Role of Plasma Angiopoietin-1 and VEGF Levels as Potential Biomarkers in Chronic Central Serous Chorioretinopathy with Macular Neovascularization
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Clinical Examination
4.3. Sample Collection
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, X.; Komuku, Y.; Araki, T.; Terasaki, H.; Miki, A.; Kuwayama, S.; Nishi, T.; Kinoshita, T.; Gomi, F. Risk factors and characteristics of central serous chorioretinopathy with later development of macular neovascularisation detected on OCT angiography: A retrospective multicentre observational study. BMJ Open Ophthalmol. 2022, 7, e000976. [Google Scholar] [CrossRef] [PubMed]
- Loo, R.H.; Scott, I.U.; Flynn, H.W. Factors associated with reduced visual acuity during long-term follow-up of patients with idiopathic central serous chorioretinopathy. Retina 2002, 22, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Bonini Filho, M.A.; de Carlo, T.E.; Ferrara, D.; Adhi, M.; Baumal, C.R.; Witkin, A.J.; Reichel, E.; Duker, J.S.; Waheed, N.K. Association of choroidal neovascularization and central serous chorioretinopathy with optical coherence tomography angiography. JAMA Ophthalmol. 2015, 133, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Savastano, M.C.; Rispoli, M.; Lumbroso, B. The incidence of neovascularization in central serous chorioretinopathy by optical coherence tomography angiography. Retina 2021, 41, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Lin, W.; Zhou, S.; Meng, X. Optical coherence tomography angiography of flat irregular pigment epithelial detachments in central serous chorioretinopathy. Br. J. Ophthalmol. 2021, 105, 233–238. [Google Scholar] [CrossRef]
