Propylthiouracil Induced Rat Model Reflects Heterogeneity Observed in Clinically Non-Obese Subjects with Nonalcoholic Fatty Liver Disease
Abstract
:1. Introduction
2. Results
2.1. Clinical and Biochemical Characteristics of NO-NAFLD Patients
2.2. Distinctive Serum Metabolites Characteristics Were Identified between Clinical NO-NAFLD and OB-NAFLD Subjects
2.3. The Pathological Features Characterized in the HFD+PTU-Induced NAFLD Rat Model
2.4. The Consistency of Metabolites Characteristics between HFD+PTU-Induced Rat Model and Clinical NO-NAFLD Subjects
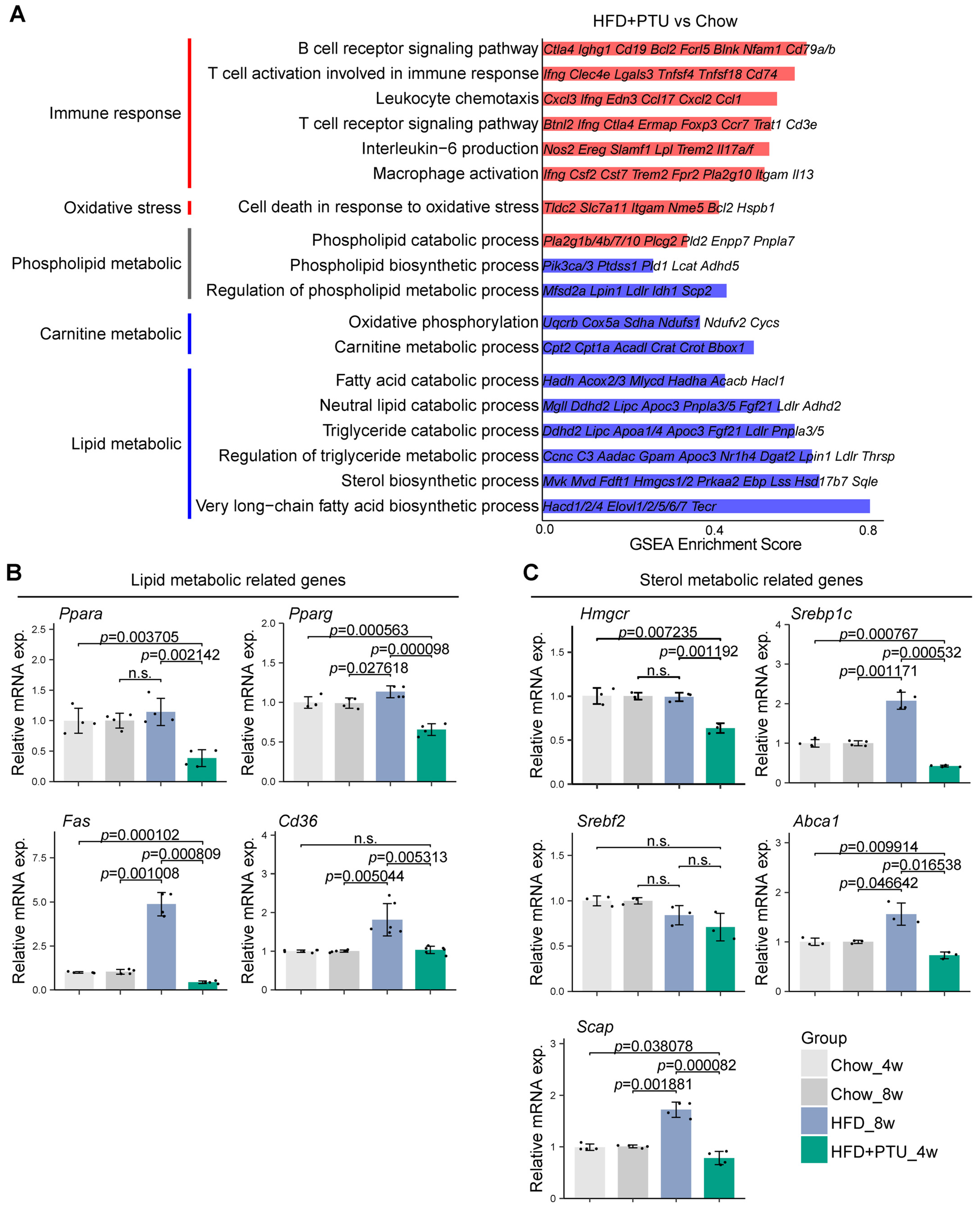
2.5. Liver Transcriptomes Analysis of the HFD+PTU-Induced Rat Model
3. Discussion
4. Materials and Methods
4.1. Patients Selection
4.2. Animal Processing Methods
4.3. Biochemical Parameters Measurement
4.3.1. Clinical Samples
4.3.2. Animal Samples
4.4. Metabolomic Analysis
4.4.1. Polar Metabolites Extraction
4.4.2. Lipid Extraction
4.4.3. HPLC-MS
4.4.4. Data Processing
4.5. RNAseq Analysis
4.6. RNA Isolation and Quantitative Real-Time PCR
4.7. Experimental Model Liver Histology
4.8. Statistic Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rinella, M.E.; Lazarus, J.V.; Ratizu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 2023, 78, 1966–1987. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; van Dongen, C.; Henry, L. The Global Epidemiology of Nonalcoholic Fatty Liver Disease (NAFLD) and Nonalcoholic Steatohepatitis (NASH): A Systematic Review. Hepatology 2023, 77, 1335–1347. [Google Scholar] [CrossRef] [PubMed]
- Le, M.H.; Le, D.M.; Baez, T.C.; Wu, Y.; Ito, T.; Lee, E.Y.; Lee, K.; Stave, C.D.; Henry, L.; Barnett, S.D.; et al. Global Incidence of Non-Alcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis of 63 Studies and 1,201,807 Persons. J. Hepatol. 2023, 79, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Milić, S.; Lulić, D.; Štimac, D. Non-alcoholic fatty liver disease and obesity: Biochemical, metabolic and clinical presentations. World J. Gastroenterol. 2014, 20, 9330–9337. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, X.; Dong, L.; Yang, Y.; Kong, D. Epidemiology of Lean/Non-Obese Nonalcoholic Fatty Liver Disease in China: A Systematic Review and Meta-Analysis. SMJ 2023, 44, 848–863. [Google Scholar] [CrossRef]
