Unveiling the Role of Schwann Cell Plasticity in the Pathogenesis of Diabetic Peripheral Neuropathy
Abstract
1. Introduction
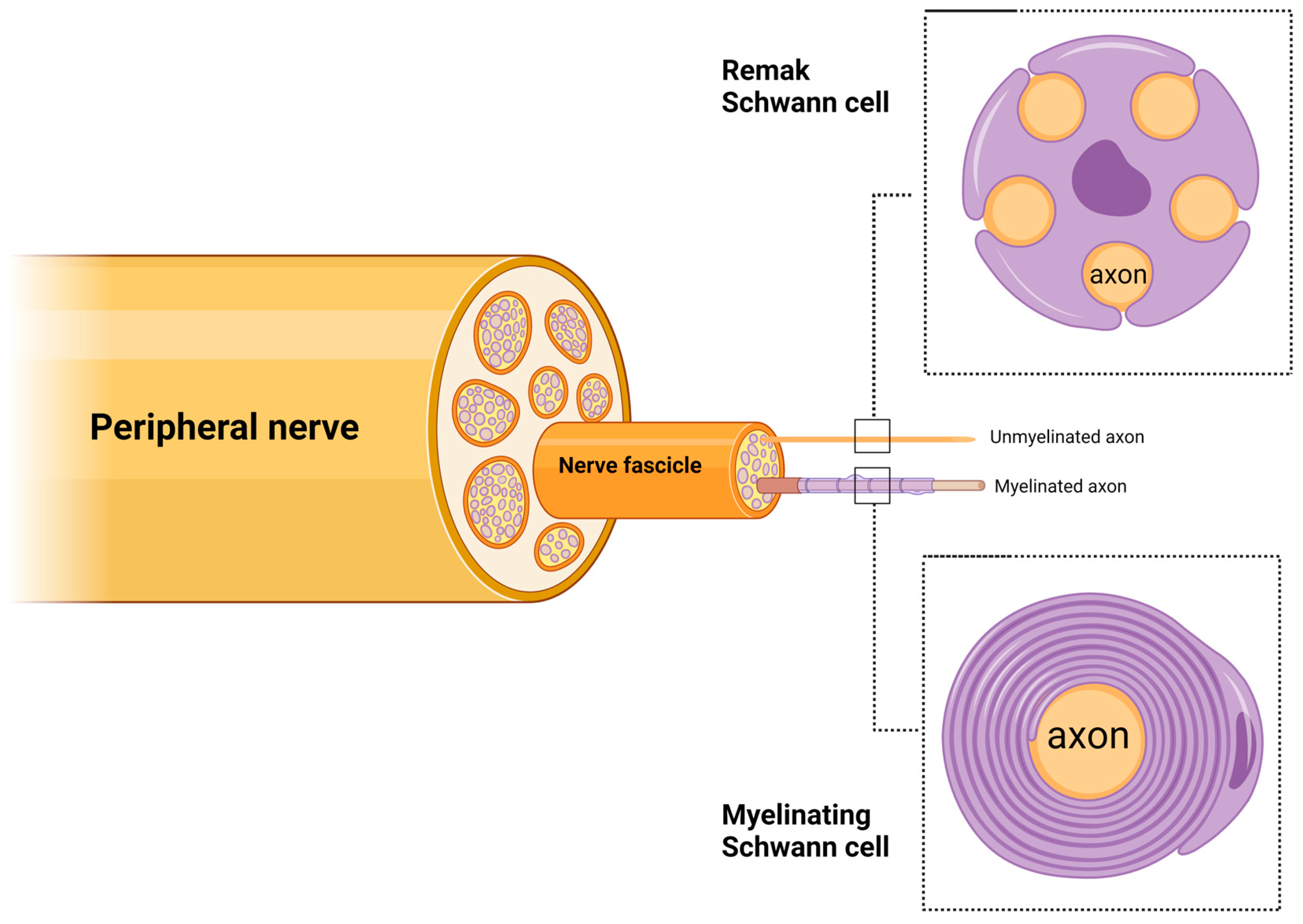
2. Schwann Cell Roles in Peripheral Nerves
3. Schwann Cell Plasticity during Tissue Regeneration
4. Pathological Changes in Schwann Cell under Diabetic Conditions
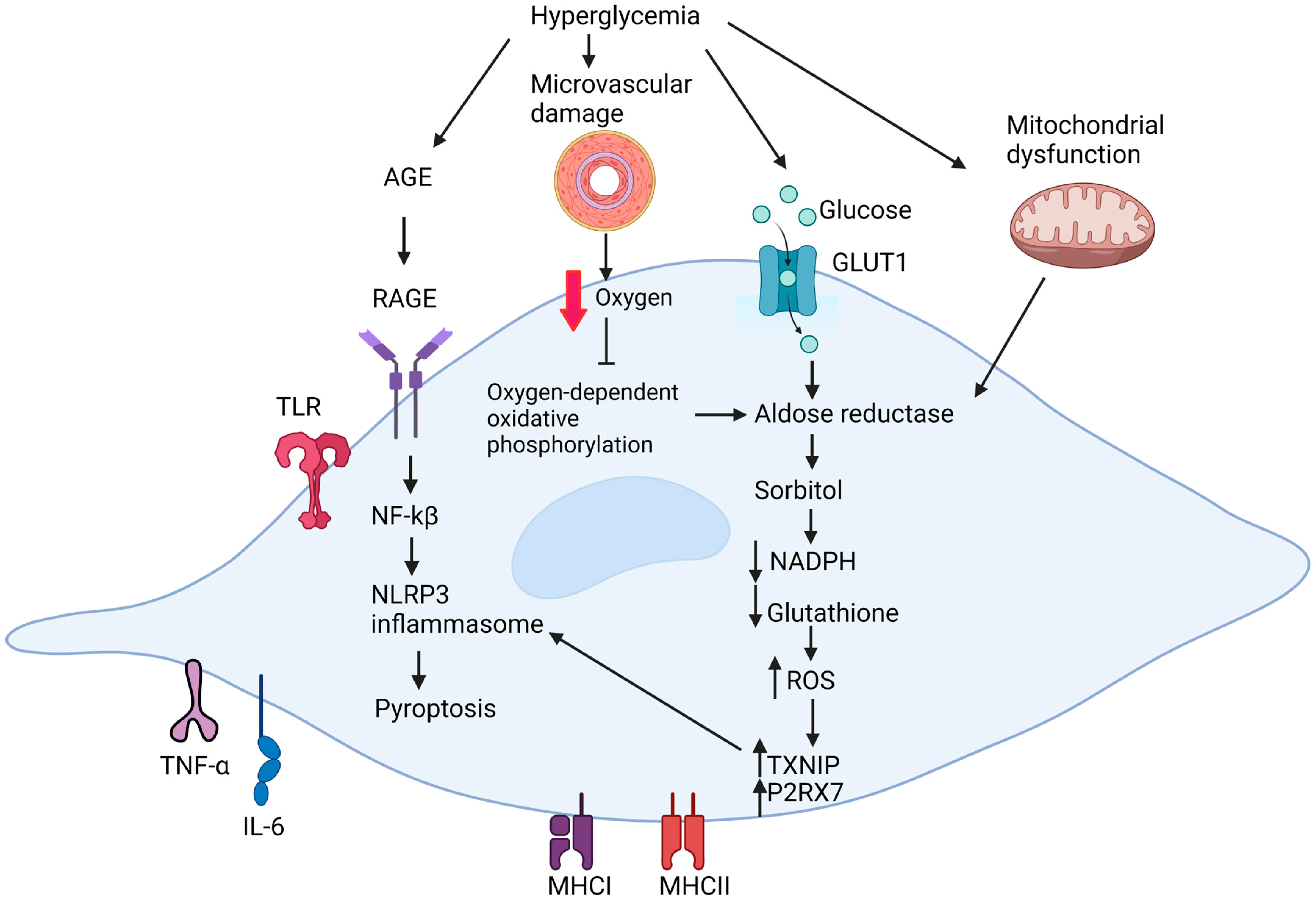
4.1. Metabolic Dysfunction in Diabetic Schwann Cells
4.2. Effect of Microvascular Damage on Schwann Cells
4.3. Inflammatory Dyregulation in Diabetic Schwann Cells
5. Schwann Cell Dysfunctions Compromise the Functional Integrity of Peripheral Nerves in Diabetic Neuropathy
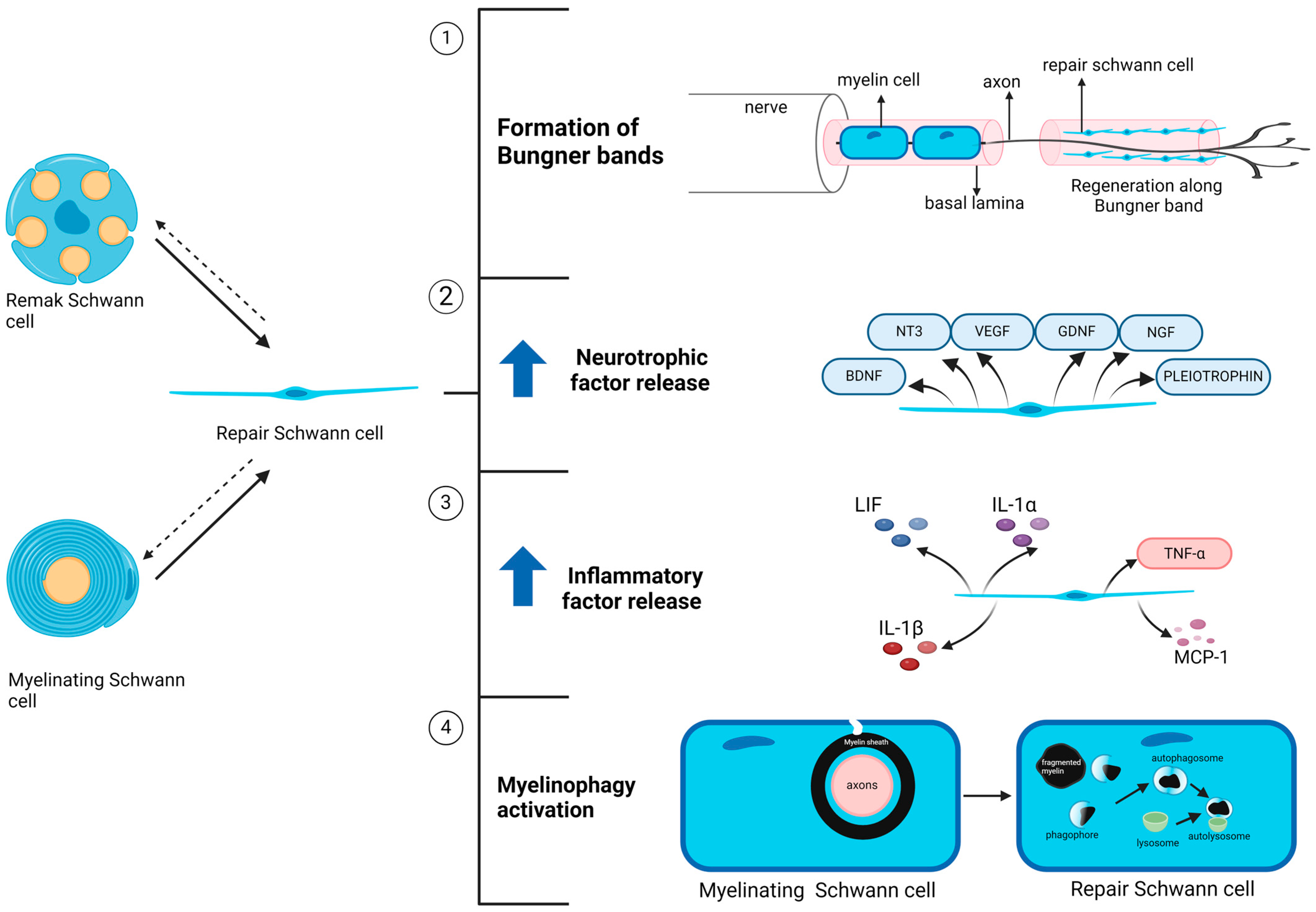
6. Dysfunctional Schwann Cell Plasticity in Diabetic Neuropathy: Diabetes-Induced Schwann Cell Reprogramming into Repair Phenotypes
6.1. Phenotypic Alteration in Diabetic Schwann Cells Resembles the Nerve Injury Response
6.2. Diabetes Elevates the Activity of Repair Regulatory Pathways in Schwann Cells
7. Failure of Repair Schwann Cells to Rescue Tissue Damage in Diabetic Neuropathy
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abbott, C.A.; Malik, R.A.; Van Ross, E.R.E.; Kulkarni, J.; Boulton, A.J.M. Prevalence and characteristics of painful diabetic neuropathy in a large community-based diabetic population in the U.K. Diabetes Care 2011, 34, 2220–2224. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Xing, P.; Cai, X.; Luo, D.; Li, R.; Lloyd, C.; Sartorius, N.; Li, M. Prevalence and Risk Factors for Diabetic Peripheral Neuropathy in Type 2 Diabetic Patients From 14 Countries: Estimates of the INTERPRET-DD Study. Front. Public Health 2020, 8, 534372. [Google Scholar] [CrossRef] [PubMed]
- Truini, A.; Biasiotta, A.; Di Stefano, G.; La Cesa, S.; Leone, C.; Cartoni, C.; Leonetti, F.; Casato, M.; Pergolini, M.; Petrucci, M.T.; et al. Peripheral nociceptor sensitization mediates allodynia in patients with distal symmetric polyneuropathy. J. Neurol. 2013, 260, 761–766. [Google Scholar] [CrossRef]
- Feldman, E.L.; Nave, K.-A.; Jensen, T.S.; Bennett, D.L.H. New Horizons in Diabetic Neuropathy: Mechanisms, Bioenergetics, and Pain. Neuron 2017, 93, 1296–1313. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, B.C.; Cheng, H.T.; Stables, C.L.; Smith, A.L.; Feldman, E.L. Diabetic neuropathy: Clinical manifestations and current treatments. Lancet Neurol. 2012, 11, 521–534. [Google Scholar] [CrossRef]
- Iqbal, Z.; Azmi, S.; Yadav, R.; Ferdousi, M.; Kumar, M.; Cuthbertson, D.J.; Lim, J.; Malik, R.A.; Alam, U. Diabetic Peripheral Neuropathy: Epidemiology, Diagnosis, and Pharmacotherapy. Clin. Ther. 2018, 40, 828–849. [Google Scholar] [CrossRef]
- Schreiber, A.K.; Nones, C.F.; Reis, R.C.; Chichorro, J.G.; Cunha, J.M. Diabetic neuropathic pain: Physiopathology and treatment. World J. Diabetes 2015, 6, 432–444. [Google Scholar] [CrossRef]
- Verrotti, A.; Prezioso, G.; Scattoni, R.; Chiarelli, F. Autonomic neuropathy in diabetes mellitus. Front. Endocrinol. 2014, 5, 205. [Google Scholar] [CrossRef]
- Zenker, J.; Ziegler, D.; Chrast, R. Novel pathogenic pathways in diabetic neuropathy. Trends Neurosci. 2013, 36, 439–449. [Google Scholar] [CrossRef]
- Sima, A.A.F.; Zhang, W. Mechanisms of diabetic neuropathy: Axon dysfunction. Handb. Clin. Neurol. 2014, 126, 429–442. [Google Scholar]
- Kobayashi, M.; Zochodne, D.W. Diabetic neuropathy and the sensory neuron: New aspects of pathogenesis and their treatment implications. J. Diabetes Investig. 2018, 9, 1239–1254. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wang, X.J.; Luo, X.; Zhang, J.; Zhao, X.; Chen, Q. Diabetic peripheral neuropathy based on Schwann cell injury: Mechanisms of cell death regulation and therapeutic perspectives. Front. Endocrinol. 2024, 15, 1427679. [Google Scholar] [CrossRef]
- Nocera, G.; Jacob, C. Mechanisms of Schwann cell plasticity involved in peripheral nerve repair after injury. Cell. Mol. Life Sci. 2020, 77, 3977–3989. [Google Scholar] [CrossRef]
- Jessen, K.R.; Arthur-Farraj, P. Repair Schwann cell update: Adaptive reprogramming, EMT, and stemness in regenerating nerves. Glia 2019, 67, 421–437. [Google Scholar] [CrossRef] [PubMed]
- David, S.; Aguayo, A.J. Axonal elongation into peripheral nervous system “bridges” after central nervous system injury in adult rats. Science 1981, 214, 931–933. [Google Scholar] [CrossRef]
- Richardson, P.M.; McGuinness, U.M.; Aguayo, A.J. Axons from CNS neurones regenerate into PNS grafts. Nature 1980, 284, 264–265. [Google Scholar] [CrossRef] [PubMed]
