Novel Indirect Antioxidant Activity Independent of Nrf2 Exerted by Lactic Acid Bacteria
Abstract
1. Introduction
2. Results
2.1. Heat-Killed LAB Showed Antioxidant Activities in Zebrafish Larvae
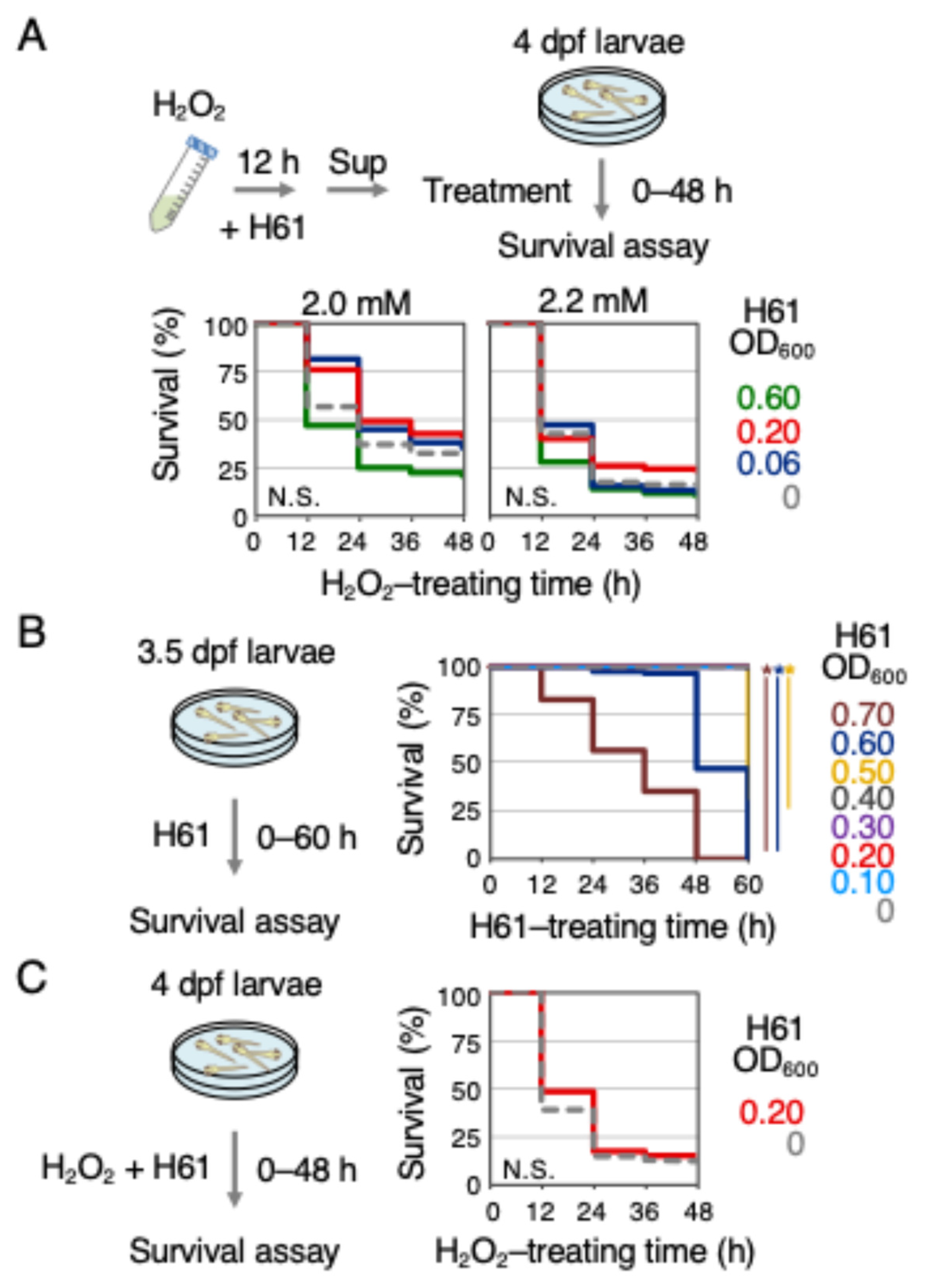
2.2. H61 Suspension Exhibited Indirect but Not Direct Antioxidant Activity
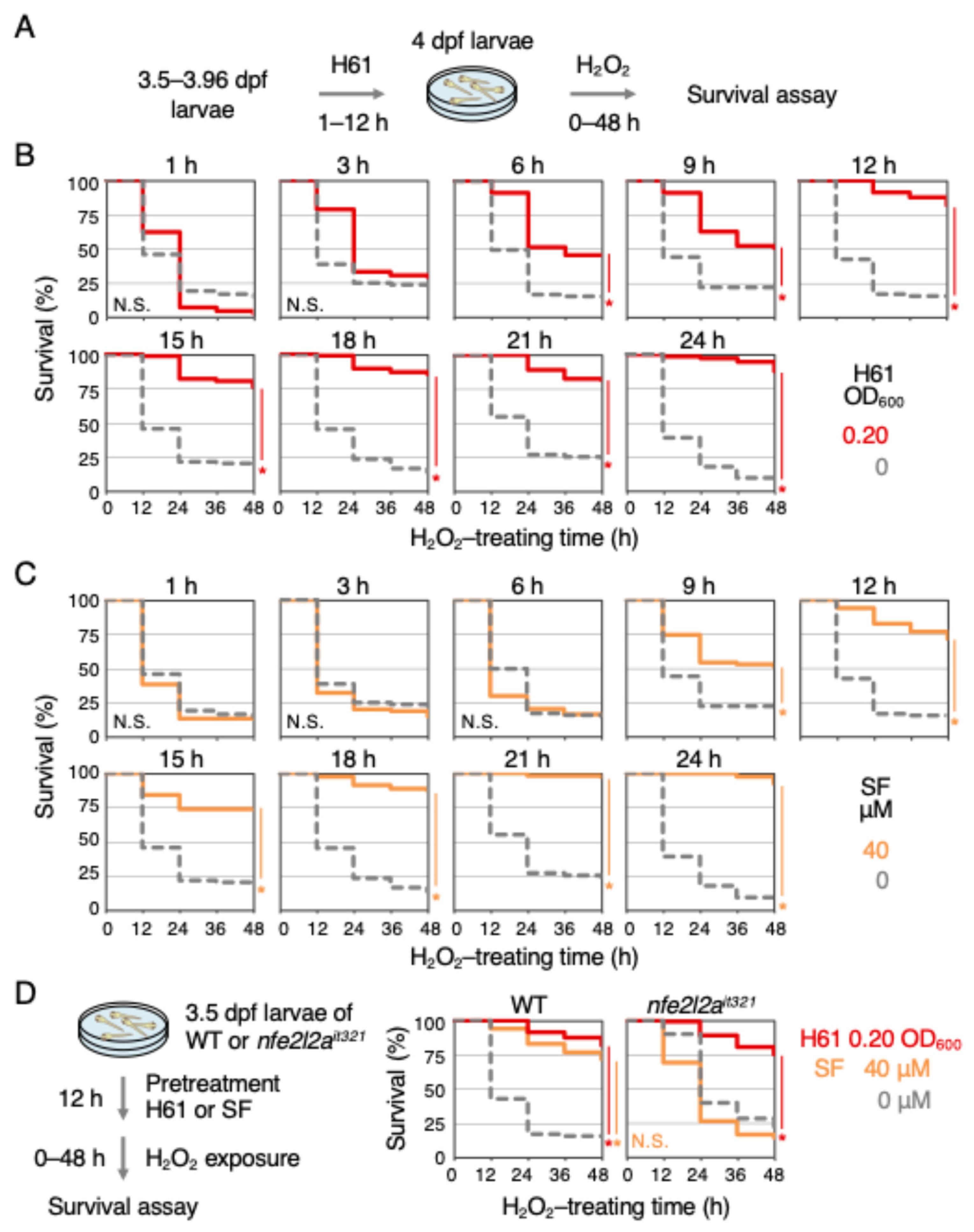
2.3. Indirect Antioxidant Activity Induced by Strain H61 Was Independent of the Keap1-Nrf2 Pathway
2.4. Strain H61 Did Not Exhibit Antioxidant Activity against Arsenite
3. Discussion
4. Materials and Methods
4.1. Zebrafish and Chemicals
4.2. LAB Preparation
4.3. Survival Assays
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petcu, C.D.; Tăpăloagă, D.; Mihai, O.D.; Gheorghe-Irimia, R.A.; Negoiță, C.; Georgescu, I.M.; Tăpăloagă, P.R.; Borda, C.; Ghimpețeanu, O.M. Harnessing natural antioxidants for enhancing food shelf life: Exploring sources and applications in the food industry. Foods 2023, 12, 3176. [Google Scholar] [CrossRef] [PubMed]
- Jena, A.B.; Samal, R.R.; Bhol, N.K.; Duttaroy, A.K. Cellular Red-Ox system in health and disease: The latest update. Biomed. Pharmacother. 2023, 162, 114606. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.A.; Kwak, M.K. The Nrf2 system as a potential target for the development of indirect antioxidants. Molecules 2010, 15, 7266–7291. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Itoh, K.; Suzuki, T.; Osanai, H.; Nishikawa, K.; Katoh, Y.; Takagi, Y.; Yamamoto, M. Identification of the interactive interface and phylogenic conservation of the Nrf2-Keap1 system. Genes Cells 2002, 7, 807–820. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Li, L.; Iwamoto, N.; Nakajima-Takagi, Y.; Kaneko, H.; Nakayama, Y.; Eguchi, M.; Wada, Y.; Kumagai, Y.; Yamamoto, M. The antioxidant defense system Keap1-Nrf2 comprises a multiple sensing mechanism for responding to a wide range of chemical compounds. Mol. Cell. Biol. 2009, 29, 493–502. [Google Scholar] [CrossRef]
