Immunostimulatory Activity of a Mixture of Platycodon grandiflorum, Pyrus serotine, Chaenomeles sinensis, and Raphanus sativus in RAW264.7 Macrophages
Abstract
1. Introduction
2. Results
2.1. Identification of PPCRE by Ultra-Performance Liquid Chromatography–Mass Spectrometry/MS (UPLC-MS/MS) Analysis
2.2. Cytotoxicity of PPCRE on RAW264.7 Macrophages
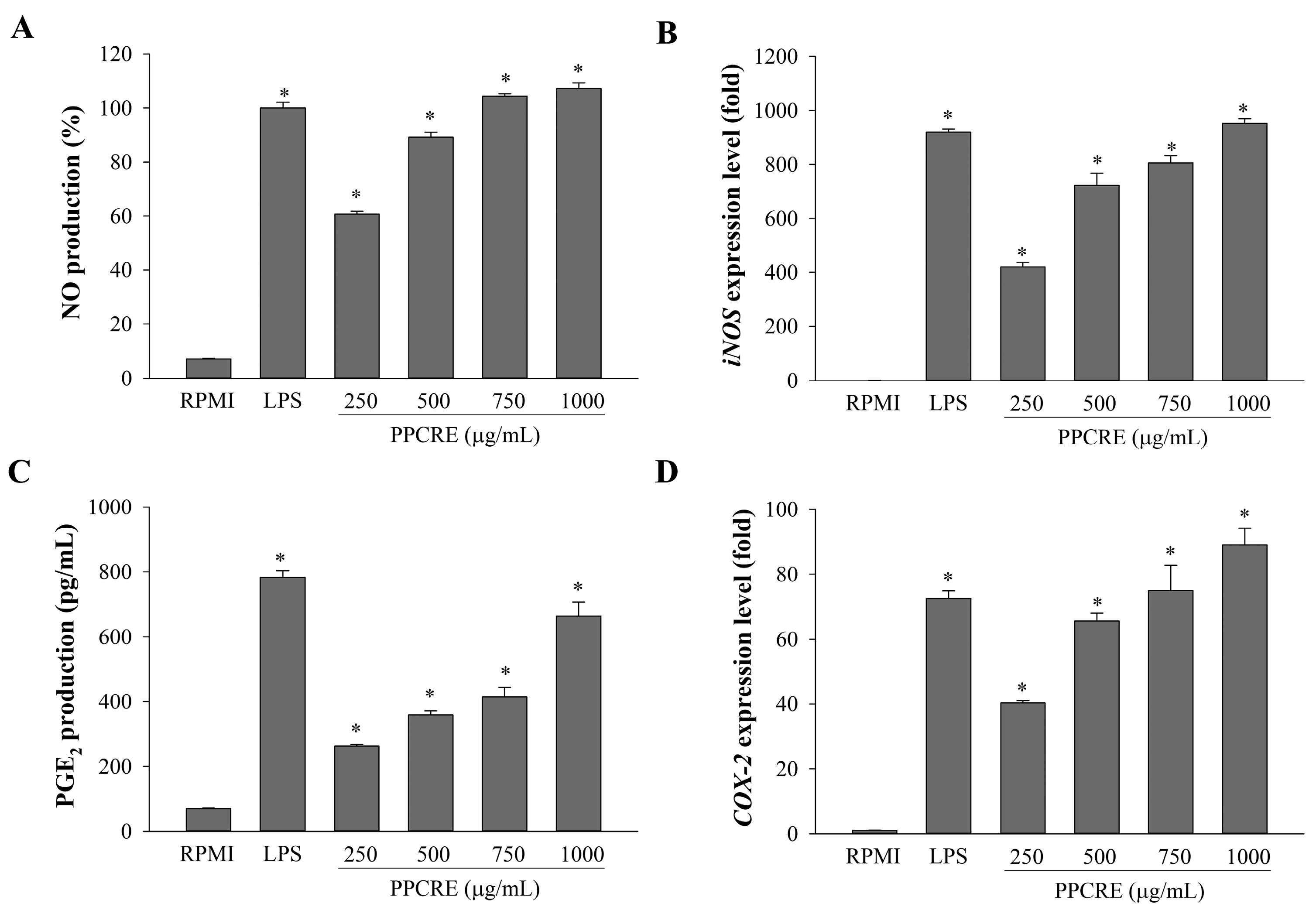
2.3. Enhancement of NO Production and iNOS mRNA Expression of PPCRE
2.4. PPCRE Induces PGE2 Production and COX-2 mRNA Expression
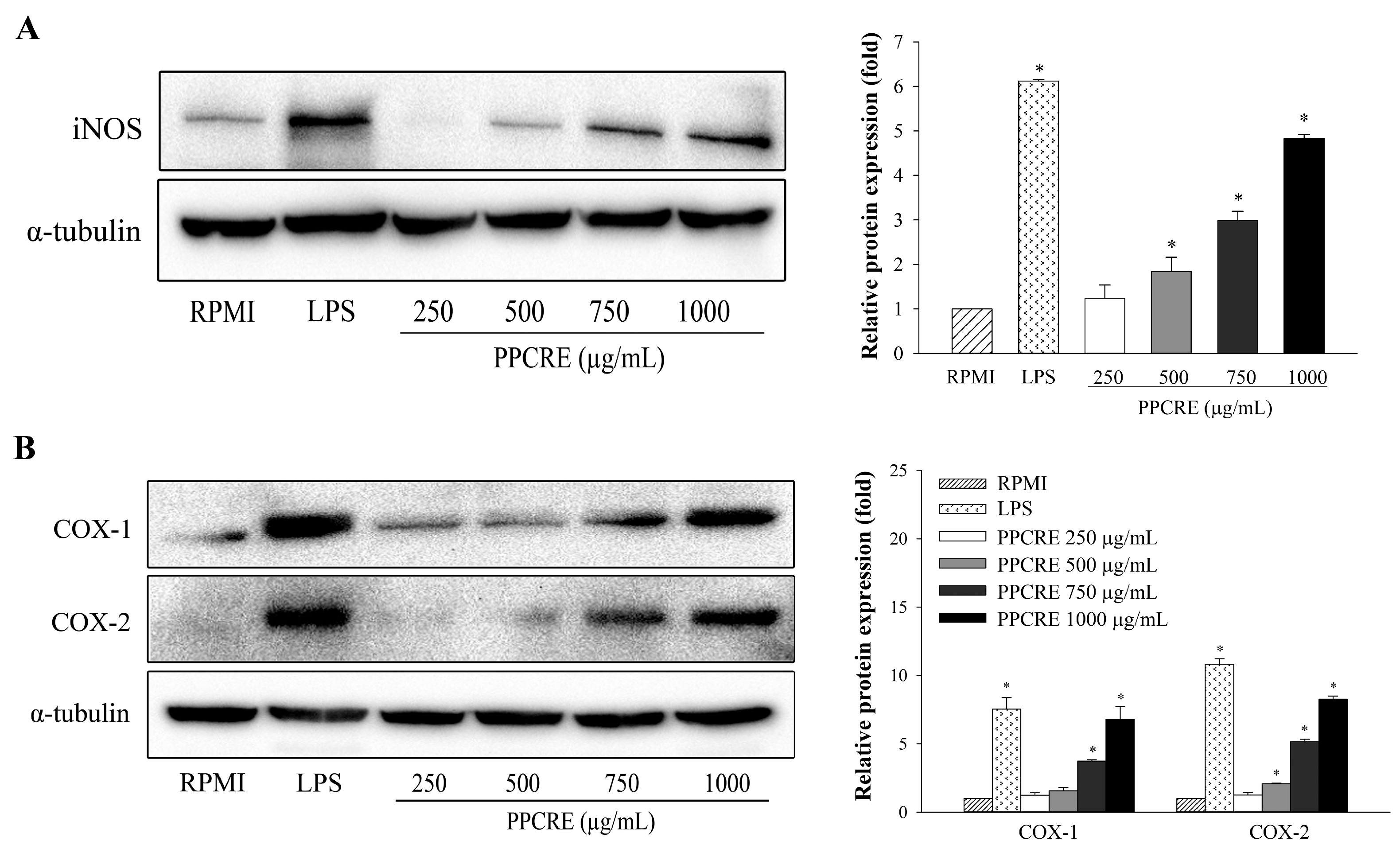
2.5. PPCRE Enhances Protein Expression Levels of iNOS, COX-1, and COX-2
2.6. PPCRE Enhances the Secretion of Pro-Inflammatory Cytokines
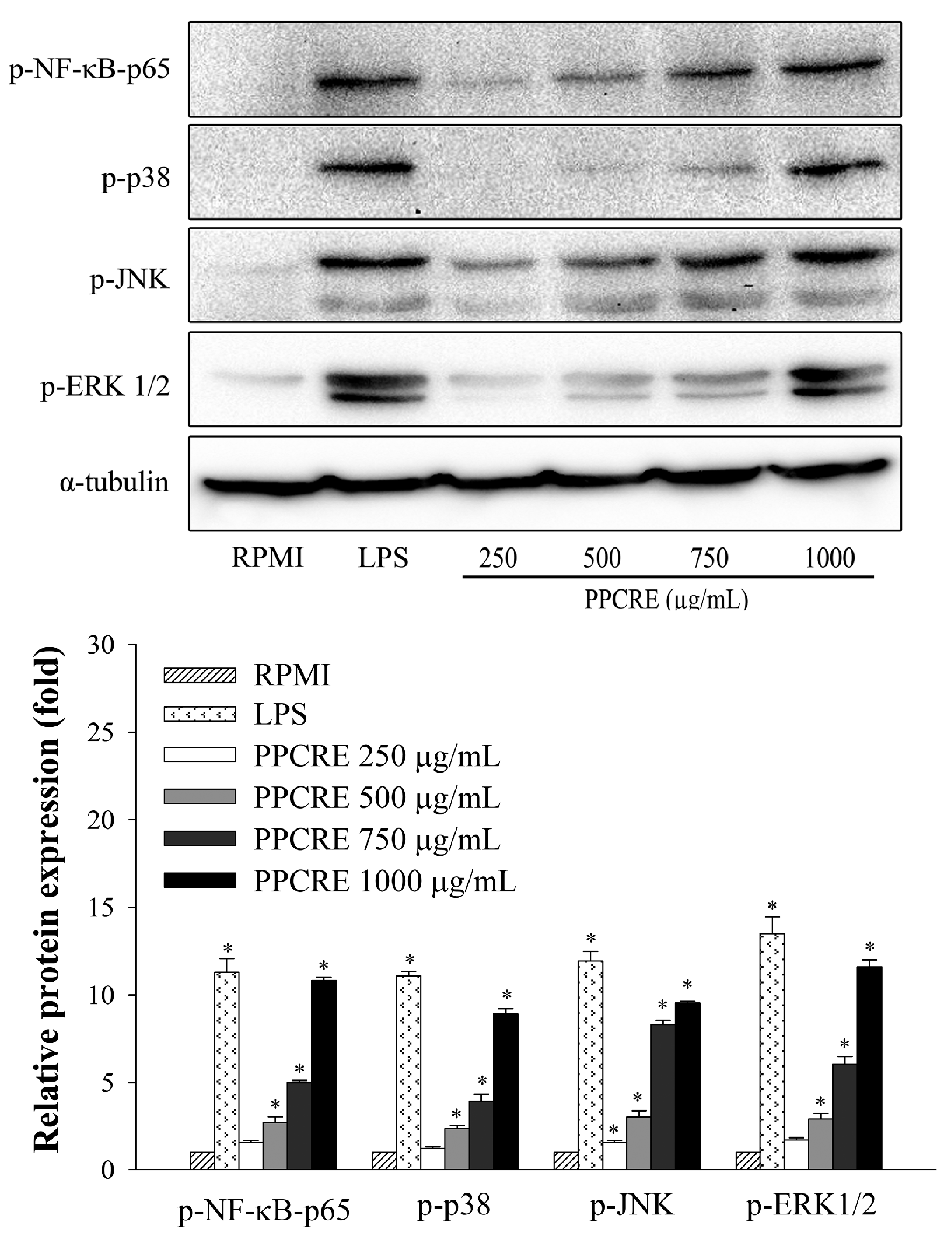
2.7. PPCRE Enhances MAPK and NF-κB Activation in RAW264.7 Cells
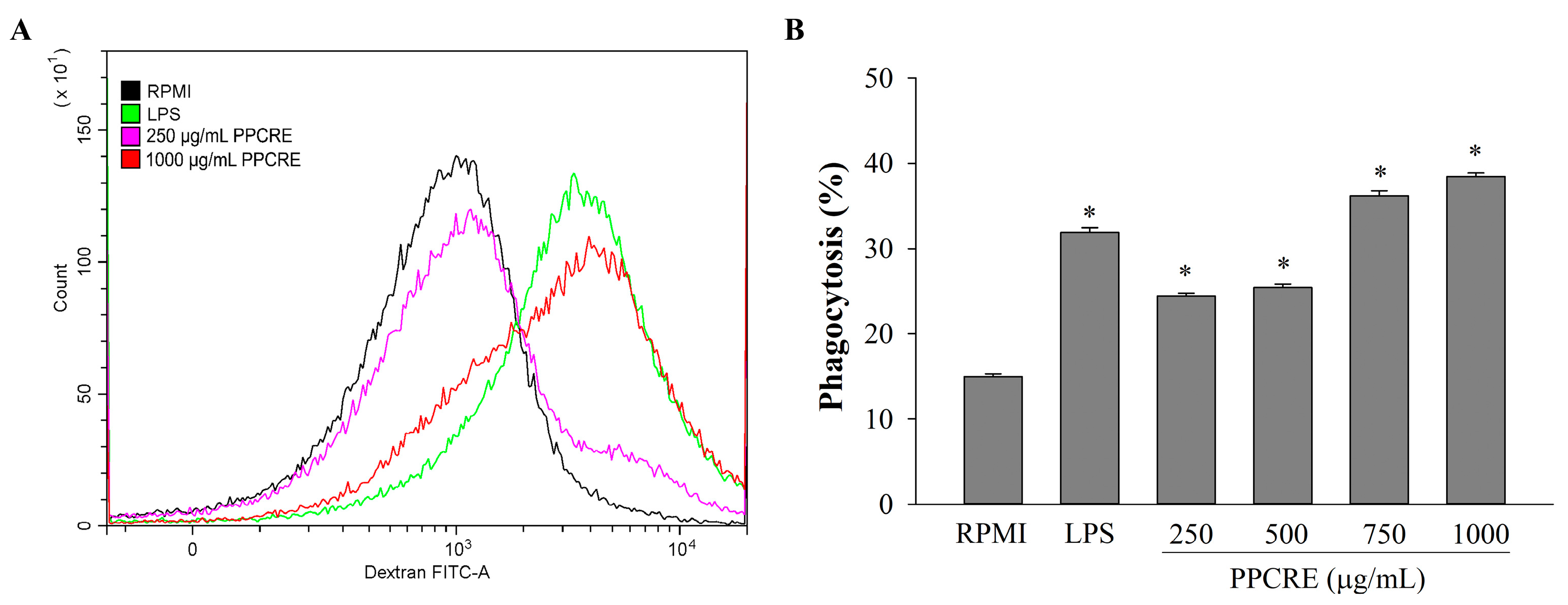
2.8. Effects of PPCRE on the Phagocytic Uptake of Macrophages
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparation of PPCRE
4.3. Analysis of Active Compounds by UPLC-MS/MS
4.4. Cell Culture
4.5. Cell Viability Analysis
4.6. Assay of NO and PGE2 Production
4.7. Cytokine Assay
4.8. qRT-PCR Analysis
4.9. Phagocytic Uptake Assays
4.10. Western Blot Analysis
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, Y.-S.; Kim, E.-K.; Nawarathna, W.P.; Dong, X.; Shin, W.-B.; Park, J.-S.; Moon, S.-H.; Park, P.-J. Immune-stimulatory effects of Althaea rosea flower extracts through the MAPK signaling pathway in RAW264.7 cells. Molecules 2017, 22, 679. [Google Scholar] [CrossRef]
- He, C.; Lin, H.Y.; Wang, C.C.; Zhang, M.; Lin, Y.Y.; Huang, F.Y.; Lin, Y.Z.; Tan, G.H. Exopolysaccharide from Paecilomyces lilacinus modulates macrophage activities through the TLR4/NF-κB/MAPK pathway. Mol. Med. Rep. 2019, 20, 4943–4952. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, C.H.; Yeo, J.-Y.; Hwang, K.W.; Park, S.-Y. Immunostimulatory activity of stem bark of Kalopanax pictus in RAW 264.7 macrophage. J. Herb. Med. 2022, 32, 100504. [Google Scholar] [CrossRef]
