Genome-Wide Identification, Phylogenetic, and Expression Analysis of Jasmonate ZIM-Domain Gene Family in Medicago Sativa L.
Abstract
:1. Introduction
2. Results
2.1. Identification of JAZ Genes in the Alfalfa Genome
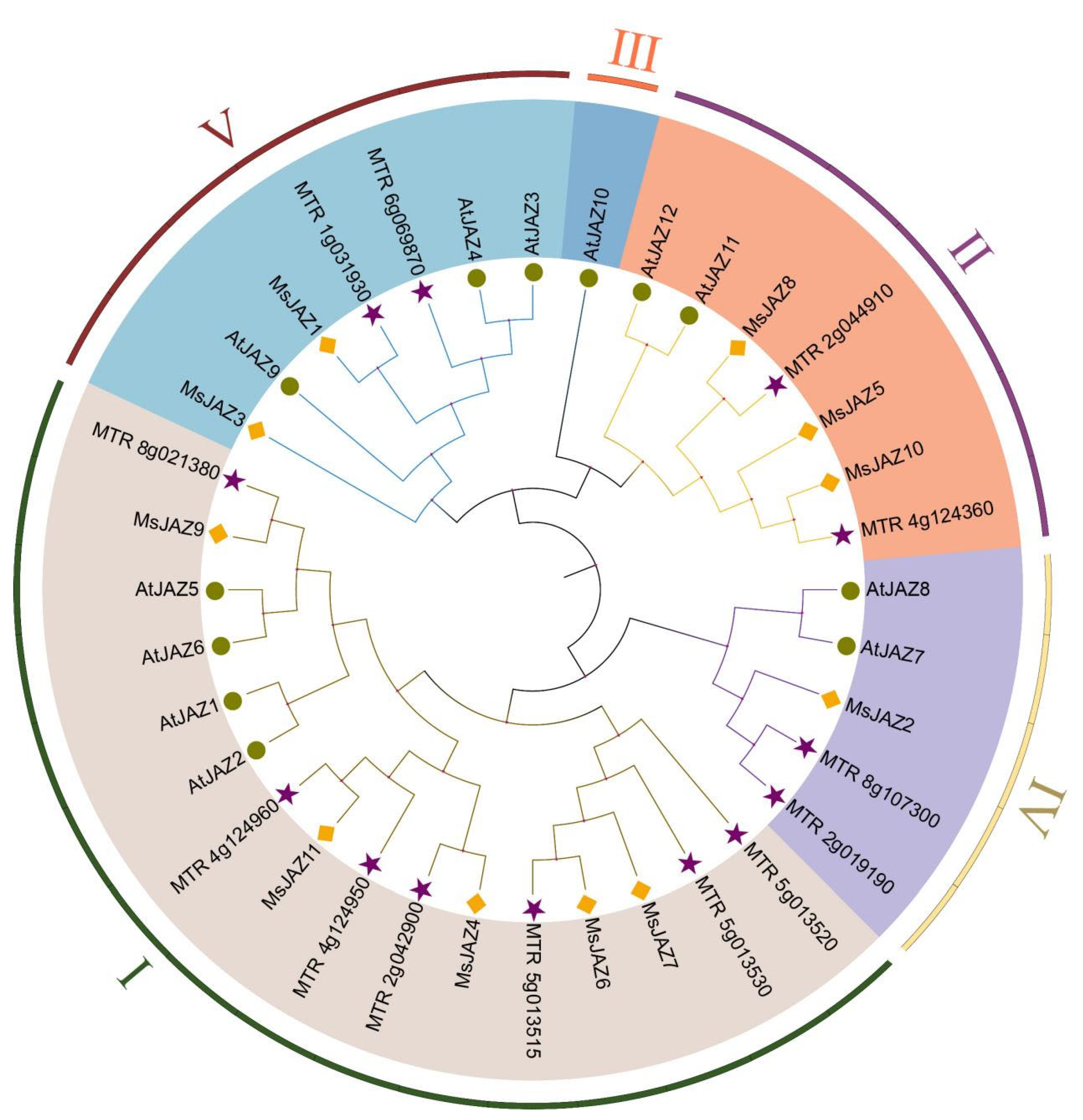
2.2. Phylogenetic Relationships Analysis of MsJAZs
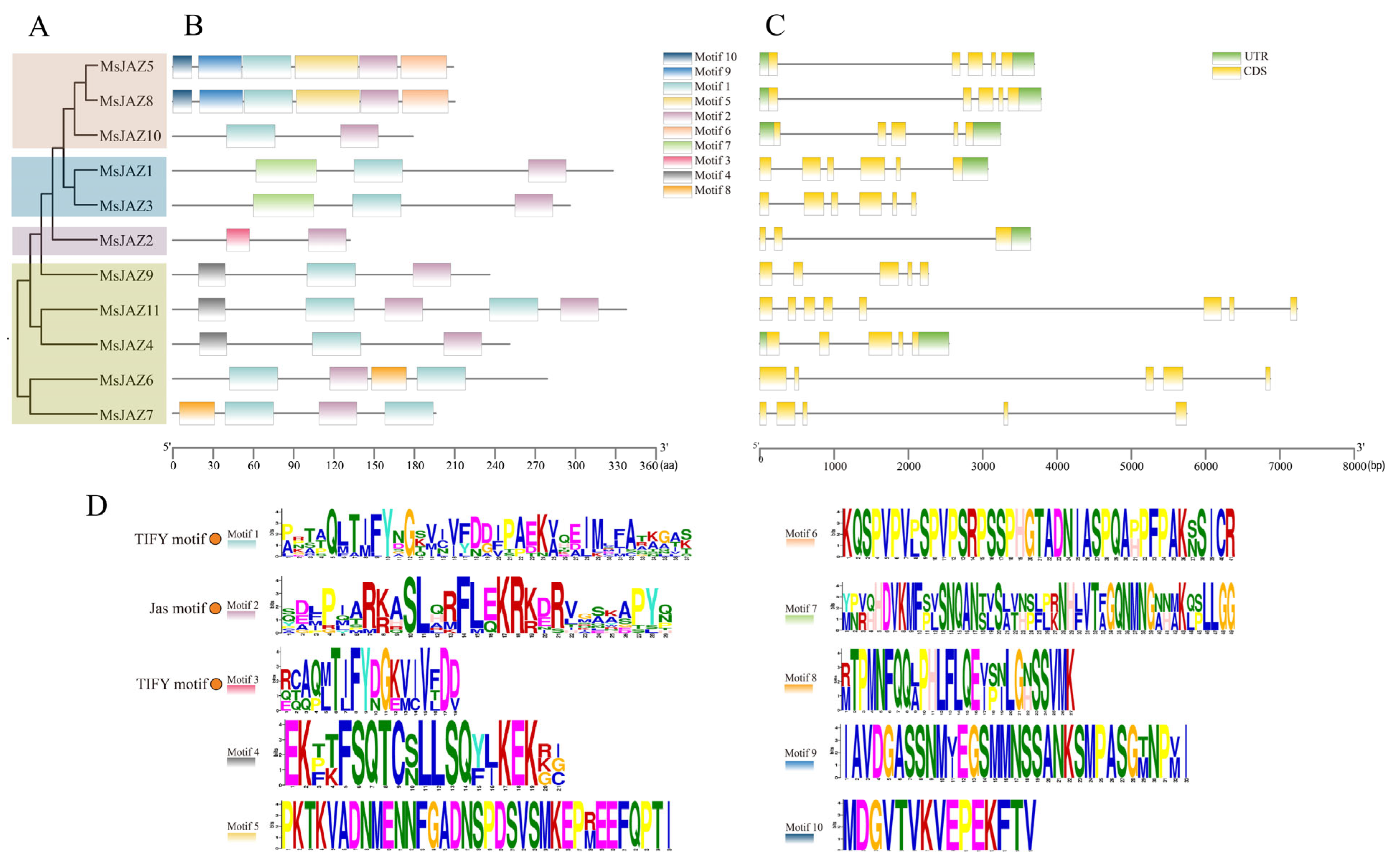
2.3. Gene Structure and Conserved Motif Analysis
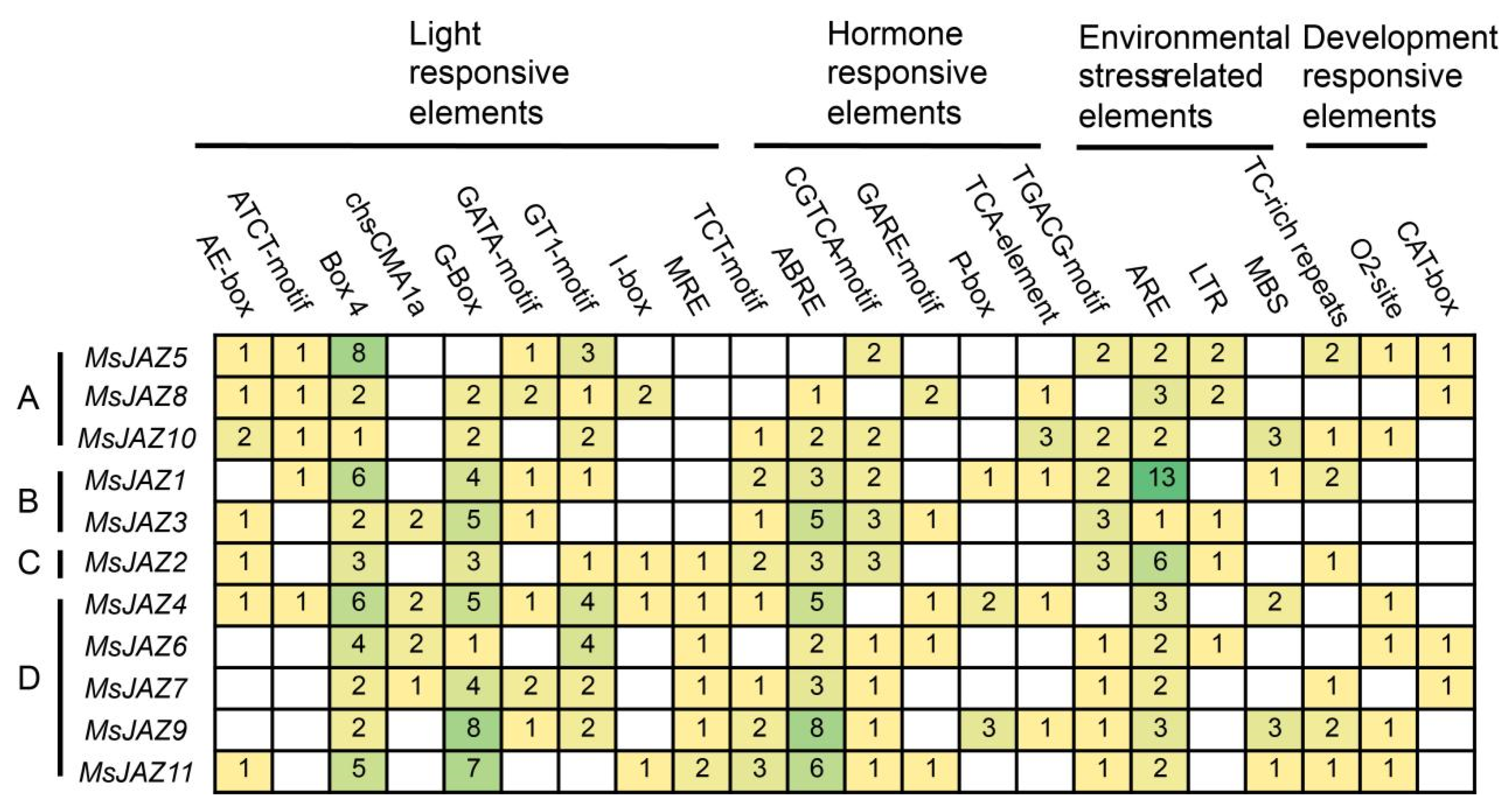
2.4. Prediction of Cis-Acting Elements in MsJAZs Promoters
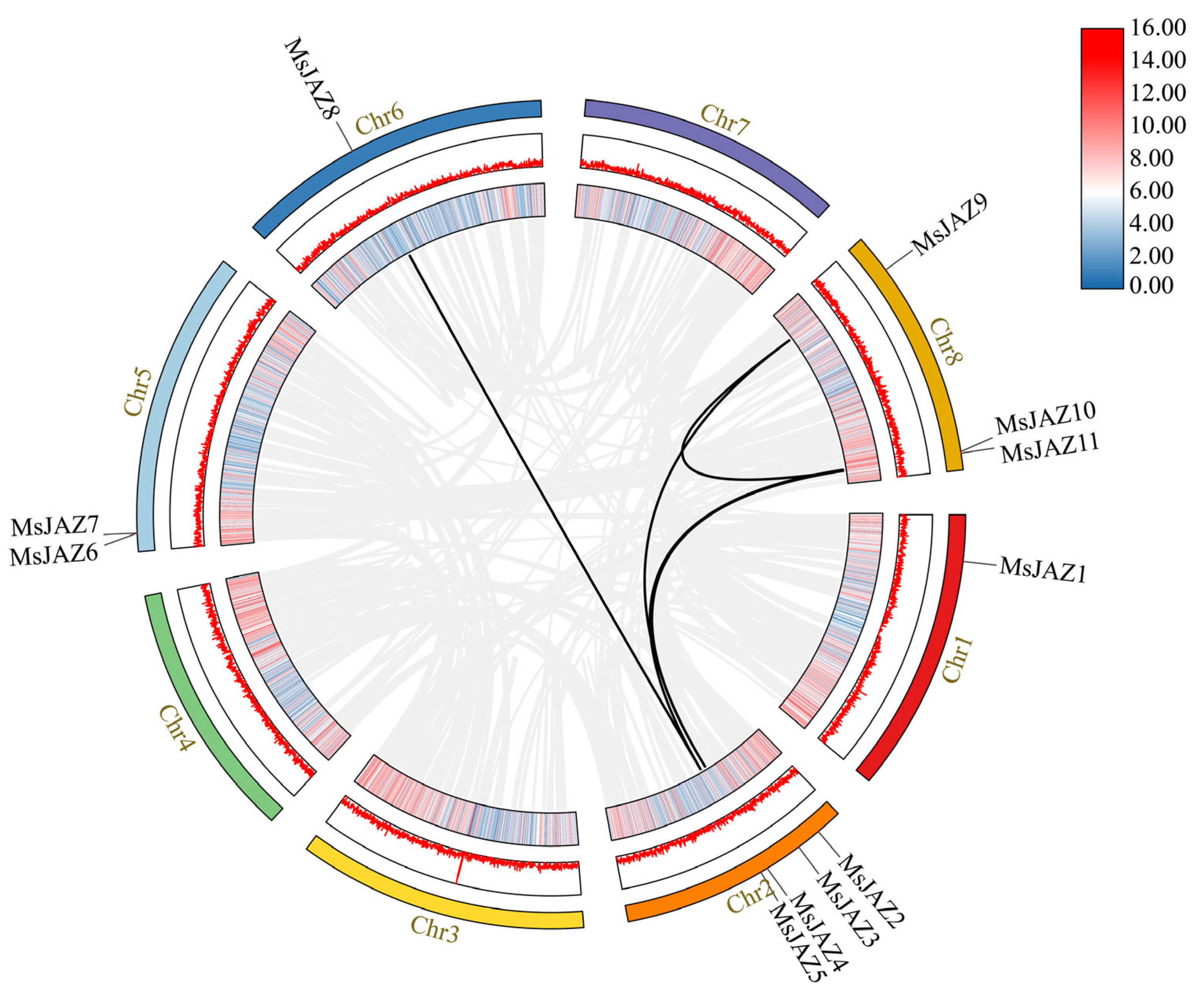
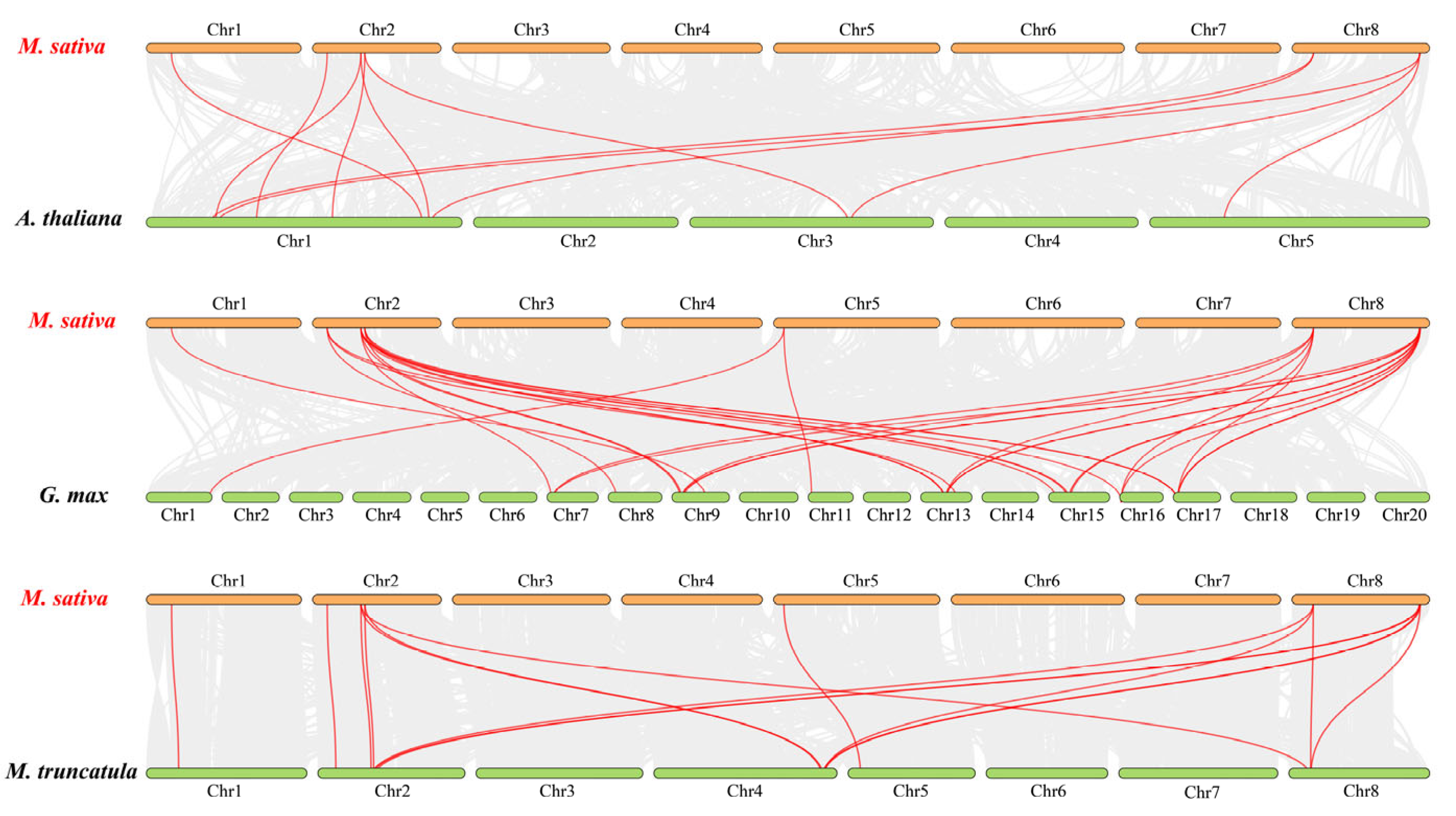
2.5. Analysis of the Gene Duplication and Synteny of the MsJAZs
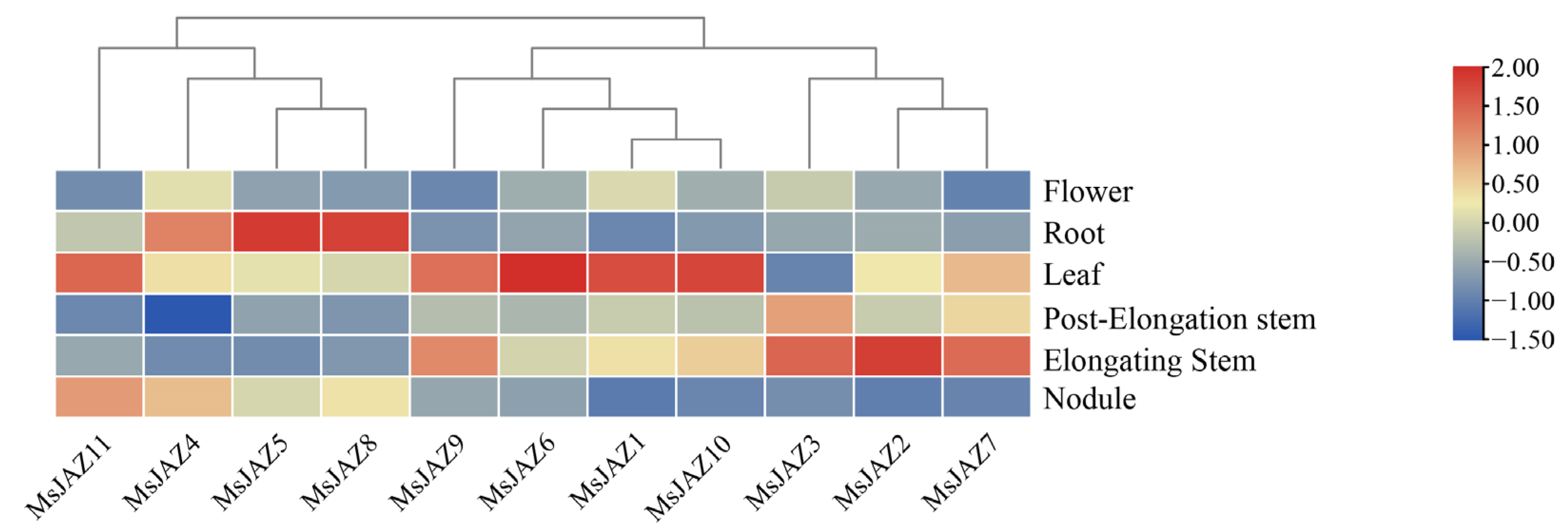
2.6. Expression Analysis of MsJAZ Genes in Different Tissues
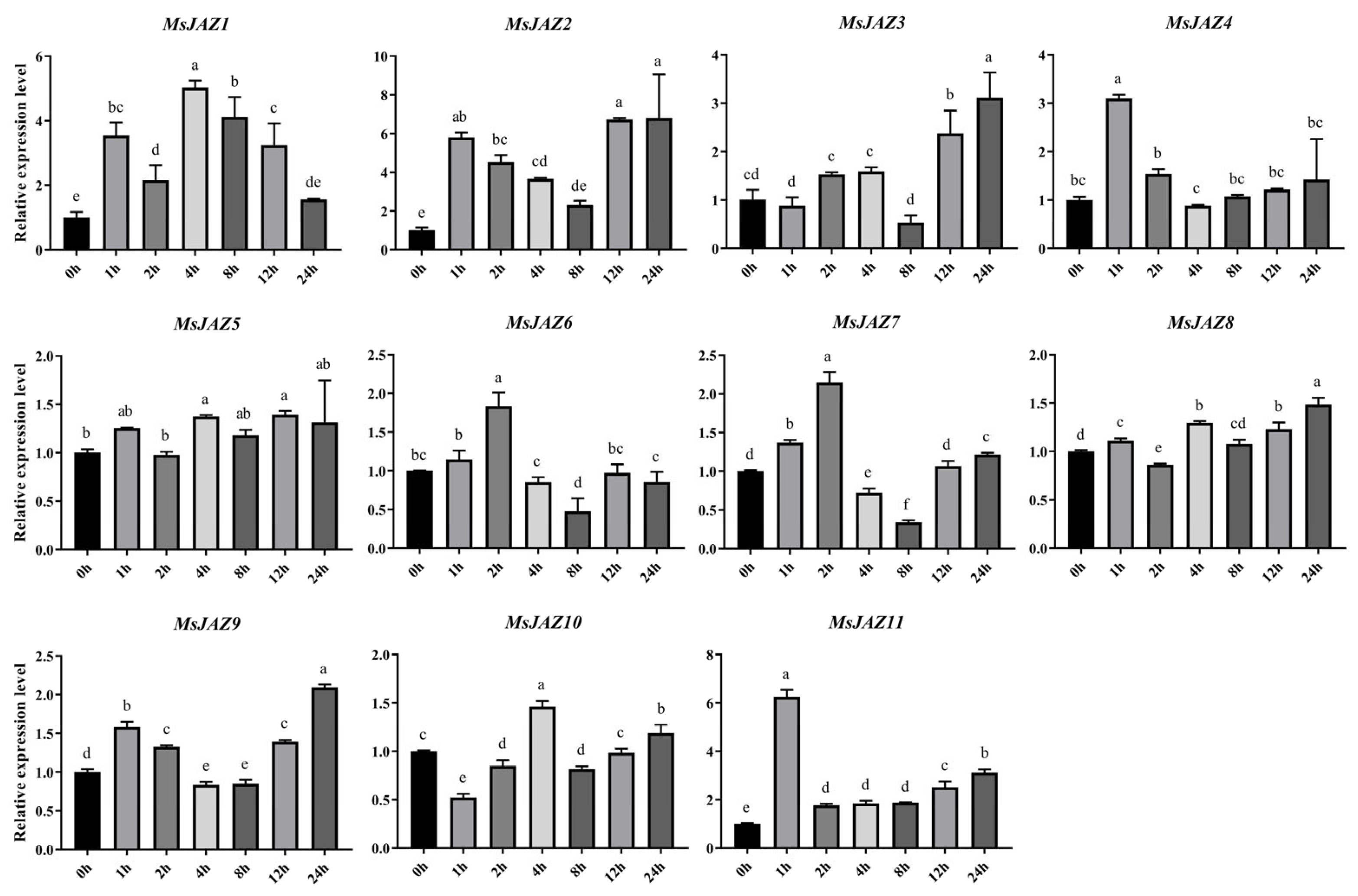
2.7. Expression Analysis of MsJAZ Genes in Salt Stress Treatments
2.8. Analysis of Salt Resistance of Arabidopsis with Overexpression of MsJAZ1
3. Discussion
4. Methods and Materials
4.1. Plant Materials and Growth Conditions
4.2. Identification of JAZ Family Members in Alfalfa
4.3. Chromosome Location Analysis and Phylogenetic Tree Construction
4.4. Gene Structure and Conserved Domains Analysis
4.5. Cis-Acting Elements Analysis of MsJAZ Promoter
4.6. Gene Duplication and Synteny Analysis
4.7. Transcriptome Data Analysis
4.8. Cloning and Expression Vector Construction of MsJAZ1
4.9. Detection of Salt Tolerance in Transgenic Arabidopsis
4.10. Real-Time Quantitative PCR (RT-qPCR) Analysis
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, J.K. Abiotic stress signaling and responses in plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Xiong, L.; Shi, H.; Yang, S.; Herrera-Estrella, L.R.; Xu, G.; Chao, D.Y.; Li, J.; Wang, P.Y.; Qin, F.; et al. Plant abiotic stress response and nutrient use efficiency. Sci. China Life Sci. 2020, 63, 635–674. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.K. Abiotic stress responses in plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Wani, S.H.; Kumar, V.; Shriram, V.; Sah, S.K. Phytohormones and their metabolic engineering for abiotic stress tolerance in crop plants. Crop J. 2016, 4, 162–176. [Google Scholar] [CrossRef]
