Seeding Aggregation Assays in Lewy Bodies Disorders: A Narrative State-of-the-Art Review
Abstract
1. Introduction
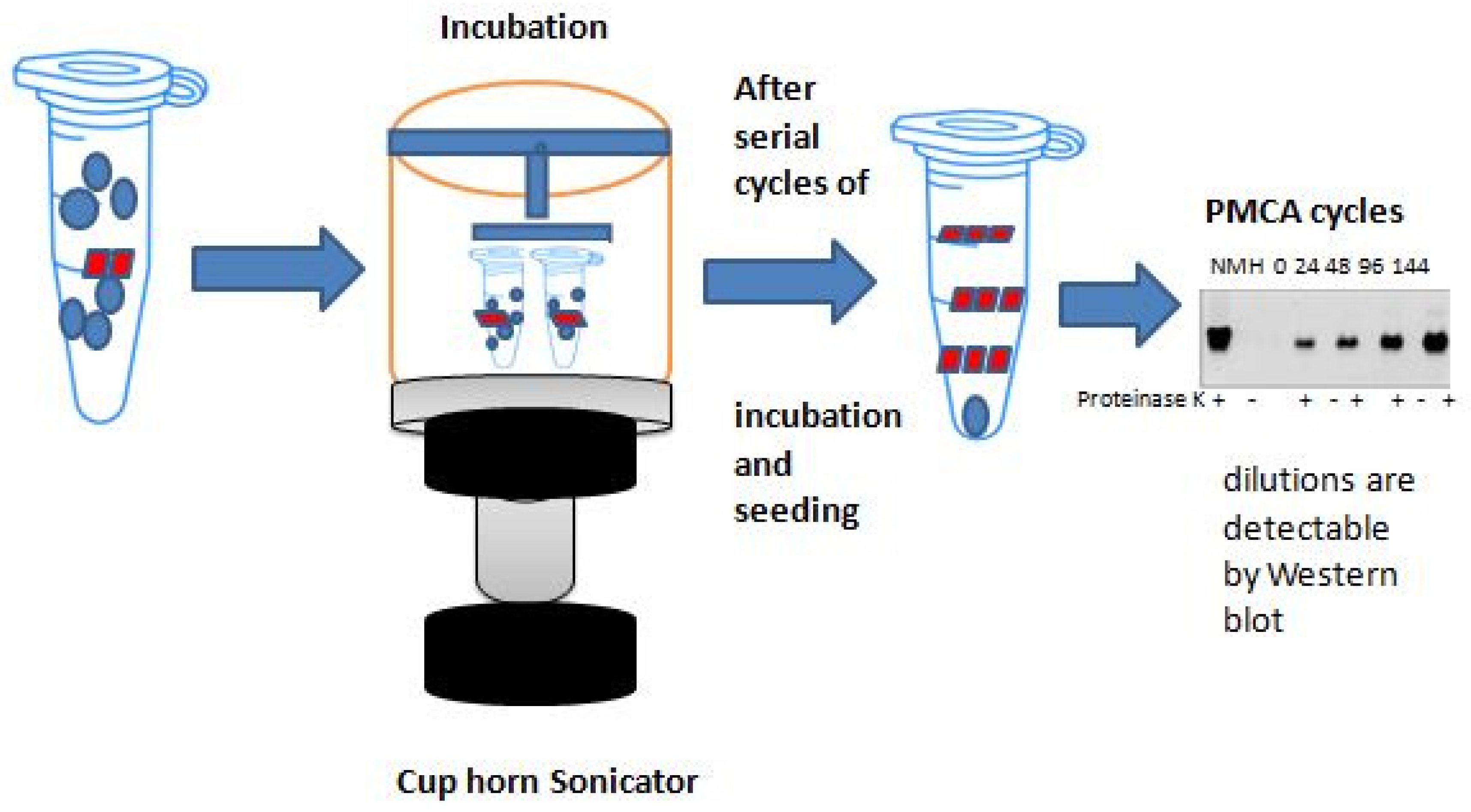
2. Basic Principles of Protein Amplification Methods
α-syn-SAAs Protocols
3. SAAs in Genetic PD
4. SAAs in MSA Variants
5. SAAs in Prodromal LBD
6. SAAs in SWEDDs
7. Distinguishing between Synucleinopathies
8. Challenges and Limitations
9. Future Directions
10. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paciotti, S.; Bellomo, G.; Gatticchi, L.; Parnetti, L. Are We Ready for Detecting α-Synuclein Prone to Aggregation in Patients? The Case of “Protein-Misfolding Cyclic Amplification” and “Real-Time Quaking-Induced Conversion” as Diagnostic Tools. Front. Neurol. 2018, 9, 415. [Google Scholar] [CrossRef]
- Bougea, A. Synuclein in neurodegeneration. Adv. Clin. Chem. 2021, 103, 97–134. [Google Scholar] [CrossRef] [PubMed]
- Erro, R.; Schneider, S.A.; Stamelou, M.; Quinn, N.P.; Bhatia, K.P. What do patients with scans without evidence of dopaminergic deficit (SWEDD) have? New evidence and continuing controversies. J. Neurol. Neurosurg. Psychiatry 2016, 87, 319–323. [Google Scholar] [CrossRef]
- Koga, S.; Sekiya, H.; Kondru, N.; Ross, O.A.; Dickson, D.W. Neuropathology and molecular diagnosis of Synucleinopathies. Mol. Neurodegener. 2021, 16, 83. [Google Scholar] [CrossRef]
- Singer, W. Recent advances in establishing fluid biomarkers for the diagnosis and differentiation of alpha-synucleinopathies—A mini review. Clin. Auton. Res. Off. J. Clin. Auton. Res. Soc. 2022, 32, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.S.; Teodorczuk, A.; Watson, R. Dementia with Lewy bodies: Challenges in the diagnosis and management. Aust. N. Z. J. Psychiatry 2019, 53, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Tolosa, E.; Garrido, A.; Scholz, S.W.; Poewe, W. Challenges in the diagnosis of Parkinson’s disease. Lancet Neurol. 2021, 20, 385–397. [Google Scholar] [CrossRef]
- Bougea, A. New markers in Parkinson’s disease. Adv. Clin. Chem. 2020, 96, 137–178. [Google Scholar] [CrossRef] [PubMed]
- Concha-Marambio, L.; Farris, C.M.; Holguin, B.; Ma, Y.; Seibyl, J.; Russo, M.J.; Kang, U.J.; Hutten, S.J.; Merchant, K.; Shahnawaz, M.; et al. Seed Amplification Assay to Diagnose Early Parkinson’s and Predict Dopaminergic Deficit Progression. Mov. Disord. Off. J. Mov. Disord. Soc. 2021, 36, 2444–2446. [Google Scholar] [CrossRef]
- Chahine, L.M.; Beach, T.G.; Adler, C.H.; Hepker, M.; Kanthasamy, A.; Appel, S.; Pritzkow, S.; Pinho, M.; Mosovsky, S.; Serrano, G.E.; et al. Central and peripheral α-synuclein in Parkinson disease detected by seed amplification assay. Ann. Clin. Transl. Neurol. 2023, 10, 696–705. [Google Scholar] [CrossRef]
- Atarashi, R.; Satoh, K.; Sano, K.; Fuse, T.; Yamaguchi, N.; Ishibashi, D.; Matsubara, T.; Nakagaki, T.; Yamanaka, H.; Shirabe, S.; et al. Ultrasensitive human prion detection in cerebrospinal fluid by real-time quaking-induced conversion. Nat. Med. 2011, 17, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Cazzaniga, F.A.; Bistaffa, E.; De Luca, C.M.G.; Bufano, G.; Indaco, A.; Giaccone, G.; Moda, F. Sporadic Creutzfeldt-Jakob disease: Real-Time Quaking Induced Conversion (RT-QuIC) assay represents a major diagnostic advance. Eur. J. Histochem. EJH 2021, 65, 3298. [Google Scholar] [CrossRef] [PubMed]
- Vascellari, S.; Orrù, C.D.; Caughey, B. Real-Time Quaking- Induced Conversion Assays for Prion Diseases, Synucleinopathies, and Tauopathies. Front. Aging Neurosci. 2022, 14, 853050. [Google Scholar] [CrossRef] [PubMed]
- Siderowf, A.; Concha-Marambio, L.; Lafontant, D.E.; Farris, C.M.; Ma, Y.; Urenia, P.A.; Nguyen, H.; Alcalay, R.N.; Chahine, L.M.; Foroud, T.; et al. Assessment of heterogeneity among participants in the Parkinson’s Progression Markers Initiative cohort using α-synuclein seed amplification: A cross-sectional study. Lancet Neurol. 2023, 22, 407–417. [Google Scholar] [CrossRef]
- Fairfoul, G.; McGuire, L.I.; Pal, S.; Ironside, J.W.; Neumann, J.; Christie, S.; Joachim, C.; Esiri, M.; Evetts, S.G.; Rolinski, M.; et al. Alpha-synuclein RT-QuIC in the CSF of patients with alpha-synucleinopathies. Ann. Clin. Transl. Neurol. 2016, 3, 812–818. [Google Scholar] [CrossRef]