- Jessen, K.R.; Mirsky, R. The Success and Failure of the Schwann Cell Response to Nerve Injury. Front. Cell. Neurosci. 2019, 13, 33. [Google Scholar] [CrossRef]
- Menorca, R.M.G.; Fussell, T.S.; Elfar, J.C. Peripheral Nerve Trauma: Mechanisms of Injury and Recovery. Hand Clin. 2013, 29, 317. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Brennan, A.; Liu, N.; Yarden, Y.; Lefkowitz, G.; Mirsky, R.; Jessen, K.R. Neu differentiation factor is a neuron-glia signal and regulates survival, proliferation, and maturation of rat schwann cell precursors. Neuron 1995, 15, 585–596. [Google Scholar] [CrossRef]
- Jessen, K.R.; Brennan, A.; Morgan, L.; Mirsky, R.; Kent, A.; Hashimoto, Y.; Gavrilovic, J. The Schwann cell precursor and its fate: A study of cell death and differentiation during gliogenesis in rat embryonic nerves. Neuron 1994, 12, 509–527. [Google Scholar] [CrossRef]
- Feltri, M.L.; Poitelon, Y.; Previtali, S.C. How Schwann Cells Sort Axons: New concepts. Neuroscientist 2016, 22, 252–265. [Google Scholar] [CrossRef] [PubMed]
- Poliak, S.; Peles, E. The local differentiation of myelinated axons at nodes of ranvier. Nat. Rev. Neurosci. 2003, 4, 968–980. [Google Scholar] [CrossRef] [PubMed]
- Simons, M.; Trotter, J. Wrapping it up: The cell biology of myelination. Curr. Opin. Neurobiol. 2007, 17, 533–540. [Google Scholar] [CrossRef]
- Morell, P.; Quarles, R.H. The Myelin Sheath. In Basic Neurochemistry: Molecular, Cellular and Medical Aspects, 6th ed.; Lippincott-Raven: New York, NY, USA, 1999. [Google Scholar]
- Jessen, K.R.; Mirsky, R. The origin and development of glial cells in peripheral nerves. Nat. Rev. Neurosci. 2005, 6, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Harty, B.L.; Monk, K.R. Unwrapping the unappreciated: Recent progress in Remak Schwann cell biology. Curr. Opin. Neurobiol. 2017, 47, 131–137. [Google Scholar] [CrossRef]
- Oliveira, J.T.; Yanick, C.; Wein, N.; Gomez Limia, C.E. Neuron-Schwann cell interactions in peripheral nervous system homeostasis, disease, and preclinical treatment. Front. Cell. Neurosci. 2023, 17, 1248922. [Google Scholar] [CrossRef]
- Keswani, S.C.; Buldanlioglu, U.; Fischer, A.; Reed, N.; Polley, M.; Liang, H.; Zhou, C.; Jack, C.; Leitz, G.J.; Hoke, A. A novel endogenous erythropoietin mediated pathway prevents axonal degeneration. Ann. Neurol. 2004, 56, 815–826. [Google Scholar] [CrossRef]
- Ren, J.; Zhu, B.; Gu, G.; Zhang, W.; Li, J.; Wang, H.; Wang, M.; Song, X.; Wei, Z.; Feng, S. Schwann cell-derived exosomes containing MFG-E8 modify macrophage/microglial polarization for attenuating inflammation via the SOCS3/STAT3 pathway after spinal cord injury. Cell Death Dis. 2023, 14, 1–17. [Google Scholar] [CrossRef]
- Hyung, S.; Kim, J.Y.; Yu, C.J.; Jung, H.S.; Hong, J.W. Neuroprotective Effect of Glial Cell-Derived Exosomes on Neurons. Immunotherapy 2019, 5, 156. [Google Scholar] [CrossRef]
- Court, F.A.; Hendriks, W.T.J.; MacGillavry, H.D.; Alvarez, J.; Van Minnen, J. Schwann Cell to Axon Transfer of Ribosomes: Toward a Novel Understanding of the Role of Glia in the Nervous System. J. Neurosci. 2008, 28, 11024–11029. [Google Scholar] [CrossRef]
- Mietto, B.S.; Jhelum, P.; Schulz, K.; David, S. Schwann Cells Provide Iron to Axonal Mitochondria and Its Role in Nerve Regeneration. J. Neurosci. 2021, 41, 7300–7313. [Google Scholar] [CrossRef]
- Deck, M.; Van Hameren, G.; Campbell, G.; Bernard-Marissal, N.; Devaux, J.; Berthelot, J.; Lattard, A.; Médard, J.J.; Gautier, B.; Guelfi, S.; et al. Physiology of PNS axons relies on glycolytic metabolism in myelinating Schwann cells. PLoS ONE 2022, 17, e0272097. [Google Scholar] [CrossRef] [PubMed]
- Viader, A.; Golden, J.P.; Baloh, R.H.; Schmidt, R.E.; Hunter, D.A.; Milbrandt, J. Schwann cell mitochondrial metabolism supports long-term axonal survival and peripheral nerve function. J. Neurosci. 2011, 31, 10128–10140. [Google Scholar] [CrossRef]
- Shen, Y.A.A.; Chen, Y.; Dao, D.Q.; Mayoral, S.R.; Wu, L.; Meijer, D.; Ullian, E.M.; Chan, J.R.; Lu, Q.R. Phosphorylation of LKB1/Par-4 establishes Schwann cell polarity to initiate and control myelin extent. Nat. Commun. 2014, 5, 4991. [Google Scholar] [CrossRef] [PubMed]
- Beirowski, B.; Babetto, E.; Golden, J.P.; Chen, Y.J.; Yang, K.; Gross, R.W.; Patti, G.J.; Milbrandt, J. Metabolic regulator LKB1 is crucial for Schwann cell-mediated axon maintenance. Nat. Neurosci. 2014, 17, 1351–1361. [Google Scholar] [CrossRef] [PubMed]
- Pooya, S.; Liu, X.; Kumar, V.B.S.; Anderson, J.; Imai, F.; Zhang, W.; Ciraolo, G.; Ratner, N.; Setchell, K.D.; Yoshida, Y.; et al. The tumour suppressor LKB1 regulates myelination through mitochondrial metabolism. Nat. Commun. 2014, 5, 4993. [Google Scholar] [CrossRef] [PubMed]