- Fuse, Y.; Kobayashi, M. Conservation of the Keap1-Nrf2 system: An evolutionary journey through stressful space and time. Molecules 2017, 22, 436. [Google Scholar] [CrossRef]
- Mukaigasa, K.; Tsujita, T.; Nguyen, V.T.; Li, L.; Yagi, H.; Fuse, Y.; Nakajima-Takagi, Y.; Kato, K.; Yamamoto, M.; Kobayashi, M. Nrf2 activation attenuates genetic endoplasmic reticulum stress induced by a mutation in the phosphomannomutase 2 gene in zebrafish. Proc. Natl. Acad. Sci. USA 2018, 115, 2758–2763. [Google Scholar] [CrossRef]
- Mukaigasa, K.; Nguyen, L.T.; Li, L.; Nakajima, H.; Yamamoto, M.; Kobayashi, M. Genetic evidence of an evolutionarily conserved role for Nrf2 in the protection against oxidative stress. Mol. Cell. Biol. 2012, 32, 4455–4461. [Google Scholar] [CrossRef]
- Fuse, Y.; Nguyen, V.T.; Kobayashi, M. Nrf2-dependent protection against acute sodium arsenite toxicity in zebrafish. Toxicol. Appl. Pharmacol. 2016, 305, 136–142. [Google Scholar] [CrossRef]
- Endo, Y.; Muraki, K.; Fuse, Y.; Kobayashi, M. Evaluation of antioxidant activity of spice-derived phytochemicals using zebrafish. Int. J. Mol. Sci. 2020, 21, 1109. [Google Scholar] [CrossRef]
- Watanabe, A.; Muraki, K.; Tamaoki, J.; Kobayashi, M. Soy-derived equol induces antioxidant activity in zebrafish in an Nrf2-independent manner. Int. J. Mol. Sci. 2022, 23, 5243. [Google Scholar] [CrossRef] [PubMed]
- Abdul Hakim, B.N.; Xuan, N.J.; Oslan, S.N.H. A comprehensive review of bioactive compounds from lactic acid bacteria: Potential functions as functional food in dietetics and the food industry. Foods 2023, 12, 2850. [Google Scholar] [CrossRef] [PubMed]
