The Role of the Extracellular Matrix in the Pathogenesis and Treatment of Pulmonary Emphysema
Abstract
1. Introduction
2. Elastic Fiber Injury in Pulmonary Emphysema
2.1. The Effect of Elastic Fibers on the Distribution of Lung Mechanical Forces
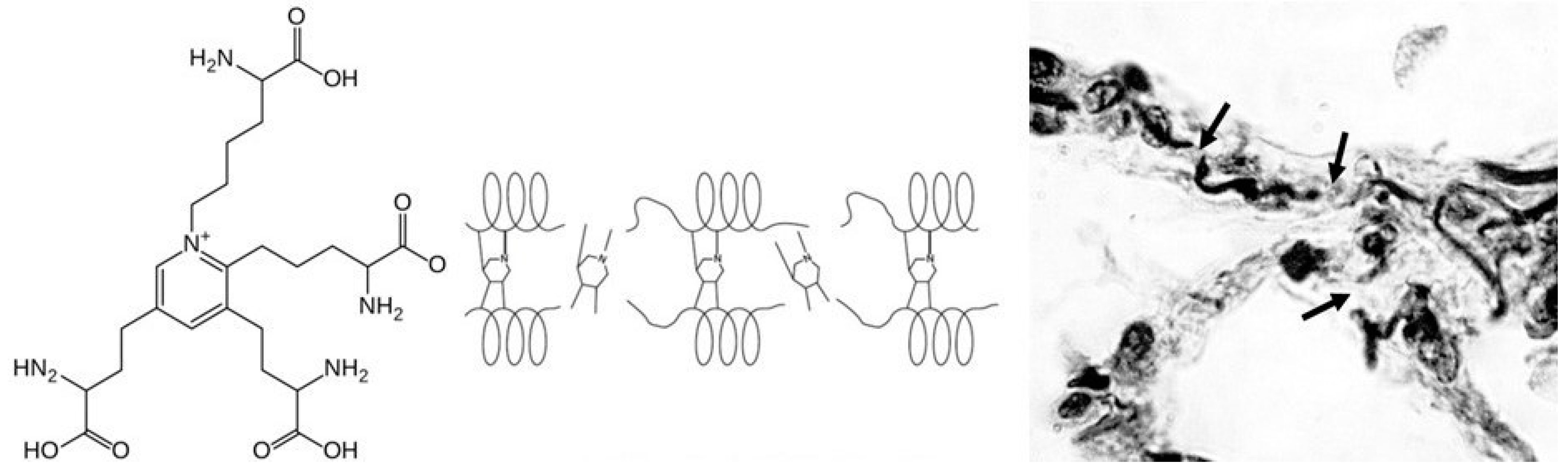
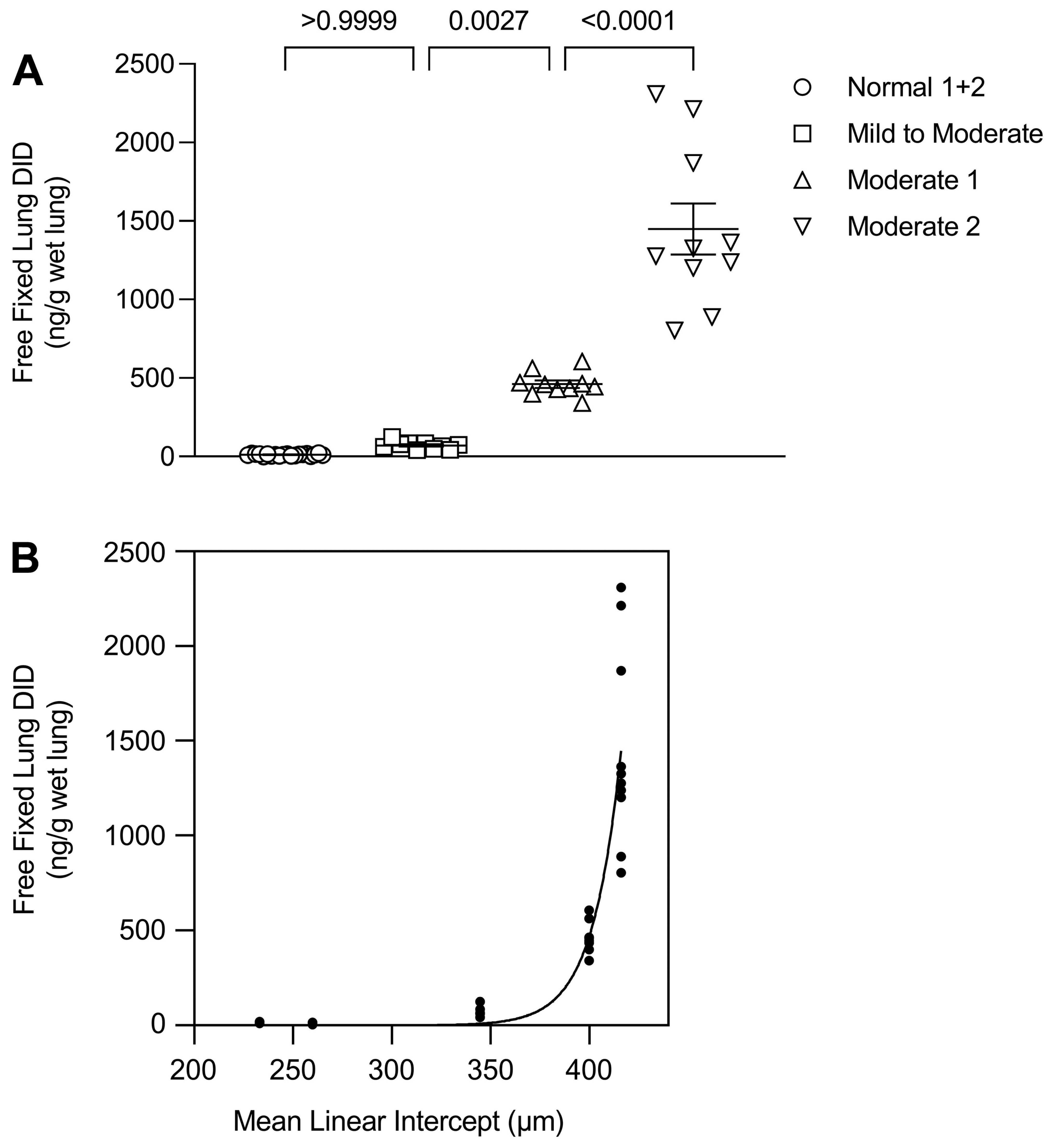
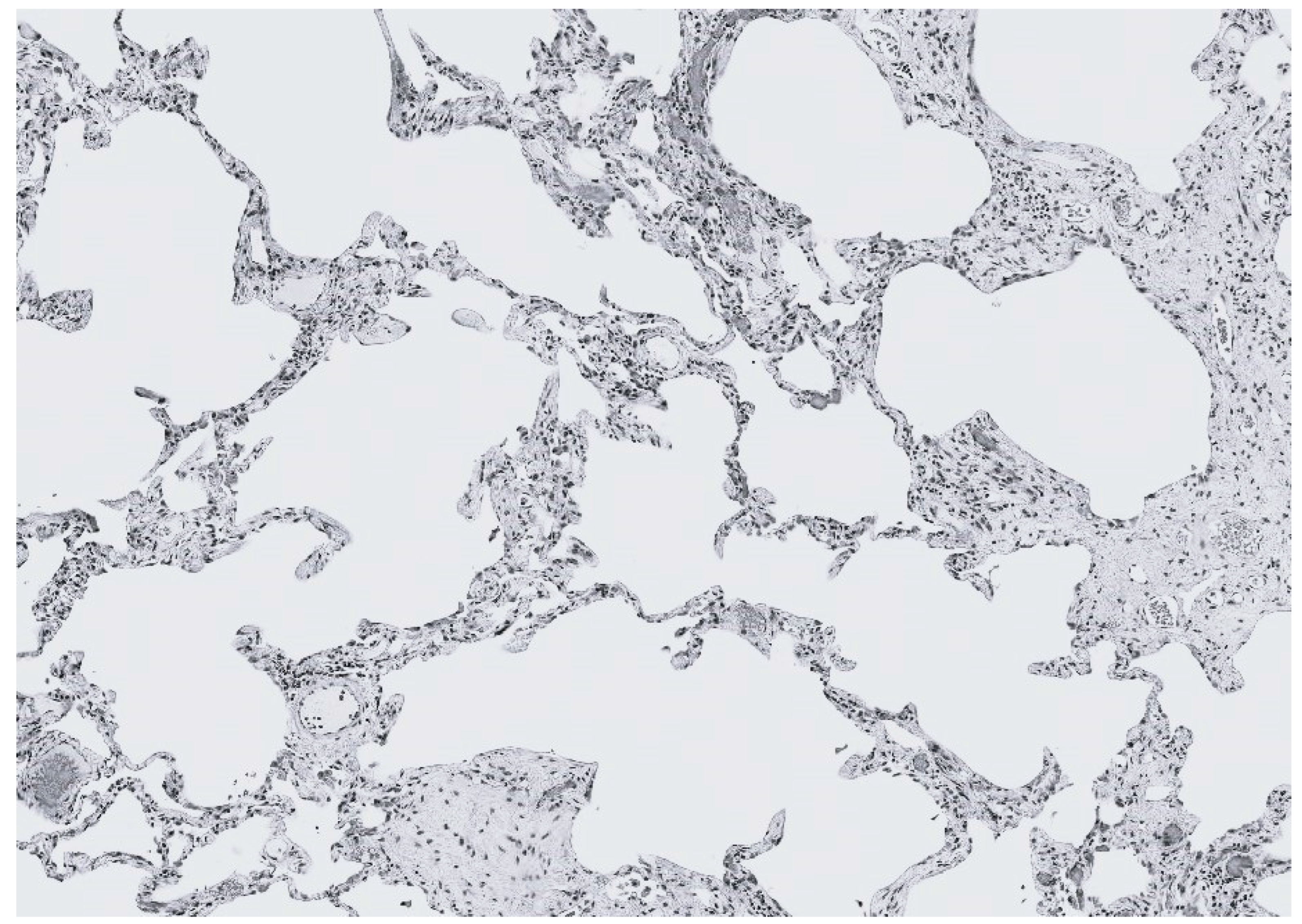
2.2. DID as a Biomarker of Elastic Fiber Injury
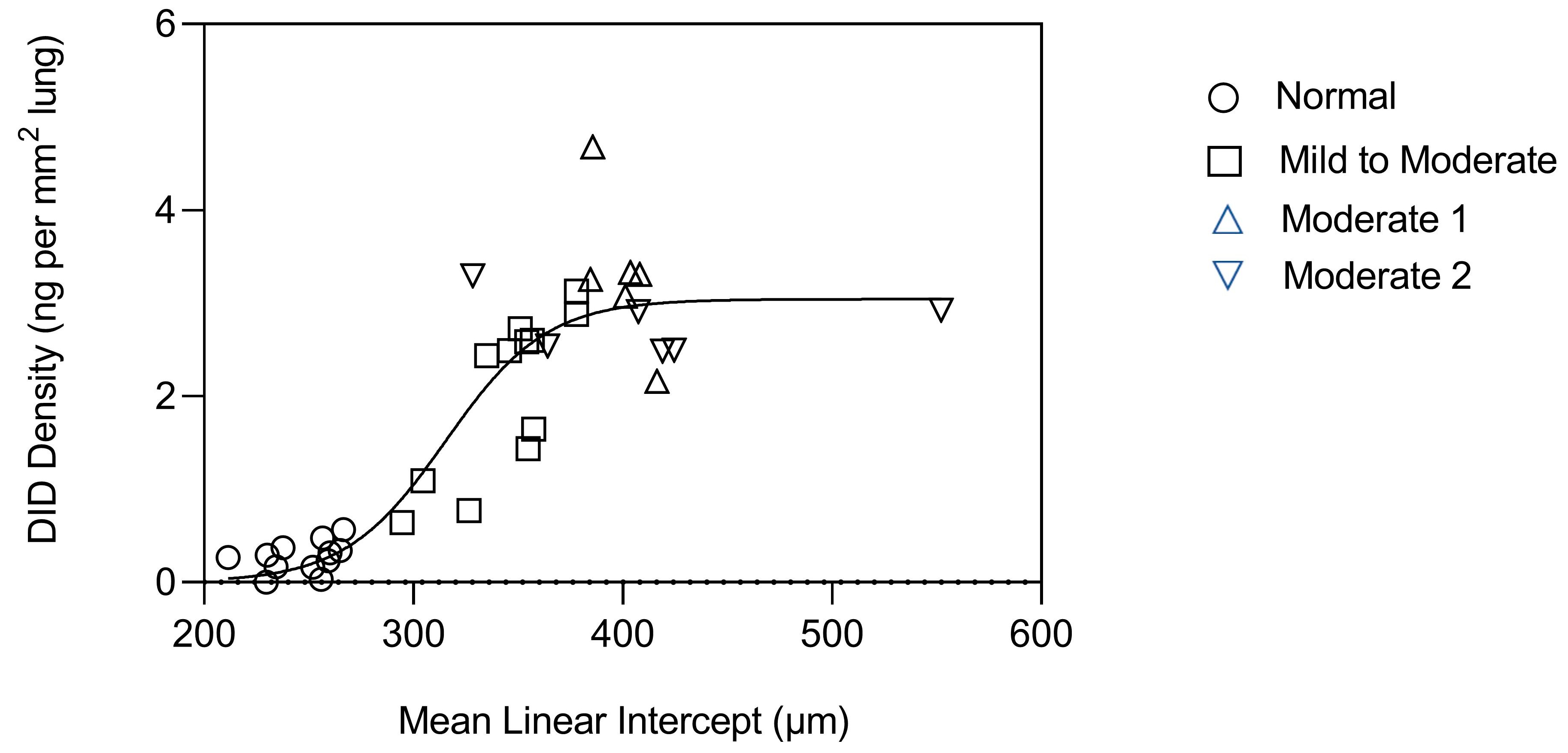
2.3. A Percolation Model of Structural Changes in Elastic Fibers
2.4. The Proinflammatory Activity of Structurally Modified Elastic Fibers
3. The Relationship between Collagen and Airspace Enlargement
3.1. Collagen Degradation in Alveolar Wall Injury and Repair
3.2. Combined Pulmonary Fibrosis and Emphysema
4. The Relationship between Hyaluronan and Elastic Fibers
4.1. Hyaluronan Prevents Elastic Fiber Degradation
4.2. Effect of HA on the Mechanical Properties of Elastin
5. Therapeutic Considerations
6. Future Directions
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rabe, K.F.; Watz, H. Chronic obstructive pulmonary disease. Lancet 2017, 389, 1931–1940. [Google Scholar] [CrossRef] [PubMed]
- Churg, A.; Wright, J.L. Proteases and emphysema. Curr. Mol. Med. 2006, 6, 289–308. [Google Scholar] [CrossRef] [PubMed]
- Burgess, J.K.; Mauad, T.; Tjin, G.; Karlsson, J.C.; Westergren-Thorsson, G. The extracellular matrix—The under-recognized element in lung disease? J. Pathol. 2016, 240, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, T.; O’Reilly, P.; Antony, V.B.; Gaggar, A.; Thannickal, V.J. Matrix Remodeling in Pulmonary Fibrosis and Emphysema. Am. J. Respir. Cell Mol. Biol. 2016, 54, 751–760. [Google Scholar] [CrossRef] [PubMed]
- White, E.S. Lung extracellular matrix and fibroblast function. Ann. Am. Thorac. Soc. 2015, 12 (Suppl. S1), S30–S33. [Google Scholar] [CrossRef]
