MiR-146a Is Mutually Regulated by High Glucose-Induced Oxidative Stress in Human Periodontal Ligament Cells
Abstract
:1. Introduction
2. Results
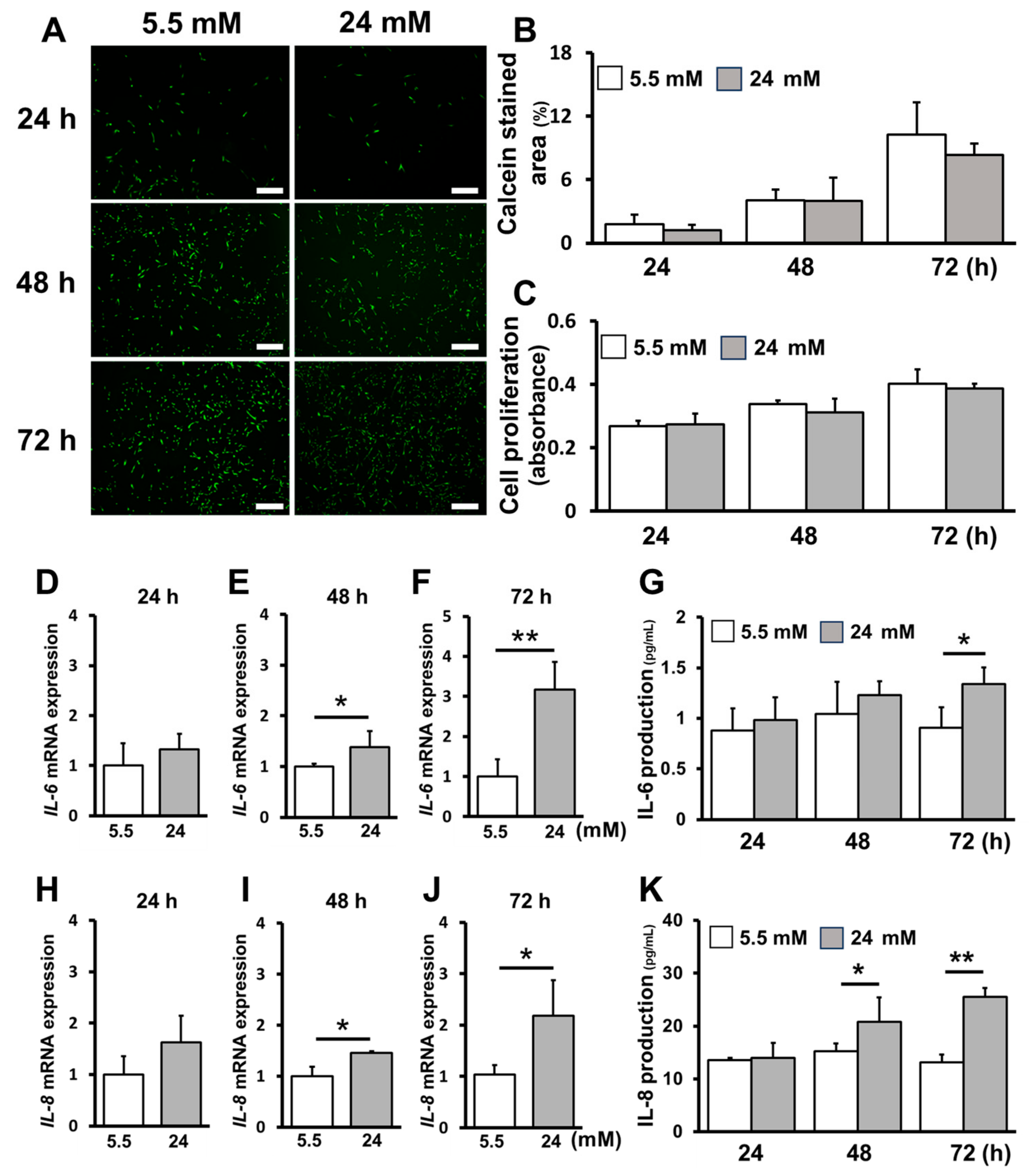
2.1. High-Glucose Conditions Increased Inflammatory Cytokine Production without Affecting Cell Proliferation
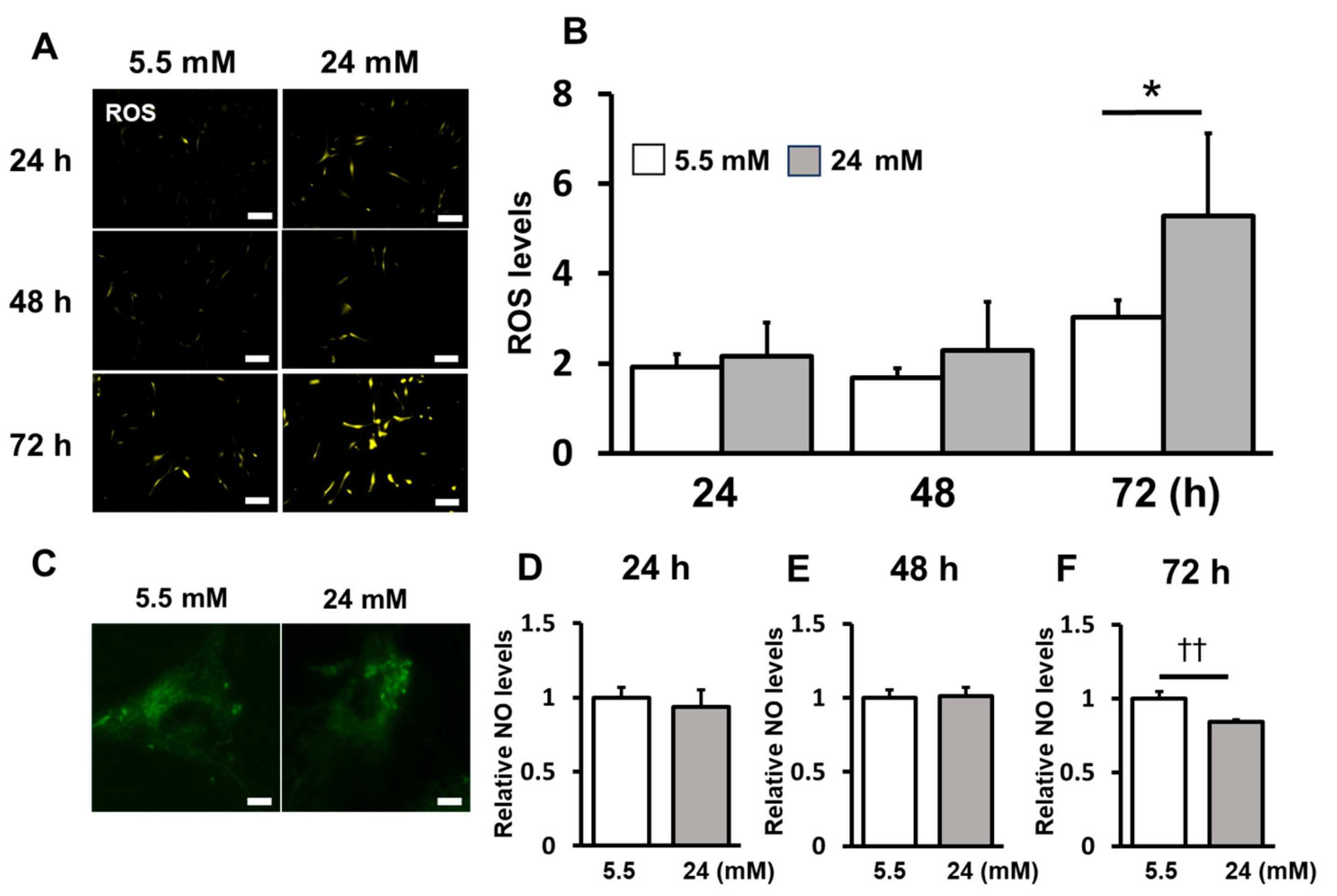
2.2. ROS Induction Was Enhanced under the High-Glucose Condition
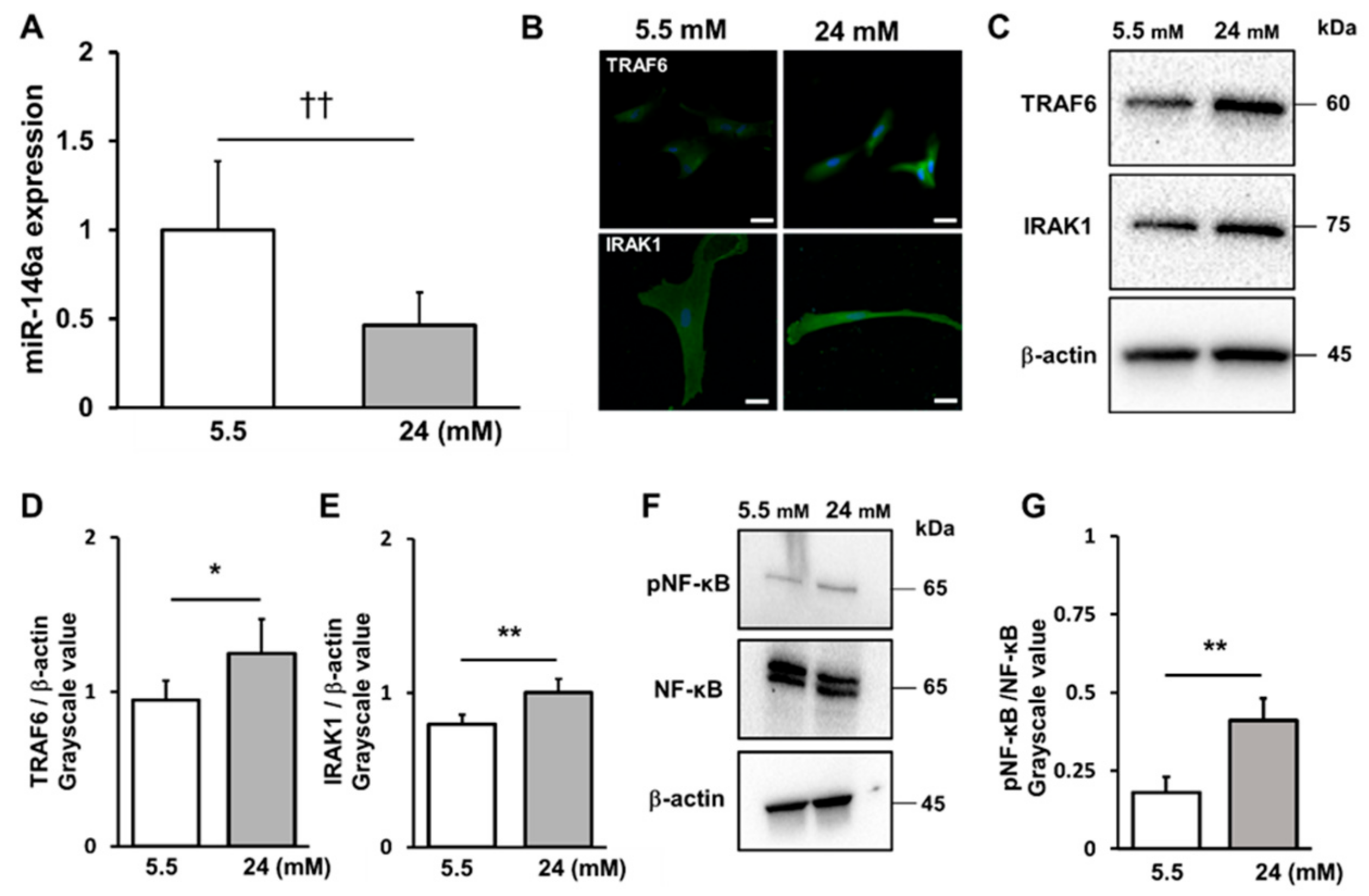
2.3. High-Glucose Conditions Decreased miR-146a Expression and Increased TRAF6 and IRAK1 and NF-κB Expression
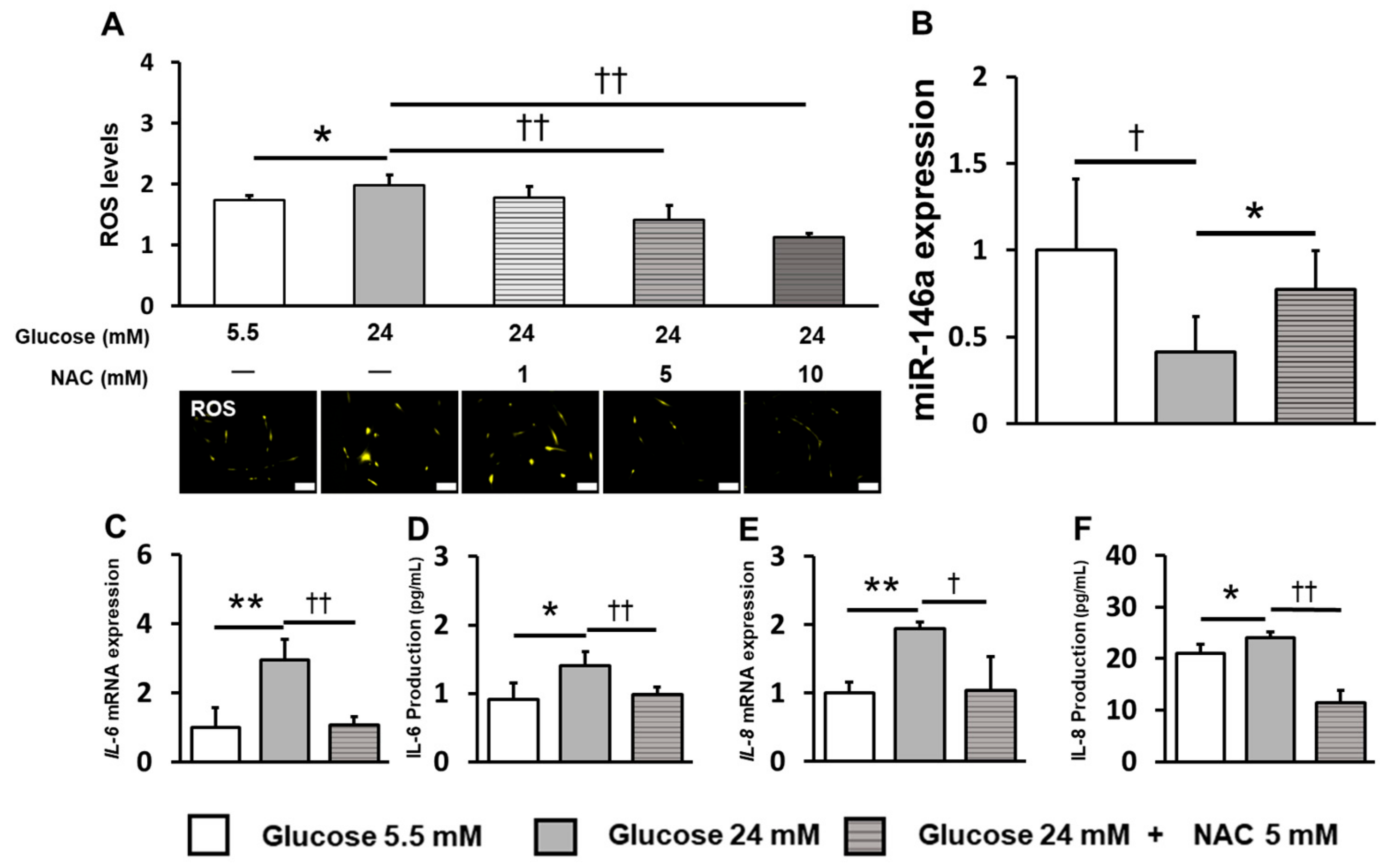
2.4. Inhibition of ROS Induction Restored miR-146a Expression and Reduced Inflammatory Cytokine Production
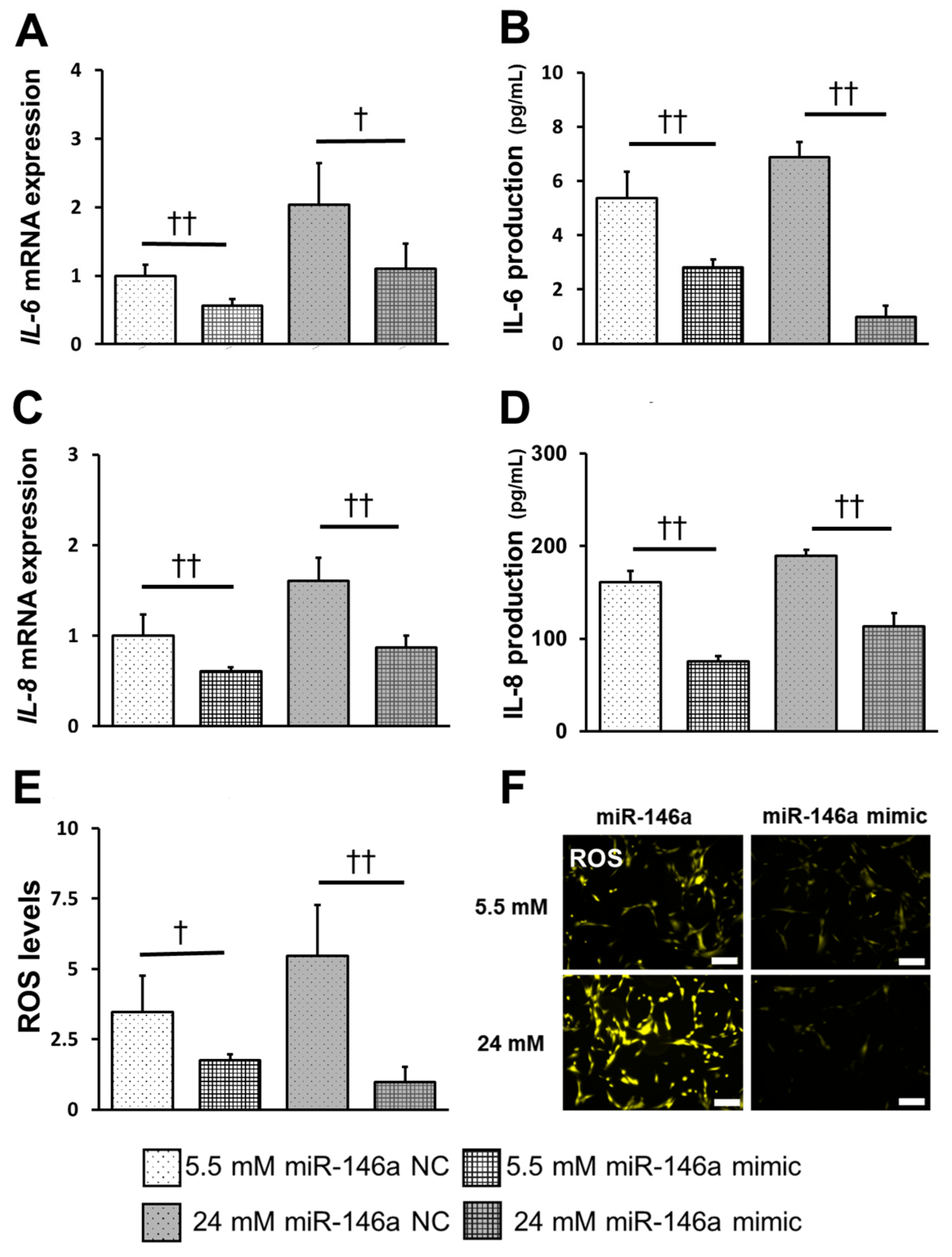
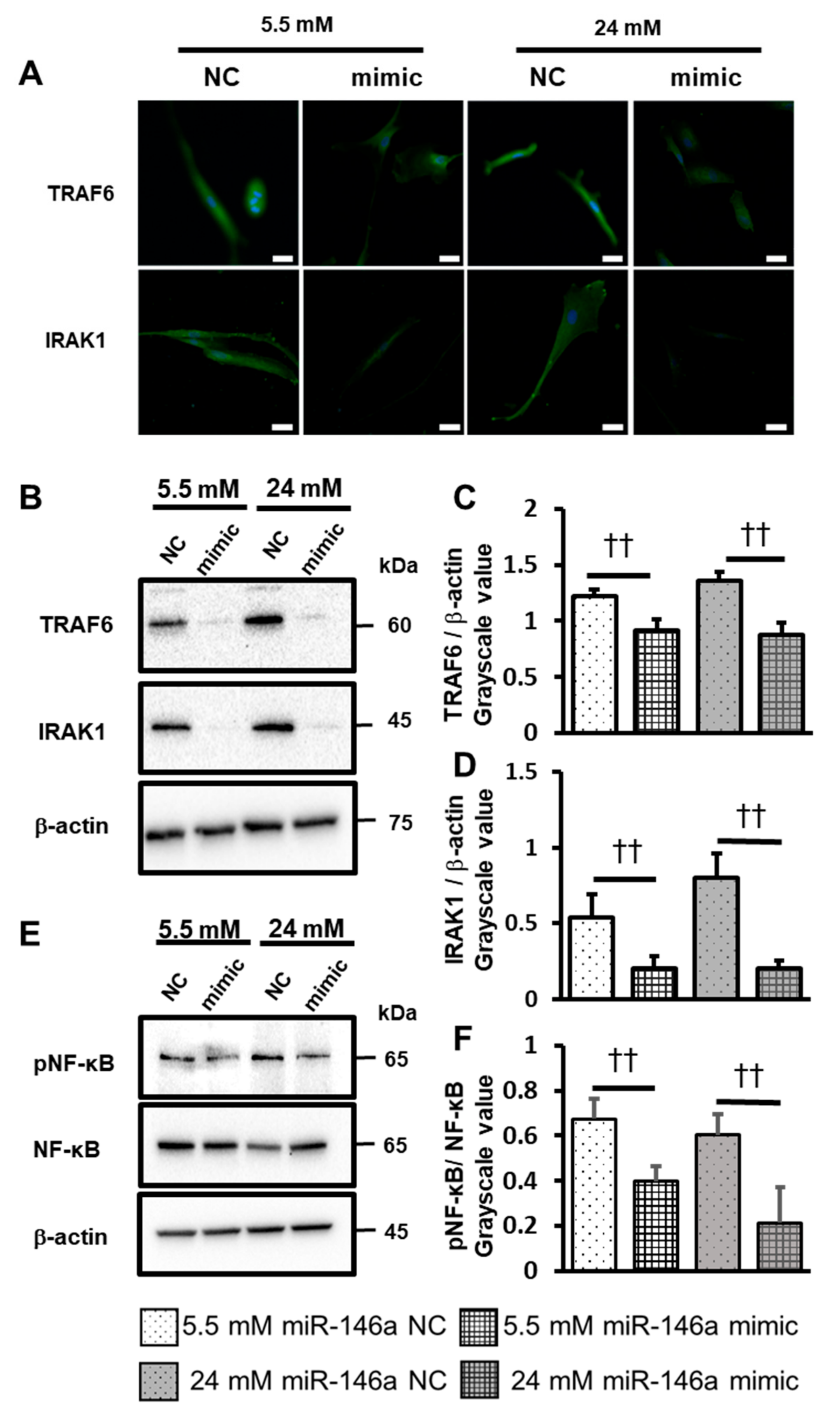
2.5. Overexpression of miR-146a Decreased Inflammatory Cytokine Production, ROS Induction, and TRAF6, IRAK1 and NF-κB Expression
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Cell Proliferation Assay
4.3. Real-Time Polymerase Chain Reaction (PCR)
4.4. ELISA
4.5. ROS and NO Detection
4.6. Western Blot Analysis
4.7. Immunofluorescence Staining
4.8. Transfection of miRNA Mimics
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liang, Y.; Li, J.; Xiao, H.; He, Y.; Zhang, L.; Yan, Y. Identification of stress-related microRNA biomarkers in type 2 diabetes mellitus: A systematic review and meta-analysis. J. Diabetes 2018, 12, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Nelson, R.G.; Shlossman, M.; Budding, L.M.; Pettitt, D.J.; Saad, M.F.; Genco, R.J.; Knowler, W.C. Periodontal Disease and NIDDM in Pima Indians. Diabetes Care 1990, 13, 836–840. [Google Scholar] [CrossRef] [PubMed]
