Toxicological Evaluation of Kaempferol and Linearolactone as Treatments for Amoebic Liver Abscess Development in Mesocricetus auratus
Abstract
1. Introduction
2. Results
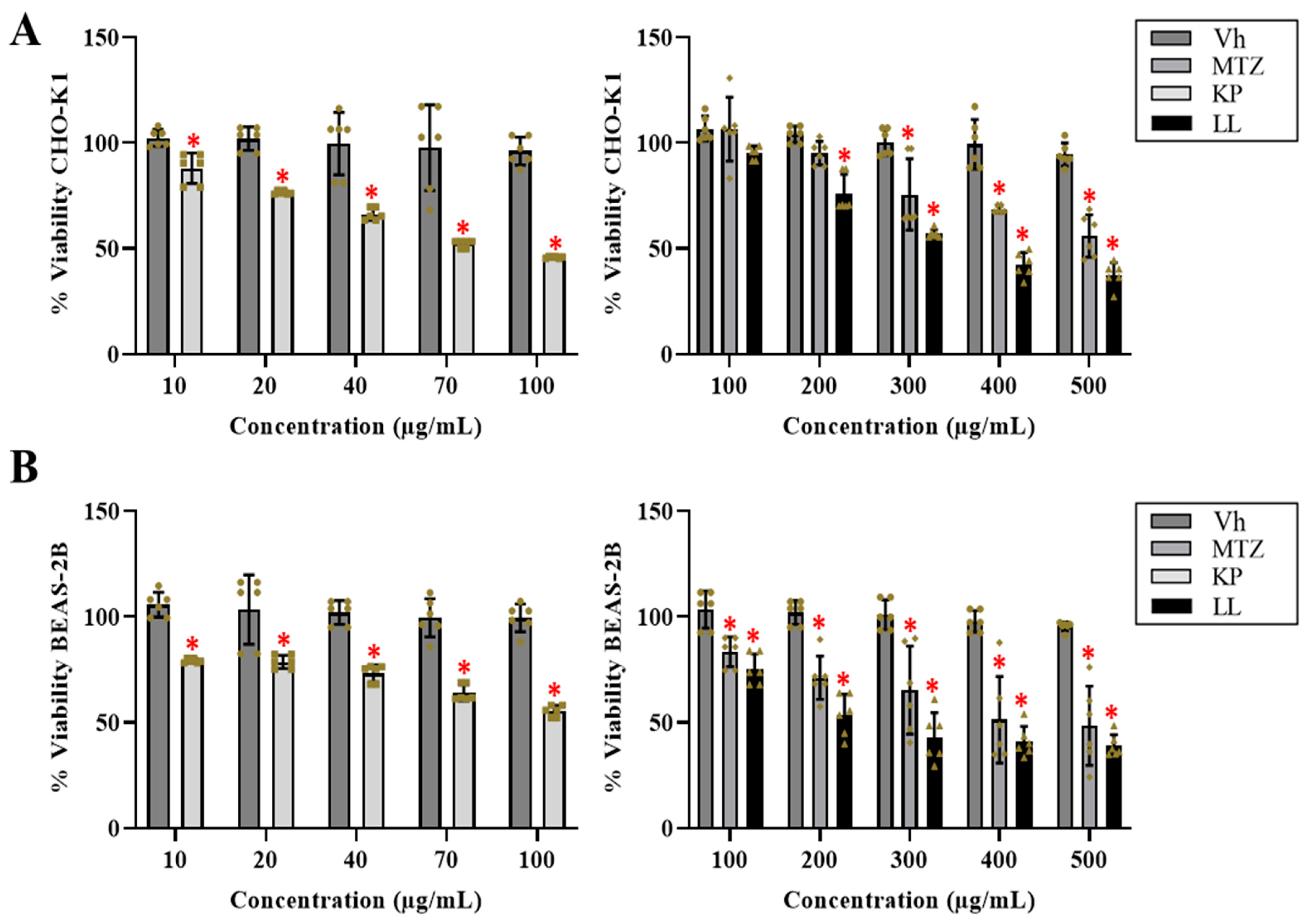
2.1. Cytotoxic Effect of the Active Principles KP and LL in Cell Lines
2.2. Treatment with KP or LL Selectively Induced Cell Death and Morphological Alterations in Trophozoites but Not in Normal Cells
2.3. The Effect of Treatment with KP or LL in the M. auratus ALA Model
2.4. Paraclinical Analysis of Post-Treatment Liver and Kidney Function
2.4.1. Evaluation of the Hepatic Function
2.4.2. Evaluation of the Renal Function
2.4.3. Determination of the Liver Injury Ratios
2.4.4. Determination of the ALA Prognosis Ratios
2.4.5. Determination of the Drugs Toxicity Ratios
2.4.6. Determination of the Kidney Damage Ratios
2.4.7. Evaluation of the Hematic Biometry
2.4.8. Determination of the Hematic Ratios
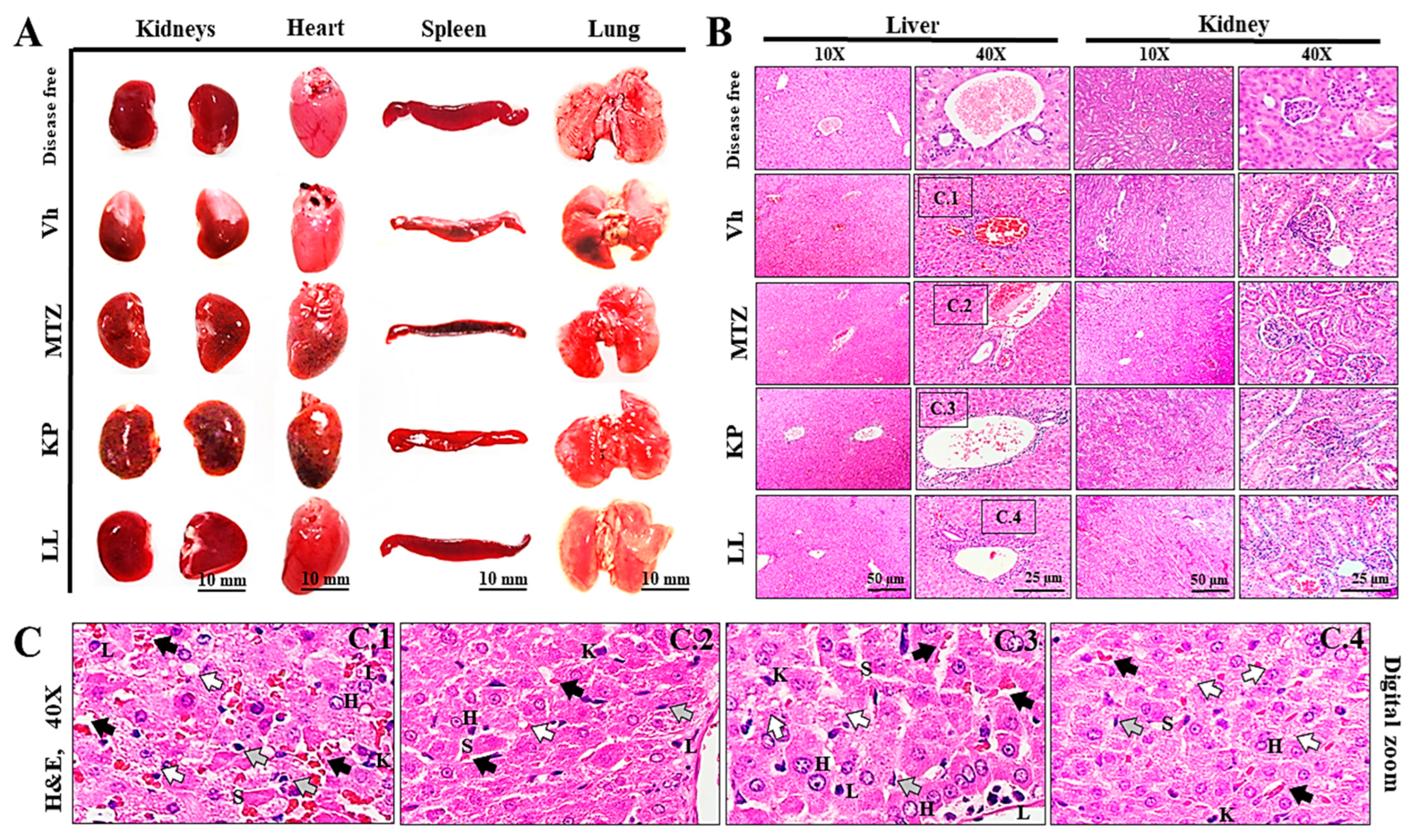