- van Rijssen, T.J.; van Dijk, E.H.C.; Yzer, S.; Ohno-Matsui, K.; Keunen, J.E.E.; Schlingemann, R.O.; Sivaprasad, S.; Querques, G.; Downes, S.M.; Fauser, S.; et al. Central serous chorioretinopathy: Towards an evidence-based treatment guideline. Prog. Retin. Eye Res. 2019, 73, 100770. [Google Scholar] [CrossRef]
- Salehi, M.; Wenick, A.S.; Law, H.A.; Evans, J.R.; Gehlbach, P. Interventions for central serous chorioretinopathy: A network meta-analysis. Cochrane Database Syst. Rev. 2015, 12, CD011841. [Google Scholar] [CrossRef]
- Spaide, R.F. Optical coherence tomography angiography signs of vascular abnormalization with antiangiogenic therapy for choroidal neovascularization. Am. J. Ophthalmol. 2015, 160, 6–16. [Google Scholar] [CrossRef]
- Sacconi, R.; Tomasso, L.; Corbelli, E.; Carnevali, A.; Querques, L.; Casati, S.; Bandello, F.; Querques, G. Early response to the treatment of choroidal neovascularization complicating central serous chorioretinopathy: A OCT-angiography study. Eye 2019, 33, 1809–1817. [Google Scholar] [CrossRef]
- Kishi, S.; Matsumoto, H. A new insight into pachychoroid diseases: Remodeling of choroidal vasculature. Graefe’s Arch. Clin. Exp. Ophthalmol. 2022, 260, 3405–3417. [Google Scholar] [CrossRef]
- Park, J.B.; Kim, K.; Kang, M.S.; Kim, E.S.; Yu, S.Y. Central serous chorioretinopathy: Treatment. Taiwan J. Ophthalmol. 2022, 12, 394–408. [Google Scholar] [CrossRef] [PubMed]
- Karska-Basta, I.; Pociej-Marciak, W.; Chrzaszcz, M.; Kubicka-Trząska, A.; Dębicka-Kumela, M.; Gawęcki, M.; Romanowska-Dixon, B.; Sanak, M. Imbalance in the levels of angiogenic factors in patients with acute and chronic central serous chorioretinopathy. J. Clin. Med. 2021, 10, 1087. [Google Scholar] [CrossRef] [PubMed]
