Identification of Genomic Regions Conferring Enhanced Zn and Fe Concentration in Wheat Varieties and Introgression Lines Derived from Wild Relatives
Abstract
:1. Introduction
2. Results
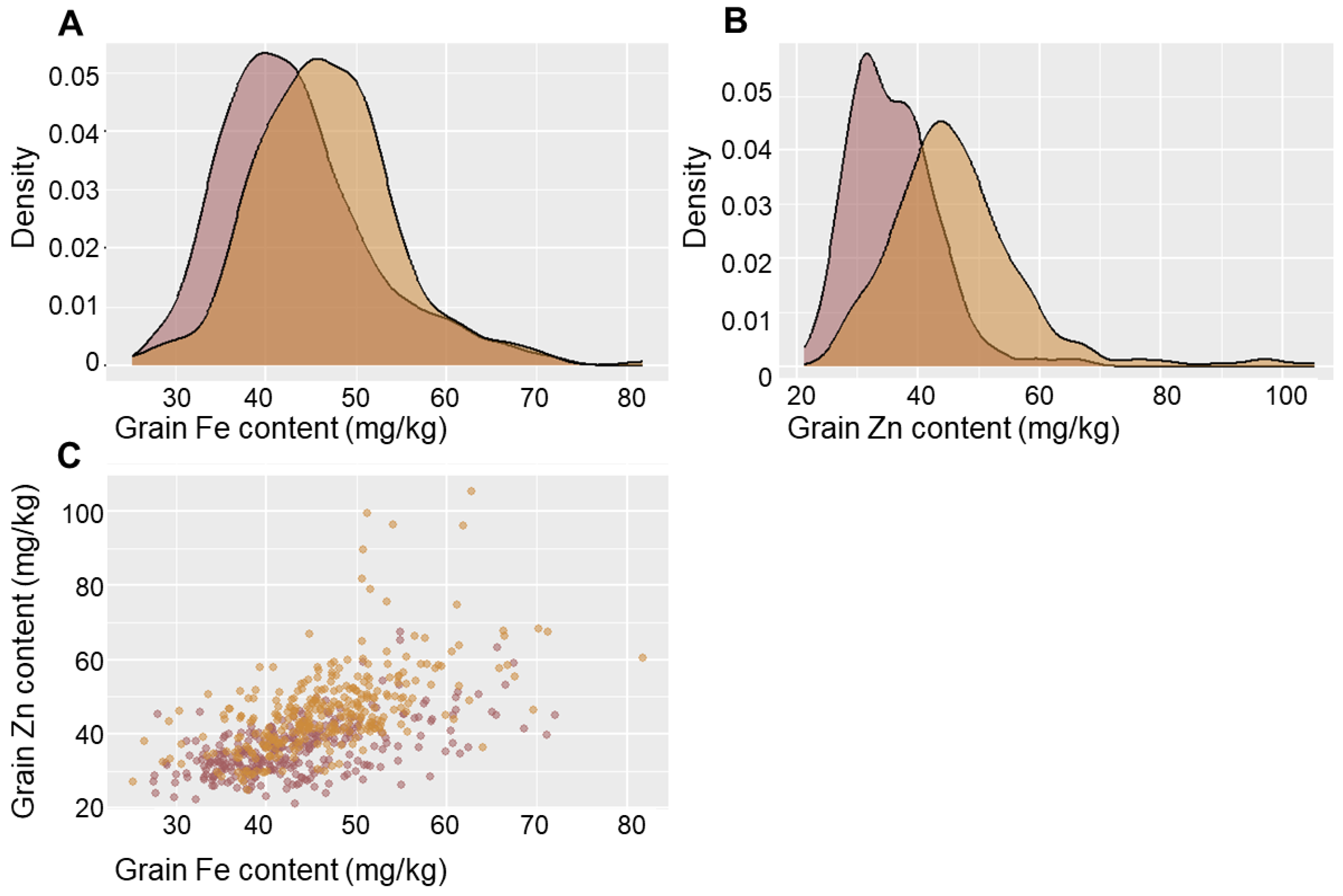
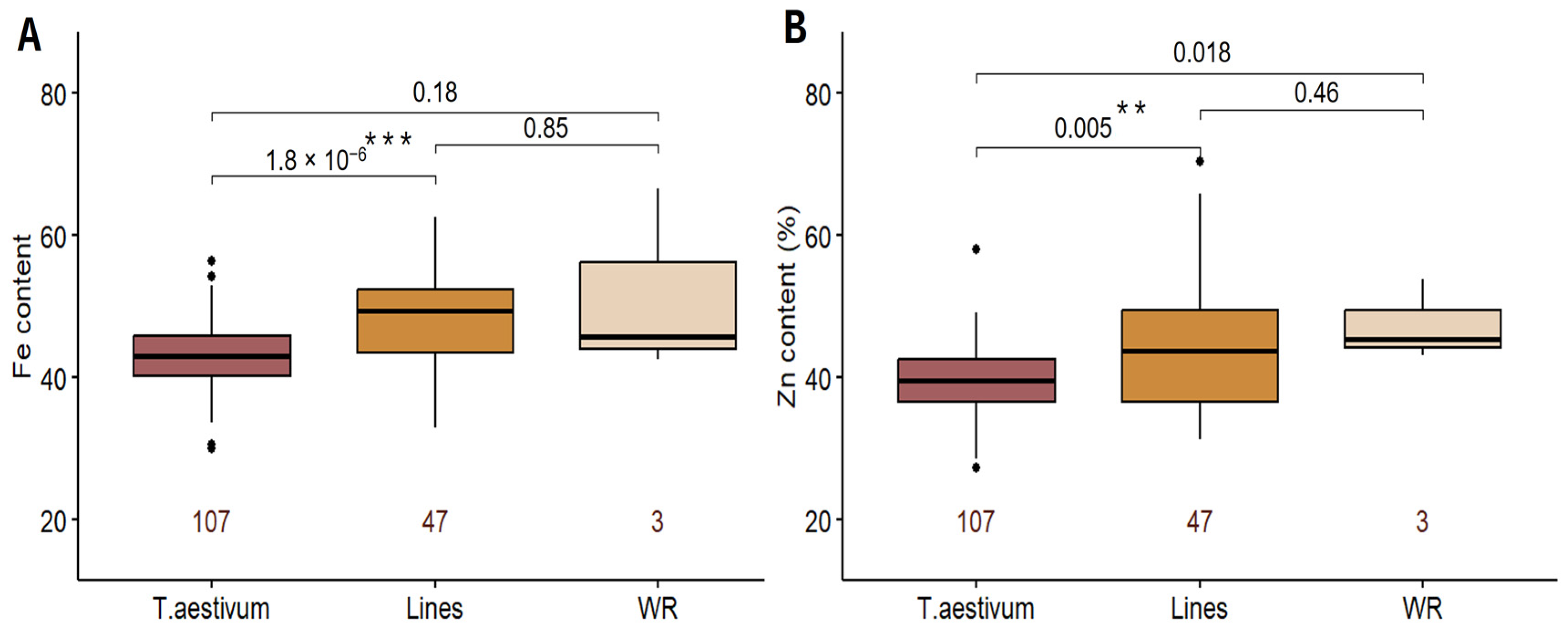
2.1. Phenotypic Variation in GFeC and GZnC
2.2. Results of GWAS
2.3. Significant MTAs Inherited from Wild Wheat Relatives
2.4. Candidate Genes
3. Discussion
3.1. Comparison of Putative QTL Localization with Known Data
3.2. Wheat Relatives as Sources of Potentially Valuable Loci for Increased Zinc and Iron Content in Grain
3.3. Candidate Genes
4. Materials and Methods
4.1. Plant Material Phenotyping
4.2. Statistical Analysis
4.3. Genotyping and Genome-Wide Association Study
4.4. Gene Annotation and Prioritization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO; IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World 2020. Transforming Food Systems for Affordable Healthy Diets; FAO: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Mertz, W. The Essential Trace Elements. Science 1981, 213, 1332–1338. [Google Scholar] [CrossRef]
- Islam, M.R.; Akash, S.; Jony, M.H.; Alam, M.N.; Nowrin, F.T.; Rahman, M.M.; Rauf, A.; Thiruvengadam, M. Exploring the Potential Function of Trace Elements in Human Health: A Therapeutic Perspective. Mol. Cell. Biochem. 2023, 478, 2141–2171. [Google Scholar] [CrossRef]
- Natekar, P.; Deshmukh, C.; Limaye, D.; Ramanathan, V.; Pawar, A. A Micro Review of a Nutritional Public Health Challenge: Iron Deficiency Anemia in India. Clin. Epidemiol. Glob. Health 2022, 14, 100992. [Google Scholar] [CrossRef]
- WHO. Guideline on Haemoglobin Cutoffs to Define Anaemia in Individuals and Populations; WHO: Geneva, Switzerland, 2024. [Google Scholar]
- Paes Leme Coutinho, G.G.; Goloni-Bertollo, E.M.; Pavarino Bertelli, É.C. Iron Deficiency Anemia in Children: A Challenge for Public Health and for Society. Sao Paulo Med. J. 2005, 123, 88–92. [Google Scholar] [CrossRef]
- Hambidge, M. Human Zinc Deficiency. J. Nutr. 2000, 130, 1344S–1349S. [Google Scholar] [CrossRef]
- Jomova, K.; Makova, M.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Rhodes, C.J.; Valko, M. Essential Metals in Health and Disease. Chem. Biol. Interact. 2022, 367, 110173. [Google Scholar] [CrossRef]
- Prashanth, L.; Kattapagari, K.; Chitturi, R.; Baddam, V.R.; Prasad, L. A Review on Role of Essential Trace Elements in Health and Disease. J. Dr. NTR Univ. Health Sci. 2015, 4, 75–85. [Google Scholar] [CrossRef]
- Shewry, P.R. Wheat. J. Exp. Bot. 2009, 60, 1537–1553. [Google Scholar] [CrossRef]
- O’Connor, A. An Overview of the Role of Bread in the Uk Diet. Nutr. Bull. 2012, 37, 193–212. [Google Scholar] [CrossRef]
- Tadesse, W.; Sanchez-Garcia, M.; Assefa, S.G.; Amri, A.; Bishaw, Z.; Ogbonnaya, F.C.; Baum, M. Genetic Gains in Wheat Breeding and Its Role in Feeding the World. Crop Breed. Genet. Genom. 2019, 1, e190005. [Google Scholar] [CrossRef]
- Aghalari, Z.; Dahms, H.U.; Sillanpää, M. Evaluation of Nutrients in Bread: A Systematic Review. J. Health Popul. Nutr. 2022, 41, 50. [Google Scholar] [CrossRef]
