The Induction of Combined Hyperthermal Ablation Effect of Irreversible Electroporation with Polydopamine Nanoparticle-Coated Electrodes
Abstract
1. Introduction
2. Results and Discussion
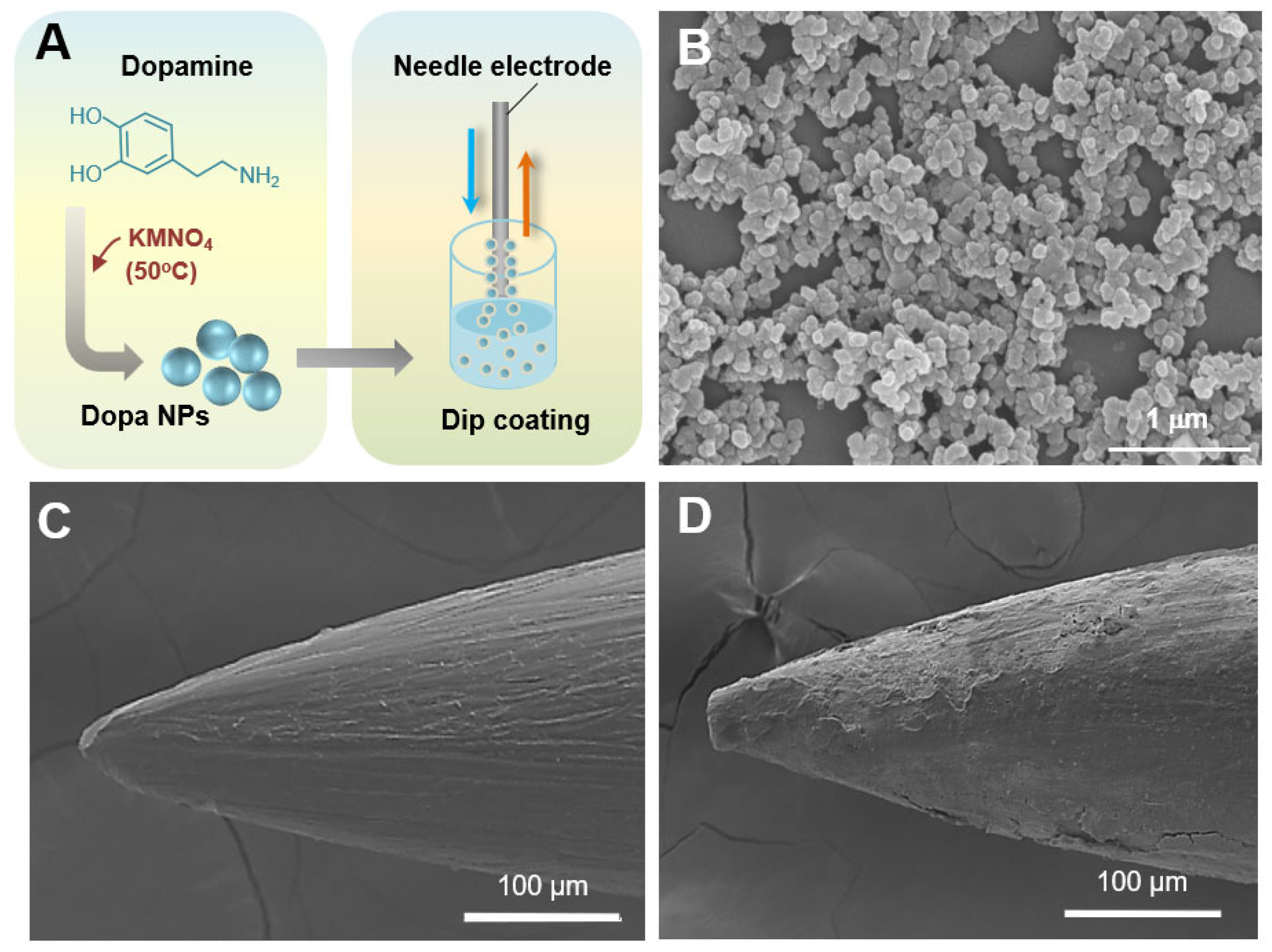
2.1. Synthesis and Characteristics of Dopa NPs
2.2. Cell Compatibility of Dopa NPs
2.3. Surface Analysis of Dopa NP-Coated Electrodes
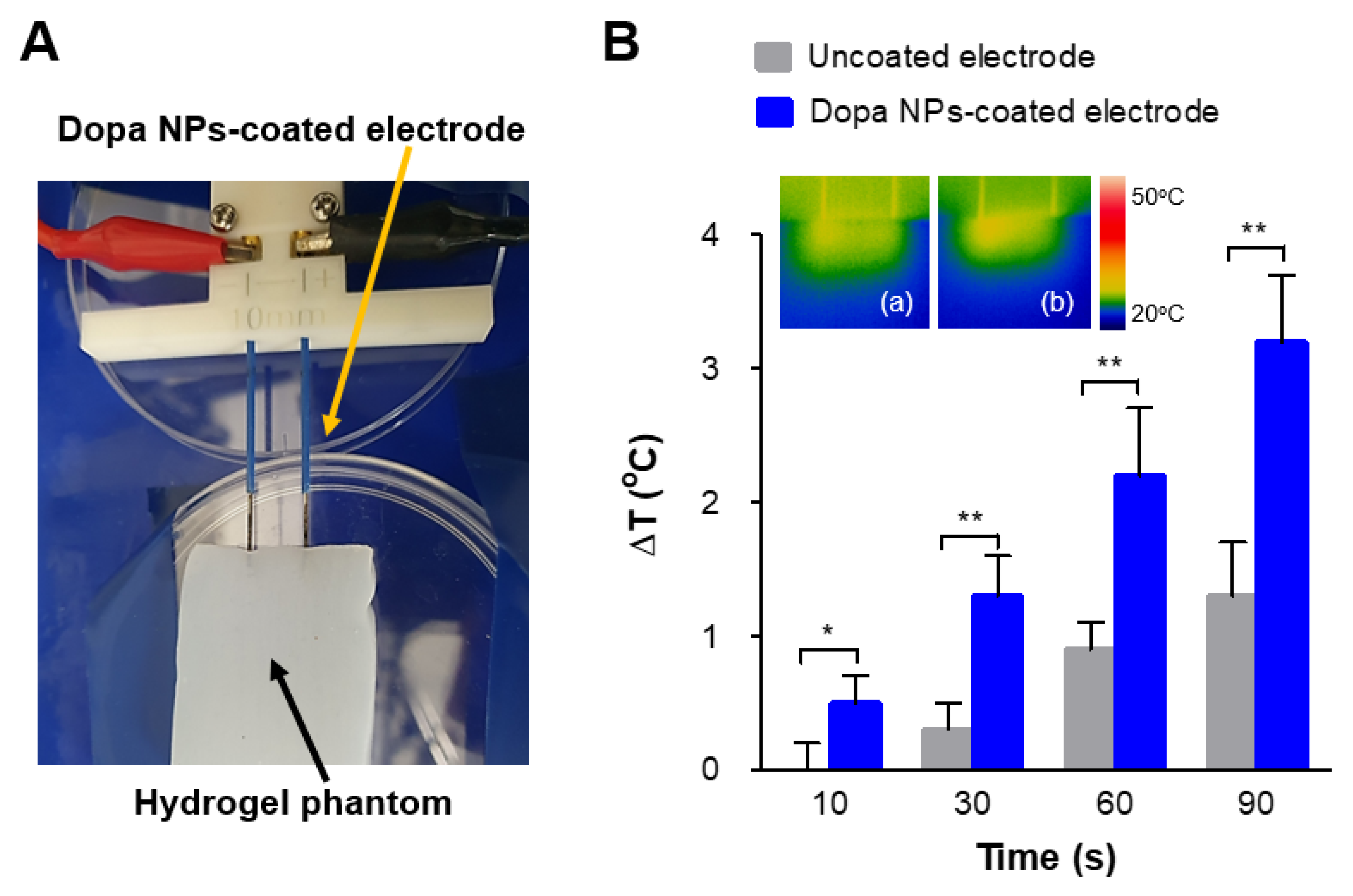
2.4. Enhanced Thermal Effect of IRE with Dopa NP-Coated Electrodes in a Phantom Model
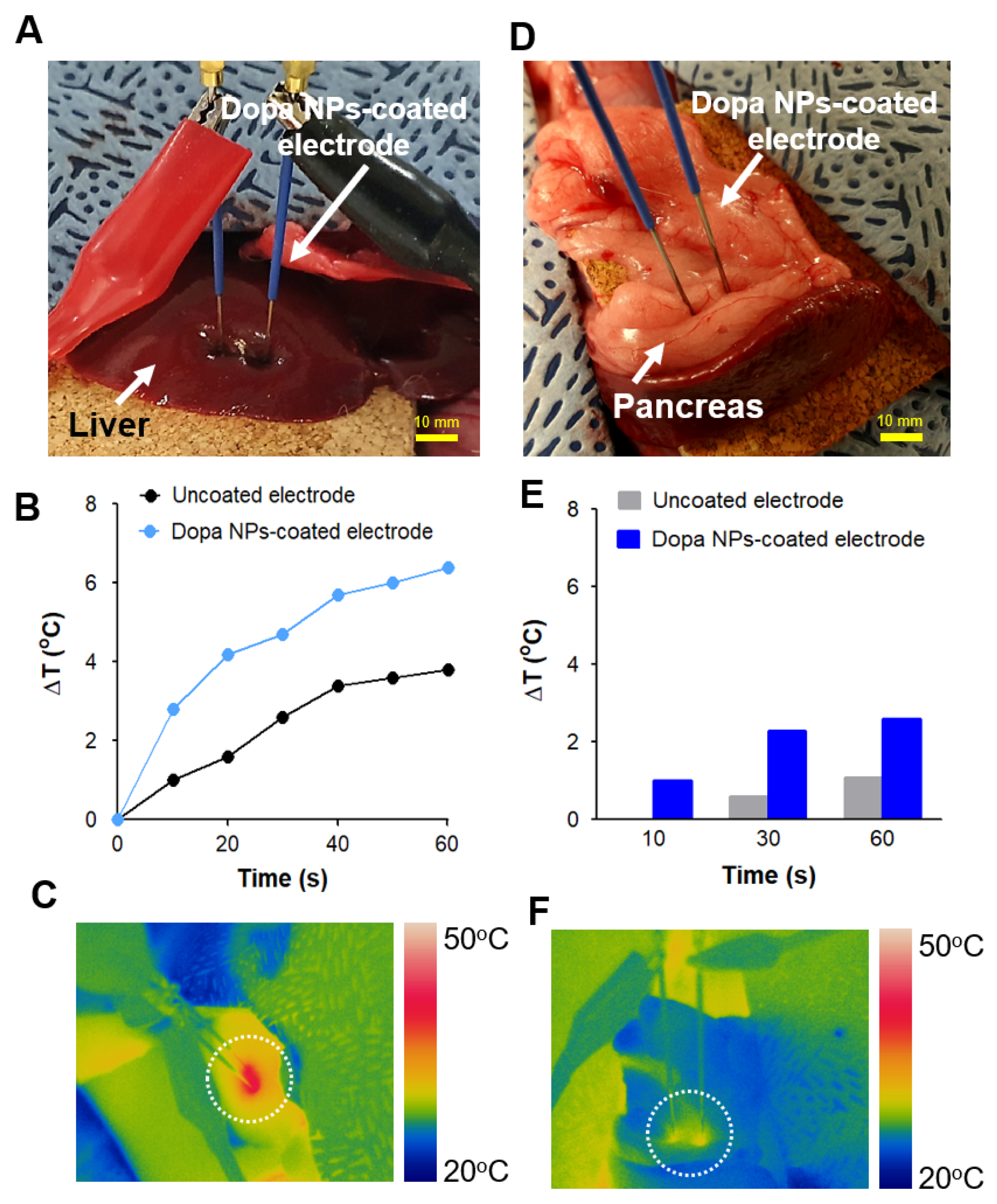
2.5. Enhanced In Vivo Thermal Effect of IRE with Dopa NP-Coated Electrodes
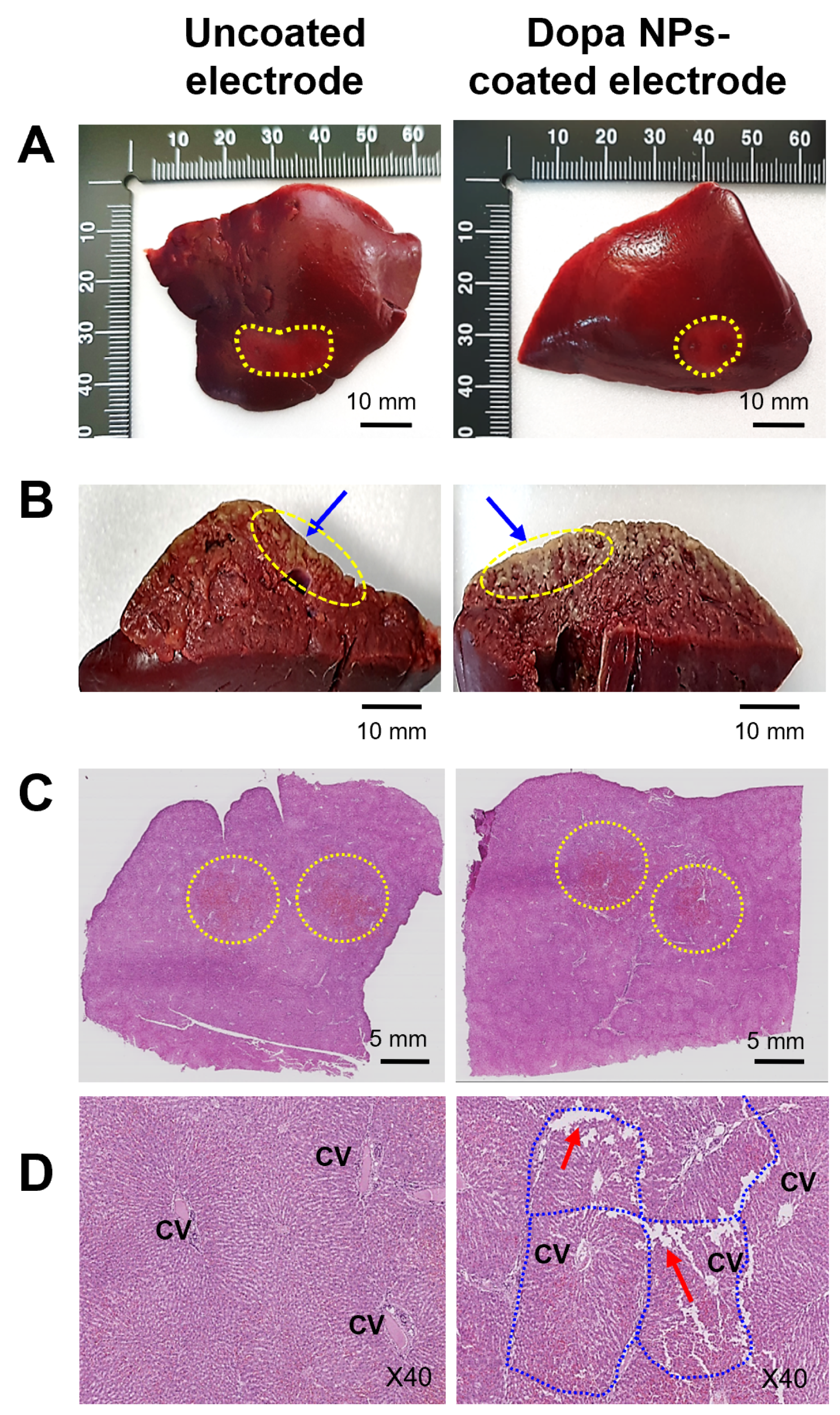
2.6. Histological Observations
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Polydopamine Nanoparticles
4.3. Physicochemical and Morphological Analysis of Dopa NPs
4.4. Cytotoxicity Test of Dopa NPs against Hepatic Carcinoma Cell
4.5. Preparation of Dopa NP-Coated Electrodes
4.6. Monitoring of Hyperthermal Effects of IRE on Hydrogel Phantom Model
4.7. Animal Care and Ethical Approval
4.8. In Vivo Animal Study
4.9. Histopathological Examination of IRE-Treated Liver in Rabbit Model
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gajewska-Naryniecka, A.; Szwedowicz, U.; Łapińska, Z.; Rudno-Rudzińska, J.; Kielan, W.; Kulbacka, J. Irreversible Electroporation in Pancreatic Cancer—An Evolving Experimental and Clinical Method. Int. J. Mol. Sci. 2023, 24, 4381. [Google Scholar] [CrossRef]
