Intrathecal Immunoselective Nanopheresis for Alzheimer’s Disease: What and How? Why and When?
Abstract
1. Introduction
1.1. Overview of Alzheimer’s Disease
1.1.1. Prevalence and Risk Factors
- Age: the greatest risk factor for Alzheimer’s is age, with most cases diagnosed after the age of 65. Early-onset Alzheimer’s (before age 65) is rarer, and often linked to genetic pathogenic variants.
- Genetic Factors: several genetic risk factors have been identified. The apolipoprotein E (APOE) gene is the most well-known, particularly the APOE4 allele, which increases the risk of developing AD and is associated with earlier onset. Familial AD, a form of early-onset AD, is linked to pathogenic variants in the APP, PSEN1, and PSEN2 genes.
- Lifestyle and environmental factors: cardiovascular health significantly impacts Alzheimer’s risk, with hypertension, hyperlipidemia, diabetes, obesity, and smoking increasing the likelihood of developing the disease. On the other hand, physical exercise, a healthy diet (such as the Mediterranean diet), and cognitive engagement are associated with lower risk. Sex Differences: women are disproportionately affected by AD, and while part of this is due to women living longer than men, biological and hormonal factors may also contribute to this increased risk.
1.1.2. Pathophysiology
Amyloid-Beta
Tau
Neuroinflammation
Synaptic Dysfunction and Neuronal Death
1.1.3. Treatment
1.2. CNS-Targeted Drug Delivery
1.3. Nanofiltration and Nanoporous Membranes in Biomedical Applications
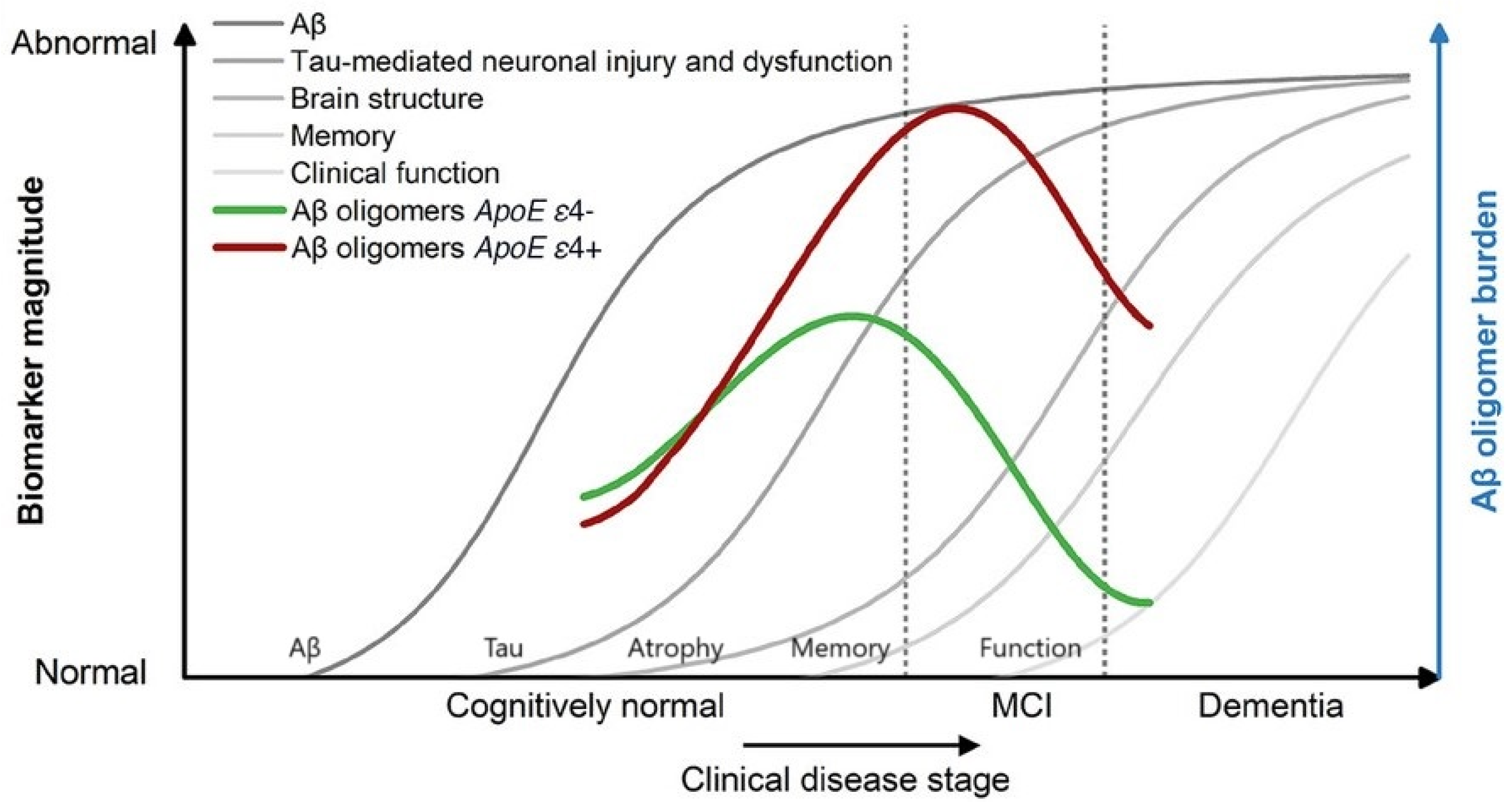
1.4. Targeting Soluble Amyloid-β Oligomers (SAβOs) in Alzheimer’s Disease
1.4.1. Strategies for Clearing SAβOs from the Periphery
Plasmapheresis
Dialysis
Immunotherapy
1.4.2. Strategies for Clearing SAβOs Directly from the CNS
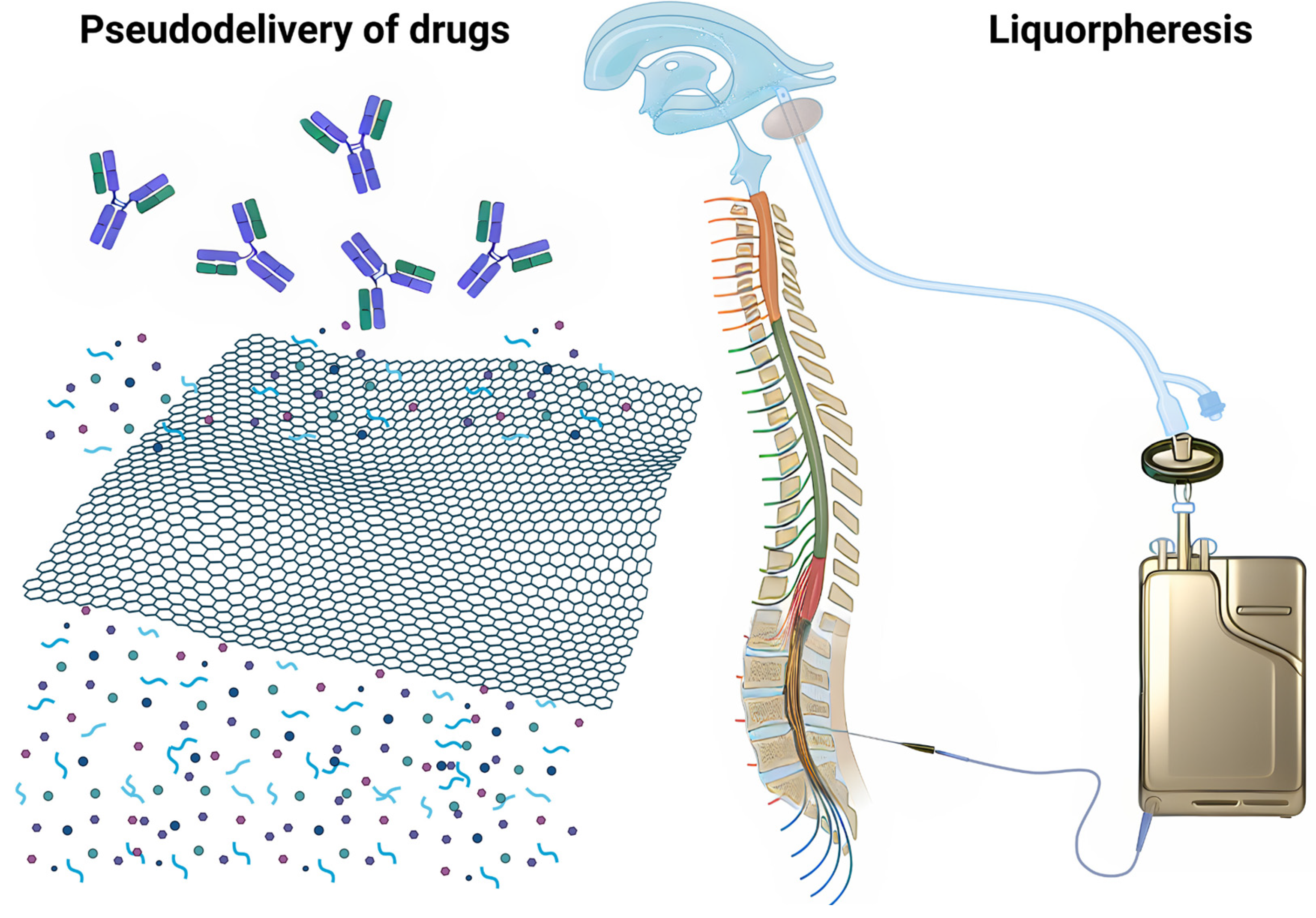
2. What Is Intrathecal Immunoselective Nanopheresis?
2.1. States of mAbs
- 4.