- Hakanpaa, L.; Sipila, T.; Leppanen, V.M.; Gautam, P.; Nurmi, H.; Jacquemet, G.; Eklund, L.; Ivaska, J.; Alitalo, K.; Saharinen, P. Endothelial destabilization by angiopoietin-2 via integrin β1 activation. Nat. Commun. 2015, 6, 5962. [Google Scholar] [CrossRef] [PubMed]
- Nambu, H.; Umeda, N.; Kachi, S.; Oshima, Y.; Akiyama, H.; Nambu, R.; Campochiaro, P.A. Angiopoietin 1 prevents retinal detachment in an aggressive model of proliferative retinopathy, but has no effect on established neovascularization. J. Cell. Physiol. 2005, 204, 227–235. [Google Scholar] [CrossRef]
- Lee, J.; Park, D.Y.; Park, D.Y.; Park, I.; Chang, W.; Nakaoka, Y.; Komuro, I.; Yoo, O.J.; Koh, G.Y. Angiopoietin-1 suppresses choroidal neovascularization and vascular leakage. Investig. Ophthalmol. Vis. Sci. 2014, 55, 2191–2199. [Google Scholar] [CrossRef]
- Mrejen, S.; Balaratnasingam, C.; Kaden, T.R.; Bottini, A.; Dansingani, K.; Bhavsar, K.V.; Yannuzzi, N.A.; Patel, S.; Chen, K.C.; Yu, S.; et al. Long-term Visual Outcomes and Causes of Vision Loss in Chronic Central Serous Chorioretinopathy. Ophthalmology 2019, 126, 576–588. [Google Scholar] [CrossRef]
- Guo, J.; Tang, W.; Xu, S.; Liu, W.; Xu, G. OCTA evaluation of treatment-naïve flat irregular PED (FIPED)-associated CNV in chronic central serous chorioretinopathy before and after half-dose PDT. Eye 2021, 35, 2871–2878. [Google Scholar] [CrossRef]
- Lee, G.I.; Kim, A.Y.; Kang, S.W.; Cho, S.C.; Park, K.H.; Kim, S.J.; Kim, K.T. Risk Factors and Outcomes of Choroidal Neovascularization Secondary to Central Serous Chorioretinopathy. Sci. Rep. 2019, 9, 3927. [Google Scholar] [CrossRef]
