Novel Chiral Self-Assembled Nano-Fluorescence Materials with AIE Characteristics for Specific Enantioselective Recognition of L-Lysine
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization
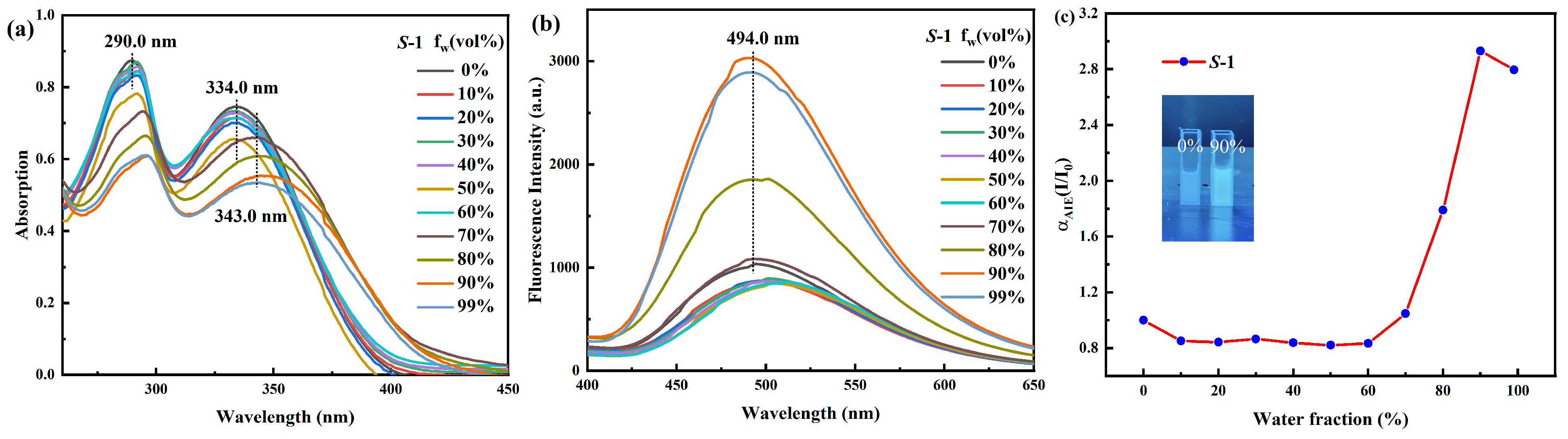
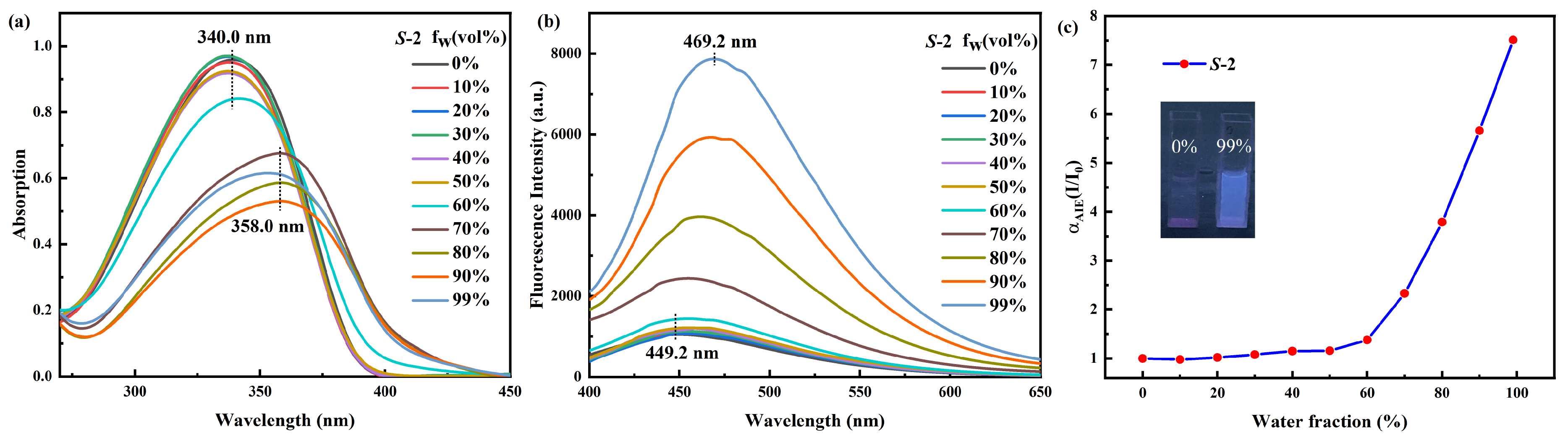
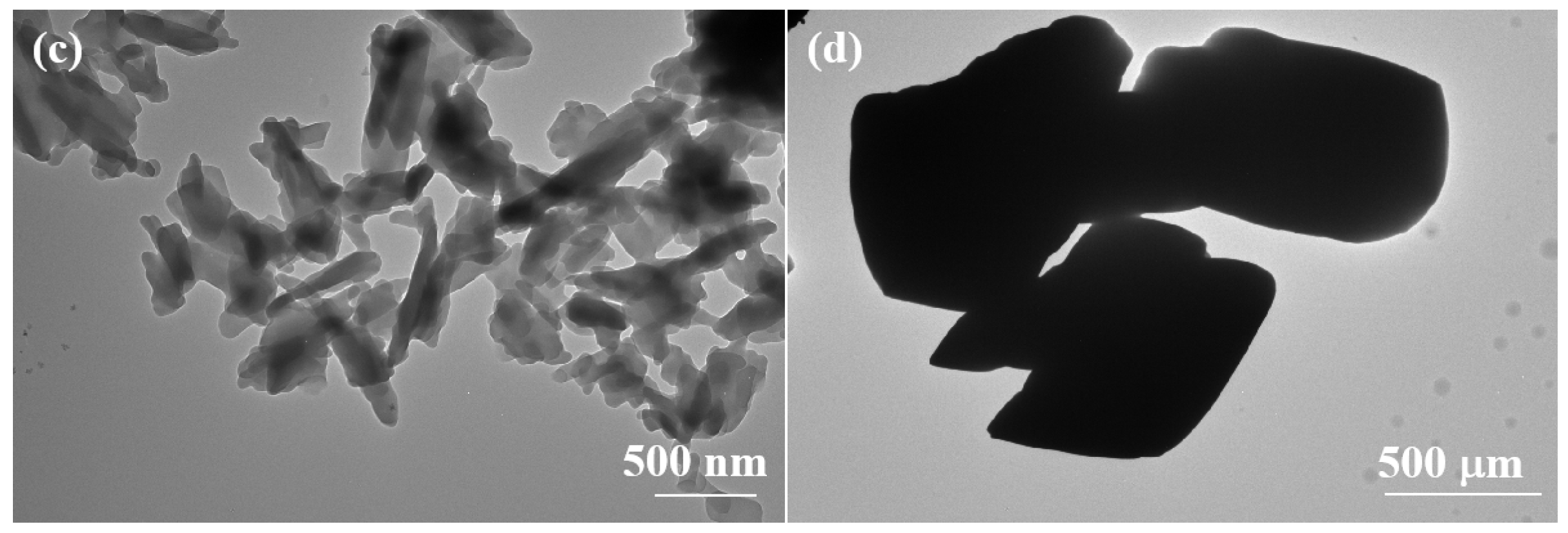
2.2. Self-Identification
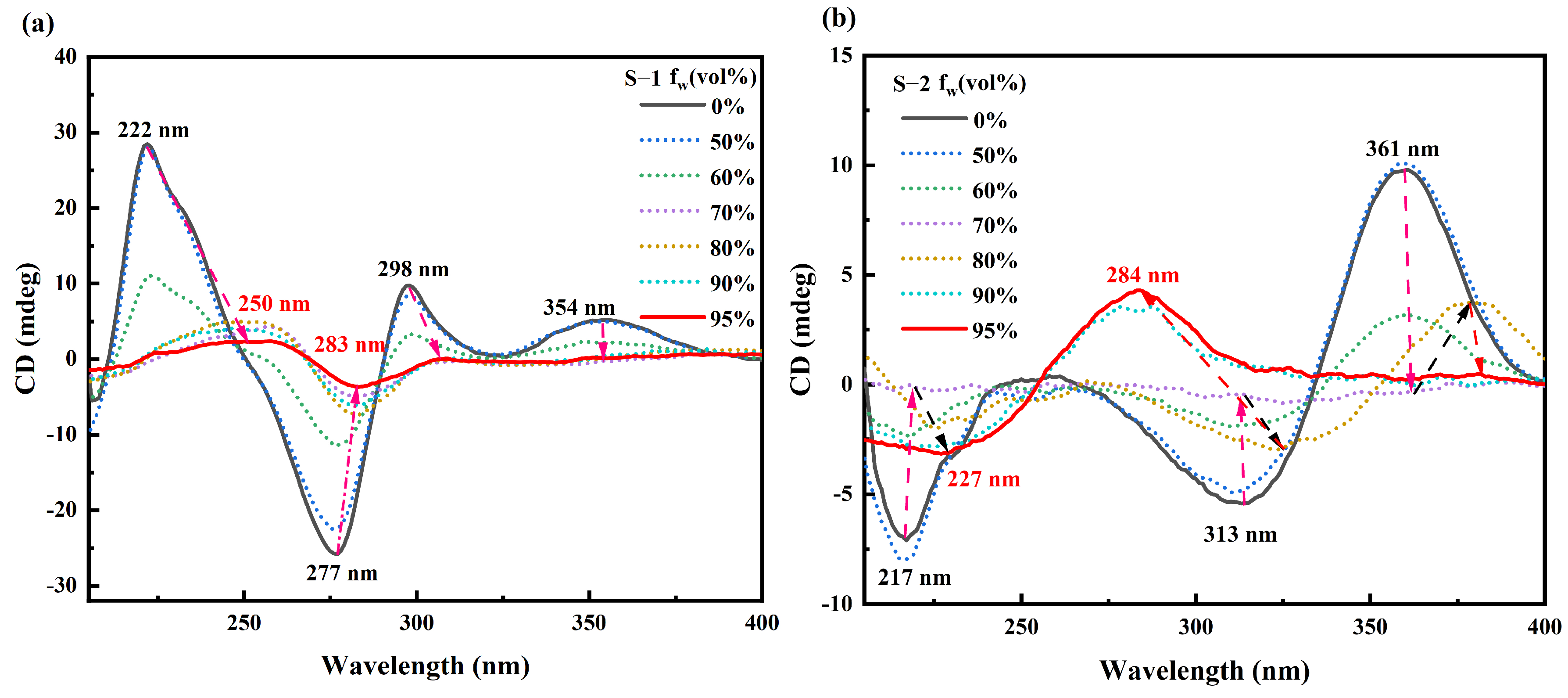
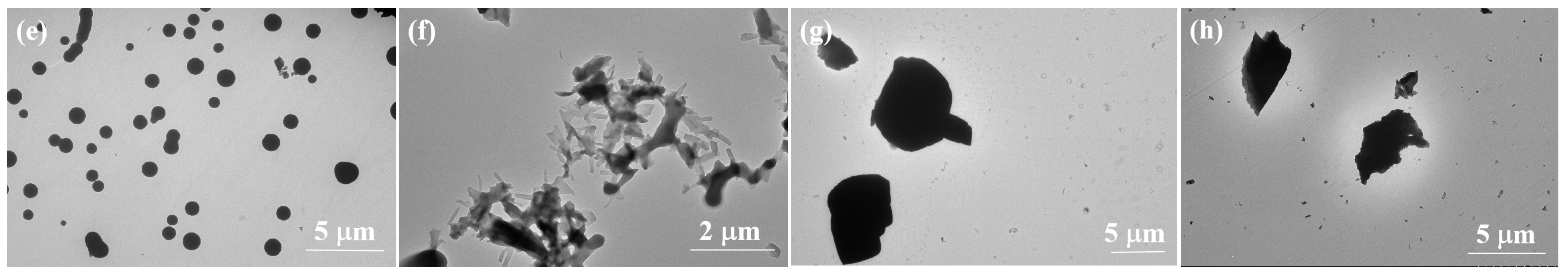
2.3. Aggregation-Induced Annihilation of CD Signals
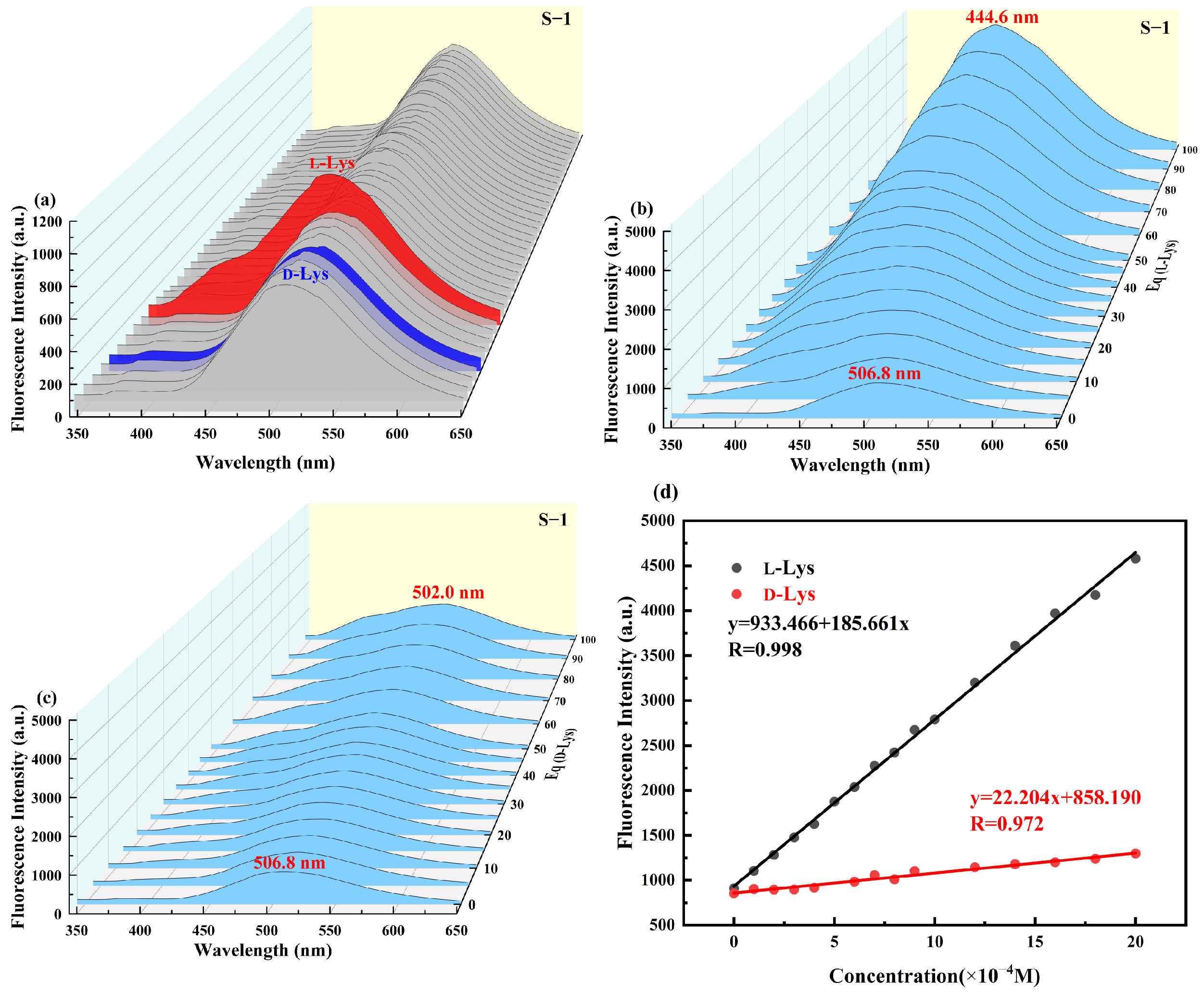
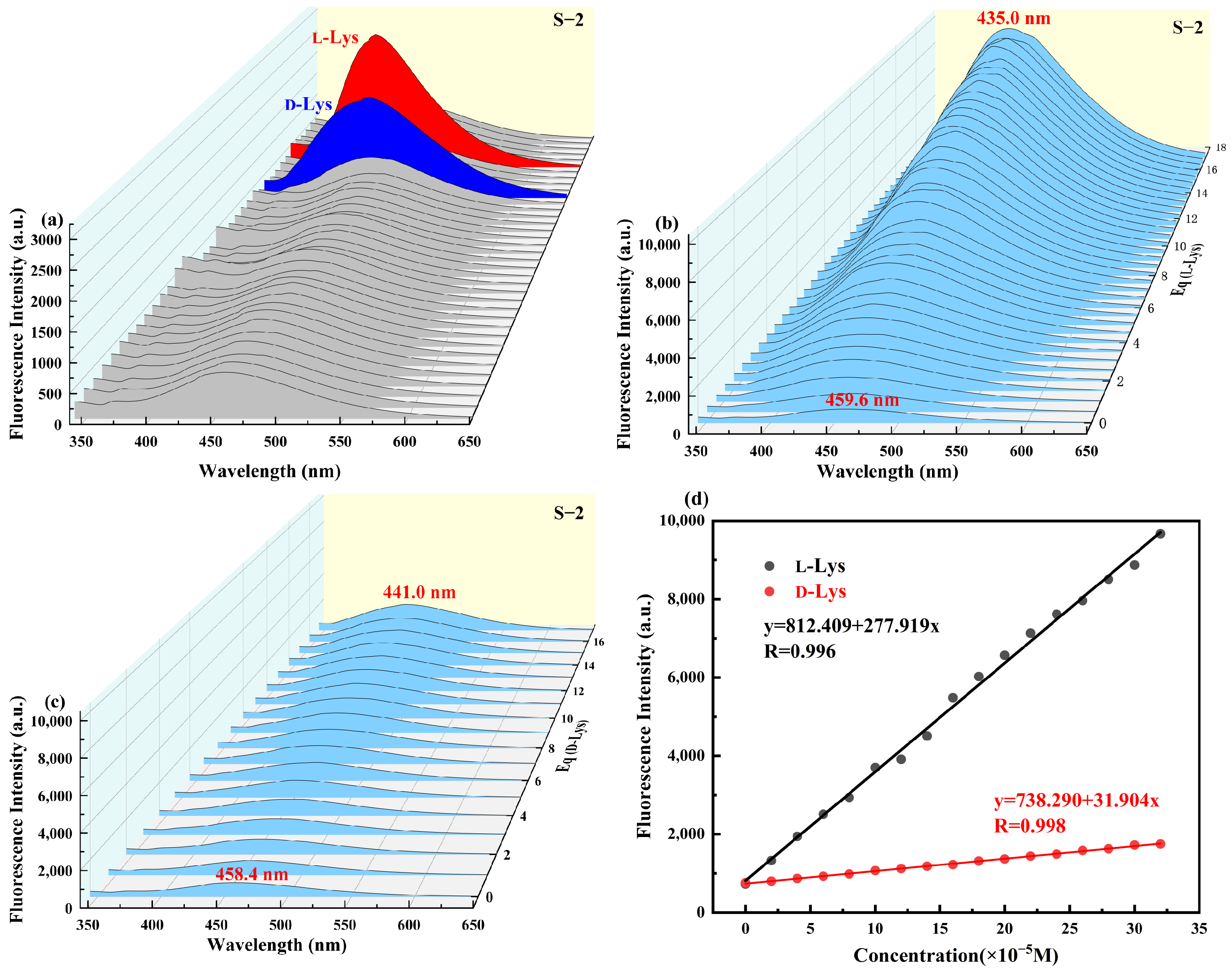
2.4. Recognition Applications
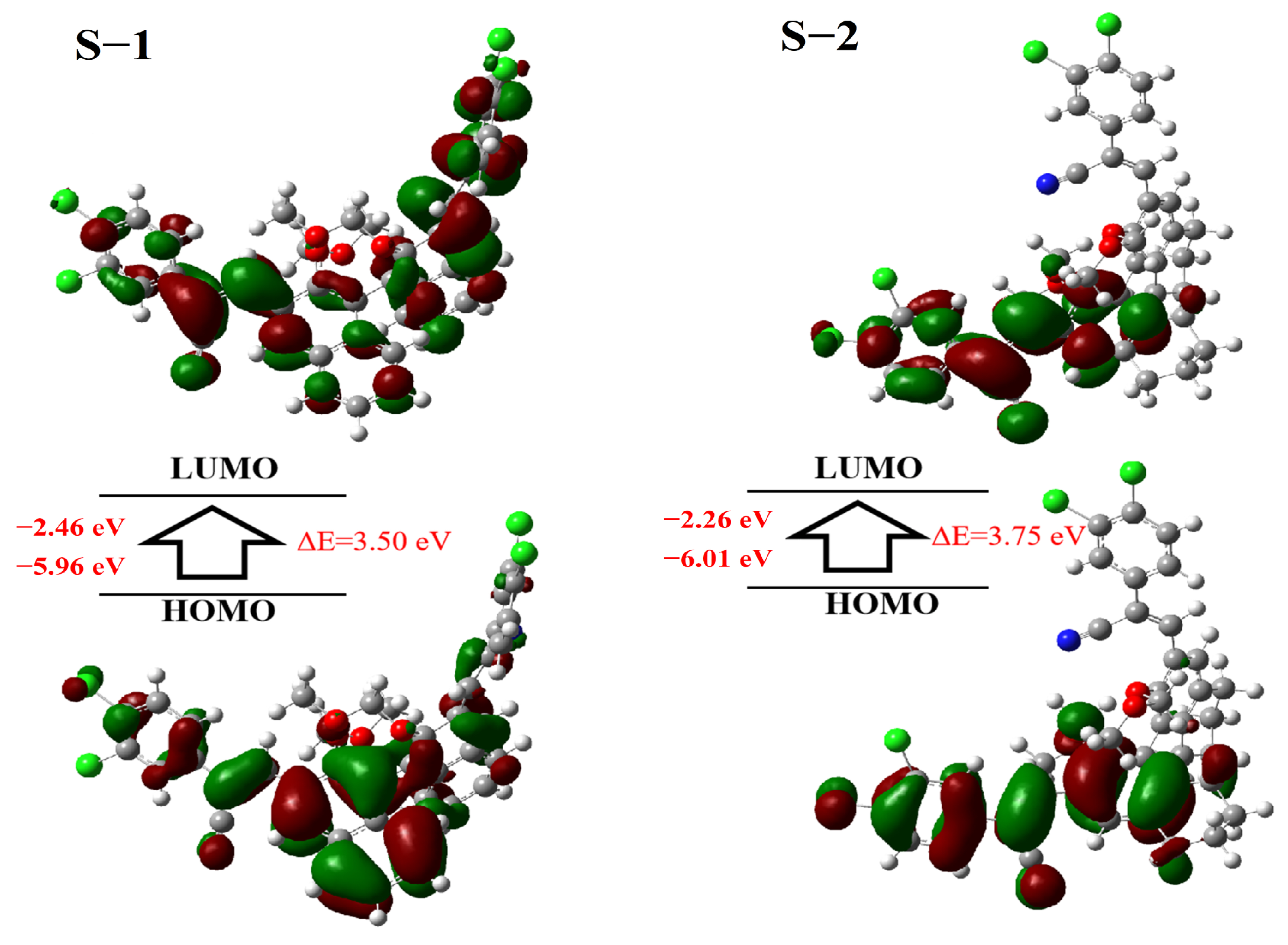
2.5. DFT Calculations
3. Materials and Methods
3.1. Synthesis of Probe S-1
3.2. Synthesis of Probe S-2
3.3. Preparation of Fluorescent Probe Solutions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yan, Z.-P.; Liao, K.; Han, H.-B.; Su, J.; Zheng, Y.-X.; Zuo, J.-L. Chiral Iridium(iii) Complexes with Four-Membered Ir–S–P–S Chelating Rings for High-Performance Circularly Polarized OLEDs. Chem. Commun. 2019, 55, 8215–8218. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.-X.; Ni, C.