- Tang, A.; Ng, C.H.; Phang, P.H.; Chan, K.E.; Chin, Y.H.; Fu, C.E.; Zeng, R.W.; Xiao, J.; Tan, D.J.H.; Quek, J.; et al. Comparative Burden of Metabolic Dysfunction in Lean NAFLD vs Non-Lean NAFLD: A Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2023, 21, 1750–1760.e12. [Google Scholar] [CrossRef]
- Ye, Q.; Zou, B.; Yeo, Y.H.; Li, J.; Huang, D.Q.; Wu, Y.; Yang, H.; Liu, C.; Kam, L.Y.; Tan, X.X.E.; et al. Global Prevalence, Incidence, and Outcomes of Non-Obese or Lean Non-Alcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Lancet Gastroenterol. Hepatol. 2020, 5, 739–752. [Google Scholar] [CrossRef]
- Sookoian, S.; Pirola, C.J. Systematic Review with Meta-analysis: Risk Factors for Non-alcoholic Fatty Liver Disease Suggest a Shared Altered Metabolic and Cardiovascular Profile between Lean and Obese Patients. Aliment. Pharmacol. Ther. 2017, 46, 85–95. [Google Scholar] [CrossRef]
- Wongtrakul, W.; Charatcharoenwitthaya, N.; Charatcharoenwitthaya, P. Lean Non-Alcoholic Fatty Liver Disease and the Risk of All-Cause Mortality: An Updated Meta-Analysis. Ann. Hepatol. 2024, 29, 101288. [Google Scholar] [CrossRef]
- Ha, J.; Yim, S.Y.; Karagozian, R. Mortality and Liver-Related Events in Lean Versus Non-Lean Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2023, 21, 2496–2507.e5. [Google Scholar] [CrossRef]
- Souza, M.; Diaz, I.; Barchetta, I.; Mantovani, A. Gastrointestinal Cancers in Lean Individuals with Non-alcoholic Fatty Liver Disease: A Systematic Review and meta-analysis. Liver Int. 2024, 44, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wai-Sun Wong, V.; Dufour, J.F.; Schattenberg, J.M. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Flessa, C.; Nasiri-Ansari, N.; Kyrou, I.; Leca, B.M.; Lianou, M.; Chatzigeorgiou, A.; Kaltsas, G.; Kassi, E.; Randeva, H.S. Genetic and Diet-Induced Animal Models for Non-Alcoholic Fatty Liver Disease (NAFLD) Research. IJMS 2022, 23, 15791. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.; Wang, H.; Pan, X.; Little, P.J.; Xu, S.; Weng, J. Mouse Models of Nonalcoholic Fatty Liver Disease (NAFLD): Pathomechanisms and Pharmacotherapies. Int. J. Biol. Sci. 2022, 18, 5681–5697. [Google Scholar] [CrossRef] [PubMed]
- Henkel, J.; Coleman, C.D.; Schraplau, A.; Jöhrens, K.; Weiss, T.S.; Jonas, W.; Schürmann, A.; Püschel, G.P. Augmented Liver Inflammation in a Microsomal Prostaglandin E Synthase 1 (mPGES-1)-Deficient Diet-Induced Mouse NASH Model. Sci Rep. 2018, 8, 16127. [Google Scholar] [CrossRef]