- Abd Mutalib, N.; Syed Mohamad, S.A.; Jusril, N.A.; Hasbullah, N.I.; Mohd Amin, M.C.I.; Ismail, N.H. Lactic acid bacteria (LAB) and neuroprotection, what is new? An up-to-date systematic review. Pharmaceuticals 2023, 16, 712. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Q.; Chen, W. Functional evaluation model for lactic acid bacteria. In Lactic Acid Bacteria, Omics and Functional Evaluation; Chen, W., Ed.; Springer Nature Singapore Pte Ltd.: Singapore; Science Press: Beijing, China, 2019; pp. 183–237. [Google Scholar] [CrossRef]
- Yan, R.; Zeng, X.; Shen, J.; Wu, Z.; Guo, Y.; Du, Q.; Tu, M.; Pan, D. New clues for postbiotics to improve host health: A review from the perspective of function and mechanisms. J. Sci. Food Agric. 2024, 104, 6376–6387. [Google Scholar] [CrossRef] [PubMed]
- Thorakkattu, P.; Khanashyam, A.C.; Shah, K.; Babu, K.S.; Mundanat, A.S.; Deliephan, A.; Deokar, G.S.; Santivarangkna, C.; Nirmal, N.P. Postbiotics: Current trends in food and pharmaceutical industry. Foods 2022, 11, 3094. [Google Scholar] [CrossRef]
- Piqué, N.; Berlanga, M.; Miñana-Galbis, D. Health benefits of heat-killed (tyndallized) probiotics: An overview. Int. J. Mol. Sci. 2019, 20, 2534. [Google Scholar] [CrossRef]
- Ringø, E.; Hoseinifar, S.H.; Ghosh, K.; Doan, H.V.; Beck, B.R.; Song, S.K. Lactic acid bacteria in finfish—An update. Front. Microbiol. 2018, 9, 1818. [Google Scholar] [CrossRef]
- Zhao, T.; Wang, H.; Liu, Z.; Liu, Y.; DeJi; Li, B.; Huang, X. Recent perspective of Lactobacillus in reducing oxidative stress to prevent disease. Antioxidants 2023, 12, 769. [Google Scholar] [CrossRef]