- Khan, M.; Aziz, A.A.; Shafi, N.A.; Abbas, T.; Khanani, A.M. Targeting angiopoietin in retinal vascular diseases: A literature review and summary of clinical trials involving faricimab. Cells 2020, 9, 1869. [Google Scholar] [CrossRef]
- Nambu, H.; Nambu, R.; Oshima, Y.; Hackett, S.F.; Okoye, G.; Wiegand, S.; Yancopoulos, G.; Zack, D.J.; Campochiaro, P.A. Angiopoietin 1 inhibits ocular neovascularization and breakdown of the blood-retinal barrier. Gene Ther. 2004, 11, 865–873. [Google Scholar] [CrossRef]
- Saharinen, P.; Eklund, L.; Alitalo, K. Therapeutic targeting of the angiopoietin-TIE pathway. Nat. Rev. Drug Discov. 2017, 16, 635–661. [Google Scholar] [CrossRef] [PubMed]
- Joussen, A.M.; Ricci, F.; Paris, L.P.; Korn, C.; Quezada-Ruiz, C.; Zarbin, M. Angiopoietin/Tie2 signalling and its role in retinal and choroidal vascular diseases: A review of preclinical data. Eye 2021, 35, 1305–1316. [Google Scholar] [CrossRef] [PubMed]
- Regula, J.T.; Lundh von Leithner, P.; Foxton, R.; Barathi, V.A.; Cheung, C.M.; Bo Tun, S.B.; Wey, Y.S.; Iwata, D.; Dostalek, M.; Moelleken, J.; et al. Targeting key angiogenic pathways with a bispecific CrossMAb optimized for neovascular eye diseases. EMBO Mol. Med. 2016, 8, 1265–1288, Erratum in EMBO Mol. Med. 2019, 11, e10666. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Lavine, J.A.; Fawzi, A.; Quaggin, S.E.; Thomson, B.R. Angiopoietin-1 Is Required for Vortex Vein and Choriocapillaris Development in Mice. Arterioscler. Thromb. Vasc. Biol. 2022, 42, 1413–1427. [Google Scholar] [CrossRef] [PubMed]
- Suri, C.; Jones, P.F.; Patan, S.; Bartunkova, S.; Maisonpierre, P.C.; Davis, S.; Sato, T.N.; Yancopoulos, G.D. Requisite role of angiopoietin-1, a ligand for the tie2 receptor, during embryonic angiogenesis. Cell 1996, 87, 1171–1180. [Google Scholar] [CrossRef]