- Ram, S.; Velu, G. Improving Wheat Nutritional Quality through Biofortification. In Wheat Quality for Improving Processing and Human Health; Igrejas, G., Ikeda, T.M., Guzmán, C., Eds.; Springer International Publishing: Geneva, Switzerland, 2020; pp. 205–224. [Google Scholar] [CrossRef]
- Monasterio, I.; Graham, R.D. Breeding for Trace Minerals in Wheat. Food Nutr. Bull. 2000, 21, 392–396. [Google Scholar] [CrossRef]
- Cakmak, I.; Ozkan, H.; Braun, H.J.; Welch, R.M.; Romheld, V. Zinc and Iron Concentrations in Seeds of Wild, Primitive, and Modern Wheats. Food Nutr. Bull. 2000, 21, 401–403. [Google Scholar] [CrossRef]
- Velu, G.; Ortiz-Monasterio, I.; Cakmak, I.; Hao, Y.; Singh, R.P. Biofortification Strategies to Increase Grain Zinc and Iron Concentrations in Wheat. J. Cereal Sci. 2014, 59, 365–372. [Google Scholar] [CrossRef]
- Salantur, A.; Karaoğlu, C. Macro-Microelements in Wheat Landraces and Their Use in Breeding. In Wheat Landraces; Zencirci, N., Baloch, F.S., Habyarimana, E., Chung, G., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 83–92. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, B.; Chen, W.; Liu, B.; Zhang, L.; Zhang, H.; Liu, D. Comparison of Zinc, Iron, and Selenium Accumulation between Synthetic Hexaploid Wheat and Its Tetraploid and Diploid Parents. Can. J. Plant Sci. 2017, 97, 692–701. [Google Scholar] [CrossRef]
- Gupta, P.K.; Balyan, H.S.; Sharma, S.; Kumar, R. Biofortification and Bioavailability of Zn, Fe and Se in Wheat: Present Status and Future Prospects. Theor. Appl. Genet. 2021, 134, 1–35. [Google Scholar] [CrossRef]
- Zeibig, F.; Kilian, B.; Frei, M. The Grain Quality of Wheat Wild Relatives in the Evolutionary Context. Theor. Appl. Genet. 2022, 135, 4029–4048. [Google Scholar] [CrossRef]
- Peleg, Z.; Saranga, Y.; Yazici, A.; Fahima, T.; Ozturk, L.; Cakmak, I. Grain Zinc, Iron and Protein Concentrations and Zinc-Efficiency in Wild Emmer Wheat under Contrasting Irrigation Regimes. Plant Soil 2008, 306, 57–67. [Google Scholar] [CrossRef]
- Chatzav, M.; Peleg, Z.; Ozturk, L.; Yazici, A.; Fahima, T.; Cakmak, I.; Saranga, Y. Genetic Diversity for Grain Nutrients in Wild Emmer Wheat: Potential for Wheat Improvement. Ann. Bot. 2010, 105, 1211–1220. [Google Scholar] [CrossRef]
- Tiwari, V.K.; Rawat, N.; Chhuneja, P.; Neelam, K.; Aggarwal, R.; Randhawa, G.S.; Dhaliwal, H.S.; Keller, B.; Singh, K. Mapping of Quantitative Trait Loci for Grain Iron and Zinc Concentration in Diploid a Genome Wheat. J. Hered. 2009, 100, 771–776. [Google Scholar] [CrossRef]
- Xu, Y.; An, D.; Li, H.; Xu, H. Review: Breeding Wheat for Enhanced Micronutrients. Can. J. Plant Sci. 2011, 91, 231–237. [Google Scholar] [CrossRef]
- Alvarez, J.B.; Guzmán, C. Interspecific and Intergeneric Hybridization as a Source of Variation for Wheat Grain Quality Improvement. Theor. Appl. Genet. 2018, 131, 225–251. [Google Scholar] [CrossRef]
- Wang, S.; Yin, L.; Tanaka, H.; Tanaka, K.; Tsujimoto, H. Wheat-Aegilops Chromosome Addition Lines Showing High Iron and Zinc Contents in Grains. Breed. Sci. 2011, 61, 189–195. [Google Scholar] [CrossRef]
