Regulation of Bone Morphogenetic Protein Receptor Type II Expression by FMR1/Fragile X Mental Retardation Protein in Human Granulosa Cells in the Context of Poor Ovarian Response
Abstract
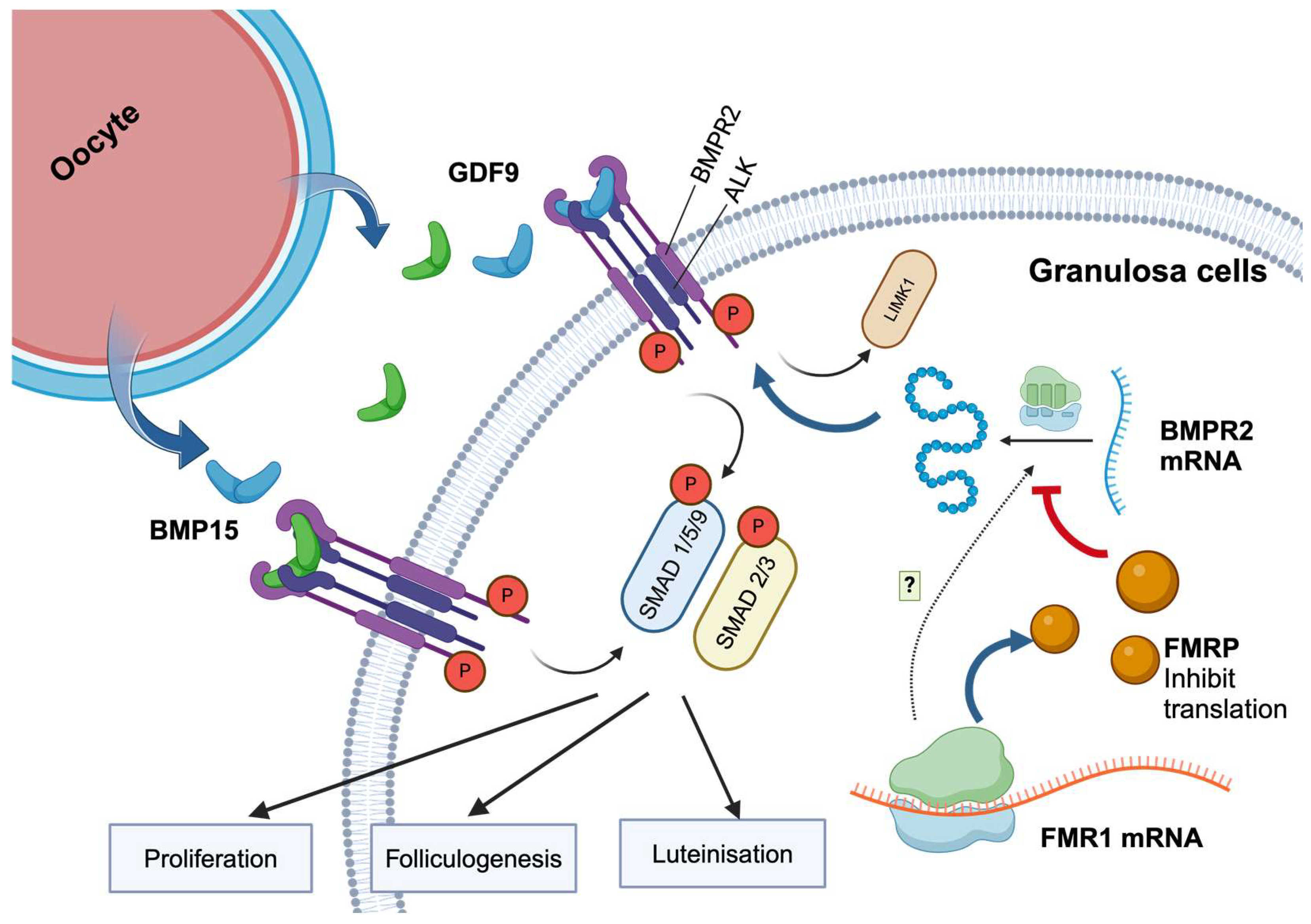
1. Introduction
2. Results
2.1. General Study Population
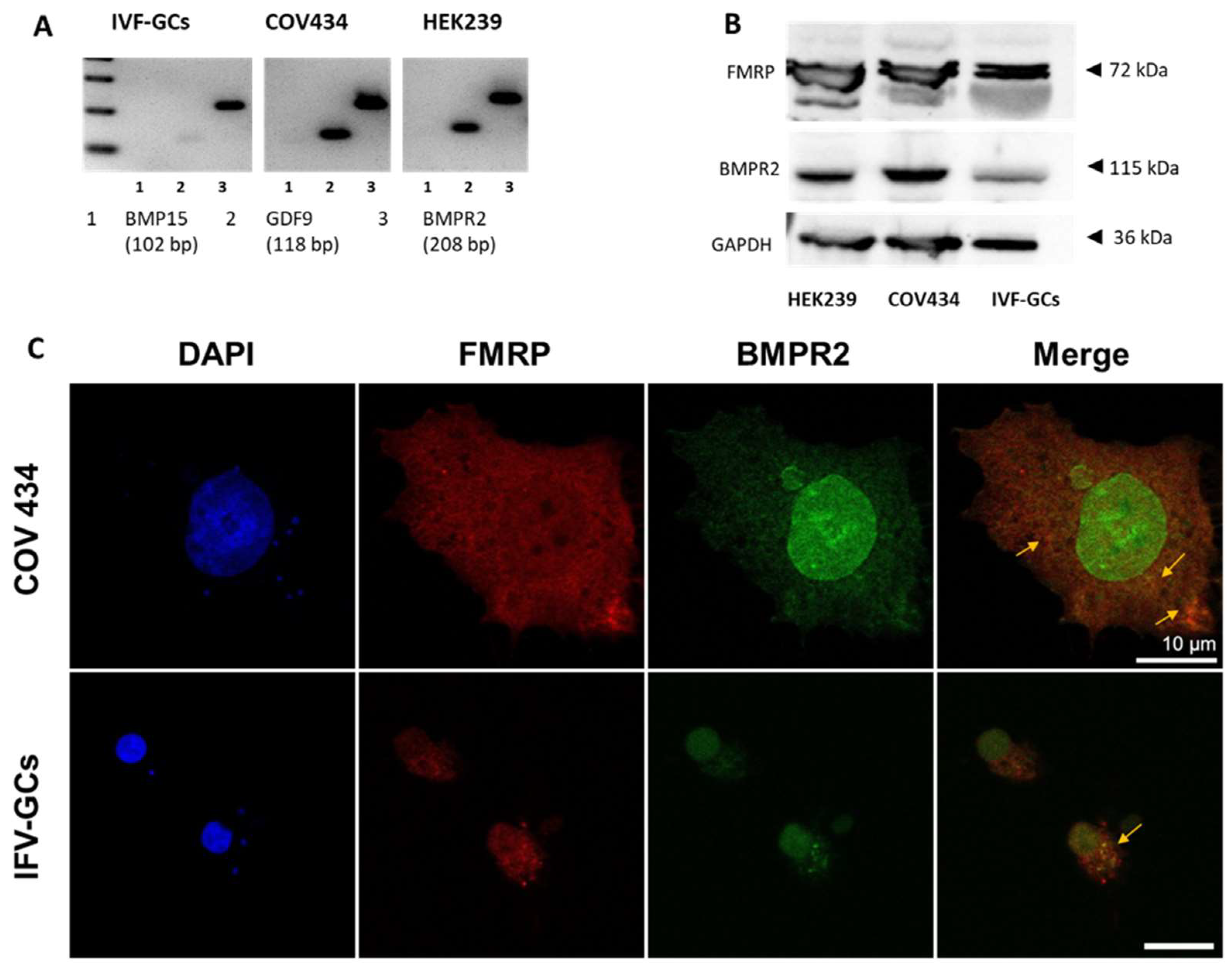
2.2. FMRP, BMPR2, and GDF9 Expression in COV434 Cells and IVF-GCs
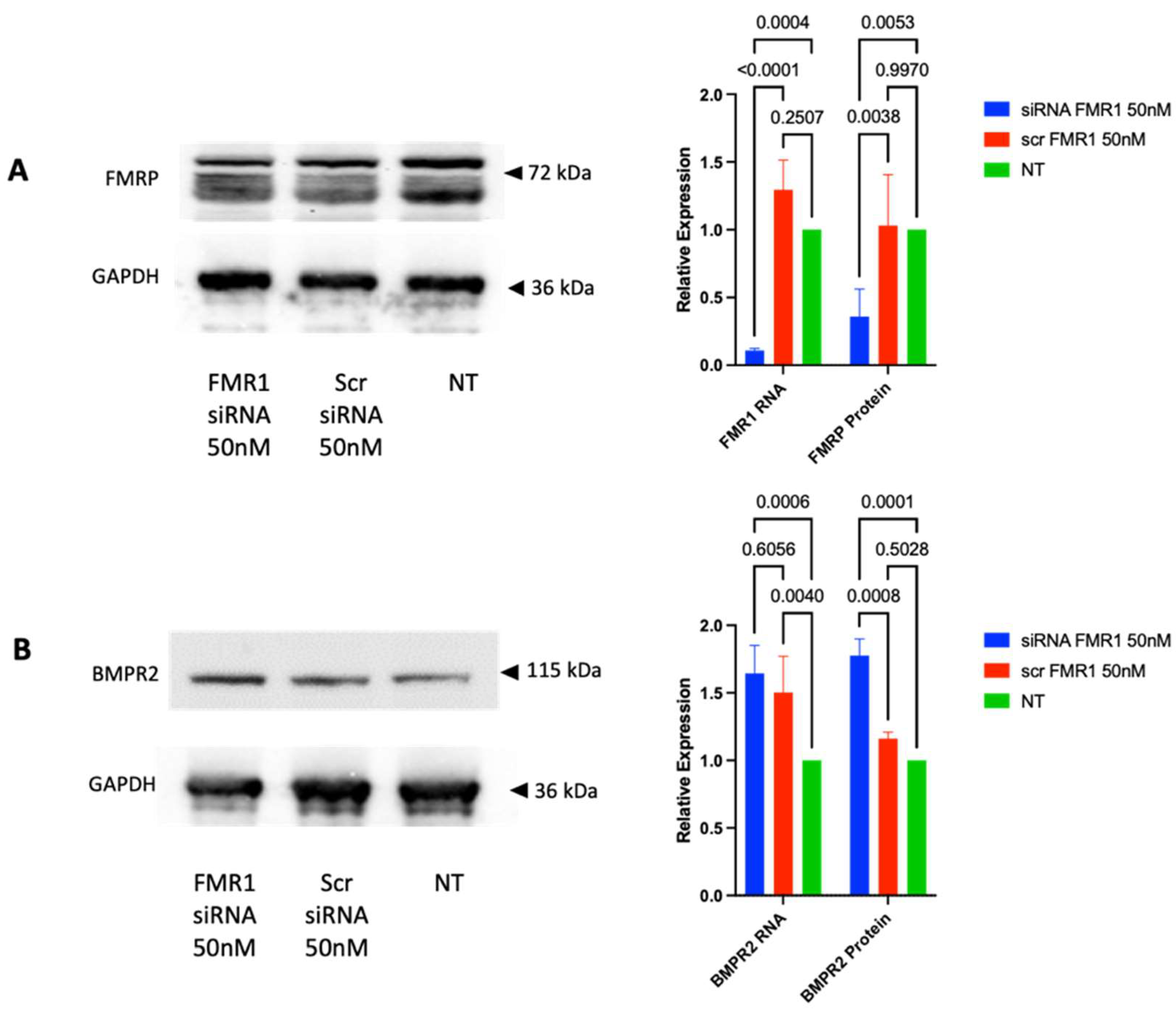
2.3. FMRP Regulates BMPR2 Expression in COV434 Cells
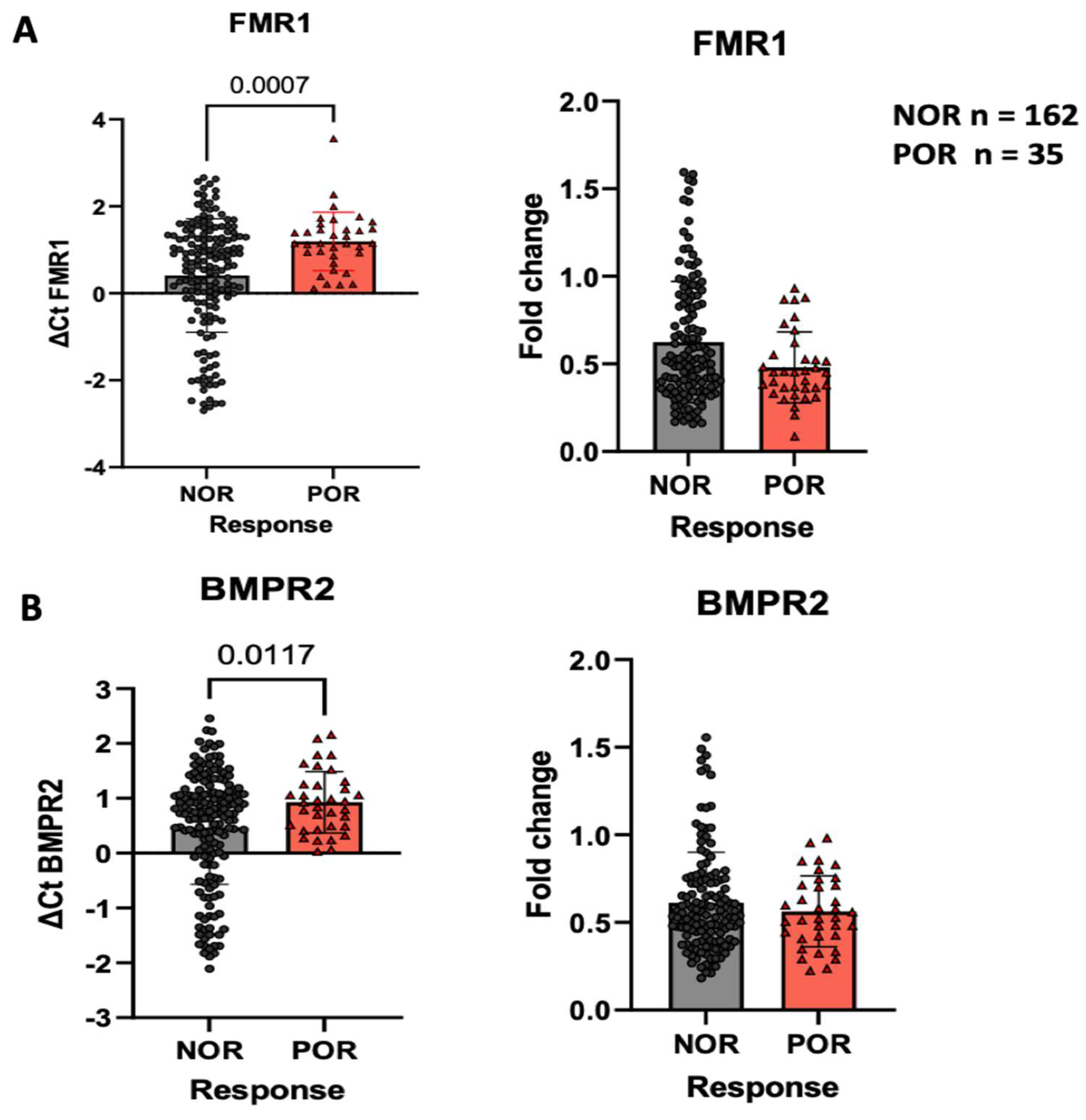
2.4. FMR1 and BMPR2 Expression in Patients with POR Compared to Those with NOR
2.5. Expression of BMPR2-Related Signaling Pathways in Patients with POR Compared to Those with NOR
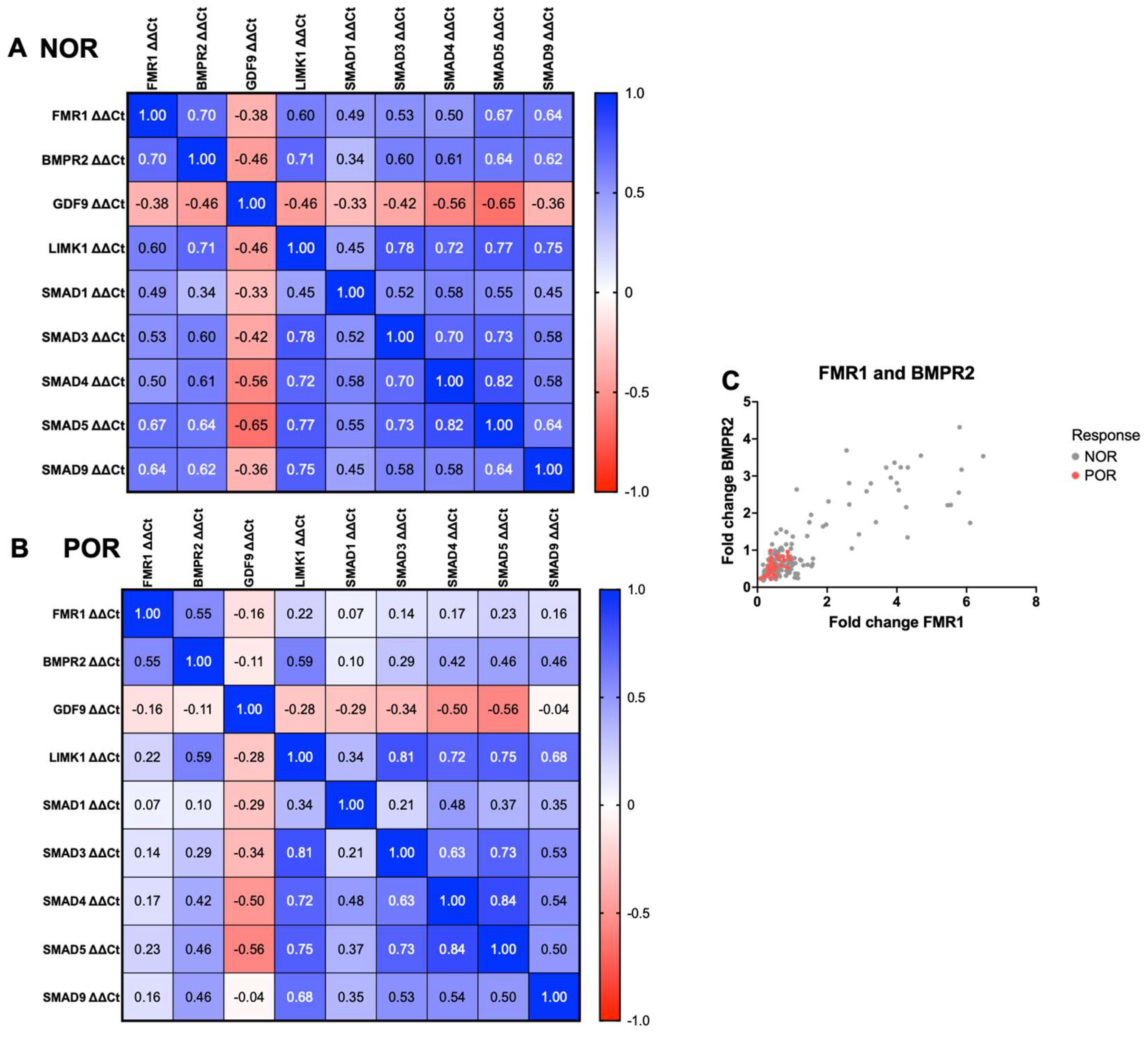
2.6. Correlation between FMR1, BMPR2, and BMPR2-Related Signaling Pathways in the NOR and POR Groups
3. Discussion
4. Materials and Methods
4.1. Patients and Ethical Approval
4.2. Ovarian Stimulation
4.3. Retrieval of GCs
4.4. CGG Repeat Length Analysis
4.5. Gene Expression Analysis
4.6. COV434 Cell Culture and Knockdown with FMR1 siRNA
4.7. Immunofluorescence Staining
4.8. Western Blotting
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hirshfield, A.N. Development of Follicles in the Mammalian Ovary. Int. Rev. Cytol. 1991, 124, 43–101. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, A.J.W.; Kawamura, K.; Cheng, Y.; Fauser, B.C.J.M. Intraovarian Control of Early Folliculogenesis. Endocr. Rev. 2015, 36, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Jirge, P.R. Poor Ovarian Reserve. J. Hum. Reprod. Sci. 2016, 9, 63. [Google Scholar] [CrossRef]
- Hagerman, R.J.; Berry-Kravis, E.; Hazlett, H.C.; Bailey, D.B.; Moine, H.; Kooy, R.F.; Tassone, F.; Gantois, I.; Sonenberg, N.; Mandel, J.L.; et al. Fragile X Syndrome. Nat. Rev. Dis. Primers 2017, 3, 17065. [Google Scholar] [CrossRef] [PubMed]
- Wittenberger, M.D.; Hagerman, R.J.; Sherman, S.L.; McConkie-Rosell, A.; Welt, C.K.; Rebar, R.W.; Corrigan, E.C.; Simpson, J.L.; Nelson, L.M. The FMR1 Premutation and Reproduction. Fertil. Steril. 2007, 87, 456–465. [Google Scholar] [CrossRef]