- Ko, M.N.; Hyun, S.B.; Ahn, K.J.; Hyun, C.-G. Immunomodulatory effects of Abelmoschus esculentus water extract through MAPK and NF-κB signaling in RAW264.7 cells. Biotechnol. Notes 2022, 3, 38–44. [Google Scholar] [CrossRef]
- Hwang, Y.P.; Lee, G.H.; Pham, T.H.; Kim, M.Y.; Kim, C.Y.; Lee, S.Y.; Han, E.H.; Choi, C.Y.; Hwang, S.D.; Ahn, S.; et al. Immune-enhancing effect of submerged culture of Ceriporia lacerata mycelia on cyclophosphamide-induced immunosuppressed mice and the underlying mechanisms in macrophages. Int. J. Mol. Sci. 2022, 23, 597. [Google Scholar] [CrossRef]
- Shen, C.-Y.; Yang, L.; Jiang, J.-G.; Zheng, C.-Y.; Zhu, W. Immune enhancement effects and extraction optimization of polysaccharides from Citrus aurantium L. var. amara Engl. Food Funct. 2017, 8, 796–807. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.-Y.; Choi, S.-I.; Han, X.; Men, X.; Jang, G.-W.; Choi, Y.-E.; Kim, S.-H.; Kang, J.-C.; Cho, J.-H.; Lee, O.-H. Enhancement of immune activities of mixtures with Sasa quelpaertensis Nakai and Ficus erecta var. sieboldii. Foods 2020, 9, 868. [Google Scholar] [CrossRef]
- Park, Y.M.; Lee, H.Y.; Shin, D.Y.; Kim, D.S.; Yoo, J.J.; Yang, H.J.; Kim, M.J.; Bae, J.S. Immune-enhancing effects of co-treatment with Kalopanax pictus Nakai Bark and Nelumbo nucifera Gaertner leaf extract in a cyclophosphamide-induced immunosuppressed rat model. Front. Nutr. 2022, 9, 898417. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-C.; Lee, G.-H.; Kim, S.-J.; Chung, W.-S.; Kim, S.-S.; Ko, S.-G.; Um, J.-Y. Immune-enhancing effect of Danggwibohyeoltang, an extract from Astragali Radix and Angelicae gigantis Radix, in vitro and in vivo. Immunopharmacol. Immunotoxicol. 2012, 34, 66–73. [Google Scholar] [CrossRef]