2.5. Treatment with LL or KP Effectively Inhibited the Development of ALA in M. auratus
2.6. Toxicological Evaluation of Hamsters Treated with KP or LL
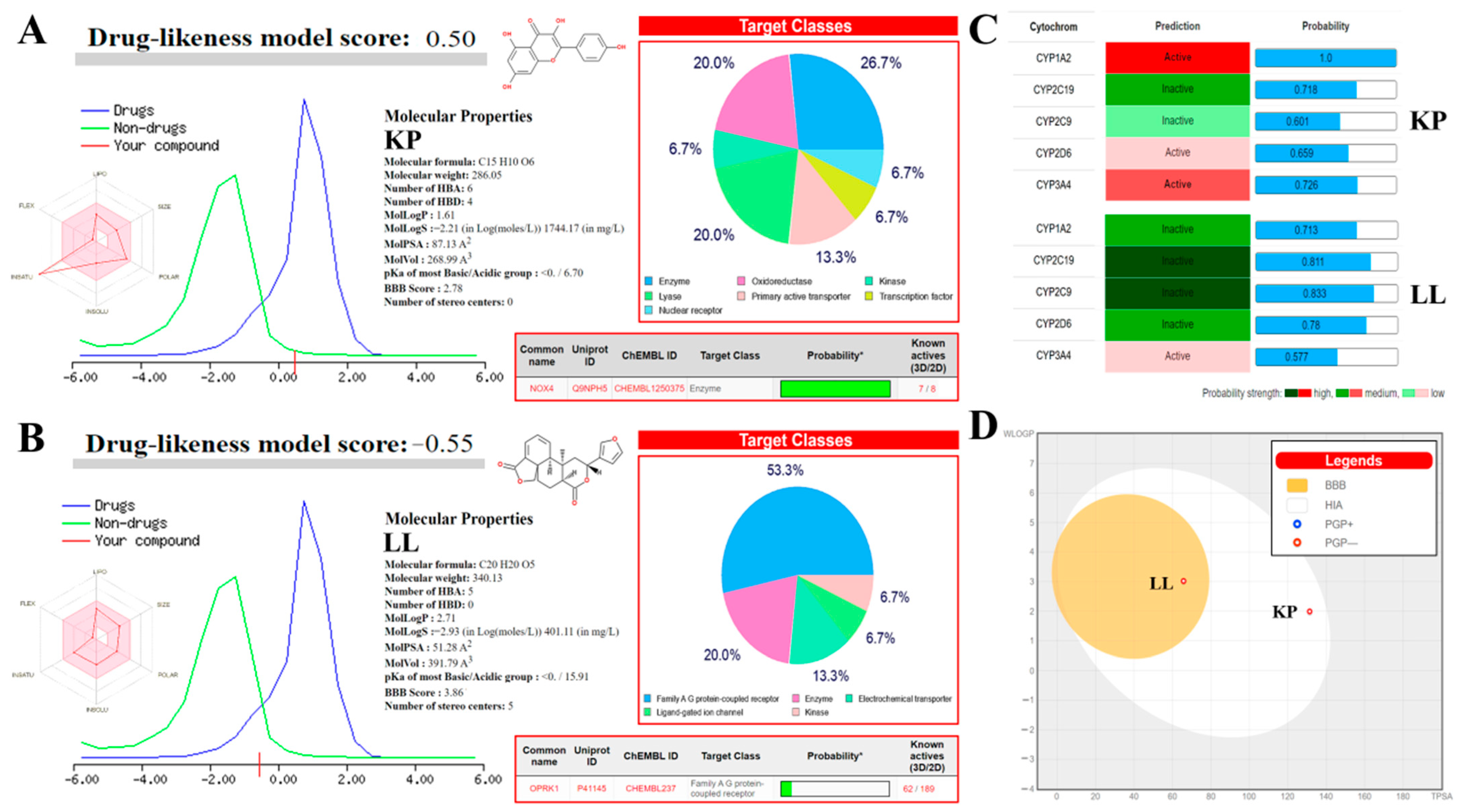
2.7. In Silico Analyses to Determine the Toxicity of KP and LL
3. Discussion
4. Materials and Methods
4.1. Chemical Compounds
4.2. Cell Cultures
4.3. Cytotoxicity Assays in Normal Cell Lines by Formazan Salts
4.4. Cell Death Determination by Flow Cytometry
4.5. Cell Morphology by Confocal Microscopy
4.6. Ultrastructural Morphology Analysis with TEM
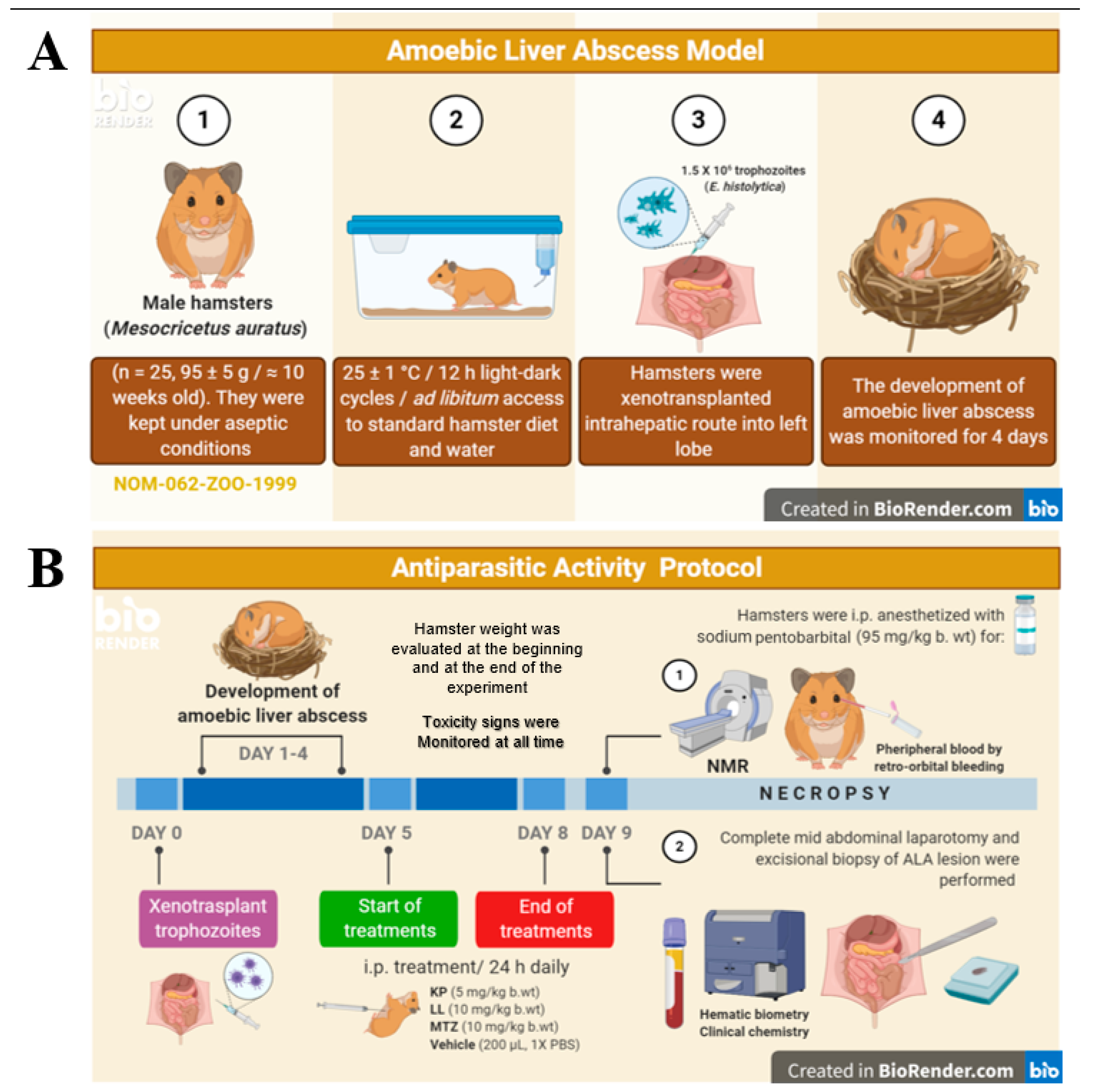
4.7. Hamster Model for ALA
4.8. ALA Induction and the Antiparasitic Protocol
4.9. MRI
4.10. Paraclinical Analysis
4.11. Histopathological Analysis
4.12. In Silico Analyses
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- WHO. Diarrheal Disease. 2017. Available online: https://www.who.int/news-room/fact-sheets/detail/diarrhoeal-disease (accessed on 23 December 2023).