-Z.; Zhang, F.-Q.; Zhu, Y.-Y.; Zeng, J.; Gu, S.-X. Chiral Amino Acid Recognition in Water: A BINOL-Based Fluorescent Probe. Chin. Chem. Lett. 2023, 34, 108345. [Google Scholar] [CrossRef]
- Munusamy, S.; Swaminathan, S.; Jothi, D.; Muralidharan, V.P.; Iyer, S.K. A Sensitive and Selective BINOL Based Ratiometric Fluorescence Sensor for the Detection of Cyanide Ions. RSC Adv. 2021, 11, 15656–15662. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: Phenomenon, Mechanism and Applications. Chem. Commun. 2009, 4332–4353. [Google Scholar] [CrossRef]
- Wang, X.; Xiang, S.; Qi, C.; Chen, M.; Su, X.; Yang, J.-C.; Tian, J.; Feng, H.-T.; Tang, B.Z. Visualization of Enantiorecognition and Resolution by Chiral AIEgens. ACS Nano 2022, 16, 8223–8232. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Zhang, H.; Quan, Y.; Li, Y.; Cheng, Y.; Ye, S. High Brightness Circularly Polarized Organic Light-Emitting Diodes Based on Nondoped Aggregation-Induced Emission (AIE)-Active Chiral Binaphthyl Emitters. Org. Lett. 2019, 21, 439–443. [Google Scholar] [CrossRef]