- Shahnawaz, M.; Tokuda, T.; Waragai, M.; Mendez, N.; Ishii, R.; Trenkwalder, C.; Mollenhauer, B.; Soto, C. Development of a Biochemical Diagnosis of Parkinson Disease by Detection of α-Synuclein Misfolded Aggregates in Cerebrospinal Fluid. JAMA Neurol. 2017, 74, 163–172. [Google Scholar] [CrossRef]
- Groveman, B.R.; Orrù, C.D.; Hughson, A.G.; Raymond, L.D.; Zanusso, G.; Ghetti, B.; Campbell, K.J.; Safar, J.; Galasko, D.; Caughey, B. Rapid and ultra-sensitive quantitation of disease-associated α-synuclein seeds in brain and cerebrospinal fluid by αSyn RT-QuIC. Acta Neuropathol. Commun. 2018, 6, 7. [Google Scholar] [CrossRef]
- Li, J.; Luo, H.; Zheng, H.; Duan, S.; Zhao, T.; Yuan, Y.; Liu, Y.; Zhang, X.; Wang, Y.; Yang, J.; et al. Clinical application of prion-like seeding in α-synucleinopathies: Early and non-invasive diagnosis and therapeutic development. Front. Mol. Neurosci. 2022, 15, 975619. [Google Scholar] [CrossRef]
- Sano, K.; Atarashi, R.; Satoh, K.; Ishibashi, D.; Nakagaki, T.; Iwasaki, Y.; Yoshida, M.; Murayama, S.; Mishima, K.; Nishida, N. Prion-Like Seeding of Misfolded α-Synuclein in the Brains of Dementia with Lewy Body Patients in RT-QUIC. Mol. Neurobiol. 2018, 55, 3916–3930. [Google Scholar] [CrossRef]
- Manne, S.; Kondru, N.; Hepker, M.; Jin, H.; Anantharam, V.; Lewis, M.; Huang, X.; Kanthasamy, A.; Kanthasamy, A.G. Ultrasensitive Detection of Aggregated α-Synuclein in Glial Cells, Human Cerebrospinal Fluid, and Brain Tissue Using the RT-QuIC Assay: New High-Throughput Neuroimmune Biomarker Assay for Parkinsonian Disorders. J. Neuroimmune Pharmacol. Off. J. Soc. NeuroImmune Pharmacol. 2019, 14, 423–435. [Google Scholar] [CrossRef]
- Han, J.-Y.; Jang, H.-S.; Green, A.J.E.; Choi, Y.P. RT-QuIC-based detection of alpha-synuclein seeding activity in brains of dementia with Lewy Body patients and of a transgenic mouse model of synucleinopathy. Prion 2020, 14, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Shahnawaz, M.; Mukherjee, A.; Pritzkow, S.; Mendez, N.; Rabadia, P.; Liu, X.; Hu, B.; Schmeichel, A.; Singer, W.; Wu, G.; et al. Discriminating α-synuclein strains in Parkinson’s disease and multiple system atrophy. Nature 2020, 578, 273–277. [Google Scholar] [CrossRef]
- Singer, W.; Schmeichel, A.M.; Shahnawaz, M.; Schmelzer, J.D.; Boeve, B.F.; Sletten, D.M.; Gehrking, T.L.; Gehrking, J.A.; Olson, A.D.; Savica, R.; et al. Alpha-Synuclein Oligomers and Neurofilament Light Chain in Spinal Fluid Differentiate Multiple System Atrophy from Lewy Body Synucleinopathies. Ann. Neurol. 2020, 88, 503–512. [Google Scholar] [CrossRef]
- Kang, U.J.; Boehme, A.K.; Fairfoul, G.; Shahnawaz, M.; Ma, T.C.; Hutten, S.J.; Green, A.; Soto, C. Comparative study of cerebrospinal fluid α-synuclein seeding aggregation assays for diagnosis of Parkinson’s disease. Mov. Disord. 2019, 34, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Concha-Marambio, L.; Weber, S.; Farris, C.M.; Dakna, M.; Lang, E.; Wicke, T.; Ma, Y.; Starke, M.; Ebentheuer, J.; Sixel-Döring, F.; et al. Accurate Detection of α-Synuclein Seeds in Cerebrospinal Fluid from Isolated Rapid Eye Movement Sleep Behavior Disorder and Patients with Parkinson’s Disease in the DeNovo Parkinson (DeNoPa) Cohort. Mov. Disord. Off. J. Mov. Disord. Soc. 2023, 38, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Brockmann, K.; Quadalti, C.; Lerche, S.; Rossi, M.; Wurster, I.; Baiardi, S.; Roeben, B.; Mammana, A.; Zimmermann, M.; Hauser, A.K.; et al. Association between CSF alpha-synuclein seeding activity and genetic status in Parkinson’s disease and dementia with Lewy bodies. Acta Neuropathol. Commun. 2021, 9, 175. [Google Scholar] [CrossRef]
- Garrido, A.; Fairfoul, G.; Tolosa, E.S.; Martí, M.J.; Green, A. α-synuclein RT-QuIC in cerebrospinal fluid of LRRK2-linked Parkinson’s disease. Ann. Clin. Transl. Neurol. 2019, 6, 1024–1032. [Google Scholar] [CrossRef]
- van Rumund, A.; Green, A.J.E.; Fairfoul, G.; Esselink, R.A.J.; Bloem, B.R.; Verbeek, M.M. α-Synuclein real-time quaking-induced conversion in the cerebrospinal fluid of uncertain cases of parkinsonism. Ann. Neurol. 2019, 85, 777–781. [Google Scholar] [CrossRef]