- Farkas, A.; Molnár, I.; Dulai, S.; Rapi, S.; Oldal, V.; Cseh, A.; Kruppa, K.; Molnár-Láng, M. Increased Micronutrient Content (Zn, Mn) in the 3Mb(4B) Wheat-Aegilops biuncialis Substitution and 3Mb.4BS Translocation Identified by GISH and FISH. Genome 2014, 57, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Ardalani, S.; Mirzaghaderi, G.; Badakhshan, H. A Robertsonian Translocation from Thinopyrum bessarabicum into Bread Wheat Confers High Iron and Zinc Contents. Plant Breed. 2016, 135, 286–290. [Google Scholar] [CrossRef]
- Savin, T.V.; Abugaliyeva, A.I.; Cakmak, I.; Kozhakhmetov, K. Mineral Composition of Wild Relatives and Introgressive Forms in Wheat Selection. Vavilov J. Genet. Breed. 2018, 22, 88–96. [Google Scholar] [CrossRef]
- Bieńkowska, T.; Suchowilska, E.; Kandler, W.; Krska, R.; Wiwart, M. Triticum polonicum L. as Potential Source Material for the Biofortification of Wheat with Essential Micronutrients. Plant Genet. Resour. Characterisation Util. 2019, 17, 213–220. [Google Scholar] [CrossRef]
- Palombieri, S.; Bonarrigo, M.; Cammerata, A.; Quagliata, G.; Astolfi, S.; Lafiandra, D.; Sestili, F.; Masci, S. Characterization of Triticum turgidum sspp. durum, turanicum, and polonicum Grown in Central Italy in Relation to Technological and Nutritional Aspects. Front. Plant Sci. 2023, 14, 1269212. [Google Scholar] [CrossRef]
- Crespo-Herrera, L.A.; Govindan, V.; Stangoulis, J.; Hao, Y.; Singh, R.P. QTL Mapping of Grain Zn and Fe Concentrations in Two Hexaploid Wheat RIL Populations with Ample Transgressive Segregation. Front. Plant Sci. 2017, 8, 01800. [Google Scholar] [CrossRef]
- Velu, G.; Singh, R.P.; Crespo-Herrera, L.; Juliana, P.; Dreisigacker, S.; Valluru, R.; Stangoulis, J.; Sohu, V.S.; Mavi, G.S.; Mishra, V.K.; et al. Genetic Dissection of Grain Zinc Concentration in Spring Wheat for Mainstreaming Biofortification in CIMMYT Wheat Breeding. Sci. Rep. 2018, 8, 13526. [Google Scholar] [CrossRef] [PubMed]
- Alomari, D.Z.; Eggert, K.; Von Wirén, N.; Polley, A.; Plieske, J.; Ganal, M.W.; Liu, F.; Pillen, K.; Röder, M.S. Whole-Genome Association Mapping and Genomic Prediction for Iron Concentration in Wheat Grains. Int. J. Mol. Sci. 2019, 20, 76. [Google Scholar] [CrossRef]
- Rathan, N.D.; Krishna, H.; Ellur, R.K.; Sehgal, D.; Govindan, V.; Ahlawat, A.K.; Krishnappa, G.; Jaiswal, J.P.; Singh, J.B.; Sv, S.; et al. Genome-Wide Association Study Identifies Loci and Candidate Genes for Grain Micronutrients and Quality Traits in Wheat (Triticum aestivum L.). Sci. Rep. 2022, 12, 7037. [Google Scholar] [CrossRef]
- Tadesse, W.; Gataa, Z.E.; Rachdad, F.E.; Baouchi, A.E.; Kehel, Z.; Alemu, A. Single- and Multi-Trait Genomic Prediction and Genome-Wide Association Analysis of Grain Yield and Micronutrient-Related Traits in ICARDA Wheat under Drought Environment. Mol. Genet. Genom. 2023, 298, 1515–1526. [Google Scholar] [CrossRef] [PubMed]
- Juliana, P.; Govindan, V.; Crespo-Herrera, L.; Mondal, S.; Huerta-Espino, J.; Shrestha, S.; Poland, J.; Singh, R.P. Genome-Wide Association Mapping Identifies Key Genomic Regions for Grain Zinc and Iron Biofortification in Bread Wheat. Front. Plant Sci. 2022, 13, 903819. [Google Scholar] [CrossRef] [PubMed]