- Shirley, D.A.; Hung, C.C.; Moonah, S. Part 5, Protozoal Infections: Chapter 94—Entamoeba histolytica (Amebiasis). In Hunter’s Tropical Medicine and Emerging Infectious Diseases, 10th ed.; Ryan, E.T., Hill, D.R., Solomon, T., Aronson, N.E., Endy, T.P., Eds.; Elsevier: Toronto, ON, Canada, 2020; pp. 699–706. ISBN 978-0-323-55512-8. [Google Scholar] [CrossRef]
- Gómez-García, C.; Marchat, L.A.; López-Cánovas, L.; Pérez-Ishiwara, D.G.; Rodríguez, M.A.; Orozco, E. Part—Parasitic Drug Resistance, Mechanisms: Chapter—Drug Resistance Mechanisms in Entamoeba histolytica, Giardia lamblia, Trichomonas vaginalis, and Opportunistic Anaerobic Protozoa. In Antimicrobial Drug Resistance. Mechanisms of Drug Resistance, 1st ed.; Mayers, D.L., Sobel, J.D., Oullette, M., Kaye, K.S., Marchaim, D., Eds.; Springer: Cham, Switzerland, 2017; pp. 613–628. ISBN 978-3-319-47264-5. [Google Scholar] [CrossRef]
- VADEMECUM. Metronidazole. 2017. Available online: https://www.vademecum.es/principios-activos-metronidazol-J01XD01 (accessed on 23 December 2023).