- Penha, F.M.; Masud, M.; Khanani, Z.A.; Thomas, M.; Fong, R.D.; Smith, K.; Chand, A.; Khan, M.; Gahn, G.; Melo, G.B.; et al. Review of real-world evidence of dual inhibition of VEGF-A and ANG-2 with faricimab in NAMD and DME. Int. J. Retina Vitr. 2024, 10, 5. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, N.; Parachuri, N.; Loewenstein, A.; Bandello, F.; Kuppermann, B.D. Global experience of faricimab in clinical settings—A review. Expert Opin. Biol. Ther. 2024, 24, 263–268. [Google Scholar] [CrossRef]
- Wong, T.Y.; Haskova, Z.; Asik, K.; Baumal, C.R.; Csaky, K.G.; Eter, N.; Ives, J.A.; Jaffe, G.J.; Korobelnik, J.F.; Lin, H.; et al. Faricimab Treat-and-Extend for Diabetic Macular Edema: Two-Year Results from the Randomized Phase 3 YOSEMITE and RHINE Trials. Ophthalmology 2024, 131, 708–723. [Google Scholar] [CrossRef]
- Panos, G.D.; Lakshmanan, A.; Dadoukis, P.; Ripa, M.; Motta, L.; Amoaku, W.M. Faricimab: Transforming the Future of Macular Diseases Treatment—A Comprehensive Review of Clinical Studies. Drug Des. Devel. Ther. 2023, 17, 2861–2873. [Google Scholar] [CrossRef]
- Khanani, A.M.; Kotecha, A.; Chang, A.; Chen, S.J.; Chen, Y.; Guymer, R.; Heier, J.S.; Holz, F.G.; Iida, T.; Ives, J.A.; et al. TENAYA and LUCERNE: Two-Year Results from the Phase 3 Neovascular Age-Related Macular Degeneration Trials of Faricimab with Treat-and-Extend Dosing in Year 2. Ophthalmology 2024, 131, 914–926. [Google Scholar] [CrossRef]
- Lim, J.W.; Kim, M.U.; Shin, M.C. Aqueous humor and plasma levels of vascular endothelial growth factor and interleukin-8 in patients with central serous chorioretinopathy. Retina 2010, 30, 1465–1471. [Google Scholar] [CrossRef] [PubMed]