- Hunter, J.; Rohr, J.; Sherman, S. Co-Occurring Diagnoses among FMR1 Premutation Allele Carriers. Clin. Genet. 2010, 77, 374–381. [Google Scholar] [CrossRef]
- Sullivan, S.D.; Welt, C.; Sherman, S. FMR1 and the Continuum of Primary Ovarian Insufficiency. Semin. Reprod. Med. 2011, 29, 299–307. [Google Scholar] [CrossRef]
- Elizur, S.E.; Lebovitz, O.; Derech-Haim, S.; Dratviman-Storobinsky, O.; Feldman, B.; Dor, J.; Orvieto, R.; Cohen, Y. Elevated Levels of FMR1 MRNA in Granulosa Cells Are Associated with Low Ovarian Reserve in FMR1 Premutation Carriers. PLoS ONE 2014, 9, e105121. [Google Scholar] [CrossRef]
- Tassone, F.; Beilina, A.; Carosi, C.; Albertosi, S.; Bagni, C.; Li, L.; Glover, K.; Bentley, D.; Hagerman, P.J. Elevated FMR1 MRNA in Premutation Carriers Is Due to Increased Transcription. RNA 2007, 13, 555–562. [Google Scholar] [CrossRef]
- Garcia-Arocena, D.; Hagerman, P.J. Advances in Understanding the Molecular Basis of FXTAS. Hum. Mol. Genet. 2010, 19, R83–R89. [Google Scholar] [CrossRef][Green Version]
- Kenneson, A.; Zhang, F.; Hagedorn, C.H.; Warren, S.T. Reduced FMRP and Increased FMR1 Transcription Is Proportionally Associated with CGG Repeat Number in Intermediate-Length and Premutation Carriers. Hum. Mol. Genet. 2001, 10, 1449–1454. [Google Scholar] [CrossRef] [PubMed]
- Berman, R.F.; Buijsen, R.A.M.; Usdin, K.; Pintado, E.; Kooy, F.; Pretto, D.; Pessah, I.N.; Nelson, D.L.; Zalewski, Z.; Charlet-Bergeurand, N.; et al. Mouse Models of the Fragile X Premutation and Fragile X-Associated Tremor/Ataxia Syndrome. J. Neurodev. Disord. 2014, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Haify, S.N.; Botta-Orfila, T.; Hukema, R.K.; Tartaglia, G.G. In Silico, in Vitro, and in Vivo Approaches to Identify Molecular Players in Fragile X Tremor and Ataxia Syndrome. Front. Mol. Biosci. 2020, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- Richter, J.D.; Zhao, X. The Molecular Biology of FMRP: New Insights into Fragile X Syndrome. Nat. Rev. Neurosci. 2021, 22, 209–222. [Google Scholar] [CrossRef]