- Tsai, J.P.; Hsieh, K.L.; Yeh, T.H.; Lee, Y.J.; Wei, C.R. The Occurrence of Metronidazole-Induced Encephalopathy in Cancer Patients: A Hospital-Based Retrospective Study. Ann. Indian Acad. Neurol. 2019, 22, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Adil, M.; Iqbal, W.; Adnan, F.; Wazir, S.; Khan, I.; Khayam, M.U.; Kamal, M.A.; Ahmad, S.; Ahmed, J.; Khan, I.N. Association of Metronidazole with Cancer: A Potential Risk Factor or Inconsistent Deductions? Curr. Drug Metab. 2018, 19, 902–909. [Google Scholar] [CrossRef]
- Quintanilla-Licea, R.; Vargas-Villarreal, J.; Verde-Star, M.J.; Rivas-Galindo, V.M.; Torres-Hernández, Á.D. Antiprotozoal Activity against Entamoeba histolytica of Flavonoids Isolated from Lippia graveolens Kunth. Molecules 2020, 25, 2464. [Google Scholar] [CrossRef]
- Llurba-Montesino, N.; Schmidt, T.J. Salvia Species as Sources of Natural Products with Antiprotozoal Activity. Int. J. Mol. Sci. 2018, 19, 264. [Google Scholar] [CrossRef]
- Velázquez-Domínguez, J.A.; Hernández-Ramírez, V.I.; Calzada, F.; Varela-Rodríguez, L.; Pichardo-Hernández, D.L.; Bautista, E.; Herrera-Martínez, M.; Castellanos-Mijangos, R.D.; Matus-Meza, A.S.; Chávez-Munguía, B.; et al. Linearolactone and kaempferol disrupt the actin cytoskeleton in Entamoeba histolytica: Inhibition of amoebic liver abscess development. J. Nat. Prod. 2020, 83, 3671–3680. [Google Scholar] [CrossRef]
- Calzada, F. Additional antiprotozoal constituents from Cuphea pinetorum, a plant used in Mayan traditional medicine to treat diarrhea. Phytother. Res. 2005, 19, 725–727. [Google Scholar] [CrossRef]
- Bolaños, V.; Díaz-Martínez, A.; Soto, J.; Marchat, L.A.; Sánchez-Monroy, V.; Ramírez-Moreno, E. Kaempferol inhibits Entamoeba histolytica growth by altering cytoskeletal functions. Mol. Biochem. Parasitol. 2015, 204, 16–25. [Google Scholar] [CrossRef]
- Calzada, F.; Yepez-Mulia, L.; Tapia-Contreras, A.; Bautista, E.; Maldonado, E.; Ortega, A. Evaluation of the antiprotozoal activity of neo-clerodane type diterpenes from Salvia polystachya against Entamoeba histolytica and Giardia lamblia. Phytother. Res. 2010, 24, 662–665. [Google Scholar] [CrossRef]
- Argüello-García, R.; Calzada, F.; Chávez-Munguía, B.; Matus-Meza, A.S.; Bautista, E.; Barbosa, E.; Velázquez, C.; Hernández-Caballero, M.E.; Ordoñez-Razo, R.M.; Velázquez-Domínguez, J.A. Linearolactone Induces Necrotic-like Death in Giardia intestinalis trophozoites: Prediction of a Likely Target. Pharmaceuticals 2022, 15, 809. [Google Scholar] [CrossRef]
- Kalra, A.; Yetiskul, E.; Wehrle, C.J.; Tuma, F. Physiology, Liver. In StatPearls [Internet], 1st ed.; Abai, B., Abu-Ghosh, A., Acharya, A.B., et al., Eds.; StatPearls Publishing: Treasure Island, FL, USA, 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK535438/ (accessed on 23 December 2023).
- Rovegno, M.; Vera, M.; Ruiz, A.; Benítez, C. Current concepts in acute liver failure. Ann. Hepatol. 2019, 18, 543–552. [Google Scholar] [CrossRef]
- Levaro-Loquio, D.; Serrano-Luna, J.; Velásquez-Torres, M.; Higuera-Martínez, G.; Arciniega-Martínez, I.M.; Reséndiz-Albor, A.A.; Pérez-Vielma, N.M.; Pacheco-Yépez, J. In Vitro Evaluation of the Antiamoebic Activity of Kaempferol against Trophozoites of Entamoeba histolytica and in the Interactions of Amoebae with Hamster Neutrophils. Int. J. Mol. Sci. 2023, 24, 11216. [Google Scholar] [CrossRef] [PubMed]
- Halle, W. The Registry of Cytotoxicity: Toxicity testing in cell cultures to predict acute toxicity (LD50) and to reduce testing in animals. Altern. Lab. Anim. ATLA 2003, 31, 89–198. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, V.; Mena-López, R.; Anaya-Velázquez, F.; Martínez-Palomo, A. Cellular bases of experimental amebic liver abscess formation. Am. J. Pathol. 1984, 117, 81–91. [Google Scholar]
- Lindstrom, N.M.; Moore, D.M.; Zimmerman, K.; Smith, S.A. Hematologic assessment in pet rats, mice, hamsters, and gerbils: Blood sample collection and blood cell identification. Vet. Clin. N. Am. Exot. Anim. Pract. 2015, 18, 21–32. [Google Scholar] [CrossRef]
- McKeon, G.P.; Nagamine, C.M.; Ruby, N.F.; Luong, R.H. Hematologic, serologic, and histologic profile of aged Siberian hamsters (Phodopus sungorus). J. Am. Assoc. Lab. Anim. Sci. 2011, 50, 308–316. [Google Scholar]
- Foreman, J.H. Hyperlipemia and Hepatic Lipidosis in Large Animals. 2023. Available online: https://www.msdvetmanual.com/digestive-system/hepatic-disease-in-large-animals/hyperlipemia-and-hepatic-lipidosis-in-large-animals (accessed on 30 May 2023).
- Flores, M.S.; Obregón-Cárdenas, A.; Tamez, E.; Rodríguez, E.; Arévalo, K.; Quintero, I.; Tijerina, R.; Bosques, F.; Galán, L. Hypocholesterolemia in patients with an amebic liver abscess. Gut Liver 2014, 8, 415–420. [Google Scholar] [CrossRef]
- Fernández-Daza, E.; Fernández, J.E.; Moreno-Mejía, I.; Moreno-Mejía, M. Aproximación al diagnóstico de enfermedades hepáticas por el laboratorio clínico. Med. Lab. 2008, 14, 533–546. [Google Scholar]
- Giannini, E.G.; Testa, R.; Savarino, V. Liver enzyme alteration: A guide for clinicians. CMAJ Can. Med. Assoc. J. 2005, 172, 367–379. [Google Scholar] [CrossRef]
- Foreman, J.H. Overview of Hepatic Disease in Large Animals. 2023. Available online: https://www.msdvetmanual.com/digestive-system/hepatic-disease-in-large-animals/overview-of-hepatic-disease-in-large-animals (accessed on 30 May 2023).
- Brown, S.A. Renal Dysfunction in Small Animals. 2022. Available online: https://www.msdvetmanual.com/urinary-system/noninfectious-diseases-of-the-urinary-system-in-small-animals/renal-dysfunction-in-small-animals (accessed on 30 May 2023).