- Rigby, M.J.; Gomez, T.M.; Puglielli, L. Glial Cell-Axonal Growth Cone Interactions in Neurodevelopment and Regeneration. Front. Neurosci. 2020, 14, 203. [Google Scholar] [CrossRef] [PubMed]
- Stierli, S.; Napoli, I.; White, I.J.; Cattin, A.L.; Cabrejos, A.M.; Calavia, N.G.; Malong, L.; Ribeiro, S.; Nihouarn, J.; Williams, R.; et al. The regulation of the homeostasis and regeneration of peripheral nerve is distinct from the CNS and independent of a stem cell population. Development 2018, 145, dev170316. [Google Scholar] [CrossRef]
- Chen, Z.L.; Yu, W.M.; Strickland, S. Peripheral regeneration. Annu. Rev. Neurosci. 2007, 30, 209–233. [Google Scholar] [CrossRef]
- Stoll, G.; Müller, H.W. Nerve injury, axonal degeneration and neural regeneration: Basic insights. Brain Pathol. 1999, 9, 313–325. [Google Scholar] [CrossRef]
- Gomez-Sanchez, J.A.; Pilch, K.S.; Van Der Lans, M.; Fazal, S.V.; Benito, C.; Wagstaff, L.J.; Mirsky, R.; Jessen, K.R. Development/Plasticity/Repair After Nerve Injury, Lineage Tracing Shows That Myelin and Remak Schwann Cells Elongate Extensively and Branch to Form Repair Schwann Cells, Which Shorten Radically on Remyelination. J. Neurosci. 2017, 37, 9086–9099. [Google Scholar] [CrossRef]
- Scheib, J.; Höke, A. Advances in peripheral nerve regeneration. Nat. Rev. Neurol. 2013, 9, 668–676. [Google Scholar] [CrossRef]
- Grothe, C.; Haastert, K.; Jungnickel, J. Physiological function and putative therapeutic impact of the FGF-2 system in peripheral nerve regeneration-Lessons from in vivo studies in mice and rats. Brain Res. Rev. 2006, 51, 293–299. [Google Scholar] [CrossRef]
- Brushart, T.M.; Aspalter, M.; Griffin, J.W.; Redett, R.; Hameed, H.; Zhou, C.; Wright, M.; Vyas, A.; Höke, A. Schwann cell phenotype is regulated by axon modality and central-peripheral location, and persists in vitro. Exp. Neurol. 2013, 247, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Wood, M.D.; Mackinnon, S.E. Pathways regulating modality-specific axonal regeneration in peripheral nerve. Exp. Neurol. 2015, 265, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Fontana, X.; Hristova, M.; Da Costa, C.; Patodia, S.; Thei, L.; Makwana, M.; Spencer-Dene, B.; Latouche, M.; Mirsky, R.; Jessen, K.R.; et al. C-Jun in Schwann cells promotes axonal regeneration and motoneuron survival via paracrine signaling. J. Cell Biol. 2012, 198, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Rotshenker, S. Wallerian degeneration: The innate-immune response to traumatic nerve injury. J. Neuroinflamm. 2011, 8, 109. [Google Scholar] [CrossRef]
- Boivin, A.; Pineau, I.; Barrette, B.; Filali, M.; Vallières, N.; Rivest, S.; Lacroix, S. Toll-like receptor signaling is critical for Wallerian degeneration and functional recovery after peripheral nerve injury. J. Neurosci. 2007, 27, 12565–12576. [Google Scholar] [CrossRef]
- Perrin, F.E.; Lacroix, S.; Avilés-Trieueros, M.; David, S. Involvement of monocyte chemoattractant protein-1, macrophage inflammatory protein-1α and interleukin-1β Wallerian degeneration. Brain 2005, 128, 854–866. [Google Scholar] [CrossRef] [PubMed]
- Subang, M.C.; Richardson, P.M. Influence of injury and cytokines on synthesis of monocyte chemoattractant protein-1 MRNA in peripheral nervous tissue. Eur. J. Neurosci. 2001, 13, 521–528. [Google Scholar] [CrossRef]
- Brück, W.; Huitinga, I.; Dykstra, C.D. Liposome-mediated monocyte depletion during Wallerian degeneration defines the role of hematogenous phagocytes in myelin removal. J. Neurosci. Res. 1996, 46, 477–484. [Google Scholar] [CrossRef]
- Carroll, S.L.; Worley, S.H. Wallerian degeneration. In The Curated Reference Collection in Neuroscience and Biobehavioral Psychology; Elsevier Science Ltd.: Amsterdam, The Netherlands, 2016; pp. 485–491. [Google Scholar]
- David, S.; Lacroix, S. Molecular approaches to spinal cord repair. Annu. Rev. Neurosci. 2003, 26, 411–440. [Google Scholar] [CrossRef]
- George, E.B.; Glass, J.D.; Griffin, J.W. Axotomy-induced axonal degeneration is mediated by calcium influx through ion-specific channels. J. Neurosci. 1995, 15, 6445–6452. [Google Scholar] [CrossRef]
- Beuche, W.; Friede, R.L. The role of non-resident cells in Wallerian degeneration. J. Neurocytol. 1984, 13, 767–796. [Google Scholar] [CrossRef]
- Gomez-Sanchez, J.A.; Carty, L.; Iruarrizaga-Lejarreta, M.; Palomo-Irigoyen, M.; Varela-Rey, M.; Griffith, M.; Hantke, J.; Macias-Camara, N.; Azkargorta, M.; Aurrekoetxea, I.; et al. Schwann cell autophagy, myelinophagy, initiates myelin clearance from injured nerves. J. Cell Biol. 2015, 210, 153–168. [Google Scholar] [PubMed]
