A Brief Overview of Cholinergic and Phosphodiesterase-5 Inhibitors in Diabetic Bladder Dysfunction
Abstract
:1. Introduction
2. Materials and Methods
3. Results
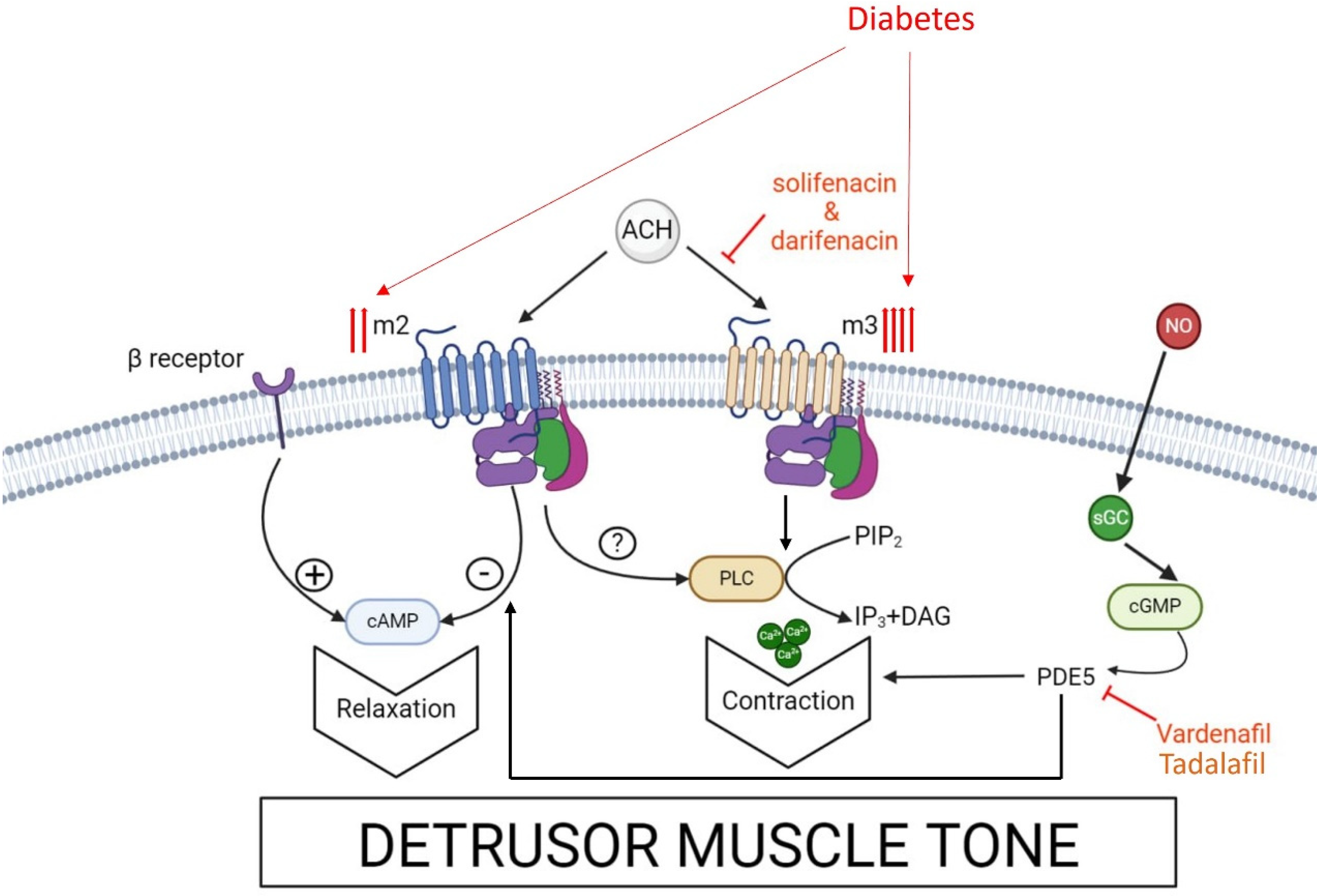
3.1. Muscarinic Receptors and Anti-Cholinergic Drugs in DBD
3.2. PDE5 Activity and PDE5 Inhibitors in DBS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BPH | benign prostate hyperplasia |
| BSM | bladder smooth muscle |
| c-GMP | cyclic-guanosine monophosphate |
| DBD | diabetic bladder dysfunction |
| DBS | diabetic bladder syndrome |
| DM | diabetes mellitus |
| GK | Goto-Kakizaki |
| IPSS | international prostate symptom score |
| LUTSs | lower urinary tract symptoms |
| OAB | overactive bladder |
| OABSS | overactive bladder symptom score |
| QOL | quality of life |
| PDE | phosphodiesterase |
References
- Burns, R.T.; Arnold, P.J.; Song, L.; Moss, K.L.; Powell, C.R. An analysis of urodynamic parameters in diabetic and nondiabetic women. Neurourol. Urodyn. 2024, 43, 1600–1608. [Google Scholar] [CrossRef] [PubMed]
- Frimodt-Moller, C. Diabetic cytopathy: Epidemiology and related disorders. Ann. Intern. Med. 1980, 92 Pt 2, 318–321. [Google Scholar] [CrossRef]
- Arrellano-Valdez, F.; Urrutia-Osorio, M.; Arroyo, C.; Soto-Vega, E. A comprehensive review of urologic complications in patients with diabetes. SpringerPlus 2014, 3, 549. [Google Scholar] [CrossRef] [PubMed]
- Golbidi, S.; Laher, I. Bladder dysfunction in diabetes mellitus. Front. Pharmacol. 2010, 1, 136. [Google Scholar] [CrossRef] [PubMed]
- Khadour, Y.A.; Ebrahem, B.M.; Alhatem, W.; Yanne, E.O.; Khadour, F.A. Predictive value of clinical risk factors for bladder dysfunction in Syrian patients with type 2 diabetes mellitus. Sci. Rep. 2024, 14, 7142. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.S.; Wessells, H.; Chancellor, M.B.; Howards, S.S.; Stamm, W.E.; Stapleton, A.E.; Steers, W.D.; Eeden, S.K.V.D.; McVary, K.T. Urologic complications of diabetes. Diabetes Care. 2005, 28, 177–185. [Google Scholar] [CrossRef]
- Daneshgari, F.; Liu, G.; Birder, L.; Hanna-Mitchell, A.T.; Chacko, S. Diabetic bladder dysfunction: Current translational knowledge. J Urol. 2009, 182, S18–S26. [Google Scholar] [CrossRef]
- Liu, G.; Daneshgari, F. Alterations in neurogenically mediated contractile responses of urinary bladder in rats with diabetes. Am. J. Physiol. Ren. Physiol. 2005, 288, F1220–F1226. [Google Scholar] [CrossRef]
- Longhurst, P.A.; Belis, J.A. Abnormalities of rat bladder contractility in streptozotocin-induced diabetes mellitus. J. Pharmacol. Exp. Ther. 1986, 238, 773–777. [Google Scholar]
- Uvelius, B. Detrusor smooth muscle in rats with alloxan-induced diabetes. J. Urol. 1986, 136, 949–952. [Google Scholar] [CrossRef]
- Role, L.W.; Berg, D.K. Nicotinic receptors in the development and modulation of CNS synapses. Neuron 1996, 16, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Caulfield, M.P.; Birdsall, N.J. International Union of Pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol. Rev. 1998, 50, 279–290. [Google Scholar] [PubMed]