- Vicente, D.; Pérez-Trallero, E. Tetracyclines, sulfonamides, and metronidazole. Enfermedades Infecc. Y Microbiol. Clínica 2010, 28, 122–130. [Google Scholar] [CrossRef]
- Bansal, Y.; Maurya, V.; Tak, V.; Bohra, G.K.; Kumar, D.; Goel, A.D.; Yadav, T.; Nag, V.L. Clinical and laboratory profile of patients with amoebic liver abscess. Trop. Parasitol. 2022, 12, 113–118. [Google Scholar] [CrossRef]
- Reyna-Sepúlveda, F.; Hernández-Guedea, M.; García-Hernández, S.; Sinsel-Ayala, J.; Muñoz-Espinoza, L.; Pérez-Rodríguez, E.; Muñoz-Maldonado, G. Epidemiology and prognostic factors of liver abscess complications in northeastern Mexico. Med. Univ. 2017, 19, 178–183. [Google Scholar] [CrossRef]
- Hall, P.; Cash, J. What is the real function of the liver ‘function’ tests? Ulst. Med. J. 2012, 81, 30–36. [Google Scholar]
- Loaeza-del-Castillo, A.; Paz-Pineda, F.; Oviedo-Cárdenas, E.; Sánchez-Ávila, F.; Vargas-Vorácková, F. AST to platelet ratio index (APRI) for the noninvasive evaluation of liver fibrosis. Ann. Hepatol. 2008, 7, 350–357. [Google Scholar] [CrossRef]
- Feng, C.X.; Chen, X.Q.; He, X.L.; Lan, L.C.; Tang, Q.; Huang, L.; Shan, Q.W. Screening for Wilson’s disease in acute liver failure: A new scoring system in children. Front. Pediatr. 2022, 10, 1003887. [Google Scholar] [CrossRef]
- Sueyoshi, S.; Sawai, S.; Satoh, M.; Seimiya, M.; Sogawa, K.; Fukumura, A.; Tsutsumi, M.; Nomura, F. Fractionation of gamma-glutamyltransferase in patients with nonalcoholic fatty liver disease and alcoholic liver disease. World J. Hepatol. 2016, 8, 1610–1616. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, T.; Fang, Y.; Wang, M.; Liu, W.; Zhao, J.; Wang, B.; Wu, Z.; Lv, Y.; Wu, R. Clinical significance of serum albumin/globulin ratio in patients with pyogenic liver abscess. Front. Surg. 2021, 8, 677799. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhu, Y.; Ni, X.; Lin, J.; Li, H.; Zheng, L.; Zhang, C.; Qi, X.; Huo, H.; Lou, X.; et al. Serum GGT/ALT ratio predicts vascular invasion in HBV-related HCC. Cancer Cell Int. 2021, 21, 517. [Google Scholar] [CrossRef]
- An, L.; Yin, W.T.; Sun, D.W. Albumin-to-alkaline phosphatase ratio as a promising indicator of prognosis in human cancers: Is it possible? BMC Cancer 2021, 21, 247. [Google Scholar] [CrossRef]
- Hulzebos, C.V.; Dijk, P.H. Bilirubin-albumin binding, bilirubin/albumin ratios, and free bilirubin levels: Where do we stand? Semin. Perinatol. 2014, 38, 412–421. [Google Scholar] [CrossRef]
- Choi, J.S.; Chung, K.S.; Lee, E.H.; Lee, S.H.; Lee, S.H.; Kim, S.Y.; Jung, J.Y.; Kang, Y.A.; Park, M.S.; Kim, Y.S.; et al. The role of bilirubin to albumin ratio as a predictor for mortality in critically ill patients without existing liver or biliary tract disease. Acute Crit. Care 2020, 35, 24–30. [Google Scholar] [CrossRef]
- Pati, S.S.; Mishra, S.K.; Mohanty, S.; Pattnaik, J.K.; Das, B.S. Influence of renal impairment on plasma concentrations of conjugated bilirubin in cases of Plasmodium falciparum malaria. Ann. Trop. Med. Parasitol. 2003, 97, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez de Cossío, A.; Rodríguez-Sánchez, R. Pruebas de laboratorio en atención primaria (II). Med. De Fam. SEMERGEN 2011, 37, 130–135. [Google Scholar] [CrossRef]
- Brookes, E.M.; Power, D.A. Elevated serum urea-to-creatinine ratio is associated with adverse inpatient clinical outcomes in non-end stage chronic kidney disease. Sci. Rep. 2022, 12, 20827. [Google Scholar] [CrossRef] [PubMed]
- Jorge Morales, B. Drogas Nefrotóxicas. Rev. Médica Clínica Las Condes 2010, 21, 623–628. [Google Scholar] [CrossRef][Green Version]
- Ghosh, S.; Sharma, S.; Gadpayle, A.K.; Gupta, H.K.; Mahajan, R.K.; Sahoo, R.; Kumar, N. Clinical, laboratory, and management profile in patients of liver abscess from northern India. J. Trop. Med. 2014, 2014, 142382. [Google Scholar] [CrossRef]
- Lin, H.; Zhou, X.; Zhang, Z. The diagnostic value of GGT-based biochemical indicators for choledocholithiasis with negative imaging results of magnetic resonance cholangiopancreatography. Contrast Media Mol. Imaging 2022, 2022, 7737610. [Google Scholar] [CrossRef]
- Elizondo, G.; Weissleder, R.; Stark, D.D.; Todd, L.E.; Compton, C.; Wittenberg, J.; Ferrucci, J.T., Jr. Amebic liver abscess: Diagnosis and treatment evaluation with MR imaging. Radiology 1987, 165, 795–800. [Google Scholar] [CrossRef]
- Priyadarshi, R.N.; Kumar, R.; Anand, U. Amebic liver abscess: Clinico-radiological findings and interventional management. World J. Radiol. 2022, 14, 272–285. [Google Scholar] [CrossRef]
- Shibayama, M.; Campos-Rodríguez, R.; Ramírez-Rosales, A.; Flores-Romo, L.; Espinosa-Cantellano, M.; Martínez-Palomo, A.; Tsutsumi, V. Entamoeba histolytica: Liver invasion and abscess production by intraperitoneal inoculation of trophozoites in hamsters, Mesocricetus auratus. Exp. Parasitol. 1998, 88, 20–27. [Google Scholar] [CrossRef]
- Denis, M.; Chadee, K. Immunopathology of Entamoeba histolytica infections. Parasitol. Today (Pers. Ed.) 1988, 4, 247–252. [Google Scholar] [CrossRef]
- Krishna, M. Anatomía microscópica del hígado. Clin. Liver Dis. 2014, 2 (Suppl. S5), 109–112. [Google Scholar] [CrossRef] [PubMed]