- Feng, T.; Wang, J. Oxidative stress tolerance and antioxidant capacity of lactic acid bacteria as probiotic: A systematic review. Gut Microbes 2020, 12, 1801944. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Wang, Y.; Xu, H.; Mei, X.; Yu, D.; Wang, Y.; Li, W. Antioxidant properties of probiotic bacteria. Nutrients 2017, 9, 521. [Google Scholar] [CrossRef]
- Fuse, Y.; Endo, Y.; Araoi, S.; Daitoku, H.; Suzuki, H.; Kato, M.; Kobayashi, M. The possible repositioning of an oral anti-arthritic drug, auranofin, for Nrf2-activating therapy: The demonstration of Nrf2-dependent anti-oxidative action using a zebrafish model. Free Rad. Biol. Med. 2018, 115, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Mu, G.; Li, H.; Tuo, Y.; Gao, Y.; Zhang, Y. Antioxidative effect of Lactobacillus plantarum Y44 on 2,2ʹ-azobis(2-methylpropionamidine) dihydrochloride (ABAP)-damaged Caco-2 cells. J. Dairy Sci. 2019, 102, 6863–6875. [Google Scholar] [CrossRef] [PubMed]
- Finamore, A.; Ambra, R.; Nobili, F.; Garaguso, I.; Raguzzini, A.; Serafini, M. Redox role of Lactobacillus casei Shirota against the cellular damage induced by 2,2ʹ-Azobis (2-Amidinopropane) Dihydrochloride-induced oxidative and inflammatory stress in enterocytes-like epithelial cells. Front. Immunol. 2018, 9, 1131. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Du, R.; Wang, L.; Zhang, H. The antioxidative effects of probiotic Lactobacillus casei Zhang on the hyperlipidemic rats. Eur. Food Res. Technol. 2010, 231, 151–158. [Google Scholar] [CrossRef]
- Wang, A.N.; Yi, X.W.; Yu, H.F.; Dong, B.; Qiao, S.Y. Free radical scavenging activity of Lactobacillus fermentum in vitro and its antioxidative effect on growing-finishing pigs. J. Appl. Microbiol. 2009, 107, 1140–1148. [Google Scholar] [CrossRef]