- Salvi, P.; Manna, M.; Kaur, H.; Thakur, T.; Gandass, N.; Bhatt, D.; Muthamilarasan, M. Phytohormone signaling and crosstalk in regulating drought stress response in plants. Plant Cell Rep. 2021, 40, 1305–1329. [Google Scholar] [CrossRef]
- Afrin, S.; Huang, J.J.; Luo, Z.Y. JA-mediated transcriptional regulation of secondary metabolism in medicinal plants. Sci. Bull. 2015, 60, 1062–1072. [Google Scholar] [CrossRef]
- Pauwels, L.; Inzé, D.; Goossens, A. Jasmonate-inducible gene: What does it mean? Trends Plant Sci. 2009, 14, 87–91. [Google Scholar] [CrossRef]
- Browse, J. Jasmonate Passes Muster: A receptor and targets for the defense hormone. Annu. Rev. Plant Biol. 2009, 60, 183–205. [Google Scholar] [CrossRef]
- Zhou, X.E.; Zhang, Y.; Yao, J.; Zheng, J.; Zhou, Y.; He, Q.; Moreno, J.; Lam, V.Q.; Cao, X.; Sugimoto, K.; et al. Assembly of JAZ-JAZ and JAZ-NINJA complexes in jasmonate signaling. Plant Commun. 2023, 4, 100639. [Google Scholar] [CrossRef]
- Ye, H.; Du, H.; Tang, N.; Li, X.; Xiong, L. Identification and expression profiling analysis of TIFY family genes involved in stress and phytohormone responses in rice. Plant Mol. Biol. 2009, 71, 291–305. [Google Scholar] [CrossRef]
- Wager, A.; Browse, J. Social network: JAZ protein interactions expand our knowledge of jasmonate signaling. Front. Plant Sci. 2012, 3, 41. [Google Scholar] [CrossRef] [PubMed]
- Major, I.T.; Yoshida, Y.; Campos, M.; Kapali, G.; Xin, X.F.; Sugimoto, K.; Ferreira, D.O.; He, S.Y.; Howe, G.A. Regulation of growth–defense balance by the JASMONATE ZIM-DOMAIN (JAZ)-MYC transcriptional module. New Phytol. 2017, 215, 1533–1547. