Hydrogel-Mediated Local Delivery of Induced Nephron Progenitor Cell-Sourced Molecules as a Cell-Free Approach for Acute Kidney Injury
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation and Profiling of iNPC-SMs
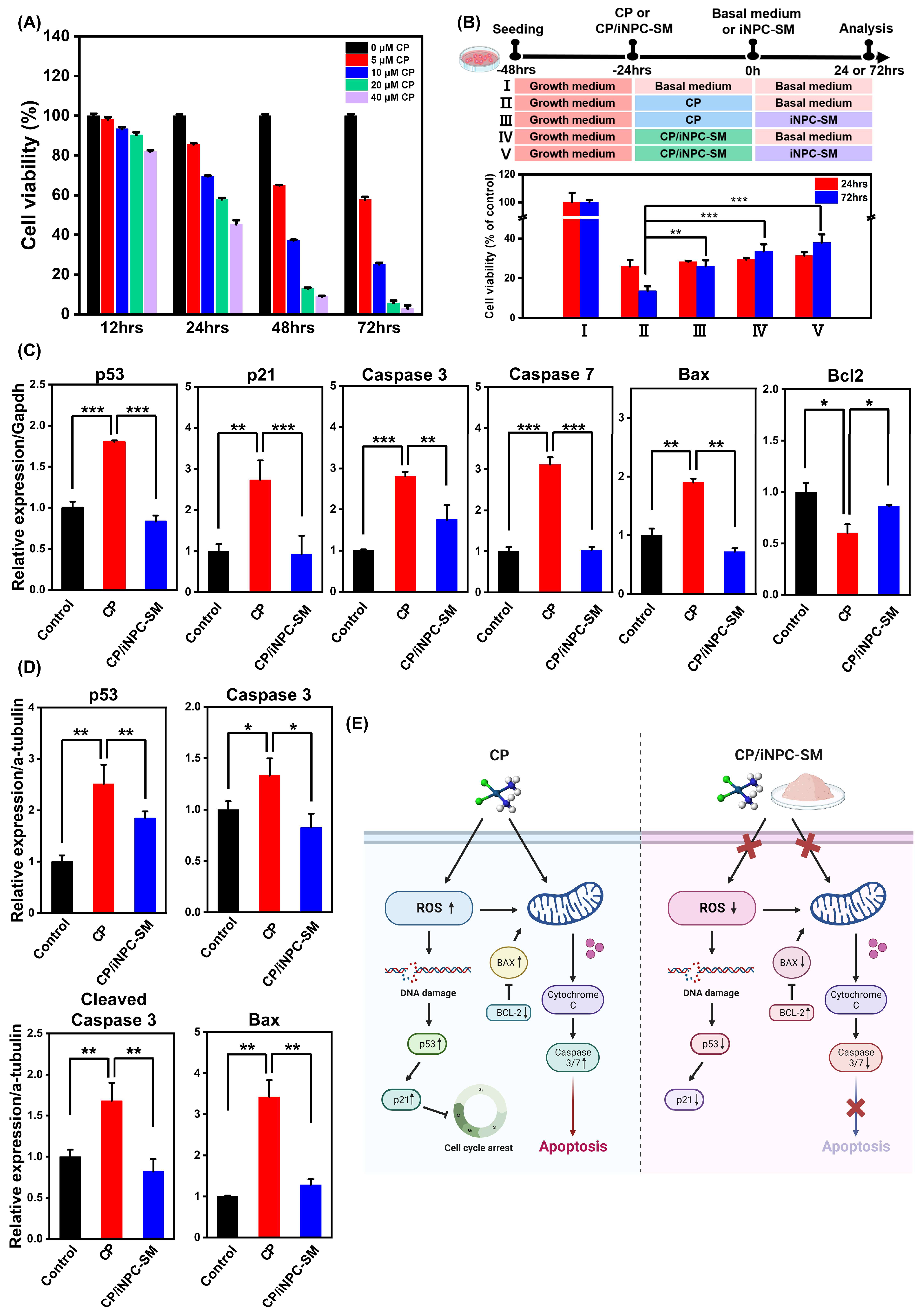
2.2. In Vitro Protective Effects of iNPC-SMs against CP-Induced Cytotoxicity
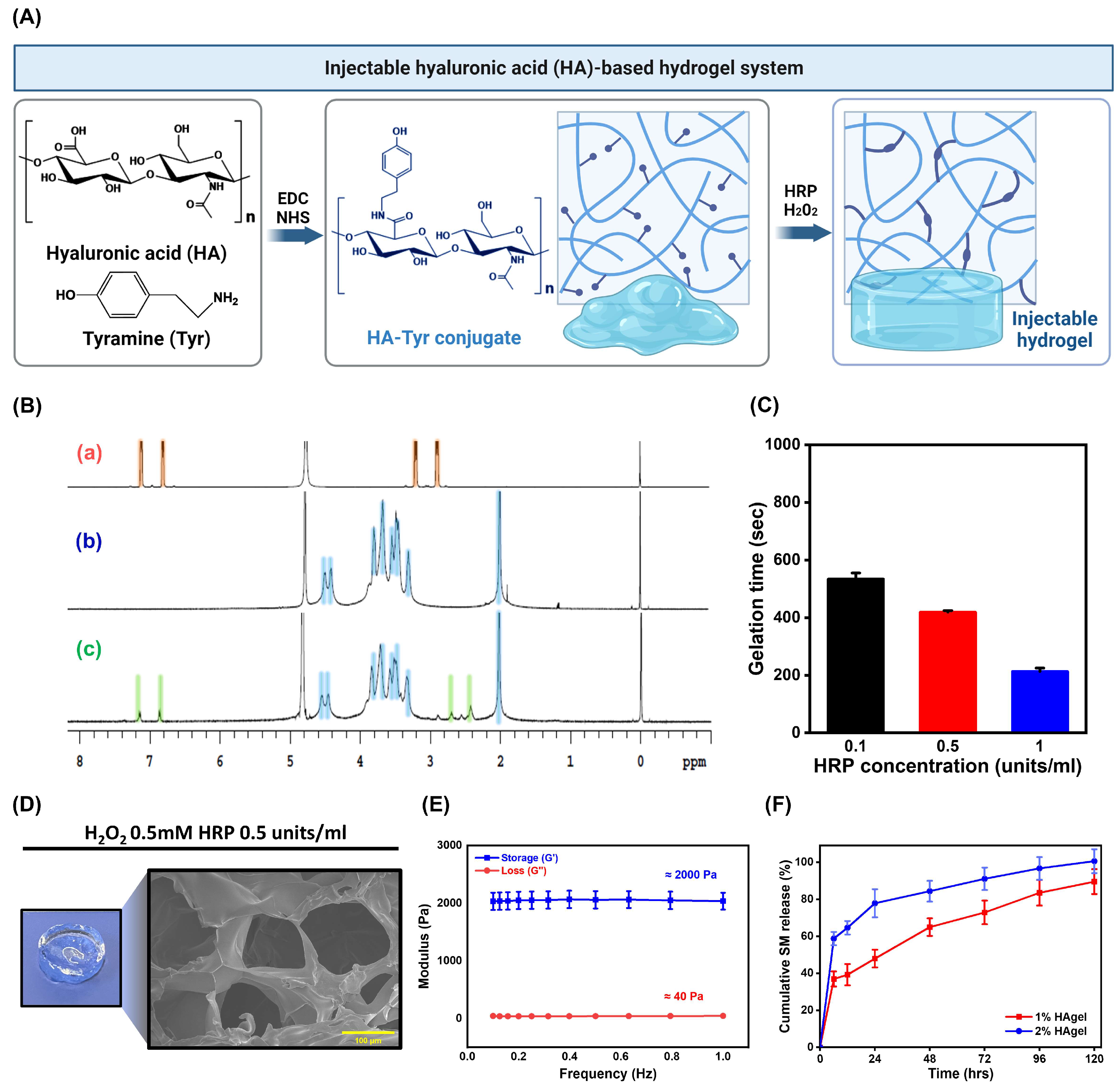
2.3. Preparation and Characterization of HA-Based Injectable Hydrogels
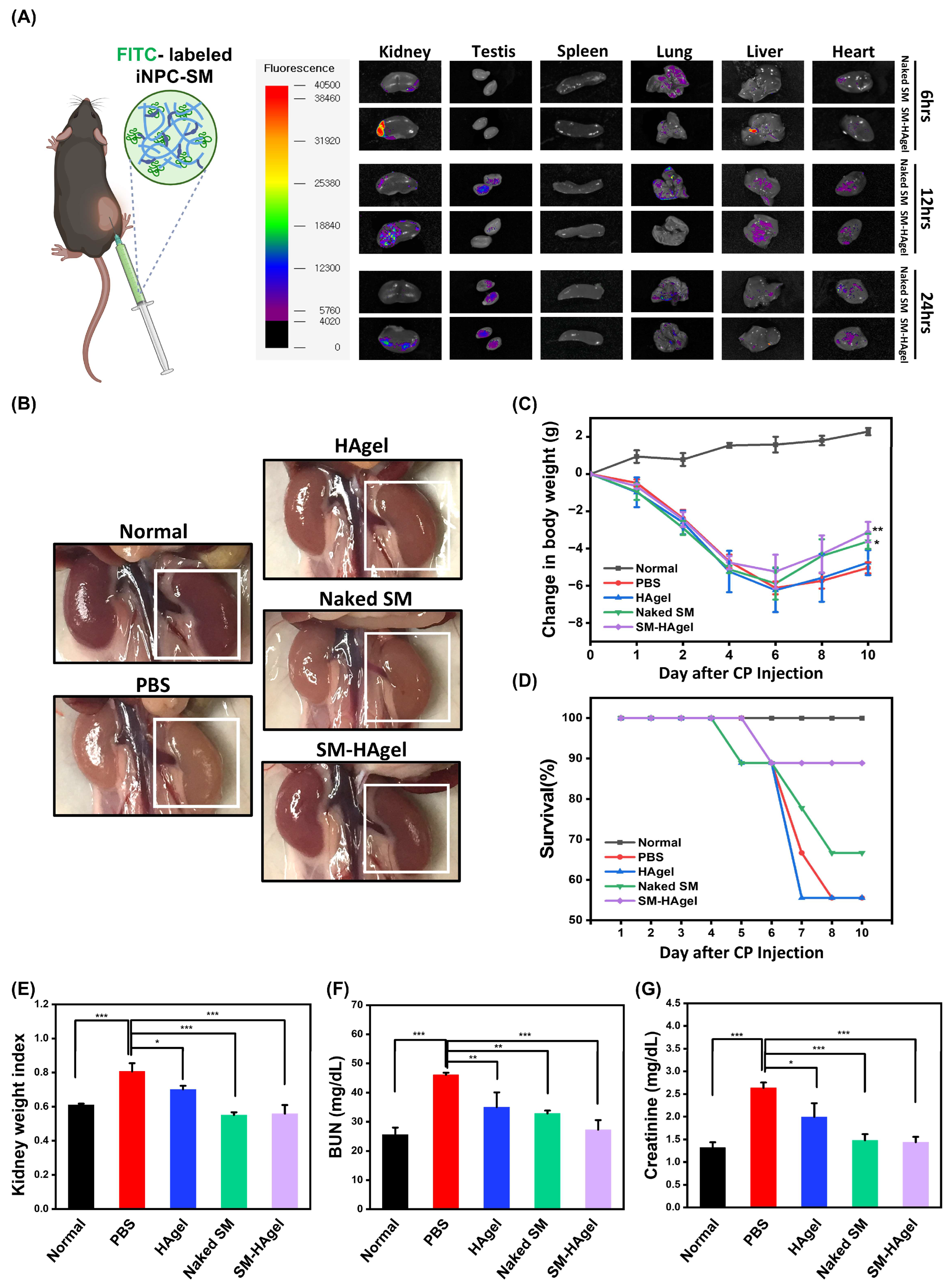
2.4. Hydrogel-Mediated Delivery of iNPC-SMs to AKI Mice
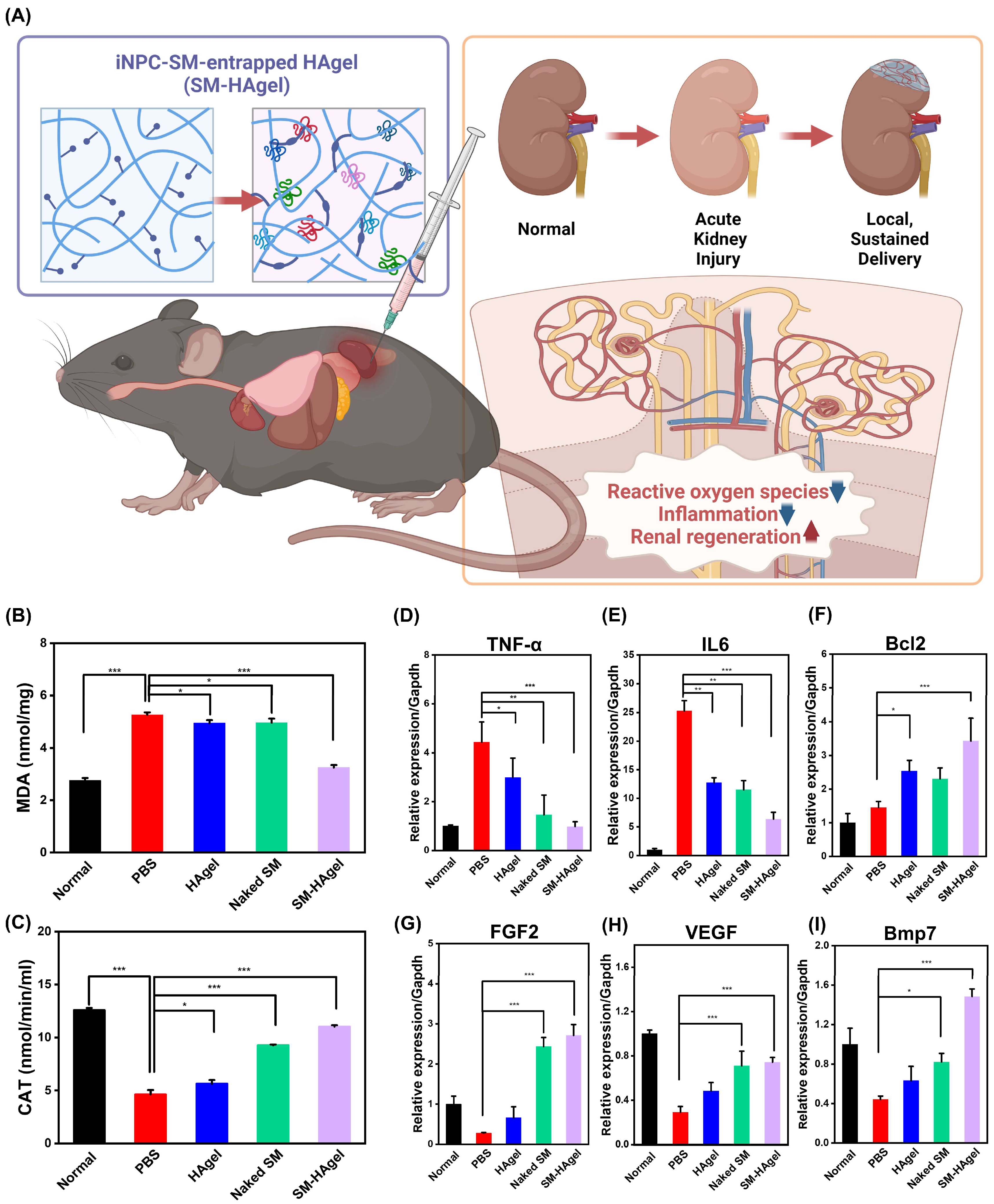
2.5. The Therapeutic Actions of iNPC-SMs in AKI Mice
3. Materials and Methods
3.1. Ethics Statement
3.2. Animals and Cells
3.3. Preparation of iNPC-SMs
3.4. ELISA
3.5. Cytokine Array
3.6. Cell Viability
3.7. Reactive Oxygen Species Assay
3.8. Real-Time PCR Analysis
3.9. Western Blot
3.10. Synthesis of Hyaluronic Acid-Tyramine Conjugate
3.11. Preparation and Characterization of HA-Hydrogel
3.12. Biodistribution Study
3.13. In Vivo Delivery and Renal Recovery
3.14. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bonventre, J.V.; Yang, L. Cellular pathophysiology of ischemic acute kidney injury. J. Clin. Investig. 2011, 121, 4210–4221. [Google Scholar] [CrossRef]
- Ronco, C.; Bellomo, R.; Kellum, J.A. Acute kidney injury. Lancet 2019, 394, 1949–1964. [Google Scholar] [CrossRef] [PubMed]
- Thurlow, J.S.; Joshi, M.; Yan, G.; Norris, K.C.; Agodoa, L.Y.; Yuan, C.M.; Nee, R. Global Epidemiology of End-Stage Kidney Disease and Disparities in Kidney Replacement Therapy. Am. J. Nephrol. 2021, 52, 98–107. [Google Scholar] [CrossRef]
- Tolwani, A. Continuous Renal-Replacement Therapy for Acute Kidney Injury. N. Engl. J. Med. 2012, 367, 2505–2514. [Google Scholar] [CrossRef] [PubMed]