- Shapiro, S.D. Matrix metalloproteinase degradation of connective tissue glycosaminoglycans in emphysema. Proc. Am. Thorac. Soc. 2006, 3, 427–432. [Google Scholar]
- Heinz, A. Elastases and elastokines: Elastin degradation and its significance in health and disease. Crit. Rev. Biochem. Mol. Biol. 2020, 55, 252–273. [Google Scholar] [CrossRef]
- Isson, T.H. MMPs in lung injury and repair: Implications for translational studies. Am. J. Physiol. Lung Cell Mol. Physiol. 2018, 314, L892–L903. [Google Scholar]
- Manicone, A.M. Role of the pulmonary epithelium and matrix metalloproteinases in the pathogenesis of chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 2012, 9, 95–101. [Google Scholar]
- Sellami, M.; Meghraoui-Kheddar, A.; Terryn, C.; Fichel, C.; Bouland, N.; Diebold, M.D.; Guenounou, M.; Héry-Huynh, S.; Le Naour, R. Induction and regulation of murine emphysema by elastin peptides. Am. J. Physiol. Lung Cell Mol. Physiol. 2016, 310, L8–L23. [Google Scholar] [CrossRef]
- Winkler, T.; Suki, B. Emergent structure-function relations in emphysema and asthma. Crit. Rev. Biomed. Eng. 2011, 39, 263–280. [Google Scholar] [CrossRef] [PubMed]
- Cantor, J.O.; Cerreta, J.M.; Keller, S.; Turino, G.M. Modulation of airspace enlargement in elastase-induced emphysema by intratracheal instillment of hyaluronidase and hyaluronic acid. Exp. Lung Res. 1995, 21, 423–436. [Google Scholar] [CrossRef]
- Cantor, J.O.; Cerreta, J.M.; Ochoa, M.; Ma, S.; Chow, T.; Grunig, G.; Turino, G.M. Aerosolized hyaluronan limits airspace enlargement in a mouse model of cigarette smoke-induced pulmonary emphysema. Exp. Lung Res. 2005, 31, 417–430. [Google Scholar] [CrossRef] [PubMed]