- Han, N.-R.; Kim, K.-C.; Kim, J.-S.; Ko, S.-G.; Park, H.-J.; Moon, P.-D. The immune-enhancing effects of a mixture of Astragalus membranaceus (Fisch.) Bunge, Angelica gigas Nakai, and Trichosanthes Kirilowii (Maxim.) or its active constituent nodakenin. J. Ethnopharmacol. 2022, 285, 114893. [Google Scholar] [CrossRef]
- Vetvicka, V.; Vetvickova, J. Immune enhancing effects of WB365, a novel combination of Ashwagandha (Withania somnifera) and Maitake (Grifola frondosa) extracts. N. Am. J. Med. Sci. 2011, 3, 320–324. [Google Scholar] [CrossRef]
- You, J.; Chang, Y.; Zhao, D.; Zhuang, J.; Zhuang, W. A mixture of functional complex extracts from Lycium barbarum and grape seed enhances immunity synergistically in vitro and in vivo. J. Food Sci. 2019, 84, 1577–1585. [Google Scholar] [CrossRef]
- Zhang, S.; Chai, X.; Hou, G.; Zhao, F.; Meng, Q. Platycodon grandiflorum (Jacq.) A. DC.: A review of phytochemistry, pharmacology, toxicology and traditional use. Phytomedicine 2022, 106, 154422. [Google Scholar] [CrossRef] [PubMed]
- Nyakudya, E.; Jeong, J.H.; Lee, N.K.; Jeong, Y.-S. Platycosides from the roots of Platycodon grandiflorum and their health benefits. Prev. Nutr. Food Sci. 2014, 19, 59–68. [Google Scholar] [CrossRef]
- Kim, M.; Hwang, I.-G.; Kim, S.-B.; Choi, A.-J. Chemical characterization of balloon flower (Platycodon grandiflorum) sprout extracts and their regulation of inflammatory activity in lipopolysaccharide-stimulated RAW264.7 murine macrophage cells. Food Sci. Nutr. 2020, 8, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yang, G.; Zhu, W.; Wen, W.; Zhang, F.; Yuan, J.; An, L. Anti-atherosclerotic activity of Platycodin D derived from roots of Platycodon grandiflorum in human endothelial cells. Biol. Pharm. Bull. 2012, 35, 1216–1221. [Google Scholar] [CrossRef]