- Nigam, P.; Gupta, A.K.; Kapoor, K.K.; Sharan, G.R.; Goyal, B.M.; Joshi, L.D. Cholestasis in amoebic liver abscess. Gut 1985, 26, 140–145. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Ghose, A.K.; Viswanadhan, V.N.; Wendoloski, J.J. A knowledge-based approach in designing combinatorial or medicinal chemistry libraries for drug discovery. 1. A qualitative and quantitative characterization of known drug databases. J. Comb. Chem. 1999, 1, 55–68. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Egan, W.J.; Merz, K.M., Jr.; Baldwin, J.J. Prediction of drug absorption using multivariate statistics. J. Med. Chem. 2000, 43, 3867–3877. [Google Scholar] [CrossRef]
- Muegge, I.; Heald, S.L.; Brittelli, D. Simple selection criteria for drug-like chemical matter. J. Med. Chem. 2001, 44, 1841–1846. [Google Scholar] [CrossRef]
- Karthika, C.; Sureshkumar, R.; Zehravi, M.; Akter, R.; Ali, F.; Ramproshad, S.; Mondal, B.; Tagde, P.; Ahmed, Z.; Khan, F.S.; et al. Multidrug resistance of cancer cells and the vital role of P-Glycoprotein. Life 2022, 12, 897. [Google Scholar] [CrossRef]
- Rajaram, R.D.; Dissard, R.; Jaquet, V.; De Seigneux, S. Potential benefits and harms of NADPH oxidase type 4 in the kidneys and cardiovascular system. Nephrol. Dial. Transplant. 2019, 34, 567–576. [Google Scholar] [CrossRef]
- Dalefield, M.L.; Scouller, B.; Bibi, R.; Kivell, B.M. The Kappa Opioid Receptor: A Promising Therapeutic Target for Multiple Pathologies. Front. Pharmacol. 2022, 13, 837671. [Google Scholar] [CrossRef] [PubMed]
- Percie du Sert, N.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; Emerson, M.; et al. Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 2020, 18, e3000411. [Google Scholar] [CrossRef]
- Marie, C.S.; Verkerke, H.P.; Paul, S.N.; Mackey, A.J.; Petri, W.A., Jr. Leptin protects host cells from Entamoeba histolytica cytotoxicity by a STAT3-dependent mechanism. Infect. Immun. 2012, 80, 1934–1943. [Google Scholar] [CrossRef]
- López-Contreras, L.; Hernández-Ramírez, V.I.; Herrera-Martínez, M.; Montaño, S.; Constantino-Jonapa, L.A.; Chávez-Munguía, B.; Talamás-Rohana, P. Structural and functional characterization of the divergent Entamoeba Src using Src inhibitor-1. Parasites Vectors 2017, 10, 500. [Google Scholar] [CrossRef][Green Version]
- Sladek, F.M. What are nuclear receptor ligands? Mol. Cell. Endocrinol. 2011, 334, 3–13. [Google Scholar] [CrossRef]
- Bosch, D.E.; Siderovski, D.P. G protein signaling in the parasite Entamoeba histolytica. Exp. Mol. Med. 2013, 45, e15. [Google Scholar] [CrossRef][Green Version]
- Basith, S.; Cui, M.; Macalino, S.; Park, J.; Clavio, N.; Kang, S.; Choi, S. Exploring G protein-coupled receptors (GPCRs) ligand space via cheminformatics approaches: Impact on rational drug design. Front. Pharmacol. 2018, 9, 128. [Google Scholar] [CrossRef]
- Valle-Solis, M.; Bolaños, J.; Orozco, E.; Huerta, M.; García-Rivera, G.; Salas-Casas, A.; Chávez-Munguía, B.; Rodríguez, M.A. A calcium/cation exchanger participates in the programmed cell death and in vitro virulence of Entamoeba histolytica. Front. Cell. Infect. Microbiol. 2018, 8, 342. [Google Scholar] [CrossRef]
- Meneses-Ruiz, D.M.; Aguilar-Díaz, H.; Bobes, R.J.; Sampieri, A.; Vaca, L.; Laclette, J.P.; Carrero, J.C. Protection against Amoebic Liver Abscess in Hamster by Intramuscular Immunization with an Autographa californica Baculovirus Driving the Expression of the Gal-Lectin LC3 Fragment. BioMed Res. Int. 2015, 2015, 760598. [Google Scholar] [CrossRef]
- Varela-Rodríguez, L.; Sánchez-Ramírez, B.; Rodríguez-Reyna, I.; Ordaz-Ortiz, J.J.; Chávez-Flores, D.; Salas-Muñoz, E.; Osorio-Trujillo, J.C.; Ramos-Martínez, E.; Talamás-Rohana, P. Biological and toxicological evaluation of Rhus trilobata Nutt. (Anacardiaceae) used traditionally in Mexico against cancer. BMC Complement Altern. Med. 2019, 19, 153. [Google Scholar] [CrossRef]
- Varela-Rodríguez, L.; Sánchez-Ramírez, B.; Saenz-Pardo-Reyes, E.; Ordaz-Ortiz, J.J.; Castellanos-Mijangos, R.D.; Hernández-Ramírez, V.I.; Cerda-García-Rojas, C.M.; González-Horta, C.; Talamás-Rohana, P. Antineoplastic Activity of Rhus trilobata Nutt. (Anacardiaceae) against Ovarian Cancer and Identification of Active Metabolites in This Pathology. Plants 2021, 10, 2074. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Munguía, B.; Salazar-Villatoro, L.; Omaña-Molina, M.; Espinosa-Cantellano, M.; Ramírez-Flores, E.; Lorenzo-Morales, J.; Martínez-Palomo, A. Acanthamoeba culbertsoni: Electron-Dense Granules in a Highly Virulent Clinical Isolate. J. Eukaryot. Microbiol. 2016, 63, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Chard, L.S.; Wang, Z.; Wang, Y. Syrian Hamster as an Animal Model for the Study on Infectious Diseases. Front. Immunol. 2019, 10, 2329. [Google Scholar] [CrossRef]
- Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación (SAGARPA). Norma Oficial Mexicana NOM-062-ZOO-1999: Especificaciones Técnicas para la Producción, Cuidado y uso de los Animales de Laboratorio. [Gobierno de México]. 2001. Available online: https://www.gob.mx/cms/uploads/attachment/file/203498/NOM-062-ZOO-1999_220801.pdf (accessed on 20 April 2023).