- Cantor, J.O.; Ma, S.; Liu, X.; Campos, M.A.; Strange, C.; Stocks, J.M.; Devine, M.S.; El Bayadi, S.G.; Lipchik, R.J.; Sandhaus, R.A.; et al. A 28-day clinical trial of aerosolized hyaluronan in alpha-1 antiprotease deficiency COPD using desmosine as a surrogate marker for drug efficacy. Respir. Med. 2021, 182, 106402. [Google Scholar] [CrossRef] [PubMed]
- Cantor, J.; Armand, G.; Turino, G. Lung hyaluronan levels are decreased in alpha-1 antiprotease deficiency COPD. Respir. Med. 2015, 109, 656–659. [Google Scholar] [CrossRef][Green Version]
- Mecham, R.P. Elastin in lung development and disease pathogenesis. Matrix Biol. 2018, 73, 6–20. [Google Scholar] [CrossRef]
- Fagiola, M.; Reznik, S.; Riaz, M.; Qyang, Y.; Lee, S.; Avella, J.; Turino, G.; Cantor, J. The relationship between elastin cross linking and alveolar wall rupture in human pulmonary emphysema. Am. J. Physiol. Lung Cell Mol. Physiol. 2023, 324, L747–L755. [Google Scholar] [CrossRef]
- Churg, A.; Cosio, M.; Wright, J.L. Mechanisms of cigarette smoke-induced COPD: Insights from animal models. Am. J. Physiol. Lung Cell Mol. Physiol. 2008, 294, L612–L631. [Google Scholar] [CrossRef]
- Barnes, P.J. Oxidative Stress in Chronic Obstructive Pulmonary Disease. Antioxidants 2022, 11, 965. [Google Scholar] [CrossRef]
- Bates, J.H.; Davis, G.S.; Majumdar, A.; Butnor, K.J.; Suki, B. Linking parenchymal disease progression to changes in lung mechanical function by percolation. Am. J. Respir. Crit. Care Med. 2007, 176, 617–623. [Google Scholar] [CrossRef]
- Trębacz, H.; Barzycka, A. Mechanical properties and functions of elastin: An overview. Biomol. Ther. 2023, 13, 574. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Daggett, V. Molecular basis for the extensibility of elastin. J. Muscle Res. Cell Motil. 2002, 23, 561–573. [Google Scholar] [CrossRef] [PubMed]