- Xiao, W.; Zhou, P.; Wang, X.; Zhao, R.; Wang, Y. Comparative characterization and immunomodulatory activities of polysaccharides extracted from the radix of Platycodon grandiflorum with different extraction methods. Molecules 2022, 27, 4759. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Hwang, W.-I.; Lim, S.-T. Antioxidant and anticancer activities of organic extracts from Platycodon grandiflorum A. De Candolle roots. J. Ethnopharmacol. 2004, 93, 409–415. [Google Scholar] [CrossRef]
- Zhao, H.L.; Harding, S.V.; Marinangeli, C.P.F.; Kim, Y.S.; Jones, P.J.H. Hypocholesterolemic and anti-obesity effects of saponins from Platycodon grandiflorum in hamsters fed atherogenic diets. J. Food Sci. 2008, 73, H195–H200. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, Y.; Yan, P.; Cheng, G.; Wang, C.; Geng, N.; Wang, X.; Liu, J. Effects of polysaccharides from Platycodon grandiflorum on immunity-enhancing activity in vitro. Molecules 2017, 22, 1918. [Google Scholar] [CrossRef] [PubMed]
- Park, E.-J.; Lee, H.-J. Immunomodulatory effects of fermented Platycodon grandiflorum extract through NF-κB signaling in RAW264.7 cells. Nutr. Res. Pract. 2020, 14, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Noh, E.-M.; Kim, J.-M.; Lee, H.Y.; Song, H.-K.; Joung, S.O.; Yang, H.J.; Kim, M.J.; Kim, K.S.; Lee, Y.-R. Immuno-enhancement effects of Platycodon grandiflorum extracts in splenocytes and a cyclophosphamide-induced immunosuppressed rat model. BMC Complement. Altern. Med. 2019, 19, 322. [Google Scholar] [CrossRef]