- Peralta, E.G.; Ashkenazi, A.; Winslow, J.W.; Ramachandran, J.; Capon, D.J. Differential regulation of PI hydrolysis and adenylyl cyclase by muscarinic receptor subtypes. Nature 1988, 334, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Fetscher, C.; Fleichman, M.; Schmidt, M.; Krege, S.; Michel, M.C. M3 muscarinic receptors mediate contraction of human urinary bladder. Br. J. Pharmacol. 2002, 136, 641–643. [Google Scholar] [CrossRef] [PubMed]
- Matsui, M.; Motomura, D.; Karasawa, H.; Fujikawa, T.; Jiang, J.; Komiya, Y.; Takahashi, S.-I.; Taketo, M.M. Multiple functional defects in peripheral autonomic organs in mice lacking muscarinic acetylcholine receptor gene for the M3 subtype. Proc. Natl. Acad. Sci. USA 2000, 97, 9579–9584. [Google Scholar] [CrossRef]
- Ehlert, F.J.; Griffin, M.T.; Abe, D.M.; Vo, T.H.; Taketo, M.M.; Manabe, T.; Matsui, M. The M2 muscarinic receptor mediates contraction through indirect mechanisms in mouse urinary bladder. J. Pharmacol. Exp. Ther. 2005, 313, 368–378. [Google Scholar] [CrossRef]
- Hegde, S.S.; Eglen, R.M. Muscarinic receptor subtypes modulating smooth muscle contractility in the urinary bladder. Life Sci. 1999, 64, 419–428. [Google Scholar] [CrossRef]
- Abrams, P.; Andersson, K.-E.; Buccafusco, J.J.; Chapple, C.; de Goat, C.W.; Fryer, A.D.; Kay, G.; Laties, A.; Nathanson, N.M.; Pasricha, P.J.; et al. Muscarinic receptors: Their distribution and function in body systems, and the implications for treating overactive bladder. Br. J. Pharmacol. 2006, 148, 565–578. [Google Scholar] [CrossRef]
- Wang, P.; Luthin, G.R.; Ruggieri, M.R. Muscarinic acetylcholine receptor subtypes mediating urinary bladder contractility and coupling to GTP binding proteins. J. Pharmacol. Exp. Ther. 1995, 273, 959–966. [Google Scholar]
- Hegde, S.S.; Choppin, A.; Bonhaus, D.; Briaud, D.; Loeb, M.; Moy, T.M.; Loury, D.; Eglen, R.M. Functional role of M2 and M3 muscarinic receptors in the urinary bladder of rats in vitro and in vivo. Br. J. Pharmacol. 1997, 120, 1409–1418. [Google Scholar] [CrossRef]
- Yoshimura, N.; Chancellor, M.B.; Andersson, K.-E.; Christ, G.J. Recent advances in understanding the biology of diabetes-associated bladder complications and novel therapy. BJU Int. 2005, 95, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Mimata, H.; Wheeler, M.A.; Fukumoto, M.A.; Takigawa, H.; Nishimoto, T.; Weiss, R.M.; Latifpour, J. Enhancement of muscarinic receptor-coupled phosphatidyl inositol hydrolysis in diabetic bladder. Mol. Cell. Biochem. 1995, 152, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Speakman, M.J.; Brading, A.F.; Gilpin, C.J.; Dixon, J.S.; Gilpin, S.A.; Gosling, J.A. Bladder outflow obstruction—A cause of denervation super sensitivity. J. Urol. 1987, 138, 1461–1466. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.I.; Wein, A.J.; Levin, R.M. Contractile responses and calcium mobilization induced by muscarinic agonists in the rat urinary bladder: Effects of age. Gen. Pharmacol. 1997, 28, 623–628. [Google Scholar] [CrossRef]