- Bhatta, M.; Stephen Baenziger, P.; Waters, B.M.; Poudel, R.; Belamkar, V.; Poland, J.; Morgounov, A. Genome-Wide Association Study Reveals Novel Genomic Regions Associated with 10 Grain Minerals in Synthetic Hexaploid Wheat. Int. J. Mol. Sci. 2018, 19, 3237. [Google Scholar] [CrossRef] [PubMed]
- Baranwal, D.; Cu, S.; Stangoulis, J.; Trethowan, R.; Bariana, H.; Bansal, U. Identification of Genomic Regions Conferring Rust Resistance and Enhanced Mineral Accumulation in a HarvestPlus Association Mapping Panel of Wheat. Theor. Appl. Genet. 2022, 135, 865–882. [Google Scholar] [CrossRef] [PubMed]
- Peleg, Z.; Cakmak, I.; Ozturk, L.; Yazici, A.; Jun, Y.; Budak, H.; Korol, A.B.; Fahima, T.; Saranga, Y. Quantitative Trait Loci Conferring Grain Mineral Nutrient Concentrations in Durum Wheat × Wild Emmer Wheat RIL Population. Theor. Appl. Genet. 2009, 119, 353–369. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Sharma, P.; Kumar, J.; Singh, V.K.; Vasistha, N.K.; Gahlaut, V.; Tyagi, V.; Verma, S.K.; Singh, S.; Dhaliwal, H.S.; et al. Genetic Analysis of Iron, Zinc and Grain Yield in Wheat-Aegilops Derivatives Using Multi-Locus GWAS. Mol. Biol. Rep. 2023, 50, 9191–9202. [Google Scholar] [CrossRef]
- Hu, X.G.; Liu, J.; Zhang, L.; Wu, B.H.; Hu, J.L.; Liu, D.C.; Zheng, Y.L. Zn and Fe Concentration Variations of Grain and Flag Leaf and the Relationship with NAM-G1 Gene in Triticum timopheevii (Zhuk.) Zhuk. ssp. timopheevii. Cereal Res. Commun. 2017, 45, 421–431. [Google Scholar] [CrossRef]
- Zeibig, F.; Kilian, B.; Özkan, H.; Pantha, S.; Frei, M. Grain Quality Traits within the Wheat (Triticum spp.) Genepool: Prospects for Improved Nutrition through de Novo Domestication. J. Sci. Food Agric. 2024, 104, 4400–4410. [Google Scholar] [CrossRef]
- Leonova, I.N.; Kiseleva, A.A.; Berezhnaya, A.A.; Orlovskaya, O.A.; Salina, E.A. Novel Genetic Loci from Triticum timopheevii Associated with Gluten Content Revealed by GWAS in Wheat Breeding Lines. Int. J. Mol. Sci. 2023, 24, 3304. [Google Scholar] [CrossRef]
- Yao, E.; Blake, V.C.; Cooper, L.; Wight, C.P.; Michel, S.; Cagirici, H.B.; Lazo, G.R.; Birkett, C.L.; Waring, D.J.; Jannink, J.-L.; et al. GrainGenes: A Data-Rich Repository for Small Grains Genetics and Genomics. Database 2022, 2022, baac034. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wong, D.; Forrest, K.; Allen, A.; Chao, S.; Huang, B.E.; Maccaferri, M.; Salvi, S.; Milner, S.G.; Cattivelli, L.; et al. Characterization of Polyploid Wheat Genomic Diversity Using a High-density 90,000 Single Nucleotide Polymorphism Array. Plant Biotechnol. J. 2014, 12, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Hassani-Pak, K.; Singh, A.; Brandizi, M.; Hearnshaw, J.; Parsons, J.D.; Amberkar, S.; Phillips, A.L.; Doonan, J.H.; Rawlings, C. KnetMiner: A Comprehensive Approach for Supporting Evidence-Based Gene Discovery and Complex Trait Analysis across Species. Plant Biotechnol. J. 2021, 19, 1670–1678. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Saripalli, G.; Gahlaut, V.; Goel, N.; Meher, P.K.; Mishra, K.K.; Mishra, P.C.; Sehgal, D.; Vikram, P.; Sansaloni, C.; et al. Genetics of Fe, Zn, β-Carotene, GPC and Yield Traits in Bread Wheat (Triticum aestivum L.) Using Multi-Locus and Multi-Traits GWAS. Euphytica 2018, 214, 219. [Google Scholar] [CrossRef]