- Kielty, C.M.; Sherratt, M.J.; Shuttleworth, C.A. Elastic fibres. J Cell Sci. 2002, 115, 2817–2828. [Google Scholar] [CrossRef] [PubMed]
- Niewoehner, D.E.; Hoidal, J.R. Lung fibrosis and emphysema: Divergent responses to a common injury? Science 1982, 217, 359–360. [Google Scholar] [CrossRef]
- Luisetti, M.; Ma, S.; Iadarola, P.; Stone, P.J.; Viglio, S.; Casado, B.; Lin, Y.Y.; Snider, G.L.; Turino, G.M. Desmosine as a biomarker of elastin degradation in COPD: Current status and future directions. Eur. Respir. J. 2008, 32, 1146–1157. [Google Scholar] [CrossRef]
- Kim, C.; Ko, Y.; Kim, S.H.; Yoo, H.J.; Lee, J.S.; Rhee, C.K.; Lee, J.H.; Lee, J.H.; Kim, T.H.; Lim, S.Y.; et al. Urinary desmosine is associated with emphysema severity and frequent exacerbation in patients with COPD. Respirology 2018, 23, 176–181. [Google Scholar] [CrossRef]
- Ma, S.; Lin, Y.Y.; Cantor, J.O.; Chapman, K.R.; Sandhaus, R.A.; Fries, M.; Edelman, J.M.; McElvaney, G.; Turino, G.M. The Effect of Alpha-1 Proteinase Inhibitor on Biomarkers of Elastin Degradation in Alpha-1 Antitrypsin Deficiency: An Analysis of the RAPID/RAPID Extension Trials. Chronic. Obstr. Pulm. Dis. 2016, 4, 34–44. [Google Scholar]
- Fagiola, M.; Gu, G.; Avella, J.; Cantor, J. Free lung desmosine: A potential biomarker for elastic fiber injury in pulmonary emphysema. Biomarkers 2022, 27, 319–324. [Google Scholar] [CrossRef]
- Bunde, A.; Kantelhart, J.W. Diffusion and conduction in percolation systems In Diffusion in Condensed Matter; Heitjans, P., Karger, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 895–914. [Google Scholar]
- Redner, S. Fractal and multifractal scaling of electrical conduction in random resistor networks. In Encyclopedia of Complexity and Systems Science; Meyers, P.A., Ed.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 3737–3754. [Google Scholar]