- Mealey, B.L.; Oates, T.W.; American Academy of Periodontology. Diabetes Mellitus and Periodontal Diseases. J. Periodontol. 2006, 77, 1289–1303. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, N.; Inoguchi, T.; Kobayashi, K.; Sonoda, N.; Nawata, H. Erythropoietin attenuated high glucose-induced apoptosis in cultured human aortic endothelial cells. Biochem. Biophys. Res. Commun. 2005, 334, 218–222. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, N.; Shi, H.; Wu, H.; Gao, Y.; He, H.; Gu, B.; Liu, H. High glucose microenvironments inhibit the proliferation and migration of bone mesenchymal stem cells by activating GSK3β. J. Bone Miner. Metab. 2015, 34, 140–150. [Google Scholar] [CrossRef]
- Jönsson, D.; Nebel, D.; Bratthall, G.; Nilsson, B.-O. The human periodontal ligament cell: A fibroblast-like cell acting as an immune cell. J. Periodontal Res. 2010, 46, 153–157. [Google Scholar] [CrossRef]
- Wu, L.; Huang, G.; Liu, L.; Zhang, Q.; Hu, J.; Pan, S. Effects of high-glucose environment on periodontal ligament cell proliferation and apoptosis via NF-κB signaling pathway. CMB. 2022, 68, 106–110. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Filipowicz, W.; Bhattacharyya, S.N.; Sonenberg, N. Mechanisms of post-transcriptional regulation by miRNAs: Are the answers in sight? Nat. Rev. Genet. 2008, 9, 102–114. [Google Scholar] [CrossRef]
- Sonkoly, E.; Ståhle, M.; Pivarcsi, A. MicroRNAs and immunity: Novel players in the regulation of normal immune function and inflammation. Semin. Cancer Biol. 2008, 18, 131–140. [Google Scholar] [CrossRef]
- Soifer, H.S.; Rossi, J.J.; Sætrom, P. MicroRNAs in Disease and Potential Therapeutic Applications. Mol. Ther. 2007, 15, 2070–2079. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Tan, J.; Liu, X.; Jin, F.; Lai, R.; Wang, X. miR-146a-5p targets Sirt1 to regulate bone mass. Bone Rep. 2021, 14, 101013. [Google Scholar] [CrossRef] [PubMed]