- Fernández, E.; Rajan, N.; Bagni, C. The FMRP Regulon: From Targets to Disease Convergence. Front. Neurosci. 2013, 7, 191. [Google Scholar] [CrossRef]
- Ascano, M.; Mukherjee, N.; Bandaru, P.; Miller, J.B.; Nusbaum, J.D.; Corcoran, D.L.; Langlois, C.; Munschauer, M.; Dewell, S.; Hafner, M.; et al. FMRP Targets Distinct MRNA Sequence Elements to Regulate Protein Expression. Nature 2012, 492, 382–386. [Google Scholar] [CrossRef]
- Kashima, R.; Roy, S.; Ascano, M.; Martinez-Cerdeno, V.; Ariza-Torres, J.; Kim, S.; Louie, J.; Lu, Y.; Leyton, P.; Bloch, K.D.; et al. Augmented Noncanonical BMP Type II Receptor Signaling Mediates the Synaptic Abnormality of Fragile X Syndrome. Sci. Signal. 2016, 9, ra58. [Google Scholar] [CrossRef]
- Patiño, L.C.; Silgado, D.; Laissue, P. A Potential Functional Association between Mutant BMPR2 and Primary Ovarian Insufficiency. Syst. Biol. Reprod. Med. 2017, 63, 145–149. [Google Scholar] [CrossRef]
- Gomez-Puerto, M.C.; Iyengar, P.V.; García de Vinuesa, A.; ten Dijke, P.; Sanchez-Duffhues, G. Bone Morphogenetic Protein Receptor Signal Transduction in Human Disease. J. Pathol. 2019, 247, 9. [Google Scholar] [CrossRef]
- Retting, K.N.; Song, B.; Yoon, B.S.; Lyons, K.M. BMP Canonical Smad Signaling through Smad1 and Smad5 Is Required for Endochondral Bone Formation. Development 2009, 136, 1093–1104. [Google Scholar] [CrossRef]
- Villalonga, E.; Mosrin, C.; Normand, T.; Girardin, C.; Serrano, A.; Žunar, B.; Doudeau, M.; Godin, F.; Bénédetti, H.; Vallée, B. LIM Kinases, LIMK1 and LIMK2, Are Crucial Node Actors of the Cell Fate: Molecular to Pathological Features. Cells 2023, 12, 805. [Google Scholar] [CrossRef] [PubMed]
- Persani, L.; Rossetti, R.; Di pasquale, E.; Cacciatore, C.; Fabre, S. The Fundamental Role of Bone Morphogenetic Protein 15 in Ovarian Function and Its Involvement in Female Fertility Disorders. Hum. Reprod. Update 2014, 20, 869–883. [Google Scholar] [CrossRef] [PubMed]