- Benoit, S.W.; Devarajan, P. Acute kidney injury: Emerging pharmacotherapies in current clinical trials. Pediatr. Nephrol. 2018, 33, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewski, W.; Dobrzyński, M.; Szymonowicz, M.; Rybak, Z. Stem cells: Past, present, and future. Stem Cell Res. Ther. 2019, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Margiana, R.; Markov, A.; Zekiy, A.O.; Hamza, M.U.; Al-Dabbagh, K.A.; Al-Zubaidi, S.H.; Hameed, N.M.; Ahmad, I.; Sivaraman, R.; Kzar, H.H.; et al. Clinical application of mesenchymal stem cell in regenerative medicine: A narrative review. Stem Cell Res. Ther. 2022, 13, 366. [Google Scholar] [CrossRef]
- Yamanaka, S. Pluripotent Stem Cell-Based Cell Therapy-Promise and Challenges. Cell Stem Cell 2020, 27, 523–531. [Google Scholar] [CrossRef]
- Hoang, D.M.; Pham, P.T.; Bach, T.Q.; Ngo, A.T.L.; Nguyen, Q.T.; Phan, T.T.K.; Nguyen, G.H.; Le, P.T.T.; Hoang, V.T.; Forsyth, N.R.; et al. Stem cell-based therapy for human diseases. Signal Transduct. Target. Ther. 2022, 7, 272. [Google Scholar] [CrossRef]
- Li, Y.; Hao, J.; Hu, Z.; Yang, Y.-G.; Zhou, Q.; Sun, L.; Wu, J. Current status of clinical trials assessing mesenchymal stem cell therapy for graft versus host disease: A systematic review. Stem Cell Res. Ther. 2022, 13, 93. [Google Scholar] [CrossRef]
- Fox, I.J.; Daley, G.Q.; Goldman, S.A.; Huard, J.; Kamp, T.J.; Trucco, M. Stem cell therapy. Use of differentiated pluripotent stem cells as replacement therapy for treating disease. Science 2014, 345, 1247391. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, K.; Haag, D.; Wernig, M. Direct somatic lineage conversion. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140368. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, Y.; Liu, J.; Qian, L. Direct cell reprogramming: Approaches, mechanisms and progress. Nat. Rev. Mol. Cell Biol. 2021, 22, 410–424. [Google Scholar] [CrossRef] [PubMed]
- Sart, S.; Jeske, R.; Chen, X.; Ma, T.; Li, Y. Engineering Stem Cell-Derived Extracellular Matrices: Decellularization, Characterization, and Biological Function. Tissue Eng. Part B Rev. 2020, 26, 402–422. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, T.; Barry, F.P. Stem Cell Therapy and Regenerative Medicine. Mayo Clin. Proc. 2009, 84, 859–861. [Google Scholar] [CrossRef]
- Hartman, H.A.; Lai, H.L.; Patterson, L.T. Cessation of renal morphogenesis in mice. Dev. Biol. 2007, 310, 379–387. [Google Scholar] [CrossRef]
- Kusaba, T.; Lalli, M.; Kramann, R.; Kobayashi, A.; Humphreys, B.D. Differentiated kidney epithelial cells repair injured proximal tubule. Proc. Natl. Acad. Sci. USA 2014, 111, 1527–1532. [Google Scholar] [CrossRef]
- Berger, K.; Bangen, J.M.; Hammerich, L.; Liedtke, C.; Floege, J.; Smeets, B.; Moeller, M.J. Origin of regenerating tubular cells after acute kidney injury. Proc. Natl. Acad. Sci. USA 2014, 111, 1533–1538. [Google Scholar] [CrossRef]
- Gao, W.W.; Zheng, J.; Yun, W.; Kang, P.J.; Park, G.; Song, G.; Kim, I.Y.; You, S. Generation of induced nephron progenitor-like cells from human urine-derived cells. Int. J. Mol. Sci. 2021, 22, 13449. [Google Scholar] [CrossRef]
- Gao, W.-W.; Chun, S.Y.; Kim, B.S.; Ha, Y.-S.; Lee, J.N.; Lee, E.H.; Kim, I.Y.; You, S.; Kwon, T.G. Locally transplanted human urine-induced nephron progenitor cells contribute to renal repair in mice kidney with diabetic nephropathy. Biochem. Biophys. Res. Commun. 2022, 629, 128–134. [Google Scholar] [CrossRef]