- Swarbrick, S.; Wragg, N.; Ghosh, S.; Stolzing, A. Systematic Review of miRNA as Biomarkers in Alzheimer’s Disease. Mol. Neurobiol. 2019, 56, 6156–6167. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Gao, J.; Wang, L.; Hao, P.; Chen, X.; Wang, Y.; Jiang, Z.; Jiang, L.; Wang, T.; Zhu, L.; et al. MicroRNA-146a negatively regulates inflammation via the IRAK1/TRAF6/NF-κB signaling pathway in dry eye. Sci Rep. 2023, 13, 11192. [Google Scholar] [CrossRef]
- Dröge, W. Free Radicals in the Physiological Control of Cell Function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef]
- Cheng, Y.-C.; Chu, L.-W.; Chen, J.-Y.; Hsieh, S.-L.; Chang, Y.-C.; Dai, Z.-K.; Wu, B.-N. Loganin Attenuates High Glucose-Induced Schwann Cells Pyroptosis by Inhibiting ROS Generation and NLRP3 Inflammasome Activation. Cells 2020, 9, 1948. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Y.; Han, N.; Chen, X.; Yu, W.; Zhang, W.; Zou, F. Profiles of oxidative stress-related microRNA and mRNA expression in auditory cells. Brain Res. 2010, 1346, 14–25. [Google Scholar] [CrossRef]
- Wang, H.-J.; Huang, Y.-L.; Shih, Y.-Y.; Wu, H.-Y.; Peng, C.-T.; Lo, W.-Y. MicroRNA-146a Decreases High Glucose/Thrombin-Induced Endothelial Inflammation by Inhibiting NAPDH Oxidase 4 Expression. Mediat. Inflamm. 2014, 2014, 1–12. [Google Scholar] [CrossRef]
- Ebrahimi, S.O.; Reiisi, S.; Shareef, S. miRNAs, oxidative stress, and cancer: A comprehensive and updated review. J. Cell. Physiol. 2020, 235, 8812–8825. [Google Scholar] [CrossRef]
- Tang, L.; Li, X.; Bai, Y.; Wang, P.; Zhao, Y. MicroRNA-146a negatively regulates the inflammatory response to Porphyromonas gingivalis in human periodontal ligament fibroblasts via TRAF6/p38 pathway. J. Periodontol. 2019, 90, 391–399. [Google Scholar] [CrossRef]