- Shirley, M. Faricimab: First Approval. Drugs 2022, 82, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Chrząszcz, M.; Pociej-Marciak, W.; Żuber-Łaskawiec, K.; Romanowska-Dixon, B.; Sanak, M.; Michalska-Małecka, K.; Petrovič, M.G.; Karska-Basta, I. Changes in Plasma VEGF and PEDF Levels in Patients with Central Serous Chorioretinopathy. Medicina 2021, 57, 1063. [Google Scholar] [CrossRef] [PubMed]
- Fung, A.T.; Yannuzzi, L.A.; Freund, K.B. Type 1 (sub-retinal pigment epithelial) neovascularization in central serous chori-oretinopathy masquerading as neovascular age-related macular degeneration. Retina 2012, 32, 1829–1837. [Google Scholar] [CrossRef]
- Chung, Y.R.; Lee, S.J.; Song, J.H. Changes in the choroidal thickness following intravitreal bevacizumab injection in chronic central serous chorioretinopathy. J. Clin. Med. 2022, 11, 3375. [Google Scholar] [CrossRef]
- Pociej-Marciak, W.; Karska-Basta, I.; Kuźniewski, M.; Kubicka-Trząska, A.; Romanowska-Dixon, B. Sudden Visual Deterioration as the First Symptom of Chronic Kidney Failure. Case Rep. Ophthalmol. 2015, 6, 394–400. [Google Scholar] [CrossRef]

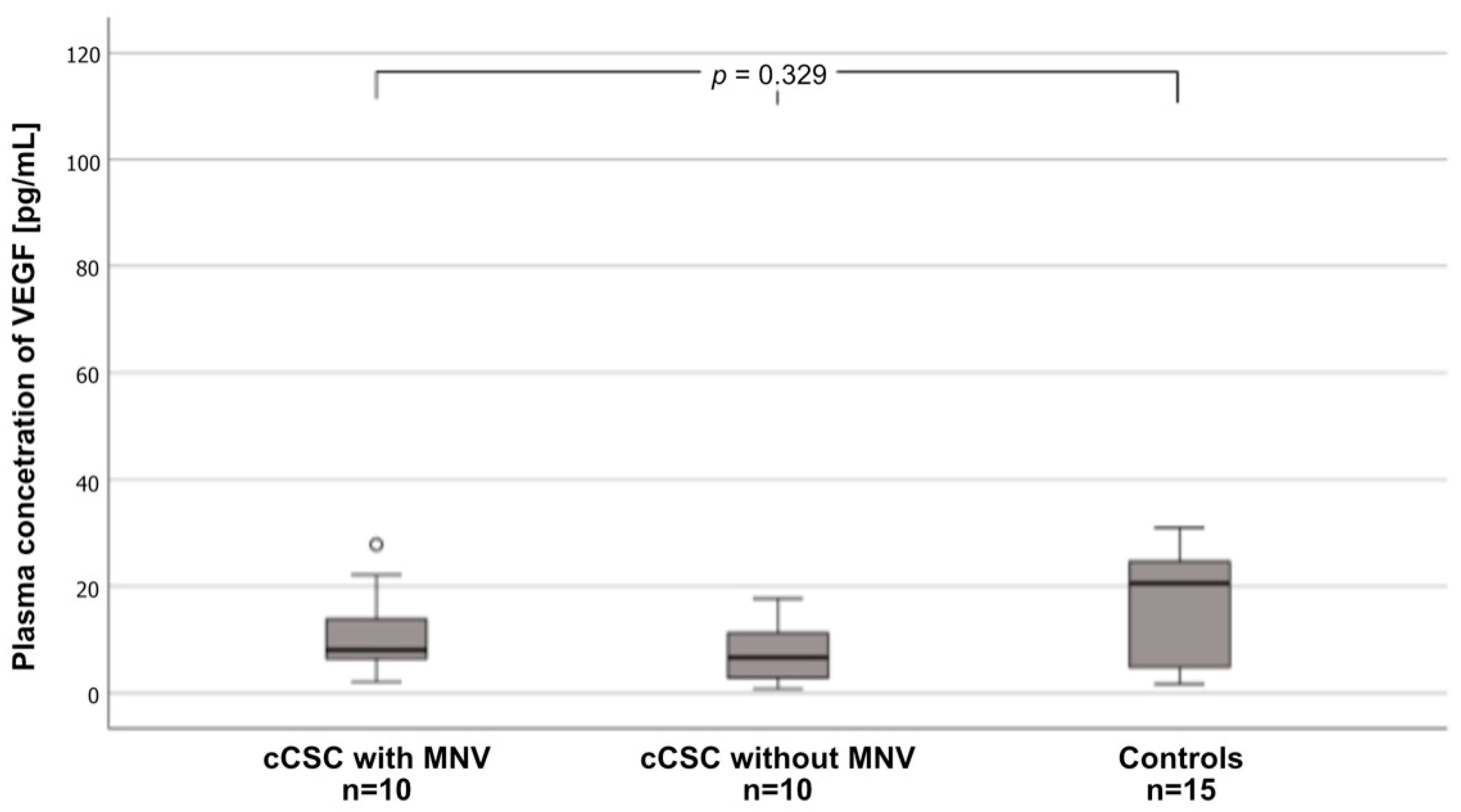
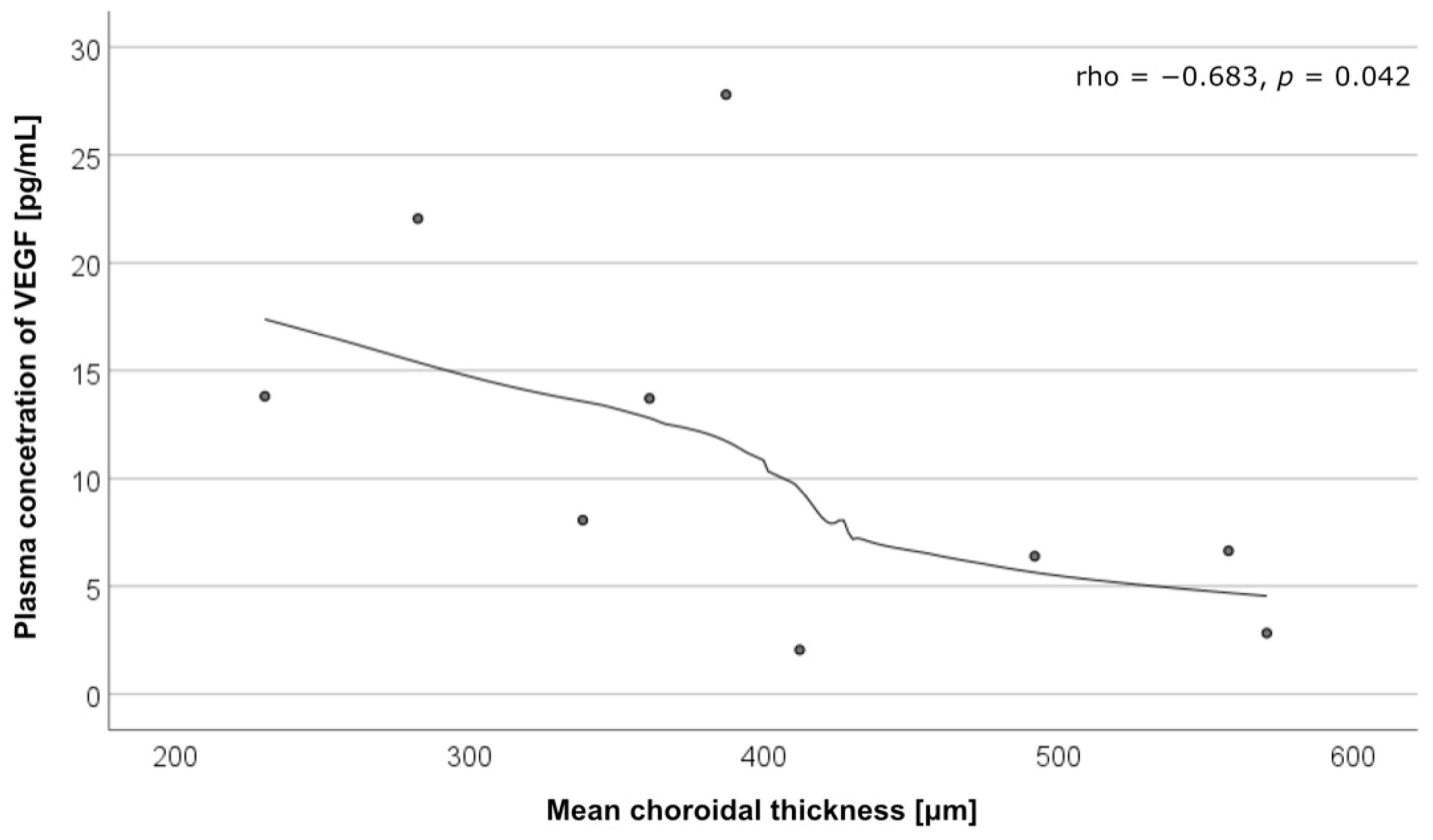
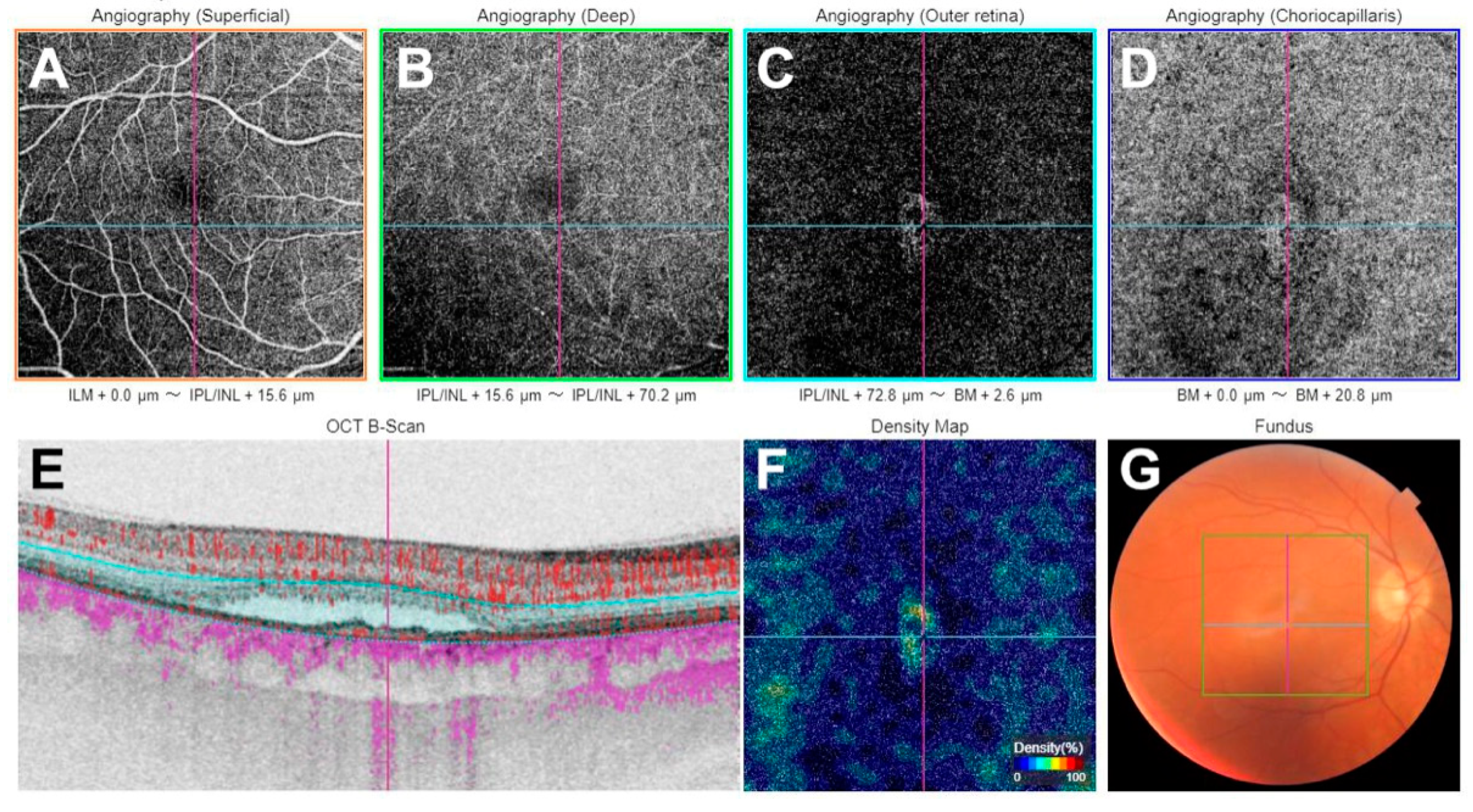
| Parameter | CSC with MNV (n = 10) | CSC without MNV (n = 10) | Controls (n = 15) | p Value | |
|---|---|---|---|---|---|
| Female sex, n (%) | 3 (30.0) | 5 (50.0) | 5 (33.3) | 0.739 | |
| Age, y | 54.8 ± 4.8 | 54.8 ± 4.7 | 45.3 ± 4.0 | 0.061 | |
| Hypertension, n (%) | 5 (50.0) | 4 (40.0) | 4 (26.7) | 0.554 | |
| Smoking, n (%) (current, former) | 1 (10.0) | 3 (30.0) | 1 (10.0) | 0.617 | |
| BCVA, n (%) | 0.5< and ≤1.0 | 4 (40.0) | 6 (60.0) | 15 (100) | 0.001 |