- Arora, S.; Cheema, J.; Poland, J.; Uauy, C.; Chhuneja, P. Genome-Wide Association Mapping of Grain Micronutrients Concentration in Aegilops tauschii. Front. Plant Sci. 2019, 10, 00054. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Zhao, C.; Sun, M.; Fu, L.; Song, J.; Liu, D.; Zhang, Y.; Zheng, J.; Pu, Z.; Liu, L.; et al. High Resolution Genome Wide Association Studies Reveal Rich Genetic Architectures of Grain Zinc and Iron in Common Wheat (Triticum aestivum L.). Front. Plant Sci. 2022, 13, 840614. [Google Scholar] [CrossRef]
- Ma, J.; Wang, Y.; Li, S.; Liu, Q.; Yuan, M.; Li, C.; Zhang, D.; Zeng, Q.; Wu, J.; Song, C.; et al. Genome-Wide Association Study Revealed the Reason for the Decrease in Grain Iron Concentration during Wheat Breeding Process in China. Field Crops Res. 2024, 309, 109326. [Google Scholar] [CrossRef]
- Wang, J.; Shi, X.; Zhou, Z.; Qin, M.; Wang, Y.; Li, W.; Yang, P.; Wu, Z.; Lei, Z. Genetic Dissection of Grain Iron Concentration in Hexaploid Wheat (Triticum aestivum L.) Using a Genome-Wide Association Analysis Method. PeerJ 2022, 10, e13625. [Google Scholar] [CrossRef] [PubMed]
- Potapova, N.A.; Timoshchuk, A.N.; Tiys, E.S.; Vinichenko, N.A.; Leonova, I.N.; Salina, E.A.; Tsepilov, Y.A. Multivariate Genome-Wide Association Study of Concentrations of Seven Elements in Seeds Reveals Four New Loci in Russian Wheat Lines. Plants 2023, 12, 3019. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, I.; Torun, A.; Özkan, H.; Millet, E.; Feldman, M.; Fahima, T.; Korol, A.; Nevo, E.; Braun, H.J. Triticum Dicoccoides: An Important Genetic Resource for Increasing Zinc and Iron Concentration in Modern Cultivated Wheat. Soil Sci. Plant Nutr. 2004, 50, 1047–1054. [Google Scholar] [CrossRef]
- Biel, W.; Jaroszewska, A.; Stankowski, S.; Sobolewska, M.; Kępińska-Pacelik, J. Comparison of Yield, Chemical Composition and Farinograph Properties of Common and Ancient Wheat Grains. Eur. Food Res. Technol. 2021, 247, 1525–1538. [Google Scholar] [CrossRef]
- Crespo-Herrera, L.A.; Velu, G.; Singh, R.P. Quantitative Trait Loci Mapping Reveals Pleiotropic Effect for Grain Iron and Zinc Concentrations in Wheat. Ann. Appl. Biol. 2016, 169, 27–35. [Google Scholar] [CrossRef]
- Liu, J.; Huang, L.; Li, T.; Liu, Y.; Yan, Z.; Tang, G.; Zheng, Y.; Liu, D.; Wu, B. Genome-Wide Association Study for Grain Micronutrient Concentrations in Wheat Advanced Lines Derived From Wild Emmer. Front. Plant Sci. 2021, 12, 651283. [Google Scholar] [CrossRef] [PubMed]
- Morgounov, A.; Li, H.; Shepelev, S.; Ali, M.; Flis, P.; Koksel, H.; Savin, T.; Shamanin, V. Genetic Characterization of Spring Wheat Germplasm for Macro-, Microelements and Trace Metals. Plants 2022, 11, 2173. [Google Scholar] [CrossRef]
- Shamanin, V.P.; Flis, P.; Savin, T.V.; Shepelev, S.S.; Kuzmin, O.G.; Chursin, A.S.; Pototskaya, I.V.; Likhenko, I.E.; Kushnirenko, I.Y.; Kazak, A.A.; et al. Genotypic and Ecological Variability of Zinc Content in the Grain of Spring Bread Wheat Varieties in the International Nursery KASIB. Vavilov J. Genet. Breed. 2021, 25, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Morgounov, A.; Gómez-Becerra, H.F.; Abugalieva, A.; Dzhunusova, M.; Yessimbekova, M.; Muminjanov, H.; Zelenskiy, Y.; Ozturk, L.; Cakmak, I. Iron and Zinc Grain Density in Common Wheat Grown in Central Asia. Euphytica 2007, 155, 193–203. [Google Scholar] [CrossRef]