- Preshaw, P.M.; Alba, A.L.; Herrera, D.; Jepsen, S.; Konstantinidis, A.; Makrilakis, K.; Taylor, R. Periodontitis and diabetes: A two-way relationship. Diabetologia 2012, 55, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Górska, R.; Gregorek, H.; Kowalski, J.; Laskus-Perendyk, A.; Syczewska, M.; Madaliński, K. Relationship between clinical parameters and cytokine profiles in inflamed gingival tissue and serum samples from patients with chronic periodontitis. J. Clin. Periodontol. 2003, 30, 1046–1052. [Google Scholar] [CrossRef] [PubMed]
- Weil, B.R.; Abarbanell, A.M.; Herrmann, J.L.; Wang, Y.; Meldrum, D.R. High glucose concentration in cell culture medium does not acutely affect human mesenchymal stem cell growth factor production or proliferation. Am. J. Physiol. Integr. Comp. Physiol. 2009, 296, R1735–R1743. [Google Scholar] [CrossRef]
- Kato, H.; Taguchi, Y.; Tominaga, K.; Kimura, D.; Yamawaki, I.; Noguchi, M.; Yamauchi, N.; Tamura, I.; Tanaka, A.; Umeda, M. High Glucose Concentrations Suppress the Proliferation of Human Periodontal Ligament Stem Cells and Their Differentiation Into Osteoblasts. J. Periodontol. 2016, 87, e44–e51. [Google Scholar] [CrossRef]
- García-Hernández, A.; Arzate, H.; Gil-Chavarría, I.; Rojo, R.; Moreno-Fierros, L. High glucose concentrations alter the biomineralization process in human osteoblastic cells. Bone 2012, 50, 276–288. [Google Scholar] [CrossRef]
- Wu, Y.; Song, L.; Li, J.; Zhu, D.; Jiang, S.; Deng, J. MicroRNA-126 Regulates Inflammatory Cytokine Secretion in Human Gingival Fibroblasts Under High Glucose via Targeting Tumor Necrosis Factor Receptor Associated Factor 6. J. Periodontol. 2017, 88, e179–e187. [Google Scholar] [CrossRef]