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Li, Y.; Tang, G.; Hui, S.; Yang, Z.; Zhao, J.; Liu, H.; Cao, J.; Yuan, M. Dynamic phytohormone profiling of rice upon rice black-streaked dwarf virus invasion. J. Plant Physiol. 2018, 228, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Thireault, C.; Shyu, C.; Yoshida, Y.; Aubin, B.S.; Campos, M.L.; Howe, G.A. Repression of jasmonate signaling by a non-TIFY JAZ protein in Arabidopsis. Plant J. 2015, 82, 669–679. [Google Scholar] [CrossRef]
- Kaji, T.; Matsumoto, K.; Okumura, T.; Nakayama, M.; Hoshino, S.; Takaoka, Y.; Wang, J.; Ueda, M. Two distinct modes of action of molecular glues in the plant hormone co-receptor COI1-JAZ system. iScience 2023, 27, 108625. [Google Scholar] [CrossRef]
- Katsir, L.; Schilmiller, A.L.; Staswick, P.E.; He, S.Y.; Howe, G.A. COI1 is a critical component of a receptor for jasmonate and the bacterial virulence factor coronatine. Proc. Natl. Acad. Sci. USA 2008, 105, 7100–7105. [Google Scholar] [CrossRef]
- Han, X.; Kui, M.; He, K.; Yang, M.; Du, J.; Jiang, Y.; Hu, Y. Jasmonate-regulated root growth inhibition and root hair elongation. J. Exp. Bot. 2023, 74, 1176–1185. [Google Scholar] [CrossRef]
- Varshney, V.; Majee, M. JA shakes hands with ABA to delay seed germination. Trends Plant Sci. 2021, 8, 764–766. [Google Scholar] [CrossRef]
- Ju, L.; Jing, Y.; Shi, P.; Liu, J.; Chen, J.; Yan, J.; Chu, J.; Chen, K.M.; Sun, J. JAZ proteins modulate seed germination through interaction with ABI5 in bread wheat and Arabidopsis. New Phytol. 2019, 223, 246–260. [Google Scholar] [CrossRef]
- Johnson, L.Y.D.; Major, L.T.; Chen, Y.; Yang, C.; Vanegas-Cano, L.J.; Howe, G.A. Diversification of JAZ-MYC signaling function in immune metabolism. New Phytol. 2023, 239, 2277–2291. [Google Scholar] [CrossRef]