- Yang, X.; Pan, X.; Jia, Z.; Bai, B.; Zhi, W.; Chen, H.; Ma, C.; Ma, D. Oral administration of Lactobacillus brevis 23017 combined with ellagic acid attenuates intestinal inflammatory injury caused by Eimeria infection by activating the Nrf2/HO-1 antioxidant pathway. Vet. Res. 2022, 53, 21. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.; Wang, Y.; Yi, C.; Tian, J.; Liu, K.; Chu, J. Protective effect of Lactobacillus plantarum on alcoholic liver injury and regulating of keap-Nrf2-ARE signaling pathway in zebrafish larvae. PLoS ONE 2019, 14, e0222339. [Google Scholar] [CrossRef]
- Srikham, K.; Daengprok, W.; Niamsup, P.; Thirabunyanon, M. Characterization of Streptococcus salivarius as new probiotics derived from human breast milk and their potential on proliferative inhibition of liver and breast cancer cells and antioxidant activity. Front. Microbiol. 2021, 12, 797445. [Google Scholar] [CrossRef]
- Kimoto-Nira, H.; Suzuki, C.; Kobayashi, M.; Sasaki, K.; Kurisaki, J.; Mizumachi, K. Anti-ageing effect of a lactococcal strain: Analysis using senescence-accelerated mice. Br. J. Nutr. 2007, 98, 1178–1186. [Google Scholar] [CrossRef]
- Oike, H.; Aoki-Yoshida, A.; Kimoto-Nira, H.; Yamagishi, N.; Tomita, S.; Sekiyama, Y.; Wakagi, M.; Sakurai, M.; Ippoushi, K.; Suzuki, C.; et al. Dietary intake of heat-killed Lactococcus lactis H61 delays age-related hearing loss in C57BL/6J mice. Sci. Rep. 2016, 6, 23556. [Google Scholar] [CrossRef]
- Kimoto-Nira, H.; Aoki, R.; Sasaki, K.; Suzuki, C.; Mizumachi, K. Oral intake of heat-killed cells of Lactococcus lactis strain H61 promotes skin health in women. J. Nutr. Sci. 2012, 1, e18. [Google Scholar] [CrossRef] [PubMed]