- Gita, J.B.; George, A.V.; Pavithra, N.; Chandrasekaran, S.C.; Latchumanadhas, K.; Gnanamani, A. Dysregulation of miR-146a by periodontal pathogens: A risk for acute coronary syndrome. J. Periodontol. 2019, 90, 756–765. [Google Scholar] [CrossRef]
- Li, S.; Li, H.; Kong, H.; Wu, S.Y.; Cheng, C.K.; Xu, J. Endogenous and microbial biomarkers for periodontitis and type 2 diabetes mellitus. Front. Endocrinol. 2023, 14, 1292596. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Shu, R.; Jiang, S.; Liu, D.; Zhang, X. Comparison of microRNA profiles of human periodontal diseased and healthy gingival tissues. Int. J. Oral Sci. 2011, 3, 125–134. [Google Scholar] [CrossRef]
- Motedayyen, H.; Ghotloo, S.; Saffari, M.; Sattari, M.; Amid, R. Evaluation of MicroRNA-146a and Its Targets in Gingival Tissues of Patients with Chronic Periodontitis. J. Periodontol. 2015, 86, 1380–1385. [Google Scholar] [CrossRef]
- Ye, E.A.; Steinle, J.J. miR-146a Attenuates inflammatory pathways mediated by TLR4/NF-κB and TNFα to protect primary human retinal microvascular endothelial cells grown in high glucose. Mediators Inflamm. 2016, 2016, 3958453. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanyam, M.; Aravind, S.; Gokulakrishnan, K.; Prabu, P.; Sathishkumar, C.; Ranjani, H.; Mohan, V. Impaired miR-146a expression links subclinical inflammation and insulin resistance in Type 2 diabetes. Mol. Cell. Biochem. 2011, 351, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Chen, S.; Gordon, A.D.; Chakrabarti, S. miR-146a mediates inflammatory changes and fibrosis in the heart in diabetes. J. Mol. Cell. Cardiol. 2017, 105, 70–76. [Google Scholar] [CrossRef]