- Wu, S.; Yang, Y.; Cheng, Y.; Wang, S.; Zhou, Z.; Zhang, P.; Zhu, X.; Wang, B.; Zhang, H.; Xie, S.; et al. Fluorogenic Detection of Mercury Ion in Aqueous Environment Using Hydrogel-based AIE Sensing Films. Aggregate 2023, 4, e287. [Google Scholar] [CrossRef]
- Sayed, S.M.; Jia, H.-R.; Jiang, Y.-W.; Zhu, Y.-X.; Ma, L.; Yin, F.; Hussain, I.; Khan, A.; Ma, Q.; Wu, F.-G.; et al. Photostable AIE Probes for Wash-Free, Ultrafast, and High-Quality Plasma Membrane Staining. J. Mater. Chem. B 2021, 9, 4303–4308. [Google Scholar] [CrossRef]
- Luo, W.; Tang, Y.; Zhang, X.; Wu, Z.; Wang, G. Multifunctional Fluorescent Materials with Reversible Mechanochromism and Distinct Photochromism. Adv. Opt. Mater. 2023, 11, 2202259. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Q.; Hu, P.; Xu, C.; Guo, W.; Wang, Z.; Mao, Z.; Yang, Z.; Liu, C.; Shi, G.; et al. Achieving Remarkable and Reversible Mechanochromism from a Bright Ionic AIEgen with High Specificity for Mitochondrial Imaging and Secondary Aggregation Emission Enhancement for Long-Term Tracking of Tumors. Mater. Chem. Front. 2020, 4, 941–949. [Google Scholar] [CrossRef]