- Immobilized mAbs: in this approach, mAbs are fixed onto a substrate within the device. The nanoporous membrane allows the target molecules in the fluid to pass through and interact with the immobilized mAbs, facilitating their removal from the fluid.
- 5.
- Soluble mAbs: alternatively, mAbs can be dissolved in a solution and contained within a capsule fitted with a nanoporous membrane. This membrane prevents the mAbs from escaping while permitting target molecules to enter the capsule. Once inside, the mAbs interact with the target molecules, effectively capturing and removing them from the fluid [19].
2.2. Application in Intrathecal Pseudodelivery
- Intrathecal Pseudodelivery: in this context, the pseudodelivery refers to the device’s function in filtering target molecules from the CSF without releasing the mAbs themselves into the fluid. The nanoporous membrane and the presence of soluble mAbs enable selective interaction with the target molecules, facilitating their removal [67].
- Liquorpheresis: this process is a type of CSF filtration or liquorpheresis, where the goal is to purify the CSF by removing unwanted substances, such as amyloid-β oligomers or other pathological agents, while leaving essential components intact.
2.3. Advantages and Potential
- Target Specificity: the combination of nanoporous membranes and mAbs allows for precise targeting of specific molecules, reducing off-target effects and increasing the efficiency of the filtration process.
- Reduced Side Effects: by focusing on the removal of specific pathological agents from the CSF, this method minimizes potential systemic side effects associated with broader therapeutic interventions.
- Enhanced Therapeutic Outcomes: improved targeting and selective clearance of harmful molecules can lead to better management of neurological diseases, potentially slowing disease progression and improving patient outcomes.