- Davalos, R.V.; Mir, L.; Rubinsky, B. Tissue ablation with irreversible electroporation. Ann. Biomed. Eng. 2005, 33, 223–231. [Google Scholar] [CrossRef]
- Wah, T.M.; Lenton, J.; Smith, J.; Bassett, P.; Jagdev, S.; Ralph, C.; Vasudev, N.; Bhattarai, S.; Kimuli, M.; Cartledge, J. Irreversible electroporation (IRE) in renal cell carcinoma (RCC): A mid-term clinical experience. Eur. Radiol. 2021, 31, 7491–7499. [Google Scholar] [CrossRef] [PubMed]
- Woeste, M.R.; Shrestha, R.; Geller, A.E.; Li, S.; Montoya-Durango, D.; Ding, C.; Hu, X.; Li, H.; Puckett, A.; Mitchell, R.A. Irreversible electroporation augments β-glucan induced trained innate immunity for the treatment of pancreatic ductal adenocarcinoma. J. Immunother. Cancer 2023, 11, e006221. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhang, Y.; Klein, R.; Nijm, G.M.; Sahakian, A.V.; Omary, R.A.; Yang, G.-Y.; Larson, A.C. Irreversible electroporation therapy in the liver: Longitudinal efficacy studies in a rat model of hepatocellular carcinoma. Cancer Res. 2010, 70, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Jourabchi, N.; Beroukhim, K.; Tafti, B.A.; Kee, S.T.; Lee, E.W. Irreversible electroporation (NanoKnife) in cancer treatment. Gastrointest. Interv. 2014, 3, 8–18. [Google Scholar] [CrossRef]
- Zhao, J.; Qiao, Y.; Zhou, M.; Wallace, M.; Gupta, S.; Li, C.; Melancon, M.P. Antitumor efficacy of irreversible electroporation and doxorubicin-loaded polymeric micelles. ACS Macro Lett. 2015, 4, 1081–1084. [Google Scholar] [CrossRef]