- Zhang, M.; Li, J.; Yu, L.; Wang, X.; Bai, M. Tuning the Fluorescence Based on the Combination of TICT and AIE Emission of a Tetraphenylethylene with D–π–A Structure. RSC Adv. 2020, 10, 14520–14524. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Feng, H.; Gong, Q.; Wu, C.; Zhou, H.; Huang, Z.; Yang, J.; Chen, X.; Zhao, N. BINOL-Based Chiral Aggregation-Induced Emission Luminogens and Their Application in Detecting Copper(ii) Ions in Aqueous Media. J. Mater. Chem. C 2015, 3, 11458–11463. [Google Scholar] [CrossRef]
- Yang, Z.; Huo, Y.; Liu, Y.; Du, G.; Zhang, W.; Zhou, L.; Zhan, L.; Ren, X.; Duan, W.; Gong, S. Axially Chiral 1,4-Dihydropyridine Derivatives: Aggregation-Induced Emission in Exciplexes and Application as Viscosity Probes. RSC Adv. 2019, 9, 32219–32225. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Li, K.; Cui, P.-C.; Li, L.-L.; Pan, S.-L.; Li, M.-Y.; Yu, X.-Q. BINOL Derivatives with Aggression-Induced Emission. J. Mater. Chem. B 2018, 6, 4413–4416. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-G.; Yan, Z.-P.; Luo, X.-F.; Yuan, L.; Liang, W.-Q.; Wang, Y.; Zheng, Y.-X.; Zuo, J.-L.; Pan, Y. Non-Doped and Doped Circularly Polarized Organic Light-Emitting Diodes with High Performances Based on Chiral Octahydro-Binaphthyl Delayed Fluorescent Luminophores. J. Mater. Chem. C 2019, 7, 7045–7052. [Google Scholar] [CrossRef]