- Jessen, K.R.; Mirsky, R.; Lloyd, A.C. Schwann cells: Development and role in nerve repair. Cold Spring Harb. Perspect. Biol. 2015, 7, 1–15. [Google Scholar] [CrossRef]
- Kuhlmann, T.; Bitsch, A.; Stadelmann, C.; Siebert, H.; Brück, W. Macrophages are eliminated from the injured peripheral nerve via local apoptosis and circulation to regional lymph nodes and the spleen. J. Neurosci. 2001, 21, 3401–3408. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, N.P.; Vægter, C.B.; Andersen, H.; Østergaard, L.; Calcutt, N.A.; Jensen, T.S. Schwann cell interactions with axons and microvessels in diabetic neuropathy. Nat. Rev. Neurol. 2017, 13, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Matough, F.A.; Budin, S.B.; Hamid, Z.A.; Alwahaibi, N.; Mohamed, J. The role of oxidative stress and antioxidants in diabetic complications. Sultan Qaboos Univ. Med. J. 2012, 12, 556–569. [Google Scholar] [CrossRef]
- Yabe-Nishimura, C. Aldose reductase in glucose toxicity: A potential target for the prevention of diabetic complications. Pharmacol. Rev. 1998, 50, 21–34. [Google Scholar]
- El-Kabbani, O.; Ruiz, F.; Darmanin, C.; Chung, R.P.T. Aldose reductase structures: Implications for mechanism and inhibition. Cell. Mol. Life Sci. 2004, 61, 750–762. [Google Scholar] [CrossRef]
- Jiang, Y.; Calcutt, N.A.; Rames, K.M.; Mizisin, A.P. Novel sites of aldose reductase immunolocalization in normal and streptozotocin-diabetic rats. J. Peripher. Nerv. Syst. 2006, 11, 274–285. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Asp. Med. 2009, 30, 1–12. [Google Scholar] [CrossRef]
- Ramana, K.V. Aldose reductase: New insights for an old enzyme. Biomol. Concepts 2011, 2, 103–114. [Google Scholar] [CrossRef]
- Obrosova, I.G.; Fathallah, L.; Lang, H.J. Interaction between osmotic and oxidative stress in diabetic precataractous lens: Studies with a sorbitol dehydrogenase inhibitor. Biochem. Pharmacol. 1999, 58, 1945–1954. [Google Scholar] [CrossRef]
- Stevens, M.J.; Lattimer, S.A.; Kamijo, M.; Van Huysen, C.; Sima, A.A.F.; Greene, D.A. Osmotically-induced nerve taurine depletion and the compatible osmolyte hypothesis in experimental diabetic neuropathy in the rat. Diabetologia 1993, 36, 608–614. [Google Scholar] [CrossRef]
- Askwith, T.; Zeng, W.; Eggo, M.C.; Stevens, M.J. Taurine reduces nitrosative stress and nitric oxide synthase expression in high glucose-exposed human Schwann cells. Exp. Neurol. 2012, 233, 154–162. [Google Scholar] [CrossRef]
- Askwith, T.; Zeng, W.; Eggo, M.C.; Stevens, M.J. Oxidative stress and dysregulation of the taurine transporter in high-glucose-exposed human Schwann cells: Implications for pathogenesis of diabetic neuropathy. Am. J. Physiol. Endocrinol. Metab. 2009, 297, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, J.; Bains, Y.; Guha, S.; Kahn, A.; Hall, D.; Bose, N.; Gugliucci, A.; Kapahi, P. The Role of Advanced Glycation End Products in Aging and Metabolic Diseases: Bridging Association and Causality. Cell Metab. 2018, 28, 337–352. [Google Scholar] [CrossRef]
- Vincent, A.M.; Perrone, L.; Sullivan, K.A.; Backus, C.; Sastry, A.M.; Lastoskie, C.; Feldman, E.L. Receptor for advanced glycation end products activation injures primary sensory neurons via oxidative stress. Endocrinology 2007, 148, 548–558. [Google Scholar] [CrossRef]
- Haslbeck, K.M.; Neundörfer, B.; Schlötzer-Schrehardt, U.; Bierhaus, A.; Schleicher, E.; Pauli, E.; Haslbeck, M.; Hecht, M.; Nawroth, P.; Heuss, D. Activation of the RAGE pathway: A general mechanism in the pathogenesis of polyneuropathies? Neurol. Res. 2007, 29, 103–110. [Google Scholar] [CrossRef]
- Sekido, H.; Suzuki, T.; Jomori, T.; Takeuchi, M.; Yabe-Nishimura, C.; Yagihashi, S. Reduced cell replication and induction of apoptosis by advanced glycation end products in rat Schwann cells. Biochem. Biophys. Res. Commun. 2004, 320, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Freeman, O.J.; Unwin, R.D.; Dowsey, A.W.; Begley, P.; Ali, S.; Hollywood, K.A.; Rustogi, N.; Petersen, R.S.; Dunn, W.B.; Cooper, G.J.; et al. Metabolic dysfunction is restricted to the sciatic nerve in experimental diabetic neuropathy. Diabetes 2016, 65, 228–238. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, C.; Vasquez, F.E.; Galeva, N.; Onyango, I.; Swerdlow, R.H.; Dobrowsky, R.T. Hyperglycemia alters the Schwann cell mitochondrial proteome and decreases coupled respiration in the absence of superoxide production. J. Proteome Res. 2010, 9, 458–471. [Google Scholar] [CrossRef][Green Version]
