathog. 2011, 50, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Parra Millan, R.; Jimenez Mejias, M.E.; Sanchez Encinales, V.; Ayerbe Algaba, R.; Gutierrez Valencia, A.; Pachon Ibanez, M.E.; Diaz, C.; Perez Del Palacio, J.; Lopez Cortes, L.F.; Pachon, J.; et al. Efficacy of Lysophosphatidylcholine in Combination with Antimicrobial Agents against Acinetobacter baumannii in Experimental Murine Peritoneal Sepsis and Pneumonia Models. Antimicrob. Agents Chemother. 2016, 60, 4464–4470. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Wang, S.; Li, D.; Wen, J.; Sun, N.; Fan, G. Population pharmacokinetics of tigecycline in critically ill patients. Front. Pharmacol. 2023, 14, 1083464. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, J.P.; Bhavnani, S.M. The Pharmacokinetics/Pharmacodynamic Relationship of Durlobactam in Combination with Sulbactam in In Vitro and In Vivo Infection Model Systems Versus Acinetobacter baumannii-calcoaceticus Complex. Clin. Infect. Dis. 2023, 76, S202–s209. [Google Scholar] [CrossRef]
- Pachon-Ibanez, M.E.; Docobo-Perez, F.; Lopez-Rojas, R.; Dominguez-Herrera, J.; Jimenez-Mejias, M.E.; Garcia-Curiel, A.; Pichardo, C.; Jimenez, L.; Pachon, J. Efficacy of rifampin and its combinations with imipenem, sulbactam, and colistin in experimental models of infection caused by imipenem-resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2010, 54, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Dinc, G.; Demiraslan, H.; Elmali, F.; Ahmed, S.S.; Alp, E.; Doganay, M. Antimicrobial efficacy of doripenem and its combinations with sulbactam, amikacin, colistin, tigecycline in experimental sepsis of carbapenem-resistant Acinetobacter baumannii. New Microbiol. 2015, 38, 67–73. [Google Scholar] [PubMed]
- Hubrecht, R.C.; Carter, E. The 3Rs and Humane Experimental Technique: Implementing Change. Animals 2019, 9, 754. [Google Scholar] [CrossRef] [PubMed]
- López-Rojas, R.; McConnell, M.J.; Jiménez-Mejías, M.E.; Domínguez-Herrera, J.; Fernández-Cuenca, F.; Pachón, J. Colistin resistance in a clinical Acinetobacter baumannii strain appearing after colistin treatment: Effect on virulence and bacterial fitness. Antimicrob. Agents Chemother. 2013, 57, 4587–4589. [Google Scholar] [CrossRef] [PubMed]
- EUCAST (The European Committee on Antimicrobial Susceptibility Testing). Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 14.0. 2024. Available online: http://www.eucast.org (accessed on 20 August 2024).
- CLSI (Clinical and Laboratory Standards Institute). Performance Standards for Antimicrobials Susceptibility Testing. In CLSI Supplement M100, 34th ed.; Clinical and Laboratory Standards Intitute: Wayne, PA, USA, 2024. [Google Scholar]