- Uchiyama, T.; Chess-Williams, R. Muscarinic receptor subtypes of the bladder and gastrointestinal tract. J. Smooth Muscle Res. 2004, 40, 237–247. [Google Scholar] [CrossRef]
- Waring, J.V.; Wendt, I.R. Effects of streptozotocin-induced diabetes mellitus on intracellular calcium and contraction of longitudinal smooth muscle from rat urinary bladder. J. Urol. 2000, 163, 323–330. [Google Scholar] [CrossRef]
- Tong, Y.C.; Chin, W.T.; Cheng, J.T. Alterations in urinary bladder M2-muscarinic receptor protein and mRNA in 2-week streptozotocin-induced diabetic rats. Neurosci. Lett. 1999, 277, 173–176. [Google Scholar] [CrossRef]
- Kubota, Y.; Nakahara, T.; Mitami, A.; Maruko, T.; Sakamoto, K.; Ishii, K. Augmentation of rat urinary bladder relaxation mediated by beta1-adrenoceptors in experimental diabetes. Eur. J. Pharmacol. 2003, 467, 191–195. [Google Scholar] [CrossRef]
- Sand, C.; Michel, M.C. Bradykinin contracts rat urinary bladder largely independently of phospholipase C. J. Pharmacol. Exp. Ther. 2014, 348, 25–31. [Google Scholar] [CrossRef]
- Andersson, K.-E.; Arner, A. Urinary bladder contraction and relaxation: Physiology and pathophysiology. Physiol. Rev. 2004, 84, 935–986. [Google Scholar] [CrossRef]
- Somlyo, A.P.; Somlyo, A.V. Ca2+ sensitivity of smooth muscle and non-muscle myosin II: Modulated by G proteins, kinases, and myosin phosphatase. Physiol. Rev. 2003, 83, 1325–1358. [Google Scholar] [CrossRef] [PubMed]
- Nishtala, P.S.; Salahudeen, S.; Hilmer, S.N. Anticholinergis: Theoretical and clinical overview. Expert Opin. Drug Saf. 2016, 15, 753–768. [Google Scholar] [CrossRef] [PubMed]
- Belis, J.A.; Curley, R.M.; Wagner, R.M.; Murty, V.N.; Winter, S.J.; Rohner, T.J., Jr. Neurogenic function of the diabetic rat bladder: Alteration by calcium channel effectors. Pharmacology 1991, 43, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Schneider, T.; Marschall-Kehrel, D.; Hanisch, J.U.; Michel, M.C. Does concomitant diabetes affect treatment responses in overactive bladder patients? Int. J. Clin. Pract. 2013, 67, 1138–1143. [Google Scholar] [CrossRef] [PubMed]
- Höfner, K.; Burkart, M.; Jacob, G.; Jonas, U. Symptomatic and quality of life response to tolterodine in subgroups of men with overactive bladder symptoms and presumed non–obstructive benign prostatic hyperplasia. World J. Urol. 2010, 28, 353–357. [Google Scholar] [CrossRef]
- Obata, J.; Matsumoto, K.; Yamanaka, H.; Ninomiya, A.; Nakamura, S. Who would benefit from Solifenacin add–on therapy to Tamsulosin for overactive bladder symptoms associated with benign prostatic hyperplasia? Low. Urin. Tract. Symptoms 2013, 5, 145–149. [Google Scholar] [CrossRef]