- Cho, B.O.; Choi, J.; Kang, H.J.; Che, D.N.; Shin, J.Y.; Kim, J.S.; Kim, S.J.; Jang, S.I. Anti-obesity effects of a mixed extract containing Platycodon grandiflorum, Apium graveolens and green tea in high-fat-diet-induced obese mice. Exp. Ther. Med. 2020, 19, 2783–2791. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.-H.; Kwak, D.Y.; So, J.-S. Inhibition of nitric oxide production and hyaluronidase activities from the combined extracts of Platycodon grandiflorum, Astragalus membranaceus, and Schisandra chinensis. J. Korean Soc. Food Sci. Nutr. 2013, 42, 844–850. [Google Scholar] [CrossRef]
- Han, Y.-K.; Kim, Y.-S.; Natarajan, S.B.; Kim, W.-S.; Hwang, J.-W.; Jeon, N.-J.; Jeong, J.-H.; Moon, S.-H.; Jeon, B.-T.; Park, P.-J. Antioxidant and anti-inflammatory effects of Chaenomeles sinensis leaf extracts on LPS-stimulated RAW264.7 cells. Molecules 2016, 21, 422. [Google Scholar] [CrossRef]
- Seong, Y.H. Protective effect of an ethanol extract mixture of Aralia elata, Chaenomeles sinensis fruit, and Glycyrrhizae radix against cerebral ischemia-reperfusion injury in rats and excitotoxic and oxidative neuronal damage. J. Biomed. Transl. Res. 2022, 23, 43–53. [Google Scholar] [CrossRef]
- Behboudian, M.H.; Lawes, G.S.; Griffiths, K.M. The influence of water deficit on water relations, photosynthesis and fruit growth in Asian pear (Pyrus serotina Rehd.). Sci. Hortic. 1994, 60, 89–99. [Google Scholar] [CrossRef]
- Hong, S.-Y.; Lansky, E.; Kang, S.-S.; Yang, M. A review of pears (Pyrus spp.), ancient functional food for modern times. BMC Complement. Altern. Med. 2021, 21, 219. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Moon, E.; Kim, S.Y.; Choi, S.U.; Lee, J.H.; Lee, K.R. 4-Methylthio-butanyl derivatives from the seeds of Raphanus sativus and their biological evaluation on anti-inflammatory and antitumor activities. J. Ethnopharmacol. 2014, 151, 503–508. [Google Scholar] [CrossRef]