- Lai, E.C. Micro RNAs are complementary to 3′ UTR sequence motifs that mediate negative post-transcriptional regulation. Nat. Genet. 2002, 30, 363–364. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, H.; Huang, H. microRNAs in inflammatory alveolar bone defect: A review. J. Periodontal Res. 2021, 56, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Shen, H.; Wang, T.; Ma, J.; Yuan, M.; Huang, J.; Wei, M.; Liu, F. TRAF6 mediates high glucose-induced endothelial dysfunction. Exp. Cell Res. 2018, 370, 490–497. [Google Scholar] [CrossRef]
- Meisgen, F.; Landén, N.X.; Wang, A.; Réthi, B.; Bouez, C.; Zuccolo, M.; Gueniche, A.; Ståhle, M.; Sonkoly, E.; Breton, L.; et al. MiR-146a Negatively Regulates TLR2-Induced Inflammatory Responses in Keratinocytes. J. Investig. Dermatol. 2014, 134, 1931–1940. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.-F.; Shu, R.; Jiang, S.-Y.; Liu, D.-L.; Ni, J.; Zhang, X.-L. MicroRNA-146 inhibits pro-inflammatory cytokine secretion through IL-1 receptor-associated kinase 1 in human gingival fibroblasts. J. Inflamm. 2013, 10, 20. [Google Scholar] [CrossRef]
- Chen, S.; Feng, B.; Thomas, A.A.; Chakrabarti, S. miR-146a regulates glucose induced upregulation of inflammatory cytokines extracellular matrix proteins in the retina and kidney in diabetes. PLoS ONE 2017, 12, e0173918. [Google Scholar] [CrossRef]
- Wang, J.C.; Zhao, Y.; Chen, S.J.; Long, J.; Jia, Q.Q.; Zhai, J.D.; Zhang, Q.; Chen, Y.; Long, H.B. AOPPs induce MCP-1 expression by increasing ROS-mediated activation of the NF-κB pathway in rat mesangial cells: Inhibition by sesquiterpene lactones. Cell. Physiol. Biochem. 2013, 32, 1867–1877. [Google Scholar] [CrossRef]
- Bereiter-Hahn, J. Behavior of mitochondria in the living cell. Int. Rev. Cytol. 1990, 122, 1–63. [Google Scholar] [PubMed]