3. How Does Intrathecal Immunoselective Nanopheresis with Soluble mAbs Work?
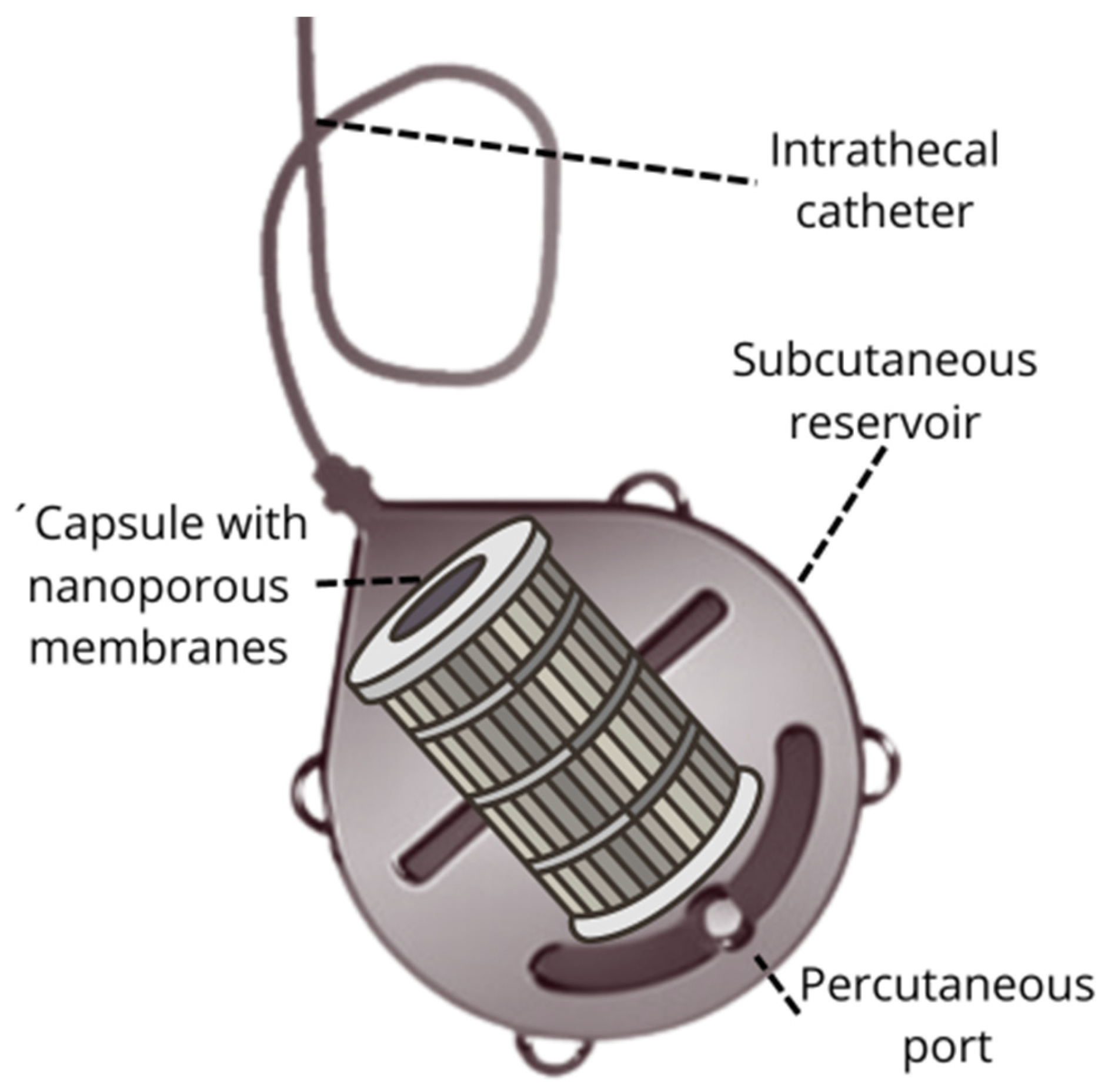
3.1. Subcutaneous Reservoir with Nanoporous Membranes
- Nanoporous Membranes: these membranes are designed to capture pathological molecules based on their size and characteristics. The ability to tailor pore sizes allows for precise targeting of different molecules, enhancing the effectiveness of the filtration process.
- Subcutaneous Placement: the reservoir is implanted beneath the skin, providing a minimally invasive approach for continuous therapy. Its placement ensures easy access for maintenance and replacement procedures.
3.2. Systems for CSF Flow
- Electromechanical Pumps: traditional systems use electromechanical pumps to regulate CSF flow. These pumps, similar to those used in intrathecal drug delivery systems, can be programmed to achieve the desired flow rate. However, they are limited by battery life, weight, and MRI compatibility issues.
- Smart Catheters: an alternative approach involves the use of “smart catheters” that can manage CSF flow without the need for electromechanical pumps. These catheters are designed to ensure continuous fluid flow, while avoiding the limitations of traditional pump systems.
- Alleviate Symptoms: reduction in the concentration of target molecules can help alleviate symptoms associated with various neurological conditions [61].
- Slow Disease Progression: by removing pathological molecules, the system may slow the progression of neurodegenerative diseases.
3.3. Preliminary and Ongoing Research
- Restoration of BBB Function: improvement in the integrity and function of the BBB [70].