- Poggiolini, I.; Gupta, V.; Lawton, M.; Lee, S.; El-Turabi, A.; Querejeta-Coma, A.; Trenkwalder, C.; Sixel-Döring, F.; Foubert-Samier, A.; Pavy-Le Traon, A.; et al. Diagnostic value of cerebrospinal fluid alpha-synuclein seed quantification in synucleinopathies. Brain J. Neurol. 2022, 145, 584–595. [Google Scholar] [CrossRef]
- Rossi, M.; Candelise, N.; Baiardi, S.; Capellari, S.; Giannini, G.; Orrù, C.D.; Antelmi, E.; Mammana, A.; Hughson, A.G.; Calandra-Buonaura, G.; et al. Ultrasensitive RT-QuIC assay with high sensitivity and specificity for Lewy body-associated synucleinopathies. Acta Neuropathol. 2020, 140, 49–62. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, J.; Chen, X.; Wang, S.; Zhang, C.; Hu, J.; Guo, D.; Liu, X. Real-time quaking-induced conversion assay is accurate for Lewy body diseases: A meta-analysis. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2022, 43, 4125–4132. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.; Orrú, C.D.; Serrano, G.E.; Galasko, D.; Hughson, A.G.; Groveman, B.R.; Adler, C.H.; Beach, T.G.; Caughey, B.; Hansson, O. Performance of αSynuclein RT-QuIC in relation to neuropathological staging of Lewy body disease. Acta Neuropathol. Commun. 2022, 10, 90. [Google Scholar] [CrossRef] [PubMed]
- Orrú, C.D.; Ma, T.C.; Hughson, A.G.; Groveman, B.R.; Srivastava, A.; Galasko, D.; Angers, R.; Downey, P.; Crawford, K.; Hutten, S.J.; et al. A rapid α-synuclein seed assay of Parkinson’s disease CSF panel shows high diagnostic accuracy. Ann. Clin. Transl. Neurol. 2020, 8, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Compta, Y.; Painous, C.; Álvarez-Mon Soto, M.; Pulido-Salgado, M.; Fernández, M.; Cámara, A.; Sánchez, V.; Bargalló, N.; Caballol, N.; Pont-Sunyer, C.; et al. Combined CSF α-SYN RT-QuIC, CSF NFL and midbrain-pons planimetry in degenerative parkinsonisms: From bedside to bench, and back again. Park. Relat. Disord. 2022, 99, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Bargar, C.; De Luca, C.M.G.; Devigili, G.; Elia, A.E.; Cilia, R.; Portaleone, S.M.; Wang, W.; Tramacere, I.; Bistaffa, E.; Cazzaniga, F.A.; et al. Discrimination of MSA-P and MSA-C by RT-QuIC analysis of olfactory mucosa: The first assessment of assay reproducibility between two specialized laboratories. Mol. Neurodegener. 2021, 16, 82. [Google Scholar] [CrossRef]
- Bongianni, M.; Ladogana, A.; Capaldi, S.; Klotz, S.; Baiardi, S.; Cagnin, A.; Perra, D.; Fiorini, M.; Poleggi, A.; Legname, G.; et al. α-Synuclein RT-QuIC assay in cerebrospinal fluid of patients with dementia with Lewy bodies. Ann. Clin. Transl. Neurol. 2019, 6, 2120–2126. [Google Scholar] [CrossRef]
- Grossauer, A.; Hemicker, G.; Krismer, F.; Peball, M.; Djamshidian, A.; Poewe, W.; Seppi, K.; Heim, B. α-Synuclein Seed Amplification Assays in the Diagnosis of Synucleinopathies Using Cerebrospinal Fluid-A Systematic Review and Meta-Analysis. Mov. Disord. Clin. Pract. 2023, 10, 737–747. [Google Scholar] [CrossRef]
- Iranzo, A.; Fairfoul, G.; Ayudhaya, A.C.N.; Serradell, M.; Gelpi, E.; Vilaseca, I.; Sanchez-Valle, R.; Gaig, C.; Santamaria, J.; Tolosa, E.; et al. Detection of α-synuclein in CSF by RT-QuIC in patients with isolated rapid-eye-movement sleep behaviour disorder: A longitudinal observational study. Lancet Neurol. 2021, 20, 203–212. [Google Scholar] [CrossRef]
- De Luca, C.M.G.; Elia, A.E.; Portaleone, S.M.; Cazzaniga, F.A.; Rossi, M.; Bistaffa, E.; De Cecco, E.; Narkiewicz, J.; Salzano, G.; Carletta, O.; et al. Efficient RT-QuIC seeding activity for α-synuclein in olfactory mucosa samples of patients with Parkinson’s disease and multiple system atrophy. Transl. Neurodegener. 2019, 8, 24. [Google Scholar] [CrossRef]
- Stefani, A.; Iranzo, A.; Holzknecht, E.; Perra, D.; Bongianni, M.; Gaig, C.; Heim, B.; Serradell, M.; Sacchetto, L.; Garrido, A.; et al. Alpha-synuclein seeds in olfactory mucosa of patients with isolated REM sleep behaviour disorder. Brain J. Neurol. 2021, 144, 1118–1126. [Google Scholar] [CrossRef]
- Perra, D.; Bongianni, M.; Novi, G.; Janes, F.; Bessi, V.; Capaldi, S.; Sacchetto, L.; Tagliapietra, M.; Schenone, G.; Morbelli, S.; et al. Alpha-synuclein seeds in olfactory mucosa and cerebrospinal fluid of patients with dementia with Lewy bodies. Brain Commun. 2021, 3, fcab045. [Google Scholar] [CrossRef] [PubMed]