- Yako, H.; Niimi, N.; Kato, A.; Takaku, S.; Tatsumi, Y.; Nishito, Y.; Kato, K.; Sango, K. Role of pyruvate in maintaining cell viability and energy production under high-glucose conditions. Sci. Rep. 2021, 11, 18910. [Google Scholar] [CrossRef]
- Mizukami, H.; Osonoi, S. Collateral Glucose-Utlizing Pathwaya in Diabetic Polyneuropathy. Int. J. Mol. Sci. 2020, 22, 94. [Google Scholar] [CrossRef]
- Thrainsdottir, S.; Malik, R.A.; Dahlin, L.B.; Wiksell, P.; Eriksson, K.F.; Rosén, I.; Petersson, J.; Greene, D.A.; Sundkvist, G. Endoneurial capillary abnormalities presage deterioration of glucose tolerance and accompany peripheral neuropathy in man. Diabetes 2003, 52, 2615–2622. [Google Scholar] [CrossRef] [PubMed]
- Giannini, C.; Dyck, P.J. Basement membrane reduplication and pericyte degeneration precede development of diabetic polyneuropathy and are associated with its severity. Ann. Neurol. 1995, 37, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Østergaard, L.; Finnerup, N.B.; Terkelsen, A.J.; Olesen, R.A.; Drasbek, K.R.; Knudsen, L.; Jespersen, S.N.; Frystyk, J.; Charles, M.; Thomsen, R.W.; et al. The effects of capillary dysfunction on oxygen and glucose extraction in diabetic neuropathy. Diabetologia 2015, 58, 666–677. [Google Scholar] [CrossRef]
- Jespersen, S.N.; Østergaard, L. The roles of cerebral blood flow, capillary transit time heterogeneity, and oxygen tension in brain oxygenation and metabolism. J. Cereb. Blood Flow Metab. 2012, 32, 264–277. [Google Scholar] [CrossRef] [PubMed]
- Tilton, R.G.; Chang, K.; Nyengaard, J.R.; Enden, M.V.D.; Ido, Y.; Williamson, J.R. Inhibition of Sorbitol Dehydrogenase: Effects on Vascular and Neural Dysfunction in Streptozocin-Induced Diabetic Rats. Diabetes 1995, 44, 234–242. [Google Scholar] [CrossRef]
- Theriault, M.; Dort, J.; Sutherland, G.; Zochodne, D.W. Local human sural nerve blood flow in diabetic and other polyneuropathies. Brain 1997, 120, 1131–1138. [Google Scholar] [CrossRef]
- Hur, J.; Sullivan, K.A.; Pande, M.; Hong, Y.; Sima, A.A.F.; Jagadish, H.V.; Kretzler, M.; Feldman, E.L. The identification of gene expression profiles associated with progression of human diabetic neuropathy. Brain 2011, 134, 3222. [Google Scholar] [CrossRef]
- Herder, C.; Lankisch, M.; Ziegler, D.; Rathmann, W.; Koenig, W.; Illig, T.; Döring, A.; Thorand, B.; Holle, R.; Giani, G.; et al. Subclinical inflammation and diabetic polyneuropathy: MONICA/KORA Survey F3 (Augsburg, Germany). Diabetes Care 2009, 32, 680–682. [Google Scholar] [CrossRef]
- Hussain, G.; Rizvi, S.A.A.; Singhal, S.; Zubair, M.; Ahmad, J. Serum levels of TNF-α in peripheral neuropathy patients and its correlation with nerve conduction velocity in type 2 diabetes mellitus. Diabetes Metab. Syndr. 2013, 7, 238–242. [Google Scholar] [CrossRef]
- Mu, Z.P.; Wang, Y.G.; Li, C.Q.; Lv, W.S.; Wang, B.; Jing, Z.H.; Song, X.J.; Lun, Y.; Qiu, M.Y.; Ma, X.L. Association Between Tumor Necrosis Factor-α and Diabetic Peripheral Neuropathy in Patients with Type 2 Diabetes: A Meta-Analysis. Mol. Neurobiol. 2017, 54, 983–996. [Google Scholar] [CrossRef]
- Hussain, G.; Rizvi, S.A.A.; Singhal, S.; Zubair, M.; Ahmad, J. Serum levels of TGF-β1 in patients of diabetic peripheral neuropathy and its correlation with nerve conduction velocity in type 2 diabetes mellitus. Diabetes Metab. Syndr. Clin. Res. Rev. 2016, 10, S135–S139. [Google Scholar] [CrossRef] [PubMed]
- Conti, G.; Scarpini, E.; Baron, P.; Livraghi, S.; Tiriticco, M.; Bianchi, R.; Vedeler, C.; Scarlato, G. Macrophage infiltration and death in the nerve during the early phases of experimental diabetic neuropathy: A process concomitant with endoneurial induction of IL-1β and p75NTR. J. Neurol. Sci. 2002, 195, 35–40. [Google Scholar] [CrossRef]
- Newton, V.L.; Guck, J.D.; Cotter, M.A.; Cameron, N.E.; Gardiner, N.J. Neutrophils Infiltrate the Spinal Cord Parenchyma of Rats with Experimental Diabetic Neuropathy. J. Diabetes Res. 2017, 2017, 4729284. [Google Scholar] [CrossRef]
- Martini, R.; Fischer, S.; López-Vales, R.; David, S. Interactions between Schwann cells and macrophages in injury and inherited demyelinating disease. Glia 2008, 56, 1566–1577. [Google Scholar] [CrossRef]
- Shamash, S.; Reichert, F.; Rotshenker, S. The Cytokine Network of Wallerian Degeneration: Tumor Necrosis Factor-α, Interleukin-1α, and Interleukin-1β. J. Neurosci. 2002, 22, 3052–3060. [Google Scholar] [CrossRef]