4. Why Is Intrathecal Immunoselective Nanopheresis Needed?
4.1. Enhanced Targe-Molecule Clearance
4.2. Reduced Risk of Systemic Side Effects
4.3. Continuous and Efficient Treatment
4.4. Integration with Other Therapeutic Approaches
4.5. Flexibility and Personalization
5. When Should Intrathecal Immunoselective Nanopheresis Be Applied?
5.1. Disease Stage
5.2. Patient Selection
5.2.1. Disease Type and Stage
5.2.2. Genetic Factors
5.2.3. Overall Health
6. Limitations, Alternative Targets, and Future Perspectives
6.1. Limitations
6.1.1. Target Limitations
6.1.2. Invasiveness and Complication Risks
6.1.3. Challenges with Long-Term Efficacy
6.1.4. Cost and Accessibility
6.2. Alternative Targets
6.2.1. Apolipoprotein E (ApoE) Isoforms
6.2.2. Soluble Amyloid Precursor Protein Beta (sAPPβ)
6.2.3. Beta-Secretase (BACE) Inhibitors
6.2.4. Amyloid-Binding Proteins
6.2.5. Pro-Inflammatory Cytokines
6.2.6. Tau Protein
6.2.7. Metalloproteins
6.3. Future Perspectives
Technological Optimization
7. Conclusions
8. Patents
Funding
Conflicts of Interest
References
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Drug and gene targeting to the brain with molecular Trojan horses. Nat. Rev. Drug Discov. 2002, 1, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Bors, L.A.; Erdő, F. Overcoming the Blood–Brain Barrier. Challenges and Tricks for CNS Drug Delivery. Sci. Pharm. 2019, 87, 6. [Google Scholar] [CrossRef]
- Banks, W.A. Blood-brain barrier transport of cytokines: A mechanism for neuropathology. Curr. Pharm. Des. 2003, 9, 1037–1048. [Google Scholar] [CrossRef]
- La Barbera, L.; Mauri, E.; D’amelio, M.; Gori, M. Functionalization strategies of polymeric nanoparticles for drug delivery in Alzheimer’s disease: Current trends and future perspectives. Front. Neurosci. 2022, 16, 939855. [Google Scholar] [CrossRef]
- Zlokovic, B.V. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 2008, 57, 178–201. [Google Scholar] [CrossRef]
- LeWitt, P.A.; Lipsman, N.; Kordower, J.H. Focused ultrasound opening of the blood–brain barrier for treatment of Parkinson’s disease. Mov. Disord. 2019, 34, 1274–1278. [Google Scholar] [CrossRef]
- Montagne, A.; Barnes, S.R.; Sweeney, M.D.; Halliday, M.R.; Sagare, A.P.; Zhao, Z.; Toga, A.W.; Jacobs, R.E.; Liu, C.Y.; Amezcua, L.; et al. Blood-brain barrier breakdown in the aging human hippocampus. Neuron 2015, 85, 296–302. [Google Scholar] [CrossRef]
- Sharma, C.; Woo, H.; Kim, S.R. Addressing Blood–Brain Barrier Impairment in Alzheimer’s Disease. Biomedicines 2022, 10, 742. [Google Scholar] [CrossRef]
- De Boer, A.G.; Gaillard, P.J. Drug targeting to the brain. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 323–355. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Day, C.M.; Abdella, S.; Garg, S. Alzheimer’s disease current therapies, novel drug delivery systems and future directions for better disease management. J. Control. Release 2024, 367, 402–424. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Ma, Z.; Yang, G.-Z. Micro/nanosystems for controllable drug delivery to the brain. Innov. 2024, 5, 100548. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Vo, T.K.; Vo, G.V. Therapeutic Strategies and Nano-Drug Delivery Applications in Management of Aging Alzheimer’s Disease. In Reviews on New Drug Targets in Age-Related Disorders; Guest, P.C., Ed.; Advances in Experimental Medicine and Biolog; Springer: Cham, Switzerland, 2021; Volume 1286, pp. 183–198. [Google Scholar] [CrossRef]
- Samipour, S.; Setoodeh, P.; Rahimpour, E.; Rahimpour, M.R. Functional nanoporous membranes for drug delivery. In Current Trends and Future Developments on (Bio-) Membranes; Elsevier: Amsterdam, The Netherlands, 2024; pp. 255–288. [Google Scholar] [CrossRef]
- Lv, L.; Zeng, Q.; Wu, S.; Xie, H.; Chen, J.; Guo, X.J.; Hao, C.; Zhang, X.; Ye, M.; Zhang, L. Exosome-Based translational nanomedicine: The therapeutic potential for drug delivery. In Mesenchymal Stem Cell Derived Exosomes; Tang, Y., Dawn, B., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 161–176. [Google Scholar] [CrossRef]
- Teleanu, R.I.; Preda, M.D.; Niculescu, A.-G.; Vladâcenco, O.; Radu, C.I.; Grumezescu, A.M.; Teleanu, D.M. Current Strategies to Enhance Delivery of Drugs across the Blood–Brain Barrier. Pharmaceutics 2022, 14, 987. [Google Scholar] [CrossRef] [PubMed]
- Shende, P.; Govardhane, S. Strategies-Based Intrathecal Targeted Drug Delivery System for Effective Therapy, Modeling, and Controlled Release Action. In Modeling and Control of Drug Delivery Systems; Azar, A.T., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 213–225. [Google Scholar] [CrossRef]
- Delhaas, E.; Huygen, F. Complications associated with intrathecal drug delivery systems. BJA Educ. 2019, 20, 51–57. [Google Scholar] [CrossRef]