- Manne, S.; Kondru, N.; Jin, H.; Serrano, G.E.; Anantharam, V.; Kanthasamy, A.; Adler, C.H.; Beach, T.G.; Kanthasamy, A.G. Blinded RT-QuIC Analysis of α-Synuclein Biomarker in Skin Tissue From Parkinson’s Disease Patients. Mov. Disord. Off. J. Mov. Disord. Soc. 2020, 35, 2230–2239. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Becker, K.; Donadio, V.; Siedlak, S.; Yuan, J.; Rezaee, M.; Incensi, A.; Kuzkina, A.; Orrú, C.D.; Tatsuoka, C.; et al. Skin α-Synuclein Aggregation Seeding Activity as a Novel Biomarker for Parkinson Disease. JAMA Neurol. 2020, 78, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Donadio, V.; Wang, Z.; Incensi, A.; Rizzo, G.; Fileccia, E.; Vacchiano, V.; Capellari, S.; Magnani, M.; Scaglione, C.; Stanzani Maserati, M.; et al. In Vivo Diagnosis of Synucleinopathies: A Comparative Study of Skin Biopsy and RT-QuIC. Neurology 2021, 96, e2513–e2524. [Google Scholar] [CrossRef]
- Mammana, A.; Baiardi, S.; Quadalti, C.; Rossi, M.; Donadio, V.; Capellari, S.; Liguori, R.; Parchi, P. RT-QuIC Detection of Pathological α-Synuclein in Skin Punches of Patients with Lewy Body Disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2021, 36, 2173–2177. [Google Scholar] [CrossRef]
- Kuzkina, A.; Bargar, C.; Schmitt, D.; Rößle, J.; Wang, W.; Schubert, A.L.; Tatsuoka, C.; Gunzler, S.A.; Zou, W.Q.; Volkmann, J.; et al. Diagnostic value of skin RT-QuIC in Parkinson’s disease: A two-laboratory study. NPJ Park. Dis 2021, 7, 99. [Google Scholar] [CrossRef]
- Kuzkina, A.; Panzer, C.; Seger, A.; Schmitt, D.; Rößle, J.; Schreglmann, S.R.; Knacke, H.; Salabasidou, E.; Kohl, A.; Sittig, E.; et al. Dermal Real-Time Quaking-Induced Conversion Is a Sensitive Marker to Confirm Isolated Rapid Eye Movement Sleep Behavior Disorder as an Early α-Synucleinopathy. Mov. Disord. Off. J. Mov. Disord. Soc. 2023, 38, 1077–1082. [Google Scholar] [CrossRef]
- Fenyi, A.; Leclair-Visonneau, L.; Clairembault, T.; Coron, E.; Neunlist, M.; Melki, R.; Derkinderen, P.; Bousset, L. Detection of alpha-synuclein aggregates in gastrointestinal biopsies by protein misfolding cyclic amplification. Neurobiol. Dis. 2019, 129, 38–43. [Google Scholar] [CrossRef]
- Luan, M.; Sun, Y.; Chen, J.; Jiang, Y.; Li, F.; Wei, L.; Sun, W.; Ma, J.; Song, L.; Liu, J.; et al. Diagnostic Value of Salivary Real-Time Quaking-Induced Conversion in Parkinson’s Disease and Multiple System Atrophy. Mov. Disord. Off. J. Mov. Disord. Soc. 2022, 37, 1059–1063. [Google Scholar] [CrossRef]
- Okuzumi, A.; Hatano, T.; Matsumoto, G.; Nojiri, S.; Ueno, S.I.; Imamichi-Tatano, Y.; Kimura, H.; Kakuta, S.; Kondo, A.; Fukuhara, T.; et al. Propagative α-synuclein seeds as serum biomarkers for synucleinopathies. Nat. Med. 2023, 29, 1448–1455. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, S.; Yang, C.; Yu, Z.; Jiang, Y.; Feng, T. Comparison of biospecimens for α-synuclein seed amplification assays in Parkinson’s disease: A systematic review and network meta-analysis. Eur. J. Neurol. 2023, 30, 3949–3967. [Google Scholar] [CrossRef]
- Manne, S.; Kondru, N.; Jin, H.; Anantharam, V.; Huang, X.; Kanthasamy, A.; Kanthasamy, A.G. α-Synuclein real-time quaking-induced conversion in the submandibular glands of Parkinson’s disease patients. Mov. Disord. Off. J. Mov. Disord. Soc. 2020, 35, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Bargar, C.; Wang, W.; Gunzler, S.A.; LeFevre, A.; Wang, Z.; Lerner, A.J.; Singh, N.; Tatsuoka, C.; Appleby, B.; Zhu, X.; et al. Streamlined alpha-synuclein RT-QuIC assay for various biospecimens in Parkinson’s disease and dementia with Lewy bodies. Acta Neuropathol. Commun. 2021, 9, 62. [Google Scholar] [CrossRef] [PubMed]
- Garrido, A.; Fairfoul, G.; Tolosa, E.; Marti, M.J.; Ezquerra, M.; Green, A.J.E. Brain and Cerebrospinal Fluid α-Synuclein Real-Time Quaking-Induced Conversion Identifies Lewy Body Pathology in LRRK2-PD. Mov. Disord. Off. J. Mov. Disord. Soc. 2023, 38, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.T. REM sleep behavior disorder (RBD). Neurobiol. Dis. 2020, 143, 104996. [Google Scholar] [CrossRef] [PubMed]
- Kluge, A.; Schaeffer, E.; Bunk, J.; Sommerauer, M.; Röttgen, S.; Schulte, C.; Roeben, B.; von Thaler, A.K.; Welzel, J.; Lucius, R.; et al. Detecting Misfolded α-Synuclein in Blood Years before the Diagnosis of Parkinson’s Disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2024, 39, 1289–1299. [Google Scholar] [CrossRef]