- Lee, H.; Jo, E.K.; Choi, S.Y.; Oh, S.B.; Park, K.; Kim, J.S.; Lee, S.J. Necrotic neuronal cells induce inflammatory Schwann cell activation via TLR2 and TLR3: Implication in Wallerian degeneration. Biochem. Biophys. Res. Commun. 2006, 350, 742–747. [Google Scholar] [CrossRef] [PubMed]
- Ydens, E.; Lornet, G.; Smits, V.; Goethals, S.; Timmerman, V.; Janssens, S. The neuroinflammatory role of Schwann cells in disease. Neurobiol. Dis. 2013, 55, 95–103. [Google Scholar] [CrossRef]
- Goethals, S.; Ydens, E.; Timmerman, V.; Janssens, S. Toll-like receptor expression in the peripheral nerve. Glia 2010, 58, 1701–1709. [Google Scholar] [CrossRef] [PubMed]
- Ydens, E.; Demon, D.; Lornet, G.; De Winter, V.; Timmerman, V.; Lamkanfi, M.; Janssens, S. Nlrp6 promotes recovery after peripheral nerve injury independently of inflammasomes. J. Neuroinflamm. 2015, 12, 143. [Google Scholar] [CrossRef]
- Sousa, M.M.; Du Yan, S.; Fernandas, R.; Guimarães, A.; Stern, D.; Saraiva, M.J. Familial amyloid polyneuropathy: Receptor for advanced glycation end products-dependent triggering of neuronal inflammatory and apoptotic pathways. J. Neurosci. 2001, 21, 7576–7586. [Google Scholar] [CrossRef] [PubMed]
- Baetas-Da-Cruz, W.; Alves, L.; Pessolani, M.C.V.; Barbosa, H.S.; Régnier-Vigouroux, A.; Corte-Real, S.; Cavalcante, L.A. Schwann cells express the macrophage mannose receptor MHC class, I.I. Do they have a role in antigen presentation? J. Peripher. Nerv. Syst. 2009, 14, 84–92. [Google Scholar] [CrossRef]
- Weis, W.I.; Taylor, M.E.; Drickamer, K. The C-type lectin superfamily in the immune system. Immunol. Rev. 1998, 163, 19–34. [Google Scholar] [CrossRef]
- Campana, W.M.; Li, X.; Dragojlovic, N.; Janes, J.; Gaultier, A.; Gonias, S.L. The low-density lipoprotein receptor-related protein is a pro-survival receptor in Schwann cells: Possible implications in peripheral nerve injury. J. Neurosci. 2006, 26, 11197–11207. [Google Scholar] [CrossRef]
- Hartlehnert, M.; Derksen, A.; Hagenacker, T.; Kindermann, D.; Schäfers, M.; Pawlak, M.; Kieseier, B.C.; Meyer Zu Hörste, G. Schwann cells promote post-traumatic nerve inflammation and neuropathic pain through MHC class II. Sci. Rep. 2017, 7, 12518. [Google Scholar] [CrossRef]
- Meyer zu Hörste, G.; Mausberg, A.K.; Müller, J.I.; Lehmann, H.C.; Löber, S.; Gmeiner, P.; Hartung, H.P.; Stüve, O.; Korth, C.; Kieseier, B.C. Quinpramine Ameliorates Rat Experimental Autoimmune Neuritis and Redistributes MHC Class II Molecules. PLoS ONE 2011, 6, e21223. [Google Scholar] [CrossRef] [PubMed]
- Tsuyuki, Y.; Fujimaki, H.; Hikawa, N.; Fujita, K.; Nagata, T.; Minami, M. IFN-gamma induces coordinate expression of MHC class I-mediated antigen presentation machinery molecules in adult mouse Schwann cells. Neuroreport 1998, 9, 2071–2075. [Google Scholar] [CrossRef] [PubMed]
- Van Rhijn, I.; Van Den Berg, L.H.; Bosboom, W.M.J.; Otten, H.G.; Logtenberg, T. Expression of accessory molecules for T-cell activation in peripheral nerve of patients with CIDP and vasculitic neuropathy. Brain 2000, 123 Pt 10, 2020–2029. [Google Scholar] [CrossRef] [PubMed]
- Spierings, E.; de Boer, T.; Wieles, B.; Adams, L.B.; Marani, E.; Ottenhoff, T.H.M. Mycobacterium leprae-Specific, HLA Class II-Restricted Killing of Human Schwann Cells by CD4+ Th1 Cells: A Novel Immunopathogenic Mechanism of Nerve Damage in Leprosy. J. Immunol. 2001, 166, 5883–5888. [Google Scholar] [CrossRef]
- Cheng, Y.C.; Chu, L.W.; Chen, J.Y.; Hsieh, S.L.; Chang, Y.C.; Dai, Z.K.; Wu, B.N. Loganin Attenuates High Glucose-Induced Schwann Cells Pyroptosis by Inhibiting ROS Generation and NLRP3 Inflammasome Activation. Cells 2020, 9, 1948. [Google Scholar] [CrossRef]
- Blevins, H.M.; Xu, Y.; Biby, S.; Zhang, S. The NLRP3 Inflammasome Pathway: A Review of Mechanisms and Inhibitors for the Treatment of Inflammatory Diseases. Front. Aging Neurosci. 2022, 14, 879021. [Google Scholar] [CrossRef]
- Padilla, A.; Descorbeth, M.; Almeyda, A.L.; Payne, K.; De Leon, M. Hyperglycemia magnifies Schwann cell dysfunction and cell death triggered by PA-induced lipotoxicity. Brain Res. 2011, 1370, 64–79. [Google Scholar] [CrossRef]
- Sato, K.; Tatsunami, R.; Yama, K.; Murao, Y.; Tampo, Y. Glycolaldehyde induces endoplasmic reticulum stress and apoptosis in Schwann cells. Toxicol. Rep. 2015, 2, 1454. [Google Scholar] [CrossRef]
- He, Y.; Hara, H.; Núñez, G. Mechanism and Regulation of NLRP3 Inflammasome Activation. Trends Biochem. Sci. 2016, 41, 1012–1021. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, S.; Yuan, Q.; Zhu, L.; Li, F.; Wang, H.; Kong, D.; Hao, J. TXNIP, a novel key factor to cause Schwann cell dysfunction in diabetic peripheral neuropathy, under the regulation of PI3K/Akt pathway inhibition-induced DNMT1 and DNMT3a overexpression. Cell Death Dis. 2021, 12, 642. [Google Scholar] [CrossRef]