- Choi, H.; Bae, J.H.; Oh, C.Y.; Jeong, S.J.; Ko, W.J.; Choi, J.B.; Seo, J.T.; Lee, D.H.; Kim, J.C.; Lee, K.W.; et al. Clinical efficacy of Solifenacin in the management of diabetes mellitus-associated versus idiopathic overactive bladder symptoms: A Multicenter Prospective Study. Int. Neurourol. J. 2018, 22, 51–57. [Google Scholar] [CrossRef]
- Oger-Roussel, S.; Behr-Roussel, D.; Caisey, S.; Kergoat, M.; Charon, C.; Audet, A.; Bernabé, J.; Alexandre, L.; Giuliano, F. Bladder and erectile dysfunctions in the Type 2 diabetic Goto–Kakizaki rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 306, R108–R117. [Google Scholar] [CrossRef]
- Lua, L.L.; Pathak, P.; Dandolu, V. Comparing anticholinergic persistence and adherence profiles in overactive bladder patients based on gender, obesity, and major anticholinergic agents. Neurourol. Urodyn. 2017, 36, 2123–2131. [Google Scholar] [CrossRef]
- Selig, D.J.; Brown, A.J.; DeLuca, J.P.; Kress, A.T.; Livezey, J.R.; Oliver, T.G.; Por, E.D.; Jean, R.T.; Thelus Jean, R. Risk of type 2 diabetes mellitus by antimuscarinic agents among adult females receiving care in the military health system. Pharmacoepidemiol. Drug Saf. 2020, 29, 1605–1615. [Google Scholar] [CrossRef]
- Huang, S.A.; Lie, J.D. Phosphodiesterase-5 (PDE5) inhibitors in the management of erectile dysfunction. Pharm. Ther. 2013, 38, 407–419. [Google Scholar]
- Fibbi, B.; Morelli, A.; Vignozzi, L.; Filippi, S.; Chavalmane, A.; de Vita, G.; Marini, M.; Gacci, M.; Vannelli, G.B.; Sandner, P.; et al. Characterization of phosphodiesterase type 5 expression and functional activity in the human male lower urinary tract. J. Sex. Med. 2010, 7, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-S.; Albersen, M.; Xin, Z.; Namiki, M.; Muller, D.; Lue, T.F. Phosphodiesterase-5 expression and function in the lower urinary tract: A critical review. Urology 2013, 81, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Filippi, S.; Morelli, A.; Sandner, P.; Fibbi, B.; Mancina, R.; Marini, M.; Gacci, M.; Vignozzi, L.; Vannelli, G.B.; Carini, M.; et al. Characterization and functional role of androgen-dependent PDE5 activity in the bladder. Endocrinology 2007, 148, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Rahnamai, M.S.; van Koeveringe, G.A.; Hohnen, R.; Ona, S.; van Kerrebroeck, P.E.V.; de Wachter, S.G.G. Distribution of phosphodiesterase type 5 (PDE5) in the lateral wall of the guinea pig urinary bladder. BJU Int. 2013, 112, 246–257. [Google Scholar] [CrossRef]
- Dhaliwal, A.; Gupta, M. PDE5 Inhibitors. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Mostafa, T. Non-sexual implications of phosphodiesterase type 5 inhibitors. Sex. Med. Rev. 2017, 5, 170–199. [Google Scholar] [CrossRef]
- Morley, D.J.; Hawley, D.M.; Ulbright, T.M.; Butler, L.G.; Culp, J.S.; Hodes, M.E. Distribution of phosphodiesterase I in normal human tissues. J. Histochem. Cytochem. 1987, 35, 75–82. [Google Scholar] [CrossRef]