- Gode, F.; Gulekli, B.; Dogan, E.; Korhan, P.; Dogan, S.; Bige, O.; Cimrin, D.; Atabey, N. Influence of Follicular Fluid GDF9 and BMP15 on Embryo Quality. Fertil. Steril. 2011, 95, 2274–2278. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.Z.; Lei, L.; Cheng, L.; Jin, Z.F.; Zu, S.J.; Shan, Z.Y.; Wang, Z.D.; Zhang, J.X.; Liu, Z.H. Expression of GDF-9, BMP-15 and Their Receptors in Mammalian Ovary Follicles. J. Mol. Histol. 2010, 41, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Albertini, D.F.; Nishimori, K.; Kumar, T.R.; Lu, N.; Matzuk, M.M. Growth Differentiation Factor-9 Is Required during Early Ovarian Folliculogenesis. Nature 1996, 383, 531–535. [Google Scholar] [CrossRef]
- Orisaka, M.; Orisaka, S.; Jiang, J.Y.; Craig, J.; Wang, Y.; Kotsuji, F.; Tsang, B.K. Growth Differentiation Factor 9 Is Antiapoptotic during Follicular Development from Preantral to Early Antral Stage. Mol. Endocrinol. 2006, 20, 2456–2468. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, T.; Han, J. Targeting FMRP: A New Window for Cancer Immunotherapy. MedComm 2023, 4, e233. [Google Scholar] [CrossRef]
- Malecki, C.; Hambly, B.D.; Jeremy, R.W.; Robertson, E.N. The RNA-Binding Fragile-X Mental Retardation Protein and Its Role beyond the Brain. Biophys. Rev. 2020, 12, 903. [Google Scholar] [CrossRef]
- Friedman-Gohas, M.; Elizur, S.E.; Dratviman-Storobinsky, O.; Aizer, A.; Haas, J.; Raanani, H.; Orvieto, R.; Cohen, Y. FMRpolyG Accumulates in FMR1 Premutation Granulosa Cells. J. Ovarian Res. 2020, 13, 22. [Google Scholar] [CrossRef]
- Gleicher, N.; Weghofer, A.; Oktay, K.; Barad, D. Relevance of Triple CGG Repeats in the FMR1 Gene to Ovarian Reserve. Reprod. Biomed. Online 2009, 19, 385–390. [Google Scholar] [CrossRef]