- Park, Y.J.; Moon, C.; Kang, J.-H.; Choi, T.-J. Antiviral effects of extracts from Celosia cristata and Raphanus sativus roots against viral hemorrhagic septicemia virus. Arch. Virol. 2017, 162, 1711–1716. [Google Scholar] [CrossRef] [PubMed]
- Son, S.-U.; Park, H.Y.; Suh, H.J.; Shin, K.-S. Evaluation of antitumor metastasis via immunostimulating activities of pectic polysaccharides isolated from radish leaves. J. Funct. Foods 2021, 85, 104639. [Google Scholar] [CrossRef]
- Jang, A.y.; Kim, M.; Rod-in, W.; Nam, Y.S.; Yoo, T.Y.; Park, W.J. In vitro immune-enhancing effects of Platycodon grandiflorum combined with Salvia plebeian via MAPK and NF-κB signaling in RAW264.7 cells. PLoS ONE 2024, 19, e0297512. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.-O.; Song, W.-S. Antioxidant activity of Pyrus serotina fruit in different cultivars and parts. Korean J. Plant Res. 2012, 25, 498–503. [Google Scholar] [CrossRef]
- Yang, E.J.; Lee, S.H. Anti-Inflammatory effects of Chaenomeles sinensis extract in an ALS animal model. Front. Biosci. 2023, 28, 326. [Google Scholar] [CrossRef]
- Di Sotto, A.; Vitalone, A.; Di Giacomo, S. Plant-derived nutraceuticals and immune system modulation: An evidence-based overview. Vaccines 2020, 8, 468. [Google Scholar] [CrossRef] [PubMed]
- Déciga-Campos, M.; Beltrán-Villalobos, K.L.; Aguilar-Mariscal, H.; González-Trujano, M.E.; Ángeles-López, G.E.; Ventura-Martínez, R. Synergistic herb-herb interaction of the antinociceptive and anti-inflammatory effects of Syzygium aromaticum and Rosmarinus officinalis combination. Evid. Based Complement. Alternat. Med. 2021, 2021, 8916618. [Google Scholar] [CrossRef]