- Nagasaki, S.; Nakano, S.; Masuda, M.; Ono, K.; Miki, Y.; Shibahara, Y.; Sasano, H. Phosphodiesterase type 9 (PDE9) in the human lower urinary tract: An immunohistochemical study. BJU Int. 2012, 109, 934–940. [Google Scholar] [CrossRef]
- Rybalkin, S.D.; Yan, C.; Bornfeldt, K.E.; Beavo, J.A. Cyclic GMP phosphodiesterases and regulation of smooth muscle function. Circ. Res. 2003, 93, 280–291. [Google Scholar] [CrossRef]
- Andersson, K.-E.; Persson, K. Nitric oxide synthase and nitric oxide-mediated effects in lower urinary tract smooth muscles. World J. Urol. 1994, 12, 274–280. [Google Scholar] [CrossRef]
- Andersson, K.-E.; Persson, K. Nitric oxide synthase and the lower urinary tract: Possible implications for physiology and pathophysiology. Scand. J. Urol. Nephrol. 1995, 175, 43–53. [Google Scholar]
- Carvajal, J.A.; Germain, A.M.; Huidobro-Toro, J.P.; Weiner, C.P. Molecular mechanism of cGMP-mediated smooth muscle relaxation. J. Cell. Physiol. 2000, 184, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Hanna-Mitchell, A.T.; Birder, L.A. New insights into the pharmacology of the bladder. Curr. Opin. Urol. 2008, 18, 347–352. [Google Scholar] [CrossRef]
- Andersson, K.-E.; Uckert, S.; Stief, C.; Hedlund, P. Phosphodiesterases (PDEs) and PDE inhibitors for treatment of LUTS. Neurourol. Urodyn. 2007, 26, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Kedia, G.T.; Uckert, S.; Jonas, U.; Kuczyk, M.A.; Burchardt, M. The nitric oxide pathway in the human prostate: Clinical implications in men with lower urinary tract symptoms. World J. Urol. 2008, 26, 603–609. [Google Scholar] [CrossRef]
- Erdogan, B.R.; Liu, G.; Arioglu-Inan, E.; Michel, M.C. Established and emerging treatments for diabetes-associated lower urinary tract dysfunction. Naunyn Schmiedebergs Arch. Pharmacol. 2022, 395, 887–906. [Google Scholar] [CrossRef]
- Matsuo, T.; Miyata, Y.; Araki, K.; Mukae, Y.; Otsubo, A.; Ohba, K.; Sakai, H. Efficacy of Tadalafil Therapy and Changes in Oxidative Stress Levels in Male Patients with Lower Urinary Tract Symptoms and Overactive Bladder. Low. Urin. Tract. Symptoms 2020, 12, 47–53. [Google Scholar] [CrossRef]
- Gotoh, D.; Torimoto, K.; Tatsumi, Y.; Hori, S.; Yamada, A.; Miyake, M.; Morizawa, Y.; Aoki, K.; Tanaka, N.; Hirayama, A.; et al. Tadalafil, a phosphodiesterase type 5 inhibitor, improves bladder blood supply and restores the initial phase of lower urinary tract dysfunction in diabetic rats. Neurourol. Urodyn. 2018, 37, 666–672. [Google Scholar] [CrossRef]
- Gotoh, D.; Torimoto, K.; Iwasaki, H.; Iwamoto, T.; Morizana, Y.; Hori, S.; Miyake, M.; Tanaka, N.; Hirayama, A.; Nishi, E.; et al. Tadalafil, a phosphodiesterase type 5 inhibitor, restores urethra and detrusor function in the initial phase of diabetes in rats. Low. Urin. Tract. Symptoms 2019, 11, 241–247. [Google Scholar] [CrossRef]