- Martinez-Valbuena, I.; Visanji, N.P.; Kim, A.; Lau, H.H.C.; So, R.W.L.; Alshimemeri, S.; Gao, A.; Seidman, M.A.; Luquin, M.R.; Watts, J.C.; et al. Alpha-synuclein seeding shows a wide heterogeneity in multiple system atrophy. Transl. Neurodegener. 2022, 11, 7. [Google Scholar] [CrossRef]
- Mammana, A.; Baiardi, S.; Rossi, M.; Quadalti, C.; Ticca, A.; Magliocchetti, F.; Bernhardt, A.; Capellari, S.; Parchi, P. Improving protocols for α-synuclein seed amplification assays: Analysis of preanalytical and analytical variables and identification of candidate parameters for seed quantification. Clin. Chem. Lab. Med. 2024, 62, 2001–2010. [Google Scholar] [CrossRef]
- Bellomo, G.; Paciotti, S.; Concha-Marambio, L.; Rizzo, D.; Wojdaƚa, A.L.; Chiasserini, D.; Gatticchi, L.; Cerofolini, L.; Giuntini, S.; De Luca, C.M.G.; et al. Cerebrospinal fluid lipoproteins inhibit α-synuclein aggregation by interacting with oligomeric species in seed amplification assays. Mol. Neurodegener. 2023, 18, 20. [Google Scholar] [CrossRef]
- Bashir, S.; Aiman, A.; Chaudhary, A.A.; Khan, N.; Ahanger, I.A.; Sami, N.; Almugri, E.A.; Ali, M.A.M.; Khan, S.U.; Shahid, M.; et al. Probing protein aggregation through spectroscopic insights and multimodal approaches: A comprehensive review for counteracting neurodegenerative disorders. Heliyon 2024, 10, e27949. [Google Scholar] [CrossRef]
- Brockmann, K.; Lerche, S.; Baiardi, S.; Rossi, M.; Wurster, I.; Quadalti, C.; Roeben, B.; Mammana, A.; Zimmermann, M.; Hauser, A.K.; et al. CSF α-synuclein seed amplification kinetic profiles are associated with cognitive decline in Parkinson’s disease. NPJ Park. Dis 2024, 10, 24. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.J.; Orru, C.D.; Concha-Marambio, L.; Giaisi, S.; Groveman, B.R.; Farris, C.M.; Holguin, B.; Hughson, A.G.; LaFontant, D.E.; Caspell-Garcia, C.; et al. High diagnostic performance of independent alpha-synuclein seed amplification assays for detection of early Parkinson’s disease. Acta Neuropathol. Commun. 2021, 9, 179. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Wang, Q.; Orrù, C.D.; Fernandez, M.; Compta, Y.; Ghetti, B.; Zanusso, G.; Zou, W.Q.; Caughey, B.; Beauchemin, C.A.A. Enhanced quantitation of pathological α-synuclein in patient biospecimens by RT-QuIC seed amplification assays. PLoS Pathog. 2024, 20, e1012554. [Google Scholar] [CrossRef] [PubMed]

| Author, Publication Year | Assay | Sample | Autopsy | Disease | Number of Samples (Cases/Controls) | Main Outcomes |
|---|---|---|---|---|---|---|
| Fairfoul et al., 2016 [15] | RT-QuIC | CSF | YES | DLB DLB/AD AD/iLBD PD PSP CBD | 12/20 17/20 13/20 2/20 2 3 | AD and control CSF: 100% specificity DLB and PD CSF CSF: 92% and 95% sensitivities, respectively. Negative results for PSP and CBD. |
| ΝO | PD iRBD | 20/15 3/15 | ||||
| Shahnawaz et al., 2017 [16] | PMCA | CSF | NO | PD DLB MSA | 76/97 10/97 10/97 | Achieved 88.5% sensitivity and 96.9%. specificity. The degree of clinical symptoms of PD was associated with the α-syn-PMCA results for various patients. |
| Sano et al., 2017 [19] | RT-QuIC | BH | YES | DLB | 7/2 | Brain samples with diffuse neocortical DLB showed 50% seeding dose (SD50) values of 107~1010/g. An estimated SD50 value of ~105/g brain was found for limbic DLB. DLB was distinguished from other neurological and neurodegenerative illnesses by the RT-QUIC assay. In addition. despite the Ser129 phosphorylation state, reactions seeded with oligomer-like species surprisingly recreated the prion-like seeding, but not with insoluble aggregates of r-α-syn. |
| Groveman et al., 2018 [17] | RT-QuIC | CSF | NO | PD DLB | 12/31 17/31 | Achieved 93% diagnostic sensitivity and 100% specificity. The quantification of α-syn D seeding activity in CSF samples was made possible using end-point dilution analyses, which also enabled for detection in as little as 0.2 μL. |
| Kang et al., 2019 [24] | RT-QuIC PMCA | CSF CSF | NO NO | PD PD | 105/79 105/79 | Clinical parameters, such as the severity or duration of the condition, did not correspond with assay data. |