- Yang, W.; Wu, D.; Zhang, R.; Deng, Q.; Ding, H.; Tian, Y.; Zhang, M.; Liu, G.; Wang, Z.; Li, X.; et al. Effects of Propylthiouracil-induced Hypothyroidism on Nonalcoholic Fatty Liver Disease in Rats fed a High-fat and High-cholesterol diet. Pak. Vet. J. 2015, 35, 1–6. [Google Scholar]
- DiStefano, J.K.; Gerhard, G.S. NAFLD in Normal Weight Individuals. Diabetol. Metab. Syndr. 2022, 14, 45. [Google Scholar] [CrossRef]
- Pouwels, S.; Sakran, N.; Graham, Y.; Leal, A.; Pintar, T.; Yang, W.; Kassir, R.; Singhal, R.; Mahawar, K.; Ramnarain, D. Non-Alcoholic Fatty Liver Disease (NAFLD): A Review of Pathophysiology, Clinical Management and Effects of Weight Loss. BMC Endocr. Disord. 2022, 22, 63. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Brunt, E.M.; van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.; Torbenson, M.S.; Unalp-Arida, A.; et al. Nonalcoholic Steatohepatitis Clinical Research Network. Design and Validation of a Histological Scoring System for Nonalcoholic Fatty Liver Disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Farrell, G.; Schattenberg, J.M.; Leclercq, I.; Yeh, M.M.; Goldin, R.; Teoh, N.; Schuppan, D. Mouse Models of Nonalcoholic Steatohepatitis: Toward Optimization of Their Relevance to Human Nonalcoholic Steatohepatitis. Hepatology 2019, 69, 2241–2257. [Google Scholar] [CrossRef]
- Lau, J.K.C.; Zhang, X.; Yu, J. Animal Models of Non-alcoholic Fatty Liver Disease: Current Perspectives and Recent Advances. J. Pathol. 2017, 241, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.V.; Michelotti, G.A.; Xie, G.; De Almeida, T.P.; Boursier, J.; Bohnic, B.; Guy, C.D.; Diehl, A.M. Mouse Models of Diet-Induced Nonalcoholic Steatohepatitis Reproduce the Heterogeneity of the Human Disease. PLoS ONE 2015, 10, e0127991. [Google Scholar] [CrossRef] [PubMed]
- Alshawsh, M.A.; Alsalahi, A.; Alshehade, S.A.; Saghir, S.A.M.; Ahmeda, A.F.; Al Zarzour, R.H.; Mahmoud, A.M. A Comparison of the Gene Expression Profiles of Non-Alcoholic Fatty Liver Disease between Animal Models of a High-Fat Diet and Methionine-Choline-Deficient Diet. Molecules 2022, 27, 858. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Tian, Q.; Guo, S.; Xie, D.; Cai, Y.; Wang, Z.; Chu, H.; Qiu, S.; Tang, S.; Zhang, A. Metabolomics for Clinical Biomarker Discovery and Therapeutic Target Identification. Molecules 2024, 29, 2198. [Google Scholar] [CrossRef]