| 0.1≤ and ≤0.5 | 3 (30.0) | 4 (40.0) | 0 (0) | ||
| <0.1 | 3 (30.0) | 0 (0.0) | 0 (0) | ||
| FIPED | 6 (60.0) | 4 (40.0) | 0 (0) | 0.656 | |
| PED | 4 (40.0) | 4 (40.0) | 0 (0) | 0.542 | |
| CT, µm | 394.42 [338.67–492.17] | 365.33 [329.67–393.33] | 313.33 [268.00–364.33] | 0.044 | |
| Cytokine | Mean Choroidal Thickness, µm | |
|---|---|---|
| cCSC with MNV (n = 10) | cCSC without MNV (n = 10) | |
| Angiopoietin-1 | rho = −0.261 p = 0.467 | rho = −0.285 p = 0.425 |
| VEGF | rho = −0.683 p = 0.042 | rho = 0.310 p = 0.456 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chrząszcz, M.; Pociej-Marciak, W.; Mackiewicz, N.; Romanowska-Dixon, B.; Sanak, M.; Teper, S.; Gawęcki, M.; Karska-Basta, I. Role of Plasma Angiopoietin-1 and VEGF Levels as Potential Biomarkers in Chronic Central Serous Chorioretinopathy with Macular Neovascularization. Int. J. Mol. Sci. 2024, 25, 10748. https://doi.org/10.3390/ijms251910748
Chrząszcz M, Pociej-Marciak W, Mackiewicz N, Romanowska-Dixon B, Sanak M, Teper S, Gawęcki M, Karska-Basta I. Role of Plasma Angiopoietin-1 and VEGF Levels as Potential Biomarkers in Chronic Central Serous Chorioretinopathy with Macular Neovascularization. International Journal of Molecular Sciences. 2024; 25(19):10748. https://doi.org/10.3390/ijms251910748
Chicago/Turabian StyleChrząszcz, Michał, Weronika Pociej-Marciak, Natalia Mackiewicz, Bożena Romanowska-Dixon, Marek Sanak, Sławomir Teper, Maciej Gawęcki, and Izabella Karska-Basta. 2024. "Role of Plasma Angiopoietin-1 and VEGF Levels as Potential Biomarkers in Chronic Central Serous Chorioretinopathy with Macular Neovascularization" International Journal of Molecular Sciences 25, no. 19: 10748. https://doi.org/10.3390/ijms251910748