- Goel, V.; Yang, Y.; Kanwar, S.; Banik, R.K.; Patwardhan, A.M.; Ibrahim, M.; Sivanesan, E.; Shankar, H. Adverse Events and Complications Associated With Intrathecal Drug Delivery Systems: Insights From the Manufacturer and User Facility Device Experience (MAUDE) Database. Neuromodulation: Technol. Neural Interface 2020, 24, 1181–1189. [Google Scholar] [CrossRef]
- Manuel, M.G.; Tamba, B.I.; Leclere, M.; Mabrouk, M.; Schreiner, T.G.; Ciobanu, R.; Cristina, T.Z. Intrathecal pseudodelivery of drugs in the therapy of neurodegenerative diseases: Rationale, basis, and potential applications. Pharmaceutics 2023, 15, 768. [Google Scholar] [CrossRef]
- Hilal, N.; Al-Zoubi, H.; Darwish, N.A.; Mohammad, A.W.; Abu Arabi, M.A. A comprehensive review of nanofiltration membranes: Treatment, pretreatment, modelling, and atomic force microscopy. Desalination 2004, 170, 281–308. [Google Scholar] [CrossRef]
- Shukla, A.K.; Alam, J.; Alhoshan, M. Recent advancements in polyphenylsulfone membrane modification methods for separation applications. Membranes 2022, 12, 247. [Google Scholar] [CrossRef]
- Lalia, B.S.; Kochkodan, V.; Hashaikeh, R.; Hilal, N. A review on membrane fabrication: Structure, properties, and performance relationship. Desalination 2013, 326, 77–95. [Google Scholar] [CrossRef]
- Ban, G.; Hou, Y.; Shen, Z.; Jia, J.; Chai, L.; Ma, C. Potential biomedical limitations of graphene nanomaterials. Int. J. Nanomed. 2023, 18, 1695–1708. [Google Scholar] [CrossRef] [PubMed]
- Cicchetti, A.; Berrino, A.; Casini, M.; Codella, P.; Facco, G.; Fiore, A.; Marano, G.; Marchetti, M.; Midolo, E.; Minacori, R.; et al. Health technology assessment of pathogen reduction technologies applied to plasma for clinical use. Blood Transfus. 2016, 14, 287–386. [Google Scholar] [CrossRef] [PubMed]
- Peydayesh, M. Nanofiltration membranes: Recent advances and environmental applications. Membranes 2022, 12, 518. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, T.G.; Tamba, B.I.; Mihai, C.T.; Lőrinczi, A.; Baibarac, M.; Ciobanu, R.C.; Popescu, B.O. Nanoporous membranes for the filtration of proteins from biological fluids: Biocompatibility tests on cell cultures and suggested applications for the treatment of Alzheimer’s disease. J. Clin. Med. 2022, 11, 5846. [Google Scholar] [CrossRef] [PubMed]
- Osama, L.; Handal, H.T.; El-Sayed, S.A.M.; Elzayat, E.M.; Mabrouk, M. Fabrication and optimisation of alumina nanoporous membranes for drug delivery applications: A comparative study. Nanomaterials 2024, 14, 1078. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.W.; Quaranta, V.; Glenner, G.G. Neuritic plaques and cerebrovascular amyloid in Alzheimer disease are antigenically related. Proc. Natl. Acad. Sci. USA 1985, 82, 8729–8732. [Google Scholar] [CrossRef]
- Glenner, G.G.; Wong, C.W. Alzheimer’s disease: Initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem. Biophys. Res. Commun. 1984, 120, 885–890. [Google Scholar] [CrossRef]
- Tolar, M.; Abushakra, S.; Sabbagh, M. The path forward in Alzheimer’s disease therapeutics: Reevaluating the amyloid cascade hypothesis. Alzheimer’s Dement. 2020, 16, 1553–1560. [Google Scholar] [CrossRef]
- Hillen, H. The Beta Amyloid Dysfunction (BAD) Hypothesis for Alzheimer’s Disease. Front. Neurosci. 2019, 13, 1154. [Google Scholar] [CrossRef]
- Tolar, M.; Snyder, P.; Abushakra, S.; Sabbagh, M. Neurotoxic Soluble Amyloid Oligomers Drive Alzheimer’s Pathogenesis and Represent a Clinically Validated Target for Slowing Disease Progression. Int. J. Mol. Sci. 2021, 22, 6355. [Google Scholar] [CrossRef]
- Benilova, I.; Karran, E.; De Strooper, B. The toxic Aβ oligomer and Alzheimer’s disease: An emperor in need of clothes. Nat. Neurosci. 2012, 15, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Blömeke, L.; Rehn, F.; Kraemer-Schulien, V.; Kutzsche, J.; Pils, M.; Bujnicki, T.; Lewczuk, P.; Kornhuber, J.; Freiesleben, S.D.; Schneider, L.; et al. Aβ oligomers peak in early stages of Alzheimer’s disease preceding tau pathology. Alzheimer’s Dementia: Diagn. Assess. Dis. Monit. 2024, 16, e12589. [Google Scholar] [CrossRef] [PubMed]
- Busche, M.A.; Hyman, B.T. Synergy between amyloid-β and tau in Alzheimer’s disease. Nat. Neurosci. 2020, 23, 1183–1193. [Google Scholar] [CrossRef] [PubMed]
- DeMattos, R.B.; Bales, K.R.; Cummins, D.J.; Paul, S.M.; Holtzman, D.M. Brain to Plasma Amyloid-β Efflux: A Measure of Brain Amyloid Burden in a Mouse Model of Alzheimer’s Disease. Science 2002, 295, 2264–2267. [Google Scholar] [CrossRef]
- Boada, M.; López, O.L.; Olazarán, J.; Núñez, L.; Pfeffer, M.; Paricio, M.; Lorites, J.; Piñol-Ripoll, G.; Gámez, J.E.; Anaya, F.; et al. A randomized, controlled clinical trial of plasma exchange with albumin replacement for Alzheimer’s disease: Primary results of the AMBAR Study. Alzheimer’s Dement. 2020, 16, 1412–1425. [Google Scholar] [CrossRef]