- Kumari, K.; Kumar, P.; Sharma, V.K.; Singh, S.K. Genomic Marker Assisted Identification of Genetic Loci and Genes Associated with Variation of Grain Zinc Concentration in Rice. J. Genet. 2019, 98, 111. [Google Scholar] [CrossRef]
- Tanin, M.J.; Saini, D.K.; Kumar, P.; Gudi, S.; Sharma, H.; Kaur, J.P.; Abassy, O.; Bromand, F.; Sharma, A. Iron Biofortification in Wheat: Past, Present, and Future. Curr. Plant Biol. 2024, 38, 100328. [Google Scholar] [CrossRef]
- Kobayashi, T.; Nakanishi, H.; Nishizawa, N.K. Recent Insights into Iron Homeostasis and Their Application in Graminaceous Crops. Proc. Jpn. Acad. Ser. B 2010, 86, 900–913. [Google Scholar] [CrossRef]
- Amini, S.; Arsova, B.; Hanikenne, M. The Molecular Basis of Zinc Homeostasis in Cereals. Plant. Cell Environ. 2022, 45, 1339–1361. [Google Scholar] [CrossRef] [PubMed]
- Ishimaru, Y.; Masuda, H.; Suzuki, M.; Bashir, K.; Takahashi, M.; Nakanishi, H.; Mori, S.; Nishizawa, N.K. Overexpression of the OsZIP4 Zinc Transporter Confers Disarrangement of Zinc Distribution in Rice Plants. J. Exp. Bot. 2007, 58, 2909–2915. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, S.A.; Shin, R.; Eide, D.J.; Schachtman, D.P. Differential Metal Selectivity and Gene Expression of Two Zinc Transporters from Rice. Plant Physiol. 2003, 133, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.R.; Feng, Y.; Chao, Y.E. Genomic Analysis and Expression Pattern of OsZIP1, OsZIP3, and OsZIP4 in Two Rice (Oryza sativa L.) Genotypes with Different Zinc Efficiency. Russ. J. Plant Physiol. 2008, 55, 400–409. [Google Scholar] [CrossRef]
- Yang, X.; Huang, J.; Jiang, Y.; Zhang, H.S. Cloning and Functional Identification of Two Members of the ZIP (Zrt, Irt-like Protein) Gene Family in Rice (Oryza sativa L.). Mol. Biol. Rep. 2009, 36, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Connolly, E.L. Mitochondrial Iron Transport and Homeostasis in Plants. Front. Plant Sci. 2013, 4, 348. [Google Scholar] [CrossRef]
- Morrissey, J.; Baxter, I.R.; Lee, J.; Li, L.; Lahner, B.; Grotz, N.; Kaplan, J.; Salt, D.E.; Guerinot, M. Lou The Ferroportin Metal Efflux Proteins Function in Iron and Cobalt Homeostasis in Arabidopsis. Plant Cell 2009, 21, 3326–3338. [Google Scholar] [CrossRef] [PubMed]
- Kan, M.; Fujiwara, T.; Kamiya, T. Golgi-Localized OsFPN1 Is Involved in Co and Ni Transport and Their Detoxification in Rice. Rice 2022, 15, 36. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Kawakami, Y.; Bhullar, N.K. Molecular Analysis of Iron Deficiency Response in Hexaploid Wheat. Front. Sustain. Food Syst. 2019, 3, 67. [Google Scholar] [CrossRef]