- Takaragawa, M.; Sakuraba, K.; Suzuki, Y. Heat-killed Lactococcus lactis subsp. cremoris H61 altered the iron status of young women: A randomized, double-blinded, placebo-controlled, parallel-group comparative study. Nutrients 2022, 14, 3144. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.M.; Desai, C.; Darby, T.M.; Luo, L.; Wolfarth, A.A.; Scharer, C.D.; Ardita, C.S.; Reedy, A.R.; Keebaugh, E.S.; Neish, A.S. Lactobacilli modulate epithelial cytoprotection through the Nrf2 pathway. Cell Rep. 2015, 12, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zeng, X.; Wu, Z.; Guo, Y.; Pan, D. Relationship of gene-structure-antioxidant ability of exopolysaccharides derived from lactic acid bacteria: A review. J. Agric. Food Chem. 2023, 71, 9187–9200. [Google Scholar] [CrossRef] [PubMed]
- Kimoto-Nira, H.; Moriya, N.; Hayakawa, S.; Kuramasu, K.; Ohmori, H.; Yamasaki, S.; Ogawa, M. Effects of rare sugar ᴅ-allulose on acid production and probiotic activities of dairy lactic acid bacteria. J. Dairy Sci. 2017, 100, 5936–5944. [Google Scholar] [CrossRef]
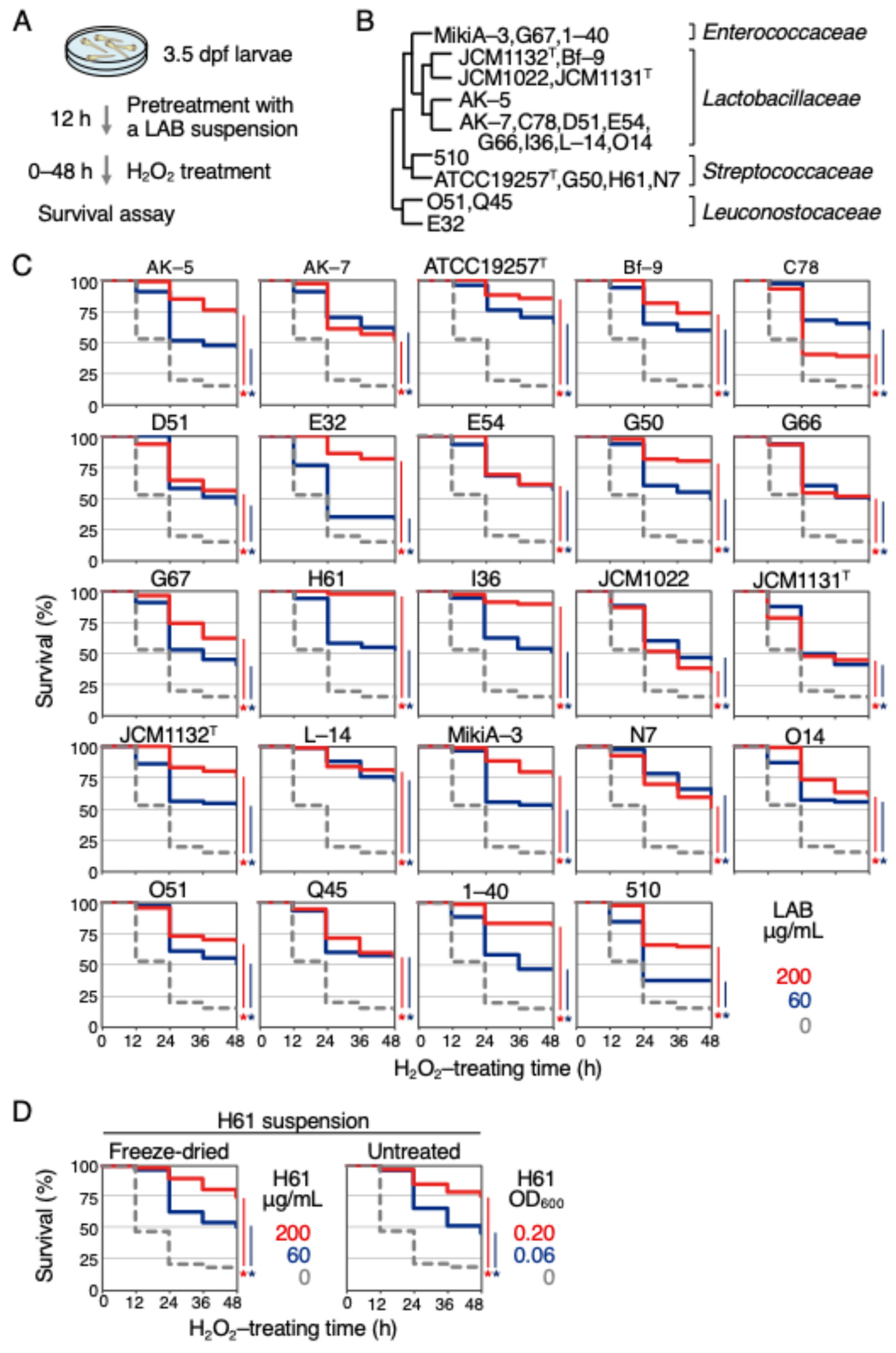

| Strains | Species | Origin |
|---|---|---|
| AK-5 | Limosilactobacillus fermentum | Fermented vegetable |
| AK-7 | Lactiplantibacillus plantarum | Fermented vegetable |
| ATCC 19257T | Lactococcus cremoris | Raw milk |
| Bf-9 | Lactobacillus helveticus | Unknown |
| C78 | Latilactobacillus curvatus | Fermented vegetable |
| D51 | Latilactobacillus sakei | Fermented vegetable |
| E32 | Weisella cibaria | Fermented vegetable |
| E54 | Lactiplantibacillus paraplantarum | Fermented vegetable |
| G50 | Lactococcus lactis subsp. lactis | Napiergrass |
| G66 | Pediococcus pentosaceus | Pasteurized milk |
| G67 | Enterococcus feacium | Pasteurized milk |
| H61 | Lactococcus cremoris | Cheese starter |