- Zhao, W.-L.; Wang, Y.-F.; Wan, S.-P.; Lu, H.-Y.; Li, M.; Chen, C.-F. Chiral Thermally Activated Delayed Fluorescence-Active Macrocycles Displaying Efficient Circularly Polarized Electroluminescence. CCS Chem. 2022, 4, 3540–3548. [Google Scholar] [CrossRef]
- Liu, T.-T.; Yan, Z.-P.; Hu, J.-J.; Yuan, L.; Luo, X.-F.; Tu, Z.-L.; Zheng, Y.-X. Chiral Thermally Activated Delayed Fluorescence Emitters-Based Efficient Circularly Polarized Organic Light-Emitting Diodes Featuring Low Efficiency Roll-Off. ACS Appl. Mater. Interfaces 2021, 13, 56413–56419. [Google Scholar] [CrossRef]
- Sun, B.; Ding, L.; Wang, X.; Tu, Z.-L.; Fan, J. Circularly Polarized Thermally Activated Delayed Fluorescence OLEDs with Nearly BT.2020 Red Emission. Chem. Eng. J. 2023, 476, 146511. [Google Scholar] [CrossRef]
- Du, G.; Pu, L. Micelle-Encapsulated Fluorescent Probe: Chemoselective and Enantioselective Recognition of Lysine in Aqueous Solution. Org. Lett. 2019, 21, 4777–4781. [Google Scholar] [CrossRef]