- Boada, M.; Ramos-Fernández, E.; Guivernau, B.; Muñoz, F.J.; Costa, M.; Ortiz, A.M.; Jorquera, J.I.; Núñez, L.; Torres, M.; Páez, A. Treatment of Alzheimer’s disease using combination therapy with plasma exchange and haemapheresis with albumin and intravenous immunoglobulin: Rationale and treatment approach of the AMBAR (Alzheimer Management By Albumin Replacement) study. Neurologia 2016, 31, 473–481. [Google Scholar] [CrossRef]
- Boada, M.; López, O.L.; Olazarán, J.; Núñez, L.; Pfeffer, M.; Puente, O.; Piñol-Ripoll, G.; Gámez, J.E.; Anaya, F.; Kiprov, D.; et al. Neuropsychological, neuropsychiatric, and quality-of-life assessments in Alzheimer’s disease patients treated with plasma exchange with albumin replacement from the randomized AMBAR study. Alzheimer’s Dement. 2022, 18, 1314–1324. [Google Scholar] [CrossRef]
- Rohrer, L.; Yunce, M.; Montine, T.J.; Shan, H. Plasma exchange in Alzheimer’s disease. Transfus. Med. Rev. 2023, 37, 10–15. [Google Scholar] [CrossRef]
- Ramirez, S.; Koerich, S.; Astudillo, N.; De Gregorio, N.; Al-Lahham, R.; Allison, T.; Rocha, N.P.; Wang, F.; Soto, C. Plasma exchange reduces Aβ levels in plasma and decreases amyloid plaques in the brain in a mouse model of Alzheimer’s disease. Int. J. Mol. Sci. 2023, 24, 17087. [Google Scholar] [CrossRef]
- Kitaguchi, N.; Tatebe, H.; Sakai, K.; Kawaguchi, K.; Matsunaga, S.; Kitajima, T.; Tomizawa, H.; Kato, M.; Sugiyama, S.; Suzuki, N.; et al. Influx of tau and amyloid-β proteins into the blood during hemodialysis as a therapeutic extracorporeal blood amyloid-β removal system for Alzheimer’s disease. J. Alzheimer’s Dis. 2019, 69, 687–707. [Google Scholar] [CrossRef]
- Kawaguchi, K.; Saigusa, A.; Yamada, S.; Gotoh, T.; Nakai, S.; Hiki, Y.; Hasegawa, M.; Yuzawa, Y.; Kitaguchi, N. Toward the treatment for Alzheimer’s disease: Adsorption is the primary mechanism of removing amyloid β protein with hollow-fiber dialyzers of the suitable materials, polysulfone and polymethyl methacrylate. J. Artif. Organs 2016, 19, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Kitaguchi, N.; Kawaguchi, K.; Yamazaki, K.; Kawachi, H.; Sakata, M.; Kaneko, M.; Kato, M.; Sakai, K.; Ohashi, N.; Hasegawa, M.; et al. Adsorptive filtration systems for effective removal of blood amyloid β: A potential therapy for Alzheimer’s disease. J. Artif. Organs 2018, 21, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, K.; Takeuchi, M.; Yamagawa, H.; Murakami, K.; Nakai, S.; Hori, H.; Ohashi, A.; Hiki, Y.; Suzuki, N.; Sugiyama, S.; et al. A potential therapeutic system for Alzheimer’s disease using adsorbents with alkyl ligands for removal of blood amyloid β. J. Artif. Organs 2013, 16, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Menéndez-González, M.; Pérez-Piñera, P.; Martínez-Rivera, M.; Muñiz, A.L.; Vega, J.A. Immunotherapy for Alzheimer’s disease: Rational basis in ongoing clinical trials. Curr. Pharm. Des. 2011, 17, 508–520. [Google Scholar] [CrossRef] [PubMed]
- Orgogozo, J.M.; Gilman, S.; Dartigues, J.F.; Laurent, B.; Puel, M.; Kirby, L.C.; Jouanny, P.; Dubois, B.; Eisner, L.; Flitman, S.; et al. Subacute meningoencephalitis in a subset of patients with AD after Aβ42 immunization. Neurology 2003, 61, 46–54. [Google Scholar] [CrossRef]
- Jin, Y.; Du, Q.; Song, M.; Kang, R.; Zhou, J.; Zhang, H.; Ding, Y. Amyloid-β-targeting immunotherapies for Alzheimer’s disease. J. Contr. Release 2024, 375, 346–365. [Google Scholar] [CrossRef]
- Sevigny, J.; Chiao, P.; Bussière, T.; Weinreb, P.H.; Williams, L.; Maier, M.; Dunstan, R.; Salloway, S.; Chen, T.; Ling, Y.; et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature 2016, 537, 50–56. [Google Scholar] [CrossRef]
- Islam, R.; Rabbi, A.; Hossain, T.; Sultana, S.; Uddin, S. Mechanistic Approach to Immunity and Immunotherapy of Alzheimer’s Disease: A Review. ACS Chem. Neurosci. 2024, in press. [Google Scholar] [CrossRef]
- Mintun, M.A.; Lo, A.C.; Duggan Evans, C.; Wessels, S.R.; Ardayfio, P.A.; Andersen, S.W.; Shcherbinin, S.; Sparks, J.; Sims, J.R.; Brys, M.; et al. Donanemab in early Alzheimer’s disease. N. Engl. J. Med. 2021, 384, 1691–1704. [Google Scholar] [CrossRef]
- van Dyck, C.H.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab in early Alzheimer’s disease. N. Engl. J. Med. 2023, 388, 9–21. [Google Scholar] [CrossRef]