- Song, X.M.; Xu, X.H.; Zhu, J.; Guo, Z.; Li, J.; He, C.; Burnstock, G.; Yuan, H.; Xiang, Z. Up-regulation of P2X7 receptors mediating proliferation of Schwann cells after sciatic nerve injury. Purinergic Signal 2015, 11, 203. [Google Scholar] [CrossRef]
- Wang, D.; Wang, H.; Gao, H.; Zhang, H.; Zhang, H.; Wang, Q.; Sun, Z. P2X7 receptor mediates NLRP3 inflammasome activation in depression and diabetes. Cell Biosci. 2020, 10, 28. [Google Scholar] [CrossRef] [PubMed]
- Dunnigan, S.K.; Ebadi, H.; Breiner, A.; Katzberg, H.D.; Lovblom, L.E.; Perkins, B.A.; Bril, V. Conduction slowing in diabetic sensorimotor polyneuropathy. Diabetes Care 2013, 36, 3684–3690. [Google Scholar] [CrossRef]
- Hamid, W.S.; Ahmed, H.S.; Osman, M.A.; Babiker, R. Nerve conduction and its correlations with duration of diabetes mellitus and glycosylated haemoglobin in type 2 diabetes mellitus (T2DM). J. Endocrinol. Metab. Diabetes S. Afr. 2021, 26, 46–51. [Google Scholar] [CrossRef]
- Guo, J.; Guo, Z.; Huang, Y.; Ma, S.; Yan, B.; Pan, C.; Jiang, Z.; Wang, F.; Zhang, Z.; Da, Y.; et al. Blockage of MLKL prevents myelin damage in experimental diabetic neuropathy. Proc. Natl. Acad. Sci. USA 2022, 119, e21215521192022. [Google Scholar] [CrossRef] [PubMed]
- Ying, Z.; Pan, C.; Shao, T.; Liu, L.; Li, L.; Guo, D.; Zhang, S.; Yuan, T.; Cao, R.; Jiang, Z.; et al. Mixed Lineage Kinase Domain-like Protein MLKL Breaks Down Myelin following Nerve Injury. Mol. Cell 2018, 72, 457–468.e5. [Google Scholar] [CrossRef]
- Zhu, L.; Hao, J.; Cheng, M.; Zhang, C.; Huo, C.; Liu, Y.; Du, W.; Zhang, X. Hyperglycemia-induced Bcl-2/Bax-mediated apoptosis of Schwann cells via mTORC1/S6K1 inhibition in diabetic peripheral neuropathy. Exp. Cell Res. 2018, 367, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Wang, N.; Li, F.; Jia, K.; An, J.; Liu, Y.; Wang, Y.; Zhu, L.; Zhao, S.; Hao, J. STAT3 phosphorylation mediates high glucose—Impaired cell autophagy in an HDAC1-dependent and -independent manner in Schwann cells of diabetic peripheral neuropathy. FASEB J. 2019, 33, 8008–8021. [Google Scholar] [CrossRef] [PubMed]
- Dyck, P.J.; Sherman, W.R.; Hallcher, L.M.; John Service, F.; O’Brien, P.C.; Grina, L.A.; Palumbo, P.J.; Swanson, C.J. Human diabetic endoneurial sorbitol, fructose, and myo-inositol related to sural nerve morphometry. Ann. Neurol. 1980, 8, 590–596. [Google Scholar] [CrossRef]
- Malik, R.A.; Tesfaye, S.; Newrick, P.G.; Walker, D.; Rajbhandari, S.M.; Siddique, I.; Sharma, A.K.; Boulton, A.J.; King, R.H.; Thomas, P.K.; et al. Sural nerve pathology in diabetic patients with minimal but progressive neuropathy. Diabetologia 2005, 48, 578–585. [Google Scholar] [CrossRef]
- Mizisin, A.P. Mechanisms of diabetic neuropathy: Schwann cells. Handb. Clin. Neurol. 2014, 126, 401–428. [Google Scholar] [PubMed]
- Mizisin, A.P.; Shelton, G.D.; Wagner, S.; Rusbridge, C.; Powell, H.C. Myelin splitting, Schwann cell injury and demyelination in feline diabetic neuropathy. Acta Neuropathol. 1998, 95, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Yagihashi, S.; Matsunaga, M. Ultrastructural Pathology of Peripheral Nerves in Patients with Diabetic Neuropathy. Tohoku J. Exp. Med. 1979, 129, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Nukada, H.; McMorran, P.D.; Baba, M.; Ogasawara, S.; Yagihashi, S. Increased susceptibility to ischemia and macrophage activation in STZ-diabetic rat nerve. Brain Res. 2011, 1373, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Lv, Q.; Chen, X.; Zou, J.; Liu, Z.; Shi, Y. CD8+ T Cell-Mediated Cytotoxicity toward Schwann Cells Promotes Diabetic Peripheral Neuropathy. Cell. Physiol. Biochem. 2013, 32, 827–837. [Google Scholar] [CrossRef]
- Hao, W.; Tashiro, S.; Hasegawa, T.; Sato, Y.; Kobayashi, T.; Tando, T.; Katsuyama, E.; Fujie, A.; Watanabe, R.; Morita, M.; et al. Hyperglycemia Promotes Schwann Cell De-differentiation and De-myelination via Sorbitol Accumulation and Igf1 Protein Down-regulation. J. Biol. Chem. 2015, 290, 17106–17115. [Google Scholar] [CrossRef]
- Cermenati, G.; Abbiati, F.; Cermenati, S.; Brioschi, E.; Volonterio, A.; Cavaletti, G.; Saez, E.; De Fabiani, E.; Crestani, M.; Garcia-Segura, L.M.; et al. Diabetes-induced myelin abnormalities are associated with an altered lipid pattern: Protective effects of LXR activation. J. Lipid Res. 2012, 53, 300–310. [Google Scholar] [CrossRef]