- Qi, T.; Huang, H.; Song, S.; Xie, D. Regulation of jasmonate-mediated stamen development and seed production by a bHLH-MYB complex in Arabidopsis. Plant Cell 2015, 27, 1620–1633. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.; Qanmber, G.; Li, F.; Wang, Z. Updated role of ABA in seed maturation, dormancy, and germination. J. Adv. Res. 2021, 35, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Delgado, C.; Mora-Poblete, F.; Ahmar, S.; Chen, J.T.; Figueroa, C.R. Jasmonates and plant salt stress: Molecular players, physiological effects, and improving tolerance by using genome-associated tools. Int. J. Mol. Sci. 2021, 22, 3082. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Hu, Y.; Wang, H.; Guo, Q.; Chen, Y.; Howe, G.A.; Yu, D. Molecular mechanism underlying the synergetic effect of jasmonate on abscisic acid signaling during seed germination in Arabidopsis. Plant Cell 2020, 32, 3846–3865. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.L.; Yao, J.; Mei, C.S.; Tong, X.H.; Zeng, L.J.; Li, Q.; Xiao, L.T.; Sun, T.; Li, J.; Deng, X.W.; et al. Plant hormone jasmonate prioritizes defense over growth by interfering with gibberellin signaling cascade. Proc. Natl. Acad. Sci. USA 2012, 109, E1192–E1200. [Google Scholar] [CrossRef]
- Kazan, K. Diverse roles of jasmonates and ethylene in abiotic stress tolerance. Trends Plant Sci. 2015, 20, 219–229. [Google Scholar] [CrossRef]
- Huang, H.; Liu, B.; Liu, L.; Song, S. Jasmonate action in plant growth and development. J. Exp. Bot. 2017, 68, 1349–1359. [Google Scholar]
- Wu, H.; Ye, H.; Yao, R.; Zhang, T.; Xiong, L. OsJAZ9 acts as a transcriptional regulator in jasmonate signaling and modulates salt stress tolerance in rice. Plant Sci. 2015, 232, 1–12. [Google Scholar] [CrossRef]
- Fu, J.; Wu, H.; Ma, S.; Xiang, D.; Liu, R.; Xiong, L. OsJAZ1 attenuates drought resistance by regulating JA and ABA signaling in rice. Front. Plant Sci. 2017, 8, 2108. [Google Scholar] [CrossRef]
- Rao, S.; Tian, Y.; Zhang, C.; Qin, Y.; Liu, M.; Niu, S.; Li, Y.; Chen, Y. The JASMONATE ZIM-domain–OPEN STOMATA1 cascade integrates jasmonic acid and abscisic acid signaling to regulate drought tolerance by mediating stomatal closure in poplar. J. Exp. Bot. 2023, 74, 443–457. [Google Scholar] [CrossRef]