- Yu, S.; Yu, S.; Liu, H.; Liao, N.; Liu, X. Enhancing mesenchymal stem cell survival and homing capability to improve cell engraftment efficacy for liver diseases. Stem Cell Res. Ther. 2023, 14, 235. [Google Scholar] [CrossRef] [PubMed]
- Scopes, R.K. Optimization of Procedures; Final Steps. In Protein Purification: Principles and Practice; Scopes, R.K., Ed.; Springer: New York, NY, USA, 1994; pp. 310–345. [Google Scholar]
- Doonan, S.; Cutler, P. General Strategies. In Protein Purification Protocols; Cutler, P., Ed.; Humana Press: Totowa, NJ, USA, 2004; pp. 1–13. [Google Scholar]
- Walker, S.J.; Zhao, S.Y. Chapter 70—Markers of Repair and Regeneration in the Marginal Kidney. In Kidney Transplantation, Bioengineering and Regeneration; Orlando, G., Remuzzi, G., Williams, D.F., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 989–996. [Google Scholar]
- Tsuji, K.; Kitamura, S. Trophic Factors from Tissue Stem Cells for Renal Regeneration. Stem Cells Int. 2015, 2015, 537204. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Park, K.; Jang, J.; Son, D.; Park, J.; Kim, J.; Yoo, J.-E.; You, S.; Kim, I.-Y. Utilizing stem cell-secreted molecules as a versatile toolbox for skin regenerative medicine. J. Control. Release 2024, 370, 583–599. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, B.D.; Valerius, M.T.; Kobayashi, A.; Mugford, J.W.; Soeung, S.; Duffield, J.S.; McMahon, A.P.; Bonventre, J.V. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell 2008, 2, 284–291. [Google Scholar] [CrossRef]