- American Veterinary Medical Association (AVMA). AVMA Guidelines for the Euthanasia of Animals, 2nd ed.; AVMA: Schaumburg, IL, USA, 2020. [Google Scholar]
- Organization for Economic Co-Operation and Development (OECD). Section 4: Test no. 425, acute oral toxicity (up-and-down procedure). In OECD Guidelines for the Testing of Chemicals; OECD, Ed.; OECD Publishing: Paris, France, 2008; pp. 1–27. [Google Scholar] [CrossRef]
- Al Shoyaib, A.; Archie, S.R.; Karamyan, V.T. Intraperitoneal Route of Drug Administration: Should it Be Used in Experimental Animal Studies? Pharm. Res. 2019, 37, 12. [Google Scholar] [CrossRef]
- Herrmann, K.; Flecknell, P. The application of humane endpoints and humane killing methods in animal research proposals: A retrospective review. Altern. Lab. Anim. ATLA 2018, 46, 317–333. [Google Scholar] [CrossRef]
- Secretaria de Salud (SSA). Norma Oficial Mexicana NOM-087-ECOL-SSA1-2002: Protección Ambiental—Salud Ambiental—Residuos Peligrosos Biológico-Infecciosos—Clasificación y Especificaciones de Manejo [Gobierno de México]. 2002. Available online: http://www.salud.gob.mx/unidades/cdi/nom/087ecolssa.html (accessed on 20 April 2023).
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, 357–364. [Google Scholar] [CrossRef]
- Sharma, A.K.; Srivastava, G.N.; Roy, A.; Sharma, V.K. ToxiM: A Toxicity Prediction Tool for Small Molecules Developed Using Machine Learning and Chemoinformatics Approaches. Front. Pharmacol. 2017, 8, 880. [Google Scholar] [CrossRef]
- Martin, T.; Harten, P.; Young, D. TEST (Toxicity Estimation Software Tool) Ver 4.1; U.S. Environmental Protection Agency: Washington, DC, USA, 2012; EPA/600/C-12/006.
- Banerjee, P.; Dunkel, M.; Kemmler, E.; Preissner, R. SuperCYPsPred-a web server for the prediction of cytochrome activity. Nucleic Acids Res. 2020, 48, W580–W585. [Google Scholar] [CrossRef]
- Maunz, A.; Gütlein, M.; Rautenberg, M.; Vorgrimmler, D.; Gebele, D.; Helma, C. lazar: A modular predictive toxicology framework. Front. Pharmacol. 2013, 4, 38. [Google Scholar] [CrossRef]

| In Vitro Studies: IC50, SI and LD50 | ||||
|---|---|---|---|---|
| Samples | Vh | MTZ | KP | LL |
| IC50 HM-1 | Innocuous | 0.16 ++ * | 31.6 + * | <7.8 + * |
| IC50 CHO-K1 | Innocuous | 500 - * | 70 +/- * | 300 - * |
| IC50 BEAS-2B | Innocuous | 400 - * | 100 +/- * | 200 - * |
| SI | ND | 3461 ++ | 2.7 +/- | >32 + |
| LD50 (Theoretical) | ND | 1025.64 | 551.75 | 824.20 |
| In Vivo Studies: Acute Toxicity and ALA Characteristics | ||||
|---|---|---|---|---|
| Treatments | Vh | MTZ | KP | LL |
| Hamster weight (g) | 91.9 ± 2.3 | 95.4 ± 5.1 | 93.5 ± 6 | 93.4 ± 7.2 |
| Toxicity signs | None | None | None | None |
| Survival rate (%) | 100 | 100 | 100 | 100 |
| ALA Weight (g) | 1.23 ± 0.15 | 0.33 ± 0.32 * | 0.86 ± 0.15 * | 0.40 ± 0.20 * |
| ALA Volume (mm3) | 2.257 ± 523.7 | 528 ± 183.5 * | 738 ± 150 * | 562.3 ± 23.3 * |
| MRI (T1/T2) | Intermediate intensity/ hyperintense | Intermediate intensity/ hypointense | Intermediate intensity/ hypointense | Intermediate intensity/ hypointense |
| Leukocyte infiltrate | ++ | − | +/− | +/− |
| Fibrosis | + | −− | −− | −− |
| Liquid | ++ | − | +/− | − |
| Edges | Irregular and poorly defined | Regular and well defined | Irregular and poorly defined | Regular and well defined |
| Morphology | Loculated ovoid | Round | Ovoid | Loculated ovoid |
| Treatments (%) Hematological Parameters | Disease-Free | Vh | MTZ | KP | LL | Reference Range (Mean) |
|---|---|---|---|---|---|---|
| Lymphocytes | 42 ± 4 | 35 ± 13 | 50 ± 13 | 39 ± 12 | 44 ± 21 | 40–85 (63) |
| Monocytes | 0 | 0 | 0 | 0 | 0 | 1–6 (3) |
| Eosinophils | 0 | 0 | 0 | 0 | 0 | 1–2 |
| Basophils | 0 | 0 | 0 | 0 | 0 | 0–5 (2) |
| Segmented neutrophils | 54 ± 2 | 64 ± 14 | 51 ± 13 | 60 ± 11 | 56 ± 21 | 25–55 (40) |
| Band neutrophils | 4 ± 2 | 2 ± 1 | 0 | 2 ± 1 | 0 | 5–13 (9) |
| Immature forms | 0 | 0 | 0 | 0 | 0 | 0 |
| Organs | Disease-Free | Vh | MTZ | KP | LL |
|---|---|---|---|---|---|
| Liver | 6.7 ± 0.07/4.04 ± 0.05 | 8.28 ± 1.1 */4.7 ± 0.5 * | 5.76 ± 1.4/4 ± 0.15 | 5.9 ± 0.8/3.88 ± 0.11 | 5.5 ± 0.9/3.94 ± 0.16 |
| Heart | 0.4 ± 0.0/0.8 ± 0.09 | 0.38 ± 0.05/1.06 ± 0.09 | 0.38 ± 0.1/1.08 ± 0.04 | 0.38 ± 0.1/1.06 ± 0.08 | 0.38 ± 0.08/1.1 ± 0.07 |
| Kidneys | 0.65 ± 0.07/1.2 ± 0.14 | 0.62 ± 0.03/1.2 ± 0.07 | 0.52 ± 0.0/1.3 ± 0.08 | 0.49 ± 0.04/1.2 ± 0.1 | 0.49 ± 0.07/1.1 ± 0.08 |
| Lungs | 0.67 ± 0.0/2.8 ± 0.2 | 0.7 ± 0.05/2.6 ± 0.2 | 0.68 ± 0.05/2.5 ± 0.2 | 0.74 ± 0.06/2.5 ± 0.05 | 0.74 ± 0.06/2.4 ± 0.1 |
| Spleen | 0.2 ± 0.0/3.4 ± 0.38 | 0.2 ± 0.0/3.30 ± 0.4 | 0.2 ± 0.05/3.3 ± 0.3 | 0.36 ± 0.05/3.7 ± 0.1 | 0.26 ± 0.05/3.4 ± 0.4 |