- Chen, L.; He, F.; Long, R.; Zhang, F.; Li, M.; Wang, Z.; Kang, J.; Yang, Q. A global alfalfa diversity panel reveals genomic selection signatures in Chinese varieties and genomic associations with root development. J. Integr. Plant Bio. 2021, 63, 1937–1951. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, F.; Zhao, G.; Li, M.; Long, R.; Kang, L.; Yang, Q.; Chen, L. Genome-wide identification and phylogenetic and expression analyses of the PLATZ gene family in Medicago sativa L. Int. J. Mol. Sci. 2023, 24, 2388. [Google Scholar] [CrossRef] [PubMed]
- Vanholme, B.; Grunewald, W.; Bateman, A.; Kohchi, T.; Gheysen, G. The tify family previously known as ZIM. Trends Plant Sci. 2007, 12, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zheng, H.; Wu, H.; Wang, C.; Liang, Z. Recent genome-wide replication promoted expansion and functional differentiation of the JAZs in soybeans. Int. J. Bio. Macromol. 2023, 238, 124064. [Google Scholar] [CrossRef] [PubMed]
- Wasternack, C.; Song, S. Jasmonates: Biosynthesis, metabolism, and signaling by proteins activating and repressing transciption. J. Exp. Bot. 2017, 68, 1303–1321. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Luthe, D. Identification and evolution analysis of the JAZ gene family in maize. BMC Genom. 2021, 22, 256. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, C.; Liu, Z.; Zhao, T.; Jiang, J.; Li, J.; Xu, X.; Yang, H. Genome-wide identification, characterization and expression analysis of the JAZ gene family in resistance to gray leaf spots in tomato. Int. J. Mol. Sci. 2021, 22, 9974. [Google Scholar] [CrossRef]
- Shen, J.; Zou, Z.; Xing, H.; Duan, Y.; Zhu, Y.; Ma, Y.; Wang, Y.; Fang, W. Genome-wide analysis reveals stress and hormone responsive patterns of JAZ family genes in Camellia Sinensis. Int. J. Mol. Sci. 2020, 21, 2433. [Google Scholar] [CrossRef]