- Oh, K.; Gupta, H.; Min, B.H.; Ganesan, R.; Sharma, S.P.; Won, S.M.; Jeong, J.J.; Lee, S.B.; Cha, M.G.; Kwon, G.H.; et al. The Identification of Metabolites from Gut Microbiota in NAFLD via Network Pharmacology. Sci. Rep. 2023, 13, 724. [Google Scholar] [CrossRef]
- Kalhan, S.C.; Guo, L.; Edmison, J.; Dasarathy, S.; McCullough, A.J.; Hanson, R.W.; Milburn, M. Plasma Metabolomic Profile in Nonalcoholic Fatty Liver Disease. Metabolism 2011, 60, 404–413. [Google Scholar] [CrossRef]
- Li, N.; Zhao, H. Role of Carnitine in Non-Alcoholic Fatty Liver Disease and Other Related Diseases: An Update. Front. Med. 2021, 8, 689042. [Google Scholar] [CrossRef]
- Vinaixa, M.; Ángel Rodríguez, M.; Rull, A.; Beltrán, R.; Bladé, C.; Brezmes, J.; Cañellas, N.; Joven, J.; Correig, X. Metabolomic Assessment of the Effect of Dietary Cholesterol in the Progressive Development of Fatty Liver Disease. J. Proteome. Res. 2010, 9, 2527–2538. [Google Scholar] [CrossRef]
- Tsoko, M.; Beauseigneur, F.; Gresti, J.; Niot, I.; Demarquoy, J.; Boichot, J.; Bézard, J.; Rochette, L.; Clouet, P. Enhancement of Activities Relative to Fatty Acid Oxidation in the Liver of Rats Depleted of L-carnitine by D-carnitine and a Gamma-butyrobetaine hydroxylase inhibitor. Biochem. Pharmacol. 1995, 49, 1403–1410. [Google Scholar] [CrossRef]
- Alves-Bezerra, M.; Cohen, D.E. Triglyceride Metabolism in the Liver. Compr. Physiol. 2017, 8, 1–22. [Google Scholar] [CrossRef]
- Cavill, R.; Jennen, D.; Kleinjans, J.; Briedé, J.J. Transcriptomic and Metabolomic Data Integration. Brief Bioinform. 2016, 17, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Pagadala, M.R.; Zein, C.O.; Dasarathy, S.; Yerian, L.M.; Lopez, R.; McCullough, A.J. Prevalence of Hypothyroidism in Nonalcoholic Fatty Liver Disease. Dig. Dis. Sci. 2012, 57, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Piantanida, E.; Ippolito, S.; Gallo, D.; Masiello, E.; Premoli, P.; Cusini, C.; Rosetti, S.; Sabatino, J.; Segato, S.; Trimarchi, F.; et al. The Interplay between Thyroid and Liver: Implications for Clinical Practice. J. Endocrinol. Investig. 2020, 43, 885–899. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhuang, X.; Zhang, J.; Li, M.; Du, S.; Tian, J.; Yuan, Y.; Ji, G.; Hu, C. Clinical Characterization and Proteomic Profiling of Lean Nonalcoholic Fatty Liver Disease. Front. Endocrinol. 2023, 14, 1171397. [Google Scholar] [CrossRef]