- Rehnitz, J.; Alcoba, D.D.; Brum, I.S.; Hinderhofer, K.; Youness, B.; Strowitzki, T.; Vogt, P.H. FMR1 and AKT/MTOR Signalling Pathways: Potential Functional Interactions Controlling Folliculogenesis in Human Granulosa Cells. Reprod. Biomed. Online 2017, 35, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Rosario, R.; Filis, P.; Tessyman, V.; Kinnell, H.; Childs, A.J.; Gray, N.K.; Anderson, R.A. FMRP Associates with Cytoplasmic Granules at the Onset of Meiosis in the Human Oocyte. PLoS ONE 2016, 11, e0163987. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.K.; Shimasaki, S. Molecular Biology and Physiological Role of the Oocyte Factor, BMP-15. Mol. Cell. Endocrinol. 2005, 234, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Godler, D.E.; Tassone, F.; Loesch, D.Z.; Taylor, A.K.; Gehling, F.; Hagerman, R.J.; Burgess, T.; Ganesamoorthy, D.; Hennerich, D.; Gordon, L.; et al. Methylation of Novel Markers of Fragile X Alleles Is Inversely Correlated with FMRP Expression and FMR1 Activation Ratio. Hum. Mol. Genet. 2010, 19, 1618. [Google Scholar] [CrossRef]
- Peprah, E.; He, W.; Allen, E.; Oliver, T.; Boyne, A.; Sherman, S.L. Examination of FMR1 Transcript and Protein Levels among 74 Premutation Carriers. J. Hum. Genet. 2010, 55, 66. [Google Scholar] [CrossRef]
- Mailick, M.R.; Hong, J.; Rathouz, P.; Baker, M.W.; Greenberg, J.S.; Smith, L.; Maenner, M. Low-Normal FMR1 CGG Repeat Length: Phenotypic Associations. Front. Genet. 2014, 5, 309. [Google Scholar] [CrossRef]
- Wang, Q.; Barad, D.H.; Darmon, S.K.; Kushnir, V.A.; Wu, Y.G.; Lazzaroni-Tealdi, E.; Zhang, L.; Albertini, D.F.; Gleicher, N. Reduced RNA Expression of the FMR1 Gene in Women with Low (CGGn<26) Repeats. PLoS ONE 2018, 13, e0209309. [Google Scholar] [CrossRef]
- Morris, K.V.; Mattick, J.S. The Rise of Regulatory RNA. Nat. Rev. Genet. 2014, 15, 423–437. [Google Scholar] [CrossRef]
- Dolskiy, A.A.; Yarushkin, A.A.; Grishchenko, I.V.; Lemskaya, N.A.; Pindyurin, A.V.; Boldyreva, L.V.; Pustylnyak, V.O.; Yudkin, D.V. MiRNA Expression and Interaction with the 3′UTR of FMR1 in FRAXopathy Pathogenesis. Non-Coding RNA Res. 2021, 6, 1–7. [Google Scholar] [CrossRef]