- Abouelhassan, Y.; Nicolau, D.P.; Abdelraouf, K. Defining optimal sulbactam regimens for treatment of Acinetobacter baumannii pneumonia and impact of blaOXA-23 on efficacy. J. Antimicrob. Chemother. 2024, 79, 2306–2316. [Google Scholar] [CrossRef] [PubMed]
- Pachón-Ibáñez, M.E.; Jiménez-Mejías, M.E.; Pichardo, C.; Llanos, A.C.; Pachón, J. Activity of tigecycline (GAR-936) against Acinetobacter baumannii strains, including those resistant to imipenem. Antimicrob. Agents Chemother. 2004, 48, 4479–4481. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cebrero-Cangueiro, T.; Labrador-Herrera, G.; Carretero-Ledesma, M.; Herrera-Espejo, S.; Álvarez-Marín, R.; Pachón, J.; Cisneros, J.M.; Pachón-Ibáñez, M.E. IgM-enriched immunoglobulin improves colistin efficacy in a pneumonia model by Pseudomonas aeruginosa. Life Sci. Alliance 2022, 5, e202101349. [Google Scholar] [CrossRef] [PubMed]
- Auerbuch, V.; Lenz, L.L.; Portnoy, D.A. Development of a competitive index assay to evaluate the virulence of Listeria monocytogenes actA mutants during primary and secondary infection of mice. Infect. Immun. 2001, 69, 5953–5957. [Google Scholar] [CrossRef]
- Recacha, E.; Machuca, J.; Díaz-Díaz, S.; García-Duque, A.; Ramos-Guelfo, M.; Docobo-Pérez, F.; Blázquez, J.; Pascual, A.; Rodríguez-Martínez, J.M. Suppression of the SOS response modifies spatiotemporal evolution, post-antibiotic effect, bacterial fitness and biofilm formation in quinolone-resistant Escherichia coli. J. Antimicrob. Chemother. 2019, 74, 66–73. [Google Scholar] [CrossRef] [PubMed]
- NRC (National Research Council). Guide for the Care and Use of Laboratory Animals; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Whitmire, J.K.; Benning, N.; Whitton, J.L. Precursor frequency, nonlinear proliferation, and functional maturation of virus-specific CD4+ T cells. J. Immunol. 2006, 176, 3028–3036. [Google Scholar] [CrossRef]
- Herrera-Espejo, S.; Vila-Domínguez, A.; Cebrero-Cangueiro, T.; Smani, Y.; Pachón, J.; Jiménez-Mejías, M.E.; Pachón-Ibáñez, M.E. Efficacy of Tamoxifen Metabolites in Combination with Colistin and Tigecycline in Experimental Murine Models of Escherichia coli and Acinetobacter baumannii. Antibiotics 2024, 13, 386. [Google Scholar] [CrossRef] [PubMed]
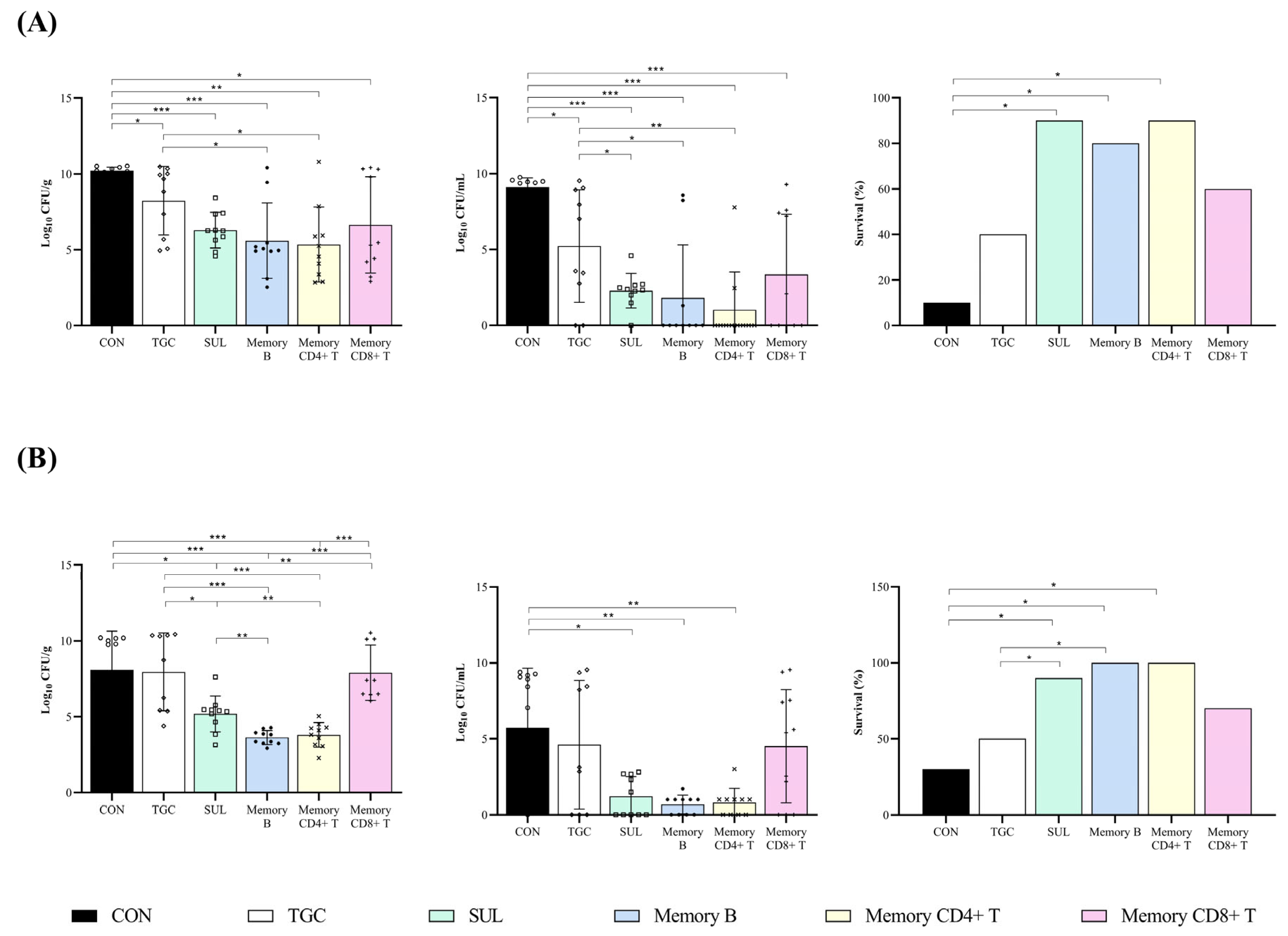
| Group | Dosage | n | Lung (Log10 CFU/g) | Blood (Log10 CFU/mL) | Survival (%) |
|---|---|---|---|---|---|
| CON | - | 10 | 10.21 ± 0.23 | 9.10 ± 0.61 | 10 |
| TGC | 5 mg/kg/q12h/sc | 10 | 8.23 ± 2.26 a | 5.23 ± 3.71 a | 40 |
| SUL | 60 mg/kg/q6h/im | 10 | 6.29 ± 1.18 a | 1.19 ± 1.30 a,b | 90 a |