- Niewoehner, J.; Bohrmann, B.; Collin, L.; Jacobsen, H.; Koch, M.; Wiesmann, M.; Brinkmann, V.; Bechter, A.; Jucker, M.; Hyman, B.T.; et al. Increased brain penetration and potency of a therapeutic antibody using a monovalent molecular shuttle. Neuron 2014, 81, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Menéndez-González, M.; Padilla-Zambrano, H.S.; Alvarez, G. Targeting beta-amyloid at the CSF. Front. Aging Neurosci. 2018, 10, 100. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, T.G.; Menéndez-González, M.; Popescu, B.O. The “Cerebrospinal Fluid Sink Therapeutic Strategy” in Alzheimer’s Disease—From theory to design of applied systems. Biomedicines 2022, 10, 1509. [Google Scholar] [CrossRef] [PubMed]
- Menéndez-González, M. Mechanical filtration of the cerebrospinal fluid: Procedures, systems, and applications. Expert Rev. Med. Devices 2023, 20, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Menéndez-González, M. Liquorpheresis: Cerebrospinal Fluid Filtration to Treat CNS Conditions; Springer: Cham, Switzerland, 2023. [Google Scholar]
- Wollinsky, K.H.; Weindler, M.; Hülser, P.J.; Geiger, P.; Matzek, N.; Mehrkens, H.H.; Kornhuber, H.H. Liquorpheresis (CSF-filtration): An effective treatment in acute and chronic severe autoimmune polyradiculoneuritis (Guillain-Barré syndrome). Eur. Arch. Psychiatry Clin. Neurosci. 1991, 241, 73–76. [Google Scholar] [CrossRef]
- Finsterer, J.; Mamoli, B. Liquorpheresis (CSF filtration) in familial amyotrophic lateral sclerosis. Spinal Cord 1999, 37, 592–593. [Google Scholar] [CrossRef][Green Version]
- Lu, V.M.; Shah, A.H.; González, M.M. The potential of liquorpheresis to treat leptomeningeal disease. World Neurosurg. 2024, 187, 93–98. [Google Scholar] [CrossRef]
- Hülser, P.J.; Wiethölter, H.; Wollinsky, K.H. Liquorpheresis eliminates blocking factors from cerebrospinal fluid in polyradiculoneuritis (Guillain-Barré syndrome). Eur. Arch. Psychiatry Clin. Neurosci. 1991, 241, 69–72. [Google Scholar] [CrossRef]
- Ngan, N.T.T.; Flower, B.; Day, J.N. Treatment of cryptococcal meningitis: How have we got here and where are we going? Drugs 2022, 82, 1237–1249. [Google Scholar] [CrossRef]
- Smilnak, G.J.; Charalambous, L.T.; Cutshaw, D.; Premji, A.M.; Giamberardino, C.D.; Ballard, C.G.; Bartuska, A.P.; Ejikeme, T.U.; Sheng, H.; Verbick, L.Z.; et al. Novel treatment of cryptococcal meningitis via neurapheresis therapy. J. Infect. Dis. 2018, 218, 1147–1154. [Google Scholar] [CrossRef]
- Zeineddine, H.A.; Honarpisheh, P.; McBride, D.; Pandit, P.K.T.; Dienel, A.; Hong, S.H.; Grotta, J.; Blackburn, S. Targeting hemoglobin to reduce delayed cerebral ischemia after subarachnoid hemorrhage. Transl. Stroke Res. 2022, 13, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, S.L.; Grande, A.W.; Swisher, C.B.; Hauck, E.F.; Jagadeesan, B.; Provencio, J.J. Prospective trial of cerebrospinal fluid filtration after aneurysmal subarachnoid hemorrhage via lumbar catheter (PILLAR). Stroke 2019, 50, 2558–2561. [Google Scholar] [CrossRef] [PubMed]
- Menéndez-González, M.; Gasparovic, C. Albumin exchange in Alzheimer’s disease: Might CSF be an alternative route to plasma? Front. Neurol. 2019, 10, 1036. [Google Scholar] [CrossRef] [PubMed]
- Coto-Vilcapoma, A.M.; Castilla-Silgado, J.; Fernández-García, B.; Pinto-Hernández, P.; Cipriani, R.; Capetillo-Zarate, E.; Menéndez-González, M.; Álvarez-Vega, M.; Tomás-Zapico, C. New, fully implantable device for selective clearance of CSF-target molecules: Proof of concept in a murine model of Alzheimer’s disease. Int. J. Mol. Sci. 2022, 23, 9256. [Google Scholar] [CrossRef] [PubMed]
- Coto-Vilcapoma, A. Nanoféresis Inmunoselectiva de Aβ en el Líquido Cefalorraquídeo Como Tratamiento de la Enfermedad de Alzheimer. Ph.D. Thesis, Universidad de Oviedo, Oviedo, Spain, 2024. [Google Scholar]
- Honig, L.S.; Barakos, J.; Dhadda, S.; Kanekiyo, M.; Reyderman, L.; Irizarry, M.; Kramer, L.D.; Swanson, C.J.; Sabbagh, M. ARIA in patients treated with lecanemab (BAN2401) in a phase 2 study in early Alzheimer’s disease. Alzheimer’s Dement. 2023, 9, e12377. [Google Scholar] [CrossRef]
- Pérez-Martín, E.; Coto-Vilcapoma, A.; Vega, J.A.; Álvarez, V. Implantable device for intrathecal pseudodelivery of immunotherapies. J. Prev. Alzheimer’s Dis. 2024, in press. [Google Scholar]
- Watamura, N.; Kakiya, N.; Fujioka, R.; Kamano, N.; Takahashi, M.; Nilsson, P.; Saito, T.; Iwata, N.; Fujisawa, S.; Saido, T.C. The dopaminergic system promotes neprilysin-mediated degradation of amyloid-β in the brain. Sci. Signal. 2024, 17, eadk1822. [Google Scholar] [CrossRef]
- Jack, C.R.; Knopman, D.S.; Jagust, W.J.; Shaw, L.M.; Aisen, P.S.; Weiner, M.W.; Petersen, R.C.; Trojanowski, J.Q. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010, 9, 119–128. [Google Scholar] [CrossRef]
- Agarwal, A.; Gupta, V.; Brahmbhatt, P.; Desai, A.; Vibhute, P.; Joseph-Mathurin, N.; Bathla, G. Amyloid-related imaging abnormalities in Alzheimer disease treated with anti-amyloid-β therapy. Radiographics 2023, 43, e230009. [Google Scholar] [CrossRef]