| Manne et al., 2019a [20] | RT-QuIC | CSF ΒH | NO YES | PD PD DLB | 15/11 11/19 5/19 | RT-QuIC: 100% sensitivity and specificity. PD and DLB BH samples: 94% sensitivity and 100% specificity. |
| Garrido et al., 2019 [27] | RT-QuIC | CSF | NO | LRRK2-PD iPD NMCs of LRRK2 | 15/10 10/10 16/10 | Achieved 90% sensitivity and 80% specificity. No clinical differences were detected between LRRK2-PD patients with positive and negative RT-QuIC. |
| Van Rumund et al., 2019 [28] | RT-QuIC | CSF | NO *1 | PD DLB MSA α-synucleinopathy with vasculopathy | 53/52 1/52 17/52 11/52 | Achieved 84% accuracy, 75% sensitivity, and 94%, specificity α-synucleinopathies from non-α-synucleinopathies and controls. |
| Bongianni et al., 2019 [36] | RT-QuIC | CSF | YES | DLB MSA LBD/AD LBD/PART LBD/CJD | 7/49 1/49 15/49 2/49 3/49 | Achievied 92.9% sensitivity and 95.9% specificity from α-synucleinopathies vs. non-alpha-synucleinopathies. |
| Han et al., 2020 [21] | RT-QuIC | BH | YES | DLB | 7/6 | α-syn seeding activity varies between patients with detectable dilutions ranging from 10−3 to 10−8 dilutions of brain tissue and is stable under exposures to the cycles of freezing, thawing, and sonication. |
| Rossi et al., 2020 [30] | RT-QuIC | CSF | YES | DLB Mixed LBD *2 MSA | 14/81 7/81 2/81 | Overall 95.3%sensitivity for DLB, PD, iRBD, and PAF. Achieved 98% specificity in a neuropathological cohort of 101 cases without LB pathology. |
| NO | DLB PD iRBD PAF MSA | 34/62 71/62 18/62 28/62 31/62 | ||||
| Shahnawaz et al., 2020 [22] | PMCA | CSF | NO | PD MSA | 94/56 75/56 | Achieved 95.4% overall sensitivity PD vs. MSA, and |
| Orrú et al., 2020 [33] | RT-QuIC | CSF | NO | PD | 108/85 | 95% of the areas under the curve were found in receiver operating characteristic analyses comparing the diagnosis of patients to healthy controls. The reaction times required to achieve 50% maximum fluorescence were linked with the scores on the REM sleep behavioral disorder questionnaire. |
| Singer et al., 2020 [23] | PMCA | CSF | NO | PD MSA DLB | 16/29 62/29 13/29 | NFL and α-syn oligomers in CSF faithfully differentiate early MSA not only from controls but also from LB synucleinopathies. |
| Concha-Marambio et al., 2021 [9] | PMCA | CSF | NO | PD SWEDD | 30/30 20/30 | Achieved 96.2% sensitivity 96.7% specificity for PD vs. controls at baseline. Achieved 96.4% sensitivity and 93.8% specificity at 4 years follow-up. |
| Iranzo et al., 2021 [38] | RT-QuIC | CSF | NO | iRBD | 52/40 | Achieved 90% sensitivity and specificity with increased risk of subsequent PD or DLB diagnosis. |
| Bargar et al., 2021 [35] | RT-QuIC | CSF | YES | PD DLB | 88/68 58/68 | Achieved 98% sensitivity and 100/5 specificity. |
| Brockmann et al., 2021 [26] | RT-QuIC | CSF | NO | sporadic PD PD GBA PD LRRK2 PD recessive *3 sporadic DLB DLB GBA NMCs *4 | 107/26 99/26 9/26 20/26 33/26 16/26 14/26 | According to RT-QuIC kinetic characteristics, patients with severe GBA mutations had the highest seeding activity among PD patients and the largest percentage of samples with four out of four positive replicates. Among DLB patients, 79% of those with wildtype DLB exhibited positive α-syn seeding, while 100% of those with GBA mutations did. Decreased CSF levels of proteins were associated with α-syn proteostasis and α-syn seeding activity. |
| Poggiolini et al., 2021 [29] | RT-QuIC | CSF | ΝO *5 | PD MSA iRBD | 74/55 24/55 45/55 | Achieved 64% sensitivity in iRBD 90% sensitivity in 3/4 longitudinal iRBD 9 out of 14 convertors to synucleionopathy with baseline RT-QuIC positive. |
| Compta et al., 2022 [34] | RT-QuIC | CSF | ΝO | PD MSA Taupathies (23 PSP, 12CBD) | 20/19 37/19 | Achieved 9% sensitivity with diagnostiv revision of MSA, 100% specificities against controls, 91% against taupathies. |
| Hall et al., 2022 [32] | RT-QuIC | CSF | NO | PD PDD MSA Controls converted to LBD | 50/47 14/47 15/47 2/47 | Standard LBD vs. no LB pathology: 100% sensitivity and 93% specificity. LB pathology in the cortex vs. cases with no LBs or LBs present only in the olfactory bulb: 97% sensitivity and 93% specificity |
| YES | standard LBD *6 non-standard LBD *7 | 25/53 23/53 | ||||