- Yu, T.; Sheu, S.-S.; Robotham, J.L.; Yoon, Y. Mitochondrial fission mediates high glucose-induced cell death through elevated production of reactive oxygen species. Cardiovasc. Res. 2008, 79, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Pessôa, B.S.; Peixoto, E.B.; Papadimitriou, A.; de Faria, J.M.L. Spironolactone improves nephropathy by enhancing glucose-6-phosphate dehydrogenase activity and reducing oxidative stress in diabetic hypertensive rat. J. Renin-Angiotensin-Aldosterone Syst. 2011, 13, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Thallas-Bonke, V.; Thorpe, S.R.; Coughlan, M.T.; Fukami, K.; Yap, F.Y.; Sourris, K.C.; Penfold, S.A.; Bach, L.A.; Cooper, M.E.; Forbes, J.M. Inhibition of NADPH Oxidase Prevents Advanced Glycation End Product–Mediated Damage in Diabetic Nephropathy Through a Protein Kinase C-α–Dependent Pathway. Diabetes 2008, 57, 460–469. [Google Scholar] [CrossRef]
- Banerjee, J.; Khanna, S.; Bhattacharya, A. MicroRNA Regulation of Oxidative Stress. Oxidative Med. Cell. Longev. 2017, 2017, 2872156. [Google Scholar] [CrossRef]
- Simone, N.L.; Soule, B.P.; Ly, D.; Saleh, A.D.; Savage, J.E.; DeGraff, W.; Cook, J.; Harris, C.C.; Gius, D.; Mitchell, J.B. Ionizing Radiation-Induced Oxidative Stress Alters miRNA Expression. PLoS ONE 2009, 4, e6377. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Kim, B.; Kim, V.N. Re-evaluation of the roles of DROSHA, Export in 5, and DICER in microRNA biogenesis. Proc. Natl. Acad. Sci. USA. 2016, 113, E1881–E1889. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Atkin, S.L.; Sahebkar, A. Potential roles of microRNAs in redox state: An update. J. Cell. Biochem. 2018, 120, 1679–1684. [Google Scholar] [CrossRef]
- Kalinina, E.V.; Ivanova-Radkevich, I.; Chernov, N.N. The role of miRNAs in the regulation of redox-dependent processes. Biochemistry 2019, 84, 1233–1246. [Google Scholar]