- Song, H.; Duan, Z.; Wang, Z.; Li, Y.; Wang, Y.; Li, C.; Mao, W.; Que, Q.; Chen, X.; Li, P. Genome-wide identification, expression pattern and subcellular localization analysis of the JAZ gene family in Toona ciliata. Ind. Crop Prod. 2022, 178, 114582. [Google Scholar] [CrossRef]
- Zheng, L.; Wan, Q.; Wang, H.; Guo, C.; Niu, X.; Zhang, X.; Zhang, R.; Chen, Y.; Luo, K. Genome-wide identification and expression of TIFY family in cassava (Manihot esculenta Crantz). Front. Plant Sci. 2022, 13, 1017840. [Google Scholar] [CrossRef]
- Shen, C.; Du, H.; Chen, Z.; Lu, H.; Zhu, F.; Chen, H.; Meng, X.; Liu, Q.; Liu, P.; Zheng, L.; et al. The chromosome-level genome sequence of the autotetraploid alfalfa and resequencing of core germplasms provide genomic resources for alfalfa research. Mol. Plant 2020, 13, 1250–1261. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Meng, Y.; Huang, D.; Qi, Y.; Chen, M. Origin and evolutionary analysis of the plant-specific TIFY transcription factor family. Genomics 2011, 98, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Shiu, S.H.; Bleecker, A.B. Expansion of the receptor-like kinase/pelle gene family and receptor-like proteins in Arabidopsis. Plant Physiol. 2003, 132, 530–543. [Google Scholar] [CrossRef] [PubMed]
- Bansal, M.S.; Eulenstein, O. The multiple gene duplication problem revisited. Bioinformatics 2008, 24, i132–i138. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Liu, Y.; Tao, J.J.; Lu, L.; Jiang, Z.H.; Wei, J.J.; Wu, C.M.; Yin, C.C.; Li, W.; Bi, Y.D.; et al. GmJAZ3 interacts with GmRR18a and GmMYC2a to regulate seed traits in soybean. J. Integr. Plant Bio. 2023, 65, 1983–2000. [Google Scholar] [CrossRef]
- Zhu, J.K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Bio. 2002, 53, 247–273. [Google Scholar] [CrossRef]
- Sun, H.; Chen, L.; Li, J.; Hu, M.; Ulah, A.; He, X.; Yang, X.; Zhang, X. The JASMONATE ZIM-Domain Gene Family Mediates JA Signaling and Stress Response in Cotton. Plant Cell Physiol. 2017, 12, 2139–2154. [Google Scholar] [CrossRef]