| Garrido et al., 2022 [27] | RT-QuIC | BH SN | YES | LRRK2-PD LTP+ LTP+ controls | 3/7 *8 7/7 | Achieved 100% accuracy RT-QuIC in SN and CSF LRRK2-PD samples. |
| BH AC | YES | LRRK2-PD LTP+ LTP+ controls | 3/8 7/8 | Achieved 100% sensitivity inSN iPD. | ||
| CSF | YES | LRRK2-PD LTP+ LTP+ controls | 2/6 6/6 | Negative in the SN control brains. | ||
| Siderowf et al., 2023 [14] | RT-QuIC | CSF | NO | PD SWEDD NMCs of GBA NMCs of LRRK2 Prodromal patients | 545/163 54/163 151/163 159/163 51/163 | Achieved 87·7% sensitivity for PD 98·6% sensitivity for PD + typical olfactory deficit 96.3% specificity for healthy controls 98·6%. |
| Concha-Marambio et al., 2023 [25] | PMCA | CSF | NO | PD DLB MSA iRBD vascular PD | 95/64 2/64 2/64 29/64 3/64 | Achieved 98% accuracy in baseline PD-CSF. Results for α-syn-SAA were more in concordance with the final diagnosis than the initial one, since 14 patients were reclassified as not having α-syn aggregation disease. When it came to synucleinopathies, α-syn-SAA and DAT-SPECT had better agreement with the final diagnosis. |
| Author | Tissue Type | Assay Type | Autopsy | LBD (Cases/Controls) | Main Outcomes |
|---|---|---|---|---|---|
| De Luca et al., 2019 [39] | OΜ | RT-QuIC | NO | PD (18/18) MSA (11/18) | High efficiency of α-syn aggregates in MSA and PD olfactory mucosa with different structural/ biochemical features |
| Stefani et al., 2021 [40] | OΜ | RT-QuIC | NO | PD (41/59) iRBD (63/59) | The specificity was high (89.8%), while the sensitivity for isolated RBD with PD was lower than controls (45.2%), with 78.6% of i RBD patients with positive α-syn. RT-QuIC and 21.4% with negative α-syn RT-QuIC (p< 0.001) reported olfactory impairment. When isolated RBD patients tested positive for olfactory mucosa α-syn RT-QuIC, the degree of olfactory impairment was greater than in those who tested negative. |
| Perra et al., 2021 [41] | OΜ | RT-QuIC | NO | probable DLB (32/38) prodromal DLB (5/38) DLB/AD (6/38) | For olfactory mucosa and CSF, the accuracy of real-time quaking-induced conversion assay results and clinical diagnoses was 86.4% and 93.8%, respectively. |
| CSF | RT-QuIC | NO | probable DLB (10/32) DLB/AD (6/32) | ||
| Bargar et al., 2021 [35] | OM | RT-QuIC | NO | PD (13/11) MSA-P (20/11) MSA-C (10/11) | A 96% IAR was achieved between laboratories using the α-syn_RT-QuIC analysis (Kappa = 0.93, 95% CI 0.83–1.00). Specifically, 9/13 patients with PD (sensitivity 69%, IAR 100%) and 18/20 patients with MSA-P (sensitivity 90%, IAR 100%) have α-syn_RT-QuIC seeding activity in their OM. Remarkably, save from one participant in the USA-lab, materials obtained from MSA-C patients did not cause α-syn_RT-QuIC seeding activity. As a result, MSA-P and MSA-C had opposing effects. |
| Manne et al., 2020 [42] | Frozen SKIN | RT-QuIC | YES | PD (25/25) | Frozen skin tissues: 96% sensitivity and 96% specificity. |
| FFPE SKIN | RT-QuIC | YES | PD (12/12) | FFPE SKIN:75% sensitivity and 83% specificity. | |
| Wang et al., 2021 [43] | Abdominal SKIN | RT-QuIC | YES | PD (47/43) LBD (7/43) MSA3/43) | PD: 82% sensitivity and 96% specificity. |
| PMCA | YES | Synucleinopathies *2 (32/8) | Achieved 93% sensitivity and 93% specificity. | ||
| Scalp SKIN | RT-QuIC | YES | PD (20/10) | The sensitivity and specificity of RT-QuIC were 95% and 100% for posterior cervical and | |
| Biopsy SKIN *3 | RT-QuIC | NO | PD (20/21) | leg skin biopsy tissues fromPD and controls without PD, respectively, | |
| PMCA | NO | PD (10/10) | sensitivity 80% and specificity 90% for PMCA. | ||
| Donadio et al., 2021 [44] | SKIN *4 | RT-QuIC | NO | PD (6/18) MSA (5/18) LBD (4/18) PAF (3/18) | When separating synucleinopathies from non-synucleinopathies and controls, both immunofluorescence and RT-QuIC had high sensitivity and specificity; however, immunofluorescence demonstrated a higher diagnostic accuracy. |
| CSF | RT-QuIC | NO | PD (2/13) MSA (2/13) LBD (2/13) PAF (2/13) | ||