- Ahmed, M.; Brace, C.L.; Lee, F.T., Jr.; Goldberg, S.N. Principles of and advances in percutaneous ablation. Radiology 2011, 258, 351–369. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Jenjob, R.; Yang, S.-G. Enhanced Therapeutic Potential of Irreversible Electroporation under Combination with Gold-Doped Mesoporous Silica Nanoparticles against EMT-6 Breast Cancer Cells. Biosensors 2022, 13, 41. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; An, J.; Surendran, S.; Lim, J.; Jeong, H.-Y.; Im, S.; Kim, J.Y.; Nam, K.T.; Sim, U. Synergistic effect of Polydopamine incorporated Copper electrocatalyst by dopamine oxidation for efficient hydrogen production. J. Colloid Interface Sci. 2023, 650, 1406–1414. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Moon, H.; Hong, S. Recent advances in melanin-like nanomaterials in biomedical applications: A mini review. Biomater. Res. 2019, 23, 24. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Baskaran, R.; Ozlu, B.; Davaa, E.; Kim, J.J.; Shim, B.S.; Yang, S.G. T1-Positive Mn2+-Doped Multi-Stimuli Responsive poly(L-DOPA) Nanoparticles for Photothermal and Photodynamic Combination Cancer Therapy. Biomedicines 2020, 8, 417. [Google Scholar] [CrossRef] [PubMed]