| M-B | 2 × 106 cells/iv | 10 | 5.59 ± 2.48 a,b | 1.81 ± 3.50 a,b | 80 a |
| M-CD4+ T | 2 × 106 cells/iv | 10 | 5.35 ± 2.47 a,b | 1.02 ± 2.49 a,b | 90 a |
| M-CD8+ T | 2 × 106 cells/iv | 10 | 6.64 ± 3.18 a | 3.35 ± 3.97 a | 60 |
| Group | Dosage | n | Lung (Log10 CFU/g) | Blood (Log10 CFU/mL) | Survival (%) |
|---|---|---|---|---|---|
| CON | - | 10 | 8.08 ± 2.56 | 5.84 ± 3.87 | 30 |
| TGC | 5 mg/kg/q12h/sc | 9 | 7.96 ± 2.56 | 4.61 ± 4.24 | 50 |
| SUL | 60 mg/kg/q6h/im | 10 | 5.19 ± 1.19 a,b,d | 1.19 ± 1.31 a | 90 a |
| M-B | 2 × 106 cells/iv | 10 | 3.63 ± 0.46 a,b,c,d | 0.67 ± 0.61 a | 100 a,b |
| M-CD4+ T | 2 × 106 cells/iv | 10 | 3.81 ± 0.81 a,b,c,d | 0.80 ± 0.92 a | 100 a,b |
| M-CD8+ T | 2 × 106 cells/iv | 10 | 7.67 ± 1.87 | 4.00 ± 3.52 | 70 |
| Antimicrobials | CS01 | CR17 | ||
|---|---|---|---|---|
| MIC (mg/L) | MBC (mg/L) | MIC (mg/L) | MBC (mg/L) | |
| Amikacin | 1 | 2 | 1 | 1 |
| Gentamycin | 2 | 2 | ≤0.50 | 0.50 |
| Meropenem | 64 | 128 | >256 | >256 |
| Ceftazidime | 64 | 128 | 64 | 128 |
| Cefepime | 32 | 64 | 8 | 32 |
| Sulbactam | 4 | 8 | 2 | 4 |
| Colistin | ≤0.50 | ≤0.50 | 64 | 128 |
| Ciprofloxacin | 32 | 32 | 16 | 128 |
| Tigecycline | ≤0.50 | 2 | 4 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cebrero-Cangueiro, T.; Herrera-Espejo, S.; Paniagua, M.; Labrador-Herrera, G.; Cisneros, J.M.; Pachón, J.; Pachón-Ibáñez, M.E. Could the Adoptive Transfer of Memory Lymphocytes be an Alternative Treatment for Acinetobacter baumannii Infections? Int. J. Mol. Sci. 2024, 25, 10550. https://doi.org/10.3390/ijms251910550
Cebrero-Cangueiro T, Herrera-Espejo S, Paniagua M, Labrador-Herrera G, Cisneros JM, Pachón J, Pachón-Ibáñez ME. Could the Adoptive Transfer of Memory Lymphocytes be an Alternative Treatment for Acinetobacter baumannii Infections? International Journal of Molecular Sciences. 2024; 25(19):10550. https://doi.org/10.3390/ijms251910550
Chicago/Turabian StyleCebrero-Cangueiro, Tania, Soraya Herrera-Espejo, María Paniagua, Gema Labrador-Herrera, José Miguel Cisneros, Jerónimo Pachón, and María Eugenia Pachón-Ibáñez. 2024. "Could the Adoptive Transfer of Memory Lymphocytes be an Alternative Treatment for Acinetobacter baumannii Infections?" International Journal of Molecular Sciences 25, no. 19: 10550. https://doi.org/10.3390/ijms251910550
APA StyleCebrero-Cangueiro, T., Herrera-Espejo, S., Paniagua, M., Labrador-Herrera, G., Cisneros, J. M., Pachón, J., & Pachón-Ibáñez, M. E. (2024). Could the Adoptive Transfer of Memory Lymphocytes be an Alternative Treatment for Acinetobacter baumannii Infections? International Journal of Molecular Sciences, 25(19), 10550. https://doi.org/10.3390/ijms251910550