- Huang, J.; Zhao, H.; Yu, X.; Limeng, Y.; Fan, C.; Liao, P.; Zhang, B.; Li, C.; Du, G.; Dong, Q.; et al. An Oxime-Based Fluorescent-ON Probe for the Rapid Detection of Arginine and Lysine in Aqueous Solution. Dyes Pigments 2023, 217, 111414. [Google Scholar] [CrossRef]
- Yu, J.; Fan, J.; Song, Y.; Zhao, Y.; Lin, Z.; Jiang, L.; Li, H. Near-Infrared Fluorescent Probe with Large Stokes Shift for Specific Detection of Lysine. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 308, 123734. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Xie, Z.; Lam, J.W.Y.; Cheng, L.; Tang, B.Z.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; et al. Aggregation-Induced Emission of 1-Methyl-1,2,3,4,5-Pentaphenylsilole. Chem. Commun. 2001, 1740–1741. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Deng, Q.; Li, Y.; Zheng, M.; Chai, Z.; Wan, C.; Zheng, Z.; Li, L.; Huang, F.; Tang, B. A Rapid and Ultrasensitive Tetraphenylethylene-Based Probe with Aggregation-Induced Emission for Direct Detection of α-Amylase in Human Body Fluids. Anal. Chem. 2018, 90, 13775–13782. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Pu, J.; Wang, S.; Jiang, S.; Yang, W.; Cao, D.; Feng, Y.-S. High Solid-State CPL Active Materials Based on Chiral BINOL-Dicyanodistyrylbenzene. Dye. Pigment. 2023, 217, 111422. [Google Scholar] [CrossRef]
- Nowak-Król, A.; Geppert, P.T.; Naveen, K.R. Boron-Containing Helicenes as New Generation of Chiral Materials: Opportunities and Challenges of Leaving the Flatland. Chem. Sci. 2024, 15, 7408–7440. [Google Scholar] [CrossRef]
- Li, X.; Hu, W.; Wang, Y.; Quan, Y.; Cheng, Y. Strong CPL of Achiral AIE-Active Dyes Induced by Supramolecular Self-Assembly in Chiral Nematic Liquid Crystals (AIE-N*-LCs). Chem. Commun. 2019, 55, 5179–5182. [Google Scholar] [CrossRef]
- Wang, R.; Song, K.; Wei, Z.; Sun, Y.; Sun, X.; Hu, Y. The Intramolecular Charge Transfer Mechanism by Which Chiral Self-Assembled H8-BINOL Vesicles Enantioselectively Recognize Amino Alcohols. Int. J. Mol. Sci. 2024, 25, 5606. [Google Scholar] [CrossRef]
- Tang, S.; Wei, Z.; Guo, J.; Sun, X.; Hu, Y. Enantioselective Recognition of L-Lysine by ICT Effect with a Novel Binaphthyl-Based Complex. Micromachines 2023, 14, 500. [Google Scholar] [CrossRef]