- Schreiner, T.G.; Adam, M.; Popescu, B.O.; Szilagyi, A.; Stanciu, G.D.; Tamba, B.I.; Ciobanu, R.C. A Nanostructured Protein Filtration Device for Possible Use in the Treatment of Alzheimer’s Disease—Concept and Feasibility after In Vivo Tests. Bioengineering 2023, 10, 1303. [Google Scholar] [CrossRef]
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Long, J.M.; Holtzman, D.M. Alzheimer disease: An update on pathobiology and treatment strategies. Cell 2019, 179, 312–339. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Kanekiyo, T.; Xu, H.; Bu, G. Apolipoprotein E and Alzheimer disease: Risk, mechanisms, and therapy. Nat. Rev. Neurol. 2013, 9, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Castellano, J.M.; Kim, J.; Stewart, F.R.; Jiang, H.; DeMattos, R.B.; Patterson, B.W.; Fagan, A.M.; Morris, J.C.; Mawuenyega, K.G.; Cruchaga, C.; et al. Human apoE isoforms differentially regulate brain amyloid-β peptide clearance. Sci. Transl. Med. 2011, 3, 89ra57. [Google Scholar] [CrossRef] [PubMed]
- Vassar, R.; Kuhn, P.H.; Haass, C.; Kennedy, M.E.; Rajendran, L.; Wong, P.C.; Lichtenthaler, S.F. Function, therapeutic potential, and cell biology of BACE proteases: Current status and future prospects. J. Biol. Chem. 2014, 289, 30373–30379. [Google Scholar] [CrossRef]
- Yan, R.; Vassar, R. Targeting the beta secretase BACE1 for Alzheimer’s disease therapy. Lancet Neurol. 2014, 13, 319–329. [Google Scholar] [CrossRef]
- Wyss-Coray, T.; Rogers, J. Inflammation in Alzheimer disease-a brief review of the basic science and clinical literature. Cold Spring Harb. Perspect. Med. 2012, 2, a006346. [Google Scholar] [CrossRef]
- Congdon, E.E.; Ji, C.; Tetlow, A.M.; Jiang, Y.; Sigurdsson, E.M. Tau-targeting therapies for Alzheimer disease: Current status and future directions. Nat. Rev. Neurol. 2023, 19, 715–736. [Google Scholar] [CrossRef]
- Bush, A.I.; Tanzi, R.E. Therapeutics for Alzheimer’s disease based on the metal hypothesis. Neurotherapeutics 2008, 5, 421–432. [Google Scholar] [CrossRef]
| Agent | Target Population | Mechanism of Action | Main Findings |
|---|---|---|---|
| Aducanumab | Early-stage Alzheimer’s disease (prodromal or mild AD) | Monoclonal antibody targeting amyloid-beta to reduce amyloid plaque deposition | Phase III showed reduction in amyloid plaques but mixed cognitive outcomes; approved by FDA, with controversial efficacy. |
| Lecanemab | Early-stage Alzheimer’s disease, preclinical AD with amyloid pathology | Monoclonal antibody that targets soluble amyloid-beta protofibrils for clearance | Phase III trial (CLARITY AD) showed a 27% reduction in cognitive decline and amyloid burden. Approved by FDA for early Alzheimer’s disease. |
| Donanemab | Early-stage Alzheimer’s disease, participants with amyloid plaques | Monoclonal antibody that specifically targets amyloid plaques in the brain | Phase III results showed a slowing of cognitive decline and significant reduction in amyloid plaques. Participants with intermediate tau levels showed the most benefit. |
| Solanezumab | Mild-to-moderate Alzheimer’s disease | Monoclonal antibody that targets soluble forms of amyloid-beta to reduce amyloid deposition | Phase III trials did not show significant cognitive benefits, despite plaque reduction. |
| Crenezumab | Mild-to-moderate Alzheimer’s disease and familial AD | Monoclonal antibody that targets multiple forms of amyloid-beta, including oligomers | Phase II trials showed plaque reduction but failed to show cognitive benefits in Phase III. |
| Gantenerumab | Mild-to-moderate Alzheimer’s disease | Monoclonal antibody targeting amyloid-beta to promote clearance of plaques | Failed to meet primary outcomes in Phase III, despite reduction in amyloid plaques. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menendez-Gonzalez, M. Intrathecal Immunoselective Nanopheresis for Alzheimer’s Disease: What and How? Why and When? Int. J. Mol. Sci. 2024, 25, 10632. https://doi.org/10.3390/ijms251910632
Menendez-Gonzalez M. Intrathecal Immunoselective Nanopheresis for Alzheimer’s Disease: What and How? Why and When? International Journal of Molecular Sciences. 2024; 25(19):10632. https://doi.org/10.3390/ijms251910632
Chicago/Turabian StyleMenendez-Gonzalez, Manuel. 2024. "Intrathecal Immunoselective Nanopheresis for Alzheimer’s Disease: What and How? Why and When?" International Journal of Molecular Sciences 25, no. 19: 10632. https://doi.org/10.3390/ijms251910632
APA StyleMenendez-Gonzalez, M. (2024). Intrathecal Immunoselective Nanopheresis for Alzheimer’s Disease: What and How? Why and When? International Journal of Molecular Sciences, 25(19), 10632. https://doi.org/10.3390/ijms251910632