| Samples | MTZ | KP | LL |
|---|---|---|---|
| SwissADME©: Physicochemical Properties | |||
| Density: | 1.5 ± 0.1 g/cm3 | 1.7 ± 0.1 g/cm3 | 1.3 ± 0.1 g/cm3 |
| Refraction index: | 1.612 | 1.785 | 1.612 |
| Polarizability: | 16.2 ± 0.5 × 10−24 cm3 | 28.3 ± 0.5 × 10−24 cm3 | 35.0 ± 0.5 × 10−24 cm3 |
| Surface tension: | 60.5 ± 7.0 dyne/cm | 98.9 ± 3.0 dyne/cm | 54.6 ± 5.0 dyne/cm |
| Number heavy atoms: | 12 | 21 | 25 |
| Number of bonds: | 12 | 23 | 29 |
| Number of rings: | 1 | 3 | 5 |
| Number aromatic heavy atoms: | 5 | 16 | 5 |
| Fraction Csp3: | 0.50 | 0.00 | 0.50 |
| Number rotatable bonds: | 3 | 1 | 1 |
| Total charge: | 0.0 | 0.0 | 0.0 |
| Molar refractivity: | 43.25 Å | 76.01 Å | 88.42 Å |
| SwissADME©: Pharmacokinetics/Molinspiration©: Bioactivity score | |||
| GI absorption: | High | High | High |
| P-gp substrate: | No | No | No |
| Log Kp: | −7.36 cm/s | −6.70 cm/s | −6.37 cm/s |
| GPCR ligand: | −1.09 | −0.10 | 0.65 |
| Ion channel modulator: | −0.87 | −0.21 | 0.16 |
| Kinase inhibitor: | −0.59 | 0.21 | −0.13 |
| Nuclear receptor ligand: | −1.74 | 0.32 | 0.66 |
| Protease inhibitor: | −1.68 | −0.27 | 0.04 |
| Enzyme inhibitor: | −0.32 | 0.26 | 0.47 |
| SwissADME©: Medicinal Chemistry | |||
| PAINS: | 0 alert | 1 alert (catechol A) | 0 alert |
| Brenk: | 2 alerts (nitro group) | 1 alert (catechol A) | 1 alert (>2 esters) |
| Leadlikeness: | No (M.W. < 250) | Yes | Yes |
| Synthetic accessibility: | 2.30 | 3.14 | 5.56 |
| T.E.S.T.© and LAZAR©: Toxicological properties | |||
| LD50 Fathead minnow (96 h): | 424.1 mg/L | 1.28 mg/L | ND |
| LD50 Daphnia magna (48 h): | 39.14 mg/L | 3.62 mg/L | ND |
| IGC50 T. pyriformis (48 h): | 270.22 | 10.54 mg/L | ND |
| LD50 Rat (Oral): | 2444 | 2018 mg/kg | ND |
| Bioconcentration factor: | 1.914 | 8.032 | ND |
| Developmental toxicity: | Not | Yes | ND |
| AMES mutagenicity: | Yes (p = 0.67) | Yes (p = 0.42) | ND |
| Carcinogenicity (rodents): | ND | No (p = 0.43) | ND |
| Adverse effects (rat): | ND | 1320 mg/kg/day | ND |
| Estrogen Receptor RBA: | 5.089 × 10−4 | 0.004 | ND |
| Estrogen Receptor Binding: | Yes | Yes | ND |
| Groups | n | Treatment | Administration Route | Doses/Time |
|---|---|---|---|---|
| I (normal control, without ALA) | 5 | Disease-free | i.p. | N/A |
| II (negative control, non-treatment) | 5 | Vh (1× PBS) | i.p. | 200 µL/day |
| III (positive control, antiamoebic drug) | 5 | MTZ | i.p. | 10 mg/kg body weight/day |
| IV (sample) | 5 | KP | i.p. | 5 mg/kg body weight/day |
| V (sample) | 5 | LL | i.p. | 10 mg/kg body weight/day |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varela-Rodríguez, L.; Calzada, F.; Velázquez-Domínguez, J.A.; Hernández-Ramírez, V.I.; Varela-Rodríguez, H.; Bautista, E.; Herrera-Martínez, M.; Pichardo-Hernández, D.L.; Castellanos-Mijangos, R.D.; Chávez-Munguía, B.; et al. Toxicological Evaluation of Kaempferol and Linearolactone as Treatments for Amoebic Liver Abscess Development in Mesocricetus auratus. Int. J. Mol. Sci. 2024, 25, 10633. https://doi.org/10.3390/ijms251910633
Varela-Rodríguez L, Calzada F, Velázquez-Domínguez JA, Hernández-Ramírez VI, Varela-Rodríguez H, Bautista E, Herrera-Martínez M, Pichardo-Hernández DL, Castellanos-Mijangos RD, Chávez-Munguía B, et al. Toxicological Evaluation of Kaempferol and Linearolactone as Treatments for Amoebic Liver Abscess Development in Mesocricetus auratus. International Journal of Molecular Sciences. 2024; 25(19):10633. https://doi.org/10.3390/ijms251910633
Chicago/Turabian StyleVarela-Rodríguez, Luis, Fernando Calzada, José Antonio Velázquez-Domínguez, Verónica Ivonne Hernández-Ramírez, Hugo Varela-Rodríguez, Elihú Bautista, Mayra Herrera-Martínez, Diana Laura Pichardo-Hernández, Rodrigo Daniel Castellanos-Mijangos, Bibiana Chávez-Munguía, and et al. 2024. "Toxicological Evaluation of Kaempferol and Linearolactone as Treatments for Amoebic Liver Abscess Development in Mesocricetus auratus" International Journal of Molecular Sciences 25, no. 19: 10633. https://doi.org/10.3390/ijms251910633
APA StyleVarela-Rodríguez, L., Calzada, F., Velázquez-Domínguez, J. A., Hernández-Ramírez, V. I., Varela-Rodríguez, H., Bautista, E., Herrera-Martínez, M., Pichardo-Hernández, D. L., Castellanos-Mijangos, R. D., Chávez-Munguía, B., & Talamás-Rohana, P. (2024). Toxicological Evaluation of Kaempferol and Linearolactone as Treatments for Amoebic Liver Abscess Development in Mesocricetus auratus. International Journal of Molecular Sciences, 25(19), 10633. https://doi.org/10.3390/ijms251910633