- Masuda, K.; Aizawa, N.; Watanabe, D.; Okegawa, T.; Kume, H.; Igawa, Y.; Fukuhara, H. Pathophysiological changes of the lower urinary tract behind voiding dysfunction in streptozotocin-induced long-term diabetic rats. Sci. Rep. 2020, 10, 4182. [Google Scholar] [CrossRef]
- Thurmond, P.; Yang, J.-H.; Azadzoi, K.M. LUTS in pelvic ischemia: A new concept in voiding dysfunction. Am. J. Physiol. Ren. Physiol. 2016, 310, F738–F746. [Google Scholar] [CrossRef] [PubMed]
- Michel, M.; Chess-Williams, R.; Hegde, S.S. Are blood vessels a target to treat lower urinary tract dysfunction? Naunyn Schmiedebergs Arch. Pharmacol. 2015, 388, 687–694. [Google Scholar]
- Han, J.S.; Min, Y.S.; Kim, G.H.; Chae, S.H.; Nam, Y.; Lee, J.; Lee, S.-Y.; Sohn, U.D. The change of signaling pathway on the electrical stimulated contraction in streptozotocin-induced bladder dysfunction of rats. Korean J. Physiol. Pharmacol. 2018, 22, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Kanda, M.; Eto, K.; Tanabe, N.; Sugiyama, A.; Hashimoto, K.; Ueno, A. Effects of ONO-2235, an aldose reductase inhibitor, on muscarinic receptors and contractile response of the urinary bladder in rats with streptozotocin-induced diabetes. Jpn. J. Pharmacol. 1997, 73, 221–228. [Google Scholar] [CrossRef]
- Uckert, S.; Kigl, K.; Waldkirch, E.S.; Sandner, P.; Ulbrich, E.; Oelke, M.; Stief, C.G.; Kuczyk, M.A. Significance of phosphodiesterase isoenzymes in the control of human detrusor smooth muscle function. An immunohistochemical and functional study. Urol. A 2009, 48, 764–769. [Google Scholar]
- Cesarini, V.; Guida, E.; Campolo, F.; Crescioli, C.; Baldassarre, A.D.; Pisano, C.; Balistreri, C.R.; Ruvolo, G.; Jannini, E.A.; Dolci, S. Type 5 phosphodiesterase (PDE5) and the vascular tree: From embryogenesis to aging and disease. Mech. Ageing Dev. 2020, 190, 111311. [Google Scholar] [CrossRef]
- Durik, M.; Kavousi, M.; van der Pluijm, I.; Isaacs, A.; Cheng, C.; Verdonk, K.; Loot, A.E.; Oeseburg, H.; Bhaggoe, U.M.; Leijten, F.; et al. Nucleotide excision DNA repair is associated with age-related vascular dysfunction. Circulation. 2012, 126, 468–478. [Google Scholar] [CrossRef]
- Mergia, E.; Stegbauer, J. Role of phosphodiesterase 5 and cyclic GMP in hypertension. Curr. Hypertens. Rep. 2016, 18, 39. [Google Scholar] [CrossRef]
- Foresta, C.; de Toni, L.; di Mambro, A.; Garolla, A.; Ferlin, A.; Zuccarello, D. The PDE5 inhibitor sildenafil increases circulating endothelial progenitor cells and CXCR4 expression. J. Sex. Med. 2009, 6, 369–372. [Google Scholar] [CrossRef]
- Vlachopoulos, C.; Ioakeimidis, N.; Rokkas, K.; Stefanadis, C. Cardiovascular effects of phosphodiesterase type 5 inhibitors. J. Sex. Med. 2009, 6, 658–674. [Google Scholar] [CrossRef]
- Di Luigi, L.; Corinaldesi, C.; Colletti, M.; Scolletta, S.; Antinozzi, C.; Vannelli, G.B.; Giannetta, E.; Gianfrilli, D.; Isidori, A.M.; Migliaccio, S.; et al. Phosphodiesterase Type 5 Inhibitor Sildenafil Decreases the Proinflammatory Chemokine CXCL10 in Human Cardiomyocytes and in Subjects with Diabetic Cardiomyopathy. Inflammation 2016, 39, 1238–1252. [Google Scholar] [CrossRef]