- Sung, M.J.; Kim, D.H.; Jung, Y.J.; Kang, K.P.; Lee, A.S.; Lee, S.; Kim, W.; Davaatseren, M.; Hwang, J.-T.; Kim, H.-J.; et al. Genistein protects the kidney from cisplatin-induced injury. Kidney Int. 2008, 74, 1538–1547. [Google Scholar] [CrossRef]
- Modaresi, A.; Nafar, M.; Sahraei, Z. Oxidative stress in chronic kidney disease. Iran. J. Kidney Dis. 2015, 9, 165–179. [Google Scholar]
- Gyurászová, M.; Gurecká, R.; Bábíčková, J.; Tóthová, Ľ. Oxidative Stress in the Pathophysiology of Kidney Disease: Implications for Noninvasive Monitoring and Identification of Biomarkers. Oxidative Med. Cell. Longev. 2020, 2020, 5478708. [Google Scholar] [CrossRef]
- Hosohata, K. Role of Oxidative Stress in Drug-Induced Kidney Injury. Int. J. Mol. Sci. 2016, 17, 1826. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Park, S.; Santosh, S.S.; Ganeshalingam, A.; Thiripuranathar, G.; Sathiyaseelan, A.; Vijayasarathy, S.; Swaminathan, A.; Priya, V.V.; Wang, M.H. Application of hyaluronic acid in tissue engineering, regenerative medicine, and nanomedicine: A review. Int. J. Biol. Macromol. 2022, 222 (Pt B), 2744–2760. [Google Scholar] [CrossRef]
- Furnus, C.C.; de Matos, D.G.; Martínez, A.G. Effect of hyaluronic acid on development of in vitro produced bovine embryos. Theriogenology 1998, 49, 1489–1499. [Google Scholar] [CrossRef]
- Jin, K.; Hyun Kuk, S.; Hyun Wook, L.; Eun Young, P.; Wan Young, K. Developmental Expression of Hyaluronan in Rat Kidney. Am. Soc. Nephrol. Natl. Meet. 2006, 25, 170. [Google Scholar]
- Kurisawa, M.; Chung, J.E.; Yang, Y.Y.; Gao, S.J.; Uyama, H. Injectable biodegradable hydrogels composed of hyaluronic acid-tyramine conjugates for drug delivery and tissue engineering. Chem. Commun. 2005, 34, 4312–4314. [Google Scholar] [CrossRef] [PubMed]