- Toda, Y.; Tanaka, M.; Ogawa, D.; Kurata, K.; Kurotani, K.I.; Habu, Y.; Ando, T.; Sugimoto, K.; Mitsuda, N.; Katoh, E.; et al. RICE SALT SENSITIVE3 forms a ternary complex with JAZ and class-C bHLH factors and regulates jasmonate-induced gene expression and root cell elongation. Plant Cell 2013, 5, 1709–1725. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive tree of life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Wiliams, N.; Misleh, C.; Li, W.W. MEME: Discovering and analyzing DNA and protein sequence motifs. Nucleic Acids Res. 2006, 34, W369–W373. [Google Scholar] [CrossRef] [PubMed]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Peer, Y.V.; Rouzé, P.; Rombatus, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, J.A.; Fu, F.; Bucciarelli, B.; Yang, S.S.; Samac, D.A.; Lamb, J.F.S.; Monteros, M.J.; Graham, M.A.; Gronwald, J.W.; Krom, N.; et al. The Medicago sativ gene index 1.2: A web-accessible gene expression atlas for investigating expression differences between Medicago sativa subspecies. BMC Genomics 2015, 16, 502. [Google Scholar]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Cui, J.; Wang, X.; Li, M.; Zhang, L.; Kang, J. Genome-wide identification, phylogenetic and expression analysis of expansin gene family in Medicago sativa L. Int. J. Mol. Sci. 2024, 25, 4700. [Google Scholar] [CrossRef]


| Gene Name | Gene ID | Full CDS Length (bp) | Protein | Subcellular Location | ||
|---|---|---|---|---|---|---|
| Length (aa) | Mw (kDa) | pI | ||||
| MsJAZ1 | MsG0180001147 | 984 | 327 | 34.29 | 9.45 | Cell membrane nucleus |
| MsJAZ2 | MsG0280007019 | 396 | 131 | 15.09 | 8.86 | Nucleus |
| MsJAZ3 | MsG0280007633 | 888 | 295 | 31.11 | 7.10 | Cell membrane nucleus |
| MsJAZ4 | MsG0280008436 | 753 | 250 | 27.15 | 8.83 | Nucleus |
| MsJAZ5 | MsG0280008590 | 627 | 208 | 22.36 | 8.41 | Nucleus |