| I36 | Levilactobacillus brevis | Fermented vegetable |
| JCM 1022 | Lactobacillus johnsonii | Human feces |
| JCM 1131T | Lactobacillus gasseri | Human feces |
| JCM 1132T | Lactobacillus acidophilus | Human feces |
| L-14 | Lacticaseibacillus casei | Cheese |
| MikiA-3 | Enterococcus feacalis | Fermented rice milk |
| N7 | Lactococcus lactis subsp. lactis biovar diacetylactis | Cheese starter |
| O14 | Lacticaseibacillus paracasei | Homemade kefir |
| O51 | Leuconostoc mesenteroides | Fermented vegetable |
| Q45 | Leuconostoc citreum | Fermented vegetable |
| 1-40 | Enterococcus munditii | Fermented vegetable |
| 510 | Streptococcus thermophilus | Cow milk |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato, A.; Watanabe, A.; Muraki, K.; Kimoto-Nira, H.; Kobayashi, M. Novel Indirect Antioxidant Activity Independent of Nrf2 Exerted by Lactic Acid Bacteria. Int. J. Mol. Sci. 2024, 25, 10648. https://doi.org/10.3390/ijms251910648
Sato A, Watanabe A, Muraki K, Kimoto-Nira H, Kobayashi M. Novel Indirect Antioxidant Activity Independent of Nrf2 Exerted by Lactic Acid Bacteria. International Journal of Molecular Sciences. 2024; 25(19):10648. https://doi.org/10.3390/ijms251910648
Chicago/Turabian StyleSato, Ayaka, Asami Watanabe, Kyoji Muraki, Hiromi Kimoto-Nira, and Makoto Kobayashi. 2024. "Novel Indirect Antioxidant Activity Independent of Nrf2 Exerted by Lactic Acid Bacteria" International Journal of Molecular Sciences 25, no. 19: 10648. https://doi.org/10.3390/ijms251910648
APA StyleSato, A., Watanabe, A., Muraki, K., Kimoto-Nira, H., & Kobayashi, M. (2024). Novel Indirect Antioxidant Activity Independent of Nrf2 Exerted by Lactic Acid Bacteria. International Journal of Molecular Sciences, 25(19), 10648. https://doi.org/10.3390/ijms251910648