- Gilli, R.; Kacuráková, M.; Mathlouthi, M.; Navarini, L.; Paoletti, S. FTIR studies of sodium hyaluronate and its oligomers in the amorphous solid phase and in aqueous solution. Carbohydr. Res. 1994, 263, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.; Martin, M.; El Tahchi, M.R.; Balme, S.; Faour, W.H.; Varga, B.; Cloitre, T.; Páll, O.; Cuisinier, F.J.G.; Gergely, C.; et al. Influence of Hydrolyzed Polyacrylamide Hydrogel Stiffness on Podocyte Morphology, Phenotype, and Mechanical Properties. ACS Appl. Mater. Interfaces 2019, 11, 32623–32632. [Google Scholar] [CrossRef]
- Melica, M.E.; La Regina, G.; Parri, M.; Peired, A.J.; Romagnani, P.; Lasagni, L. Substrate Stiffness Modulates Renal Progenitor Cell Properties via a ROCK-Mediated Mechanotransduction Mechanism. Cells 2019, 8, 1561. [Google Scholar] [CrossRef]
- Cherstvy, A.G.; Thapa, S.; Wagner, C.E.; Metzler, R. Non-Gaussian, non-ergodic, and non-Fickian diffusion of tracers in mucin hydrogels. Soft Matter 2019, 15, 2526–2551. [Google Scholar] [CrossRef]
- Fujiyabu, T.; Li, X.; Chung, U.-i.; Sakai, T. Diffusion Behavior of Water Molecules in Hydrogels with Controlled Network Structure. Macromolecules 2019, 52, 1923–1929. [Google Scholar] [CrossRef]
- Axpe, E.; Chan, D.; Offeddu, G.S.; Chang, Y.; Merida, D.; Hernandez, H.L.; Appel, E.A. A Multiscale Model for Solute Diffusion in Hydrogels. Macromolecules 2019, 52, 6889–6897. [Google Scholar] [CrossRef]
- Tögel, F.; Zhang, P.; Hu, Z.; Westenfelder, C. VEGF is a mediator of the renoprotective effects of multipotent marrow stromal cells in acute kidney injury. J. Cell. Mol. Med. 2009, 13, 2109–2114. [Google Scholar] [CrossRef]