| Mammana et al., 2021 [45] | SKIN cervical SKIN thigh CSF SKIN cervical SKIN thigh SKIN leg CSF | RT-QuIC RT-QuIC RT-QuIC RT-QuIC RT-QuIC RT-QuIC RT-QuIC | YES YES YES NO NO NO NO | PD (1/40) iLBD (7/40) DLB (1/40) PD (1/39) iLBD (6/49) DLB (1/39) iLBD (4/30) DLB (1/30) DLB (4/15) PD (4/15) DLB (7/11) PD (4/11) DLB (4/15 PD (5/15) DLB (11/27) PD (7/27) | A 94.1% accuracy (sensitivity, 89.2%; specificity, 96.3%) was achieved in differentiating LBD patients using the skin α-syn real-time quaking-induced conversion assay. In the 17 LBD patients examined in the cervical region, the assay’s sensitivity was 94.1%. The two real-time quaking-induced conversion assay procedures produced comparable diagnostic accuracy (skin, 97.5%; CSF, 98.7%) in patients with both skin and CSF samples. |
| Kuzkina et al., 2021 [46] | SKIN *5 | RT-QuIC | NO | PD (34/30) | High degree of inter-rater agreement between the two laboratories (92.2%) and PD diagnostic accuracy of 88.9%. Patients with more advanced illness stages and longer disease duration had higher α-syn seeding activity in RT-QuIC, which was linked with constipation, cognitive impairment, and REM sleep behavior disorder. |
| Kuzkina et al., 2023 [47] | SKIN *6 | RT-QuIC | NO | iRBD (38/23) PD (39/23) | A total of 92.2% of PD patients (70% of PD biopsies), 13.3% of controls (7.9% of control biopsies), and 97.4% of iRBD patients (78.4% of iRBD biopsies) all showed evidence of α-synuclein aggregation, with iRBD showing higher seeding activity than PD. Comparing immunohistochemistry to RT-QuIC, the latter was less specific but more sensitive. |
| Fenyi et al., 2019 [48] | GI rectum | PMCA | ΝO | PD (4/4) | All of the control samples, with the exception of one patient, did not cause α-syn aggregation in the PMCA reaction. In 10 of the 18 cases, GI biopsies from PD patients (2 from the antrum, 1 from the rectum, and 7 from the sigmoid colon) sparked an α-syn aggregation. The PMCA and immunohistochemistry results agreed well since, with the exception of two instances, all PD patients who tested positive for PMCA also tested positive for PASH. |
| GI sigmoid | PMCA | NO | PD (12/7) | ||
| GI antrum | PMCA | NO | PD2/- | ||
| Manne et al., 2019 [20] | SMG | RT-QuIC | YES | PD (13/16) iLBD (3/16) | When compared to control tissues, the enhanced levels of pathogenic α-syn seeding activity in PD and incidental LBD tissues exhibited 100% agreement. The submandibular gland exhibited a broad dynamic range of pathogenic α-syn seeding activity, according to end-point dilution kinetic studies. |
| Chahine et al., 2023 [10] | SMG | RT-QuIC | NO | PD (41/14) | Achievied 73.2% sensitivity and 78.6% specificity. |
| CSF | PMCA | NO | PD (54/21) | Achievied 92.6% sensitivity and 90.5%specificity. | |
| Luan et al., 2022 [49] | SALIVA | RT-QuIC | NO | PD (75/36) MSA (18/36) | PD: 76.0% sensitivity and 94.4% specificity MSA: 61.1% sensitivity. Patients with PD and MSA did not exhibit any significant changes in the diameter of salivary α-syn fibrils analyzed by electron microscopy or in the thioflavin T fluorescence intensity of salivary α-syn fibrils detected by RT-QuIC test. Notably, patients with PD had a notably shorter lag phase in the RT-QuIC assay than patients with MSA. This finding may have practical applications in differentiating between PD and MSA. |
| Okuzumi et al., 2023 [50] | SERUM | IP/RT-QuIC | NO | PD (221/128) MSA (39/128) DLB (10/128 RBD (9/128) Parkin-PD (17/128) | When comparing PD and MSA to controls, IP/RT-QuIC demonstrated excellent diagnostic performance, 96%. In a blinded external cohort, IP/RT-QuIC also shown strong diagnostic efficacy in separating patients with PD (86%) and MSA (80%) from controls. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bougea, A. Seeding Aggregation Assays in Lewy Bodies Disorders: A Narrative State-of-the-Art Review. Int. J. Mol. Sci. 2024, 25, 10783. https://doi.org/10.3390/ijms251910783
Bougea A. Seeding Aggregation Assays in Lewy Bodies Disorders: A Narrative State-of-the-Art Review. International Journal of Molecular Sciences. 2024; 25(19):10783. https://doi.org/10.3390/ijms251910783
Chicago/Turabian StyleBougea, Anastasia. 2024. "Seeding Aggregation Assays in Lewy Bodies Disorders: A Narrative State-of-the-Art Review" International Journal of Molecular Sciences 25, no. 19: 10783. https://doi.org/10.3390/ijms251910783
APA StyleBougea, A. (2024). Seeding Aggregation Assays in Lewy Bodies Disorders: A Narrative State-of-the-Art Review. International Journal of Molecular Sciences, 25(19), 10783. https://doi.org/10.3390/ijms251910783