- Sinha, R.A.; You, S.; Zhou, J.; Siddique, M.M.; Bay, B.; Zhu, X.; Privalsky, M.L.; Cheng, S.; Stevens, R.D.; Summers, S.A.; et al. Thyroid Hormone Stimulates Hepatic Lipid Catabolism via Activation of Autophagy. J. Clin. Investig. 2012, 122, 2428–2438. [Google Scholar] [CrossRef]
- Wang, Z.; Haange, S.; Haake, V.; Huisinga, M.; Kamp, H.; Buesen, R.; Schubert, K.; Canzler, S.; Hackermüller, J.; Rolle-Kampczyk, U.; et al. Assessing the Influence of Propylthiouracil and Phenytoin on the Metabolomes of the Thyroid, Liver, and Plasma in Rats. Metabolites 2023, 13, 847. [Google Scholar] [CrossRef]
- Hao, W.; Yang, Q.; Li, J.; Ma, Y.; Lu, L.; Xiong, J.; Li, Y.; Xu, P.; Chen, Y.; Xue, M.; et al. Effects of propylthiouracil addition in high fat diet on blood lipid, body weight and body fat of rats. J. Cap. Med. Univ. 2018, 39, 385–392. [Google Scholar]
- Gaggini, M.; Carli, F.; Rosso, C.; Buzzigoli, E.; Marietti, M.; Della Latta, V.; Ciociaro, D.; Abate, M.L.; Gambino, R.; Cassader, M.; et al. Altered Amino Acid Concentrations in NAFLD: Impact of Obesity and Insulin Resistance. Hepatology 2018, 67, 145–158. [Google Scholar] [CrossRef]
- Guven Cetin, E. The Relationship between Insulin Resistance and Liver Damage in Non-Alcoholic Fatty Liver Patients. Med. Bull. Sisli Etfal Hosp. 2020, 54, 411–415. [Google Scholar] [CrossRef]
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.M.; Shah, S.H.; Arlotto, M.; Slentz, C.A.; et al. A Branched-Chain Amino Acid-Related Metabolic Signature That Differentiates Obese and Lean Humans and Contributes to Insulin Resistance. Cell Metab. 2009, 9, 311–326. [Google Scholar] [CrossRef]
- Wu, S.; Gao, Y.; Dong, X.; Tan, G.; Li, W.; Lou, Z.; Chai, Y. Serum Metabonomics Coupled with Ingenuity Pathway Analysis Characterizes Metabolic Perturbations in Response to Hypothyroidism Induced by Propylthiouracil in Rats. J. Pharm. Biomed. Anal. 2013, 72, 109–114. [Google Scholar] [CrossRef]
- Kahle, M.; Schäfer, A.; Seelig, A.; Schultheiß, J.; Wu, M.; Aichler, M.; Leonhardt, J.; Rathkolb, B.; Rozman, J.; Sarioglu, H.; et al. High Fat Diet-Induced Modifications in Membrane Lipid and Mitochondrial-Membrane Protein Signatures Precede the Development of Hepatic Insulin Resistance in Mice. Mol. Metab. 2015, 4, 39–50. [Google Scholar] [CrossRef]