- Murphy, K.D.; Hunt, G.W.; Almond, D.P. Evidence of emergent scaling in mechanical systems. Philos. Mag. 2006, 86, 3325–3338. [Google Scholar] [CrossRef]
- Mehraban, S.; Gu, G.; Ma, S.; Liu, X.; Turino, G.; Cantor, J. The Proinflammatory Activity of Structurally Altered Elastic Fibers. Am. J. Respir. Cell Mol. Biol. 2020, 63, 699–706. [Google Scholar] [CrossRef]
- de Oliveira, M.V.; Rocha, N.N.; Santos, R.S.; Rocco, M.R.M.; de Magalhães, R.F.; Silva, J.D.; Souza, S.A.L.; Capelozzi, V.L.; Pelosi, P.; Silva, P.L.; et al. Endotoxin-induced emphysema exacerbation: A novel model of chronic obstructive pulmonary disease exacerbations causing cardiopulmonary impairment and diaphragm dysfunction. Front. Physiol. 2019, 10, 664. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Fujinawa, R.; Ota, F.; Kobayashi, S.; Angata, T.; Ueno, M.; Maeno, T.; Kitazume, S.; Yoshida, K.; Ishii, T.; et al. A single dose of lipopolysaccharide into mice with emphysema mimics human chronic obstructive pulmonary disease exacerbation as assessed by micro-computed tomography. Am. J. Respir. Cell Mol. Biol. 2013, 49, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Christopoulou, M.E.; Papakonstantinou, E.; Stolz, D. Matrix Metalloproteinases in Chronic Obstructive Pulmonary Disease. Int. J. Mol. Sci. 2023, 24, 3786. [Google Scholar] [CrossRef] [PubMed]
- Togo, S.; Holz, O.; Liu, X.; Sugiura, H.; Kamio, K.; Wang, X.; Kawasaki, S.; Ahn, Y.; Fredriksson, K.; Skold, C.M.; et al. Lung fibroblast repair functions in patients with chronic obstructive pulmonary disease are altered by multiple mechanisms. Am. J. Respir. Crit. Care Med. 2008, 178, 248–260. [Google Scholar] [CrossRef]
- Onursal, C.; Dick, E.; Angelidis, I.; Schiller, H.B.; Staab-Weijnitz, C.A. Collagen Biosynthesis, Processing, and Maturation in Lung Ageing. Front. Med. 2021, 8, 593874. [Google Scholar] [CrossRef]