- Franek, K.J.; Zhou, Z.; Zhang, W.-D.; Chen, W.Y. In vitro studies of baicalin alone or in combination with Salvia miltiorrhiza extract as a potential anti-cancer agent. Int. J. Oncol. 2005, 26, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Mugford, S.T.; Osbourn, A. Saponin Synthesis and Function. In Isoprenoid Synthesis in Plants and Microorganisms: New Concepts and Experimental Approaches; Bach, T.J., Rohmer, M., Eds.; Springer: New York, NY, USA, 2013; pp. 405–424. [Google Scholar]
- Ahn, K.S.; Noh, E.J.; Zhao, H.L.; Jung, S.H.; Kang, S.S.; Kim, Y.S. Inhibition of inducible nitric oxide synthase and cyclooxygenase II by Platycodon grandiflorum saponins via suppression of nuclear factor-κB activation in RAW 264.7 cells. Life Sci. 2005, 76, 2315–2328. [Google Scholar] [CrossRef]
- Wang, C.; Schuller Levis, G.B.; Lee, E.B.; Levis, W.R.; Lee, D.W.; Kim, B.S.; Park, S.Y.; Park, E. Platycodin D and D3 isolated from the root of Platycodon grandiflorum modulate the production of nitric oxide and secretion of TNF-α in activated RAW 264.7 cells. Int. Immunopharmacol. 2004, 4, 1039–1049. [Google Scholar] [CrossRef]
- Coleman, J.W. Nitric oxide in immunity and inflammation. Int. Immunopharmacol. 2001, 1, 1397–1406. [Google Scholar] [CrossRef]
- Tanabe, T.; Tohnai, N. Cyclooxygenase isozymes and their gene structures and expression. Prostaglandins Other Lipid Mediat. 2002, 68–69, 95–114. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, N.; Kobayashi, K. Macrophages in inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Son, H.J.; Eo, H.J.; Park, G.H.; Jeong, J.B. Heracleum moellendorffii root extracts exert immunostimulatory activity through TLR2/4-dependent MAPK activation in mouse macrophages, RAW264.7 cells. Food Sci. Nutr. 2021, 9, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Verma, I.M. NF-κB regulation in the immune system. Nat. Rev. Immunol. 2002, 2, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L.; Wu, W.; Dholakiya, S.L.; Gorasiya, S.; Wu, J.; Sitapara, R.; Patel, V.; Wang, M.; Zur, M.; Reddy, S.; et al. Assessment of phagocytic activity of cultured macrophages using fluorescence microscopy and flow cytometry. In Cytokine Bioassays: Methods and Protocols; Vancurova, I., Ed.; Springer: New York, NY, USA, 2014; pp. 137–145. [Google Scholar]