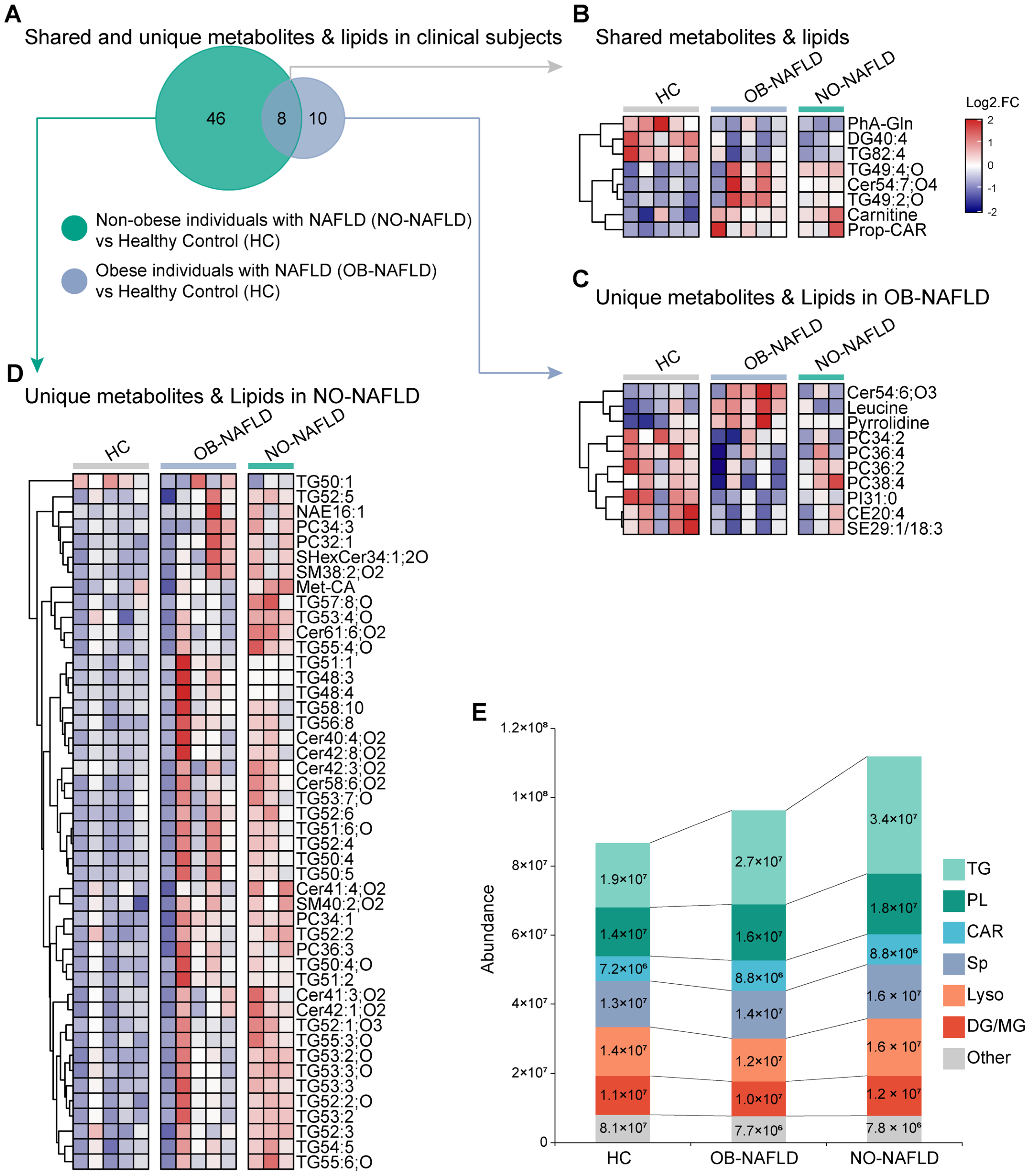


| Characteristic | HC (n = 5) | OB-NAFLD (n = 5) | NO-NAFLD (n = 3) | p Value OB-NAFLD vs. HC | p Value NO-NAFLD vs. HC | p Value NO-NAFLD vs. OB-NAFLD | |||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||||
| Age (year) | 43.00 | 9.72 | 38.40 | 5.90 | 51.00 | 7.00 | 0.398 | 0.230 | 0.064 |
| Sex (Female, %) | 20.00% | - | 20.00% | - | 33.33% | - | - | - | - |
| Body weight (kg) | 61.80 | 7.08 | 84.26 | 7.12 | 58.73 | 3.40 | 0.001 * | 0.448 | 0.001 * |
| BMI (kg/m2) | 23.50 | 2.52 | 29.70 | 1.36 | 21.96 | 1.66 | 0.003 * | 0.344 | 0.003 * |
| FBG (mmol/L) | 4.90 | 0.51 | 5.12 | 0.70 | 5.25 | 0.55 | 0.601 | 0.432 | 0.784 |
| ALT (U/L) | 11.60 | 3.21 | 31.20 | 6.76 | 23.67 | 3.51 | 0.001 * | 0.008 * | 0.084 |
| AST (U/L) | 18.10 | 1.95 | 22.80 | 4.97 | 23.33 | 1.53 | 0.103 | 0.007 * | 0.832 |
| CHOL (mmol/L) | 4.70 | 0.42 | 5.17 | 0.68 | 5.48 | 0.90 | 0.240 | 0.268 | 0.631 |
| TG (mmol/L) | 0.90 | 0.36 | 2.63 | 2.31 | 2.25 | 0.44 | 0.169 | 0.013 * | 0.737 |
| LDL(mmol/L) | 3.00 | 0.41 | 3.36 | 0.82 | 3.69 | 0.89 | 0.369 | 0.287 | 0.624 |
| HDL (mmol/L) | 1.40 | 0.11 | 1.09 | 0.26 | 1.15 | 0.34 | 0.063 | 0.353 | 0.813 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Y.; Liu, Q.; Wang, Y.; Wang, B.; An, J.; Chen, Q.; Wang, T.; Shang, J. Propylthiouracil Induced Rat Model Reflects Heterogeneity Observed in Clinically Non-Obese Subjects with Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2024, 25, 10764. https://doi.org/10.3390/ijms251910764
Jin Y, Liu Q, Wang Y, Wang B, An J, Chen Q, Wang T, Shang J. Propylthiouracil Induced Rat Model Reflects Heterogeneity Observed in Clinically Non-Obese Subjects with Nonalcoholic Fatty Liver Disease. International Journal of Molecular Sciences. 2024; 25(19):10764. https://doi.org/10.3390/ijms251910764
Chicago/Turabian StyleJin, Yu, Qiuyan Liu, Yuqin Wang, Bing Wang, Jing An, Qimeng Chen, Tao Wang, and Jing Shang. 2024. "Propylthiouracil Induced Rat Model Reflects Heterogeneity Observed in Clinically Non-Obese Subjects with Nonalcoholic Fatty Liver Disease" International Journal of Molecular Sciences 25, no. 19: 10764. https://doi.org/10.3390/ijms251910764