| MsJAZ6 | MsG0580024589 | 837 | 278 | 31.71 | 9.56 | Nucleus |
| MsJAZ7 | MsG0580024590 | 588 | 195 | 22.81 | 8.42 | Nucleus |
| MsJAZ8 | MsG0680032555 | 630 | 209 | 22.41 | 8.52 | Nucleus |
| MsJAZ9 | MsG0880042776 | 708 | 235 | 25.91 | 8.34 | Nucleus |
| MsJAZ10 | MsG0880047288 | 537 | 178 | 19.33 | 8.97 | Nucleus |
| MsJAZ11 | MsG0880047330 | 1014 | 337 | 38.03 | 8.34 | Nucleus |
| Gene1 | Gene2 | Ka | Ks | Ka/Ks | Duplication Type |
|---|---|---|---|---|---|
| MsJAZ4 | MsJAZ9 | 0.643651984 | 1.384563729 | 0.46487711 | Segmental duplication |
| MsJAZ4 | MsJAZ11 | 0.317998017 | 0.795918567 | 0.39953587 | Segmental duplication |
| MsJAZ5 | MsJAZ8 | 0.01802844 | 0.038425167 | 0.469183122 | Segmental duplication |
| MsJAZ5 | MsJAZ10 | 0.394218786 | 0.584020786 | 0.675008142 | Segmental duplication |
| MsJAZ9 | MsJAZ11 | 0.608088468 | 2.200562393 | 0.276333209 | Segmental duplication |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, J.; Jiang, X.; Li, Y.; Zhang, L.; Zhang, Y.; Wang, X.; He, F.; Li, M.; Zhang, T.; Kang, J. Genome-Wide Identification, Phylogenetic, and Expression Analysis of Jasmonate ZIM-Domain Gene Family in Medicago Sativa L. Int. J. Mol. Sci. 2024, 25, 10589. https://doi.org/10.3390/ijms251910589
Cui J, Jiang X, Li Y, Zhang L, Zhang Y, Wang X, He F, Li M, Zhang T, Kang J. Genome-Wide Identification, Phylogenetic, and Expression Analysis of Jasmonate ZIM-Domain Gene Family in Medicago Sativa L. International Journal of Molecular Sciences. 2024; 25(19):10589. https://doi.org/10.3390/ijms251910589
Chicago/Turabian StyleCui, Jing, Xu Jiang, Yajing Li, Lili Zhang, Yangyang Zhang, Xue Wang, Fei He, Mingna Li, Tiejun Zhang, and Junmei Kang. 2024. "Genome-Wide Identification, Phylogenetic, and Expression Analysis of Jasmonate ZIM-Domain Gene Family in Medicago Sativa L." International Journal of Molecular Sciences 25, no. 19: 10589. https://doi.org/10.3390/ijms251910589