- Wei, Z.; Tang, S.; Sun, X.; Hu, Y. Enantioselective Recognition of Lysine and Phenylalanine Using an Imidazole Salt-Type Fluorescent Probe Based on H8-BINOL. Molecules 2022, 27, 8470. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Wang, R.; Sun, Y.; Hu, Y.; Song, K.; Sun, X. Novel Chiral Self-Assembled Nano-Fluorescence Materials with AIE Characteristics for Specific Enantioselective Recognition of L-Lysine. Int. J. Mol. Sci. 2024, 25, 10666. https://doi.org/10.3390/ijms251910666
Wang P, Wang R, Sun Y, Hu Y, Song K, Sun X. Novel Chiral Self-Assembled Nano-Fluorescence Materials with AIE Characteristics for Specific Enantioselective Recognition of L-Lysine. International Journal of Molecular Sciences. 2024; 25(19):10666. https://doi.org/10.3390/ijms251910666
Chicago/Turabian StyleWang, Peng, Rong Wang, Yue Sun, Yu Hu, Kaiyue Song, and Xiaoxia Sun. 2024. "Novel Chiral Self-Assembled Nano-Fluorescence Materials with AIE Characteristics for Specific Enantioselective Recognition of L-Lysine" International Journal of Molecular Sciences 25, no. 19: 10666. https://doi.org/10.3390/ijms251910666