- Yi, Y.H.; Sun, X.S.; Qin, J.M.; Zhao, Q.H.; Liao, W.P.; Long, Y.S. Experimental Identification of MicroRNA Targets on the 3′ Untranslated Region of Human FMR1 Gene. J. Neurosci. Methods 2010, 190, 34–38. [Google Scholar] [CrossRef]
- Zongaro, S.; Hukema, R.; D’antoni, S.; Davidovic, L.; Barbry, P.; Catania, M.V.; Willemsen, R.; Mari, B.; Bardoni, B. The 3′ UTR of FMR1 MRNA Is a Target of MiR-101, MiR-129-5p and MiR-221: Implications for the Molecular Pathology of FXTAS at the Synapse. Hum. Mol. Genet. 2013, 22, 1971–1982. [Google Scholar] [CrossRef] [PubMed]
- Boustanai, I.; Raanani, H.; Aizer, A.; Orvieto, R.; Elizur, S.E. Granulosa Cell Dysfunction Is Associated With Diminished Ovarian Response in FMR1 Premutation Carriers. J. Clin. Endocrinol. Metab. 2022, 107, 3000–3009. [Google Scholar] [CrossRef] [PubMed]
- Godler, D.E.; Slater, H.R.; Bui, Q.M.; Ono, M.; Gehling, F.; Francis, D.; Amor, D.J.; Hopper, J.L.; Hagerman, R.; Loesch, D.Z. FMR1 Intron 1 Methylation Predicts FMRP Expression in Blood of Female Carriers of Expanded FMR1 Alleles. J. Mol. Diagn. 2011, 13, 528. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Lin, L.; Tan, H.; Wu, H.; Sherman, S.L.; Gao, F.; Jin, P.; Chen, D. Fragile X Premutation RNA Is Sufficient to Cause Primary Ovarian Insufficiency in Mice. Hum. Mol. Genet. 2012, 21, 5039. [Google Scholar] [CrossRef]
- Guo, X.; Wang, X.F. Signaling Cross-Talk between TGF-β/BMP and Other Pathways. Cell Res. 2009, 19, 71–88. [Google Scholar] [CrossRef]
- Kaivo-Oja, N.; Jeffery, L.A.; Ritvos, O.; Mottershead, D.G. Smad Signalling in the Ovary. Reprod. Biol. Endocrinol. 2006, 4, 21. [Google Scholar] [CrossRef]
- Kristensen, S.G.; Andersen, K.; Clement, C.A.; Franks, S.; Hardy, K.; Andersen, C.Y. Expression of TGF-Beta Superfamily Growth Factors, Their Receptors, the Associated SMADs and Antagonists in Five Isolated Size-Matched Populations of Pre-Antral Follicles from Normal Human Ovaries. Mol. Hum. Reprod. 2014, 20, 293–308. [Google Scholar] [CrossRef]