- Chao, B.; Jiao, J.; Yang, L.; Wang, Y.; Jiang, W.; Yu, T.; Wang, L.; Liu, H.; Zhang, H.; Wang, Z.; et al. Application of advanced biomaterials in photothermal therapy for malignant bone tumors. Biomater. Res. 2023, 27, 116. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and its derivative materials: Synthesis and promising applications in energy, environmental, and biomedical fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, G.; Monteduro, A.G.; Turco, A.; Primiceri, E.; Rizzato, S.; Depalo, N.; Curri, M.L.; Maruccio, G. Polydopamine-coated magnetic iron oxide nanoparticles: From design to applications. Nanomaterials 2022, 12, 1145. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ma, S.; Pei, Y.; Xiong, X.; Sivakumar, P.; Singler, T.J. Regulation of the Deposition Morphology of Inkjet-Printed Crystalline Materials via Polydopamine Functional Coatings for Highly Uniform and Electrically Conductive Patterns. ACS Appl. Mater. Interfaces 2016, 8, 21750–21761. [Google Scholar] [CrossRef]
- Chalmers, E.; Lee, H.; Zhu, C.; Liu, X. Increasing the Conductivity and Adhesion of Polypyrrole Hydrogels with Electropolymerized Polydopamine. Chem. Mater. 2020, 32, 234–244. [Google Scholar] [CrossRef]
- Lu, J.; Cai, L.; Dai, Y.; Liu, Y.; Zuo, F.; Ni, C.; Shi, M.; Li, J. Polydopamine-Based Nanoparticles for Photothermal Therapy/Chemotherapy and their Synergistic Therapy with Autophagy Inhibitor to Promote Antitumor Treatment. Chem. Rec. 2021, 21, 781–796. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, J.; Tian, J.; Li, H. A polydopamine nanomedicine used in photothermal therapy for liver cancer knocks down the anti-cancer target NEDD8-E3 ligase ROC1 (RBX1). J. Nanobiotechnol. 2021, 19, 323. [Google Scholar] [CrossRef]