- Kim, J.-H.; Ku, B.-H.; Ko, G.-P.; Kang, M.-J.; Son, K.-H.; Bang, M.-A.; Park, H.-Y. Enzyme feed additive with arazyme improve growth performance, meat quality, and gut microbiome of pigs. Animals 2023, 13, 423. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rod-in, W.; Kim, M.; Jang, A.-y.; Nam, Y.S.; Yoo, T.Y.; Park, W.J. Immunostimulatory Activity of a Mixture of Platycodon grandiflorum, Pyrus serotine, Chaenomeles sinensis, and Raphanus sativus in RAW264.7 Macrophages. Int. J. Mol. Sci. 2024, 25, 10660. https://doi.org/10.3390/ijms251910660
Rod-in W, Kim M, Jang A-y, Nam YS, Yoo TY, Park WJ. Immunostimulatory Activity of a Mixture of Platycodon grandiflorum, Pyrus serotine, Chaenomeles sinensis, and Raphanus sativus in RAW264.7 Macrophages. International Journal of Molecular Sciences. 2024; 25(19):10660. https://doi.org/10.3390/ijms251910660
Chicago/Turabian StyleRod-in, Weerawan, Minji Kim, A-yeong Jang, Yu Suk Nam, Tae Young Yoo, and Woo Jung Park. 2024. "Immunostimulatory Activity of a Mixture of Platycodon grandiflorum, Pyrus serotine, Chaenomeles sinensis, and Raphanus sativus in RAW264.7 Macrophages" International Journal of Molecular Sciences 25, no. 19: 10660. https://doi.org/10.3390/ijms251910660
APA StyleRod-in, W., Kim, M., Jang, A.-y., Nam, Y. S., Yoo, T. Y., & Park, W. J. (2024). Immunostimulatory Activity of a Mixture of Platycodon grandiflorum, Pyrus serotine, Chaenomeles sinensis, and Raphanus sativus in RAW264.7 Macrophages. International Journal of Molecular Sciences, 25(19), 10660. https://doi.org/10.3390/ijms251910660