- Senoura, T.; Sakashita, E.; Kobayashi, T.; Takahashi, M.; Aung, M.S.; Masuda, H.; Nakanishi, H.; Nishizawa, N.K. The Iron-Chelate Transporter OsYSL9 Plays a Role in Iron Distribution in Developing Rice Grains. Plant Mol. Biol. 2017, 95, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Plaschke, J.; Ganal, M.W.; Röder, M.S. Detection of Genetic Diversity in Closely Related Bread Wheat Using Microsatellite Markers. Theor. Appl. Genet. 1995, 91, 1001–1007. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Z. GAPIT Version 3: Boosting Power and Accuracy for Genomic Association and Prediction. Genom. Proteom. Bioinform. 2021, 19, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Zhang, H.; Tang, Z.; Xu, J.; Yin, D.; Zhang, Z.; Yuan, X.; Zhu, M.; Zhao, S.; Li, X.; et al. RMVP: A Memory-Efficient, Visualization-Enhanced, and Parallel-Accelerated Tool for Genome-Wide Association Study. Genom. Proteom. Bioinform. 2021, 19, 619–628. [Google Scholar] [CrossRef]
- Warnes, G.; Gorjanc, G.; Leisch, F.; Man, M. Genetics: Population Genetics. The Comprehensive R Archive Network. Available online: https://cran.r-project.org/web/packages/genetics/ (accessed on 22 August 2024).
- Shin, J.-H.; Blay, S.; Graham, J.; McNeney, B. LDheatmap: An R Function for Graphical Display of Pairwise Linkage Disequilibria between Single Nucleotide Polymorphisms. J. Stat. Softw. 2006, 16, 1–9. [Google Scholar] [CrossRef]
- Persephone. Available online: https://persephonesoft.com (accessed on 22 August 2024).
- Tian, T.; Liu, Y.; Yan, H.; You, Q.; Yi, X.; Du, Z.; Xu, W.; Su, Z. AgriGO v2.0: A GO Analysis Toolkit for the Agricultural Community, 2017 Update. Nucleic Acids Res. 2017, 45, W122–W129. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-González, R.H.; Borrill, P.; Lang, D.; Harrington, S.A.; Brinton, J.; Venturini, L.; Davey, M.; Jacobs, J.; Van Ex, F.; Pasha, A.; et al. The transcriptional landscape of polyploid wheat. Science 2018, 361, eaar6089. [Google Scholar] [CrossRef] [PubMed]
- Borrill, P.; Ramirez-Gonzalez, R.; Uauy, C. ExpVIP: A customizable RNA-seq data analysis and visualization platform. Plant Physiol. 2016, 170, 2172–2186. [Google Scholar] [CrossRef]

| SNP | Chr | QTL | Position RefSeq 1.1, bp | Position RefSeq 2.1, bp | Genetic Position, cM | p-Value | Allele | Effect | Allele Source |
|---|---|---|---|---|---|---|---|---|---|
| BS00025736_51 | 1B | QZn.icg-1B.1 | 39,866,100 | 44,233,164 | 56.65 | 2.7 × 10−4 | G | Zn + 4.95 | T.timopheevii, T. kiharae, T.dicoccum |
| Kukri_rep_c100936_449 | 40,199,636 | 44,565,803 | 56.88 | 1.8 × 10−4 | T | Zn + 5.29 | T. timopheevii, T. kiharae, T.dicoccum | ||
| GENE-0427_442 | 40,942,799 | 45,304,682 | 56.88 | 1.3 × 10−4 | A | Zn + 5.45 | T. timopheevii, T. kiharae, T.dicoccum | ||
| Excalibur_c17202_1833 RAC875_c826_839 | 1B | QZn.icg-1B.2 | 58,324,982 59,599,775 | 63,893,763 65,164,043 | 57.59 57.59 | 3.2 × 10−4 2.2 × 10−4 | G G | Zn − 2.76 Zn − 2.89 | T. timopheevii, T. kiharae T. timopheevii, T. kiharae |
| Kukri_c44442_274 | 2A | QZn.icg-2A | 123,548,354 | 128,154,513 | 102.43 | 3.6 × 10−9 | C | Zn + 4.05 | T. timopheevii, T. kiharae, T. dicoccum |
| Kukri_c441_891 | 126,655,033 | 131,241,681 | 102.47 | 3.8 × 10−4 | G | Zn + 5.07 Fe + 3.27 | T. timopheevii, T. kiharae | ||
| TA001792-1026 | 141,166,705 | 145,801,217 | 103.62 | 4.3 × 10−4 | G | Fe + 3.63 | T. timopheevii, T. kiharae | ||
| CAP12_s9114_61 | 2B | QZn.icg-2B | 767,132,274 | 776,298,229 | 134.46 | 3 × 10−4 | T | Fe + 4.61 | T. timopheevii, T. kiharae |