| Muscarinic Receptor | Method/Models | Results | Authors |
|---|---|---|---|
| M1 + M3 + M5 | Systematic review | System of inositol trisphosphate (IP3) activation. | [12] |
| M2 + M4 | Systematic Review | Reduction in cAMP levels—smooth muscle relaxation. | [13] |
| M3 | Human tissue specimens | Affinity in human bladder detrusor is greater for the M3 subtype. | [14] |
| M3 knockout mice | Contraction induced by carbachol mediated by M3 receptors. | [15] | |
| M2 + M3 | M2, M3, and M2/M3 double knockout mice | M2 receptors enhance contractile response to M3 receptors’ activation/minor M2 receptors’ impact on contractions. | [16] |
| M2 | Review | M2 receptors inhibit smooth muscle relaxation. | [17] |
| M1 + M2 + M3 + M4 + M5 | Systematic review | Binding of Acetylcholine on muscarinic M3 receptors (M3)/prejunctional M2 and M4 receptors inhibit the release of ach/prejunctional M1 receptors facilitate the release of Ach. | [18] |
| Drug | Models/Method | Results | Authors |
|---|---|---|---|
| Tadalafil | Human male participants | Symptoms improved in 10/44 DM patients. | [58] |
| Tadalafil | Female Sprague Dawley rats | Improvement in inter-contraction intervals and bladder blood flow. | [59] |
| Tadalafil | Female Sprague Dawley rats | Restored contractility of detrusor muscle. | [60] |
| Tadalafil | Male Wistar rats | Restored detrusor function. | [61] |
| Tadalafil | Systematic review | Improved perfusion of the bladder. | [62,63] |
| Vardenafil | Review article | Decreased contractility of bladder’s smooth muscle. | [50] |
| Udenafil | Male Sprague Dawley (SD) rats | Significant improvement in smooth muscle cell contractility. | [64] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kallinikas, G.; Haronis, G.; Kallinika, E.; Kozyrakis, D.; Rodinos, E.; Filios, A.; Filios, P.; Mityliniou, D.; Safioleas, K.; Zarkadas, A.; et al. A Brief Overview of Cholinergic and Phosphodiesterase-5 Inhibitors in Diabetic Bladder Dysfunction. Int. J. Mol. Sci. 2024, 25, 10704. https://doi.org/10.3390/ijms251910704
Kallinikas G, Haronis G, Kallinika E, Kozyrakis D, Rodinos E, Filios A, Filios P, Mityliniou D, Safioleas K, Zarkadas A, et al. A Brief Overview of Cholinergic and Phosphodiesterase-5 Inhibitors in Diabetic Bladder Dysfunction. International Journal of Molecular Sciences. 2024; 25(19):10704. https://doi.org/10.3390/ijms251910704
Chicago/Turabian StyleKallinikas, Georgios, Georgios Haronis, Eirini Kallinika, Diomidis Kozyrakis, Evangelos Rodinos, Athanasios Filios, Panagiotis Filios, Despoina Mityliniou, Konstantinos Safioleas, Anastasios Zarkadas, and et al. 2024. "A Brief Overview of Cholinergic and Phosphodiesterase-5 Inhibitors in Diabetic Bladder Dysfunction" International Journal of Molecular Sciences 25, no. 19: 10704. https://doi.org/10.3390/ijms251910704