- Wallace, S.R.; Pagano, P.J.; Kračun, D. MicroRNAs in the regulation of NADPH oxidases in vascular diabetic and ischemic pathologies: A case for alternate inhibitory strategies? Antioxidants. 2022, 12, 70. [Google Scholar] [CrossRef]
- Wan, R.J.; Li, Y.H. MicroRNA-146a/NAPDH oxidase4 decreases reactive oxygen species generation and inflammation in a diabetic nephropathy model. Mol. Med. Rep. 2018, 17, 4759–4766. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Wang, N.; Cheng, W.; Xue, Y.; Chen, W.; Qi, M. MicroRNA-146a protects against intracerebral hemorrhage by inhibiting inflammation and oxidative stress. Exp. Ther. Med. 2019, 18, 3920–3928. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fumimoto, C.; Yamauchi, N.; Minagawa, E.; Umeda, M. MiR-146a Is Mutually Regulated by High Glucose-Induced Oxidative Stress in Human Periodontal Ligament Cells. Int. J. Mol. Sci. 2024, 25, 10702. https://doi.org/10.3390/ijms251910702
Fumimoto C, Yamauchi N, Minagawa E, Umeda M. MiR-146a Is Mutually Regulated by High Glucose-Induced Oxidative Stress in Human Periodontal Ligament Cells. International Journal of Molecular Sciences. 2024; 25(19):10702. https://doi.org/10.3390/ijms251910702
Chicago/Turabian StyleFumimoto, Chihiro, Nobuhiro Yamauchi, Emika Minagawa, and Makoto Umeda. 2024. "MiR-146a Is Mutually Regulated by High Glucose-Induced Oxidative Stress in Human Periodontal Ligament Cells" International Journal of Molecular Sciences 25, no. 19: 10702. https://doi.org/10.3390/ijms251910702
APA StyleFumimoto, C., Yamauchi, N., Minagawa, E., & Umeda, M. (2024). MiR-146a Is Mutually Regulated by High Glucose-Induced Oxidative Stress in Human Periodontal Ligament Cells. International Journal of Molecular Sciences, 25(19), 10702. https://doi.org/10.3390/ijms251910702