- Webber, L.; Davies, M.; Anderson, R.; Bartlett, J.; Braat, D.; Cartwright, B.; Cifkova, R.; De Muinck Keizer-Schrama, S.; Hogervorst, E.; Janse, F.; et al. ESHRE Guideline: Management of Women with Premature Ovarian Insufficiency. Hum. Reprod. 2016, 31, 926–937. [Google Scholar] [CrossRef]
- Rehnitz, J.; Alcoba, D.D.; Brum, I.S.; Dietrich, J.E.; Youness, B.; Hinderhofer, K.; Messmer, B.; Freis, A.; Strowitzki, T.; Germeyer, A. FMR1 Expression in Human Granulosa Cells Increases with Exon 1 CGG Repeat Length Depending on Ovarian Reserve. Reprod. Biol. Endocrinol. 2018, 16, 65. [Google Scholar] [CrossRef]
- Rehnitz, J.; Capp, E.; Messmer, B.; Nguyen, X.P.; Germeyer, A.; Freis, A.; Dietrich, J.E.; Hinderhofer, K.; Strowitzki, T.; Vogt, P.H. Fmr1 and Akt/Mtor Signaling in Human Granulosa Cells: Functional Interaction and Impact on Ovarian Response. J. Clin. Med. 2021, 10, 3892. [Google Scholar] [CrossRef]
- Stoyanova, V.; Oostra, B.A. The CGG Repeat and the FMR1 Gene. Trinucleotide Repeat Protoc. 2004, 277, 173–184. [Google Scholar] [CrossRef]
- Rehnitz, J.; Youness, B.; Nguyen, X.P.; Dietrich, J.E.; Roesner, S.; Messmer, B.; Strowitzki, T.; Vogt, P.H. FMR1 Expression in Human Granulosa Cells and Variable Ovarian Response: Control by Epigenetic Mechanisms. Mol. Hum. Reprod. 2021, 27, gaab001. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, X.P.; Vilkaite, A.; Messmer, B.; Dietrich, J.E.; Hinderhofer, K.; Schäkel, K.; Strowitzki, T.; Rehnitz, J. Expression of FMRpolyG in Peripheral Blood Mononuclear Cells of Women with Fragile X Mental Retardation 1 Gene Premutation. Genes 2022, 13, 451. [Google Scholar] [CrossRef]

| NOR | POR | p Value | |||
|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | ||
| Age | 162 | 34.15 (4.16) | 35 | 38.86 (4.56) | <0.0001 |
| BMI | 162 | 23.93 (4.02) | 35 | 23.44 (3.42) | 0.503 |
| FSH (U/L) | 156 | 7.84 (2.48) | 35 | 9.81 (3.83) | 0.0002 |