- Tsujimura, T.; Idei, M.; Yoshikawa, M.; Takase, O.; Hishikawa, K. Roles and regulation of bone morphogenetic protein-7 in kidney development and diseases. World J. Stem Cells 2016, 8, 288–296. [Google Scholar] [CrossRef]
- Jooybar, E.; Abdekhodaie, M.J.; Alvi, M.; Mousavi, A.; Karperien, M.; Dijkstra, P.J. An injectable platelet lysate-hyaluronic acid hydrogel supports cellular activities and induces chondrogenesis of encapsulated mesenchymal stem cells. Acta Biomater. 2019, 83, 233–244. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, K.; Gao, W.-W.; Zheng, J.; Oh, K.T.; Kim, I.-Y.; You, S. Hydrogel-Mediated Local Delivery of Induced Nephron Progenitor Cell-Sourced Molecules as a Cell-Free Approach for Acute Kidney Injury. Int. J. Mol. Sci. 2024, 25, 10615. https://doi.org/10.3390/ijms251910615
Park K, Gao W-W, Zheng J, Oh KT, Kim I-Y, You S. Hydrogel-Mediated Local Delivery of Induced Nephron Progenitor Cell-Sourced Molecules as a Cell-Free Approach for Acute Kidney Injury. International Journal of Molecular Sciences. 2024; 25(19):10615. https://doi.org/10.3390/ijms251910615
Chicago/Turabian StylePark, Kyoungmin, Wei-Wei Gao, Jie Zheng, Kyung Taek Oh, In-Yong Kim, and Seungkwon You. 2024. "Hydrogel-Mediated Local Delivery of Induced Nephron Progenitor Cell-Sourced Molecules as a Cell-Free Approach for Acute Kidney Injury" International Journal of Molecular Sciences 25, no. 19: 10615. https://doi.org/10.3390/ijms251910615
APA StylePark, K., Gao, W.-W., Zheng, J., Oh, K. T., Kim, I.-Y., & You, S. (2024). Hydrogel-Mediated Local Delivery of Induced Nephron Progenitor Cell-Sourced Molecules as a Cell-Free Approach for Acute Kidney Injury. International Journal of Molecular Sciences, 25(19), 10615. https://doi.org/10.3390/ijms251910615