| BobWhite_c5191_362 | 767,169,885 | 776,335,465 | 134.46 | 3 × 10−4 | T | Fe + 4.60 | T. timopheevii, T. kiharae | ||
| BobWhite_c5191_563 | 767,170,156 | 776,336,169 | 134.46 | 6.6 × 10−5 | T | Zn + 2.61 Fe + 5.14 | T. timopheevii, T. kiharae | ||
| BS00046601_51 | 767,375,331 | 776,541,318 | 134.46 | 2.9 × 10−4 | C | Fe + 4.52 | T. timopheevii, T. kiharae | ||
| Excalibur_c14859_394 | 1B | 588,766,841 | 595,748,769 | 1A: 102.92 | 5.2 × 10−8 | G | Zn + 4.58 | T. timopheevii, T. kiharae | |
| Ku_c6546_718 | 2B | 206,752,135 | 214,819,365 | 97.54 | 5.6 × 10−4 | T | Zn + 3.21 | T. timopheevii, T. kiharae, T. dicoccum | |
| Tdurum_contig64416_441 | 2B | 530,046,526 | 538,048,972 | 102.23 | 8.2 × 10−8 | A | Zn − 2.07 | T. timopheevii, T. kiharae | |
| BS00067150_51 | 5A | 609,243,940 | 611,013,091 | 98.72 | 2.1 × 10−7 | A | Zn + 4.08 | T. timopheevii, T. kiharae, T. dicoccum | |
| Excalibur_c82693_359 | 5B | 604,022,712 | 607,850,798 | unmap | 3.9 × 10−5 | T | Zn − 3.83 | T. timopheevii, T. kiharae | |
| BS00091302_51 | 5B | 695,491,227 | 7B:67,683,834 | 58.88 | 3.2 × 10−6 | C | Zn + 4.28 | T. timopheevii, T. kiharae, T. dicoccum | |
| RAC875_c703_2551 | 5D | 555,048,619 | 558,933,322 | 202.99 | 7 × 10−4 | T | Fe + 5.18 | T. timopheevii, T. kiharae, T. dicoccum |
| QTL | Chr | Gene ID IWGSC RefSeq v2.2 | Gene ID IWGSC RefSeq v1.1 | Description |
|---|---|---|---|---|
| QZn.icg-1A.1 | Chr1A | TraesCS1A03G0312000 | TraesCS1A02G125500 | Zinc/iron permease |
| QZn.icg-1A.1 | Chr1A | TraesCS1A03G0341000 | TraesCS1A02G133400 | ZIP metal ion transporter family |
| QFe.icg-2B.2 | Chr2B | TraesCS2B03G1038000 | TraesCS2B02G407900 | Metal-Nicotianamine Transporter YSL2 |
| QFe.icg-2B.2 | Chr2B | TraesCS2B03G1038100 | TraesCS2B02G408000 | Metal-Nicotianamine Transporter YSL2 |
| QFe.icg-2B.2 | Chr2B | TraesCS2B03G1040500 | TraesCS2B02G408500 | Metal-Nicotianamine Transporter YSL2 |
| QZn.icg-4A.1 | Chr4A | TraesCS4A03G0233800 | TraesCS4A02G113800 | Mitochondrial iron transporter |
| QFe.icg-5B.1 | Chr5B | TraesCS5B03G0236100 | TraesCS5B02G093600 | Ferroportin1 (FPN1), iron-regulated protein 3 |
| QFe.icg-7A | Chr7A | TraesCS7A03G0836800 | TraesCS7A02G340000 | ZIP Zinc transporter |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leonova, I.N.; Kiseleva, A.A.; Salina, E.A. Identification of Genomic Regions Conferring Enhanced Zn and Fe Concentration in Wheat Varieties and Introgression Lines Derived from Wild Relatives. Int. J. Mol. Sci. 2024, 25, 10556. https://doi.org/10.3390/ijms251910556
Leonova IN, Kiseleva AA, Salina EA. Identification of Genomic Regions Conferring Enhanced Zn and Fe Concentration in Wheat Varieties and Introgression Lines Derived from Wild Relatives. International Journal of Molecular Sciences. 2024; 25(19):10556. https://doi.org/10.3390/ijms251910556
Chicago/Turabian StyleLeonova, Irina N., Antonina A. Kiseleva, and Elena A. Salina. 2024. "Identification of Genomic Regions Conferring Enhanced Zn and Fe Concentration in Wheat Varieties and Introgression Lines Derived from Wild Relatives" International Journal of Molecular Sciences 25, no. 19: 10556. https://doi.org/10.3390/ijms251910556