- Yung, J.H.M.; Giacca, A. Role of c-Jun N-terminal Kinase (JNK) in Obesity and Type 2 Diabetes. Cells 2020, 9, 706. [Google Scholar] [CrossRef]
- Jessen, K.R.; Mirsky, R. The repair Schwann cell and its function in regenerating nerves. J. Physiol. 2016, 594, 3521–3531. [Google Scholar] [CrossRef]
- Parkinson, D.B.; Bhaskaran, A.; Arthur-Farraj, P.; Noon, L.A.; Woodhoo, A.; Lloyd, A.C.; Feltri, M.L.; Wrabetz, L.; Behrens, A.; Mirsky, R.; et al. c-Jun is a negative regulator of myelination. J. Cell Biol. 2008, 181, 625–637. [Google Scholar] [CrossRef]
- Parkinson, D.B.; Bhaskaran, A.; Droggiti, A.; Dickinson, S.; D’Antonio, M.; Mirsky, R.; Jessen, K.R. Krox-20 inhibits Jun-NH2-terminal kinase/c-Jun to control Schwann cell proliferation and death. J. Cell Biol. 2004, 164, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Arthur-Farraj, P.J.; Latouche, M.; Wilton, D.K.; Quintes, S.; Chabrol, E.; Banerjee, A.; Woodhoo, A.; Jenkins, B.; Rahman, M.; Turmaine, M.; et al. c-Jun Reprograms Schwann Cells of Injured Nerves to Generate a Repair Cell Essential for Regeneration. Neuron 2012, 75, 633–647. [Google Scholar] [CrossRef]
- Tricaud, N.; Gautier, B.; Berthelot, J.; Gonzalez, S.; Van Hameren, G. Traumatic and Diabetic Schwann Cell Demyelination Is Triggered by a Transient Mitochondrial Calcium Release through Voltage Dependent Anion Channel 1. Biomedicines 2022, 10, 1447. [Google Scholar] [CrossRef]
- Liu, D.; Liang, X.; Zhang, H. Effects of High Glucose on Cell Viability and Differentiation in Primary Cultured Schwann Cells: Potential Role of ERK Signaling Pathway. Neurochem. Res. 2016, 41, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
- Napoli, I.; Noon, L.A.; Ribeiro, S.; Kerai, A.P.; Parrinello, S.; Rosenberg, L.H.; Collins, M.J.; Harrisingh, M.C.; White, I.J.; Woodhoo, A.; et al. A Central Role for the ERK-Signaling Pathway in Controlling Schwann Cell Plasticity and Peripheral Nerve Regeneration In Vivo. Neuron 2012, 73, 729–742. [Google Scholar] [CrossRef] [PubMed]
- Boerboom, A.; Dion, V.; Chariot, A.; Franzen, R. Molecular mechanisms involved in schwann cell plasticity. Front. Mol. Neurosci. 2017, 17, 38. [Google Scholar] [CrossRef] [PubMed]
- Pan, P.; Dobrowsky, R.T. Differential expression of neuregulin-1 isoforms and downregulation of erbin are associated with Erb B2 receptor activation in diabetic peripheral neuropathy. Acta Neuropathol. Commun. 2014, 2, 39. [Google Scholar] [CrossRef]
- Taveggia, C.; Zanazzi, G.; Petrylak, A.; Yano, H.; Rosenbluth, J.; Einheber, S.; Xu, X.; Esper, R.M.; Loeb, J.A.; Shrager, P.; et al. Neuregulin-1 type III determines the ensheathment fate of axons. Neuron 2005, 47, 681–694. [Google Scholar] [CrossRef]
- Fledrich, R.; Akkermann, D.; Schütza, V.; Abdelaal, T.A.; Hermes, D.; Schäffner, E.; Soto-Bernardini, M.C.; Götze, T.; Klink, A.; Kusch, K.; et al. NRG1 type I dependent autoparacrine stimulation of Schwann cells in onion bulbs of peripheral neuropathies. Nat. Commun. 2019, 10, 1467. [Google Scholar] [CrossRef]
- Syed, N.; Reddy, K.; Yang, D.P.; Taveggia, C.; Salzer, J.L.; Maurel, P.; Kim, H.A. Soluble neuregulin-1 has bifunctional, concentration-dependent effects on Schwann cell myelination. J. Neurosci. 2010, 30, 6122–6131. [Google Scholar] [CrossRef]
- McKerracher, L.; Rosen, K. MAG, myelin and overcoming growth inhibition in the CNS. Front. Mol. Neurosci. 2015, 8, 51. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd Razak, N.H.; Idris, J.; Hassan, N.H.; Zaini, F.; Muhamad, N.; Daud, M.F. Unveiling the Role of Schwann Cell Plasticity in the Pathogenesis of Diabetic Peripheral Neuropathy. Int. J. Mol. Sci. 2024, 25, 10785. https://doi.org/10.3390/ijms251910785
Abd Razak NH, Idris J, Hassan NH, Zaini F, Muhamad N, Daud MF. Unveiling the Role of Schwann Cell Plasticity in the Pathogenesis of Diabetic Peripheral Neuropathy. International Journal of Molecular Sciences. 2024; 25(19):10785. https://doi.org/10.3390/ijms251910785
Chicago/Turabian StyleAbd Razak, Nurul Husna, Jalilah Idris, Nur Hidayah Hassan, Fazlin Zaini, Noorzaid Muhamad, and Muhammad Fauzi Daud. 2024. "Unveiling the Role of Schwann Cell Plasticity in the Pathogenesis of Diabetic Peripheral Neuropathy" International Journal of Molecular Sciences 25, no. 19: 10785. https://doi.org/10.3390/ijms251910785
APA StyleAbd Razak, N. H., Idris, J., Hassan, N. H., Zaini, F., Muhamad, N., & Daud, M. F. (2024). Unveiling the Role of Schwann Cell Plasticity in the Pathogenesis of Diabetic Peripheral Neuropathy. International Journal of Molecular Sciences, 25(19), 10785. https://doi.org/10.3390/ijms251910785