- Ho, C.-C.; Ding, S.-J. The pH-controlled nanoparticles size of polydopamine for anti-cancer drug delivery. J. Mater. Sci. Mater. Med. 2013, 24, 2381–2390. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Gu, C.; Zheng, W.; Dai, F.; Wang, X.; Zheng, Z. Facile synthesis of novel size-controlled antibacterial hybrid spheres using silver nanoparticles loaded with poly-dopamine spheres. Rsc Adv. 2015, 5, 13470–13477. [Google Scholar] [CrossRef]
- Tang, Z.; Ma, Z. Ultrasensitive amperometric immunoassay for carcinoembryonic antigens by using a glassy carbon electrode coated with a polydopamine-pb (ii) redox system and a chitosan-gold nanocomposite. Microchim. Acta 2017, 184, 1135–1142. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, S.; Gong, X. Polydopamine Nanoparticles-Based Photothermal Effect Against Adhesion Formation in a Rat Model of Achilles Tendon Laceration Repair. Int. J. Nanomed. 2023, 18, 1765–1776. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Su, M. Polydopamine Nanoparticles for Combined Chemo- and Photothermal Cancer Therapy. Nanomaterials 2017, 7, 160. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; van der Mei, H.C.; Ren, Y.; Chen, H.; Chen, G.; Busscher, H.J.; Peterson, B.W. Thermo-resistance of ESKAPE-panel pathogens, eradication and growth prevention of an infectious biofilm by photothermal, polydopamine-nanoparticles in vitro. Nanomedicine 2021, 32, 102324. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, S.-M.; Davaa, E.; Jenjob, R.; Pechyen, C.; Natphopsuk, S.; Jeong, S.; Yoo, H.J.; Yang, S.-G. The Induction of Combined Hyperthermal Ablation Effect of Irreversible Electroporation with Polydopamine Nanoparticle-Coated Electrodes. Int. J. Mol. Sci. 2024, 25, 4317. https://doi.org/10.3390/ijms25084317
Jeon S-M, Davaa E, Jenjob R, Pechyen C, Natphopsuk S, Jeong S, Yoo HJ, Yang S-G. The Induction of Combined Hyperthermal Ablation Effect of Irreversible Electroporation with Polydopamine Nanoparticle-Coated Electrodes. International Journal of Molecular Sciences. 2024; 25(8):4317. https://doi.org/10.3390/ijms25084317
Chicago/Turabian StyleJeon, Sung-Min, Enkhzaya Davaa, Ratchapol Jenjob, Chiravoot Pechyen, Sitakan Natphopsuk, Seok Jeong, Hye Jin Yoo, and Su-Geun Yang. 2024. "The Induction of Combined Hyperthermal Ablation Effect of Irreversible Electroporation with Polydopamine Nanoparticle-Coated Electrodes" International Journal of Molecular Sciences 25, no. 8: 4317. https://doi.org/10.3390/ijms25084317
APA StyleJeon, S.-M., Davaa, E., Jenjob, R., Pechyen, C., Natphopsuk, S., Jeong, S., Yoo, H. J., & Yang, S.-G. (2024). The Induction of Combined Hyperthermal Ablation Effect of Irreversible Electroporation with Polydopamine Nanoparticle-Coated Electrodes. International Journal of Molecular Sciences, 25(8), 4317. https://doi.org/10.3390/ijms25084317