- Gao, S.; Chen, K.; Zhao, Y.; Rich, C.B.; Chen, L.; Li, S.J.; Toselli, P.; Stone, P.; Li, W. Transcriptional and posttranscriptional inhibition of lysyl oxidase expression by cigarette smoke condensate in cultured rat fetal lung fibroblasts. Toxicol. Sci. 2005, 87, 197–203. [Google Scholar] [CrossRef]
- Braber, S.; Koelink, P.J.; Henricks, P.A.; Jackson, P.L.; Nijkamp, F.P.; Garssen, J.; Kraneveld, A.D.; Blalock, J.E.; Folkerts, G. Cigarette smoke-induced lung emphysema in mice is associated with prolyl endopeptidase, an enzyme involved in collagen breakdown. Am. J. Physiol. Lung Cell Mol. Physiol. 2011, 300, L255–L265. [Google Scholar] [CrossRef]
- Wright, J.L.; Churg, A. Smoke-induced emphysema in guinea pigs is associated with morphometric evidence of collagen break-down and repair. Am. J. Physiol. Lung Cell Mol. Physiol. 1995, 12, 17–20. [Google Scholar] [CrossRef]
- Foronjy, R.; D’Armiento, J. The role of collagenase in emphysema. Respir. Res. 2001, 2, 348–352. [Google Scholar] [CrossRef]
- Ito, J.T.; Lourenço, J.D.; Righetti, R.F.; Tibério, I.F.L.C.; Prado, C.M.; Lopes, F.D.T.Q.S. Extracellular matrix component remodeling in respiratory diseases: What has been found in clinical and experimental studies? Cells 2019, 8, 342. [Google Scholar] [CrossRef]
- Johanson, W.G., Jr.; Pierce, A.K. Effects of elastase, collagenase, and papain on structure and function of rat lungs in vitro. J. Clin. Investig. 1972, 51, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.L.; Tazelaar, H.D.; Churg, A. Fibrosis with emphysema. Histopathology 2011, 58, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.U.; Kim, E.K.; Lim, S.Y.; Lee, J.H.; Lee, J.S.; Lee, S.D.; Oh, Y.M.; Rhee, C.K.; KOLD Study Group. Mixed Phenotype of Emphysema and Airway Wall Thickening Is Associated with Frequent Exacerbation in Chronic Obstructive Pulmonary Disease Patients. Int. J. Chronic Obstr. Pulm. Dis. 2019, 14, 3035–3042. [Google Scholar] [CrossRef]