| LH (U/L) | 161 | 5.40 (2.58) | 35 | 4.94 (1.95) | 0.319 |
| Estradiol (pg/mL) | 162 | 49.82 (29.26) | 35 | 54.96 (20.03) | 0.324 |
| AMH (ng/mL) | 161 | 3.12 (2.94) | 34 | 1.07 (0.97) | <0.0001 |
| AFC | 95 | 15.07 (10.88) | 22 | 6.18 (4.09) | 0.0003 |
| Total Oocytes | 161 | 11.03 (6.79) | 35 | 4.71 (3.19) | <0.0001 |
| MII Oocytes | 105 | 7.57 (6.19) | 24 | 3.58 (2.60) | 0.0025 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, X.P.; Vilkaite, A.; Bender, U.; Dietrich, J.E.; Hinderhofer, K.; Strowitzki, T.; Rehnitz, J. Regulation of Bone Morphogenetic Protein Receptor Type II Expression by FMR1/Fragile X Mental Retardation Protein in Human Granulosa Cells in the Context of Poor Ovarian Response. Int. J. Mol. Sci. 2024, 25, 10643. https://doi.org/10.3390/ijms251910643
Nguyen XP, Vilkaite A, Bender U, Dietrich JE, Hinderhofer K, Strowitzki T, Rehnitz J. Regulation of Bone Morphogenetic Protein Receptor Type II Expression by FMR1/Fragile X Mental Retardation Protein in Human Granulosa Cells in the Context of Poor Ovarian Response. International Journal of Molecular Sciences. 2024; 25(19):10643. https://doi.org/10.3390/ijms251910643
Chicago/Turabian StyleNguyen, Xuan Phuoc, Adriana Vilkaite, Ulrike Bender, Jens E. Dietrich, Katrin Hinderhofer, Thomas Strowitzki, and Julia Rehnitz. 2024. "Regulation of Bone Morphogenetic Protein Receptor Type II Expression by FMR1/Fragile X Mental Retardation Protein in Human Granulosa Cells in the Context of Poor Ovarian Response" International Journal of Molecular Sciences 25, no. 19: 10643. https://doi.org/10.3390/ijms251910643
APA StyleNguyen, X. P., Vilkaite, A., Bender, U., Dietrich, J. E., Hinderhofer, K., Strowitzki, T., & Rehnitz, J. (2024). Regulation of Bone Morphogenetic Protein Receptor Type II Expression by FMR1/Fragile X Mental Retardation Protein in Human Granulosa Cells in the Context of Poor Ovarian Response. International Journal of Molecular Sciences, 25(19), 10643. https://doi.org/10.3390/ijms251910643