- Hage, R.; Gautschi, F.; Steinack, C.; Schuurmans, M.M. Combined Pulmonary Fibrosis and Emphysema (CPFE) Clinical Features and Management. Int. J. Chronic Obstr. Pulm. Dis. 2021, 16, 167–177. [Google Scholar] [CrossRef]
- Kulkarni, G.S.; Nadkarni, P.P.; Cerreta, J.M.; Ma, S.; Cantor, J.O. Short-term cigarette smoke exposure potentiates endotoxin-induced pulmonary inflammation. Exp. Lung Res. 2007, 33, 1–13. [Google Scholar] [CrossRef]
- Cantor, J.O.; Cerreta, J.M.; Armand, G.; Turino, G.M. Further investigation of the use of intratracheally administered hyaluronic acid to ameliorate elastase-induced emphysema. Exp. Lung Res. 1997, 23, 229–244. [Google Scholar] [CrossRef]
- Cantor, J.O.; Shteyngart, B.; Cerreta, J.M.; Liu, M.; Armand, G.; Turino, G.M. The effect of hyaluronan on elastic fiber injury in vitro and elastase-induced airspace enlargement in vivo. Proc. Soc. Exp. Biol. Med. 2000, 225, 65–71. [Google Scholar] [PubMed]
- Murasawa, Y.; Watanabe, K.; Yoneda, M.; Zako, M.; Kimata, K.; Sakai, L.Y.; Isogai, Z. Homotypic versican G1 domain interactions enhance hyaluronan incorporation into fibrillin microfibrils. J. Biol. Chem. 2013, 288, 29170–29181. [Google Scholar] [CrossRef]
- Stecco, A.; Cowman, M.; Pirri, N.; Raghavan, P.; Pirri, C. Densification: Hyaluronan Aggregation in Different Human Organs. Bioengineering 2022, 9, 159. [Google Scholar] [CrossRef]
- Galdi, F.; Pedone, C.; McGee, C.A.; George, M.; Rice, A.B.; Hussain, S.S.; Vijaykumar, K.; Boitet, E.R.; Tearney, G.J.; McGrath, J.A.; et al. Inhaled high molecular weight hyaluronan ameliorates respiratory failure in acute COPD exacerbation: A pilot study. Respir. Res. 2021, 22, 30. [Google Scholar] [CrossRef]
- Petrigni, G.; Allegra, L. Aerosolised hyaluronic acid prevents exercise-induced bronchoconstriction, suggesting novel hypotheses on the correction of matrix defects in asthma. Pulm. Pharmacol. Ther. 2006, 19, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hahn, J.; Zhang, Y. Mechanical properties of arterial elastin with water loss. J. Biomech. Eng. 2018, 140, 0410121–0410128. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, J.; Cruz, T.; Bhattacharya, S.; Bray, B.A. Hyaluronan affects extravascular water in lungs of unanesthetized rabbits. J. Appl. Physiol. 1989, 66, 2595–2599. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Franco, A.; Giraldo-Cadavid, L.F.; Tuta-Quintero, E.; Bastidas Goyes, A.R.; Botero-Rosas, D.A. The Challenges of Spirometric Diagnosis of COPD. Can. Respir. J. 2023, 2023, 6991493. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cantor, J. The Role of the Extracellular Matrix in the Pathogenesis and Treatment of Pulmonary Emphysema. Int. J. Mol. Sci. 2024, 25, 10613. https://doi.org/10.3390/ijms251910613
Cantor J. The Role of the Extracellular Matrix in the Pathogenesis and Treatment of Pulmonary Emphysema. International Journal of Molecular Sciences. 2024; 25(19):10613. https://doi.org/10.3390/ijms251910613
Chicago/Turabian StyleCantor, Jerome. 2024. "The Role of the Extracellular Matrix in the Pathogenesis and Treatment of Pulmonary Emphysema" International Journal of Molecular Sciences 25, no. 19: 10613. https://doi.org/10.3390/ijms251910613
APA StyleCantor, J. (2024). The Role of the Extracellular Matrix in the Pathogenesis and Treatment of Pulmonary Emphysema. International Journal of Molecular Sciences, 25(19), 10613. https://doi.org/10.3390/ijms251910613



