Hepatokines and MASLD: The GLP1-Ras-FGF21-Fetuin-A Crosstalk as a Therapeutic Target
Abstract
1. Introduction
2. MASLD: Epidemiology and Diagnosis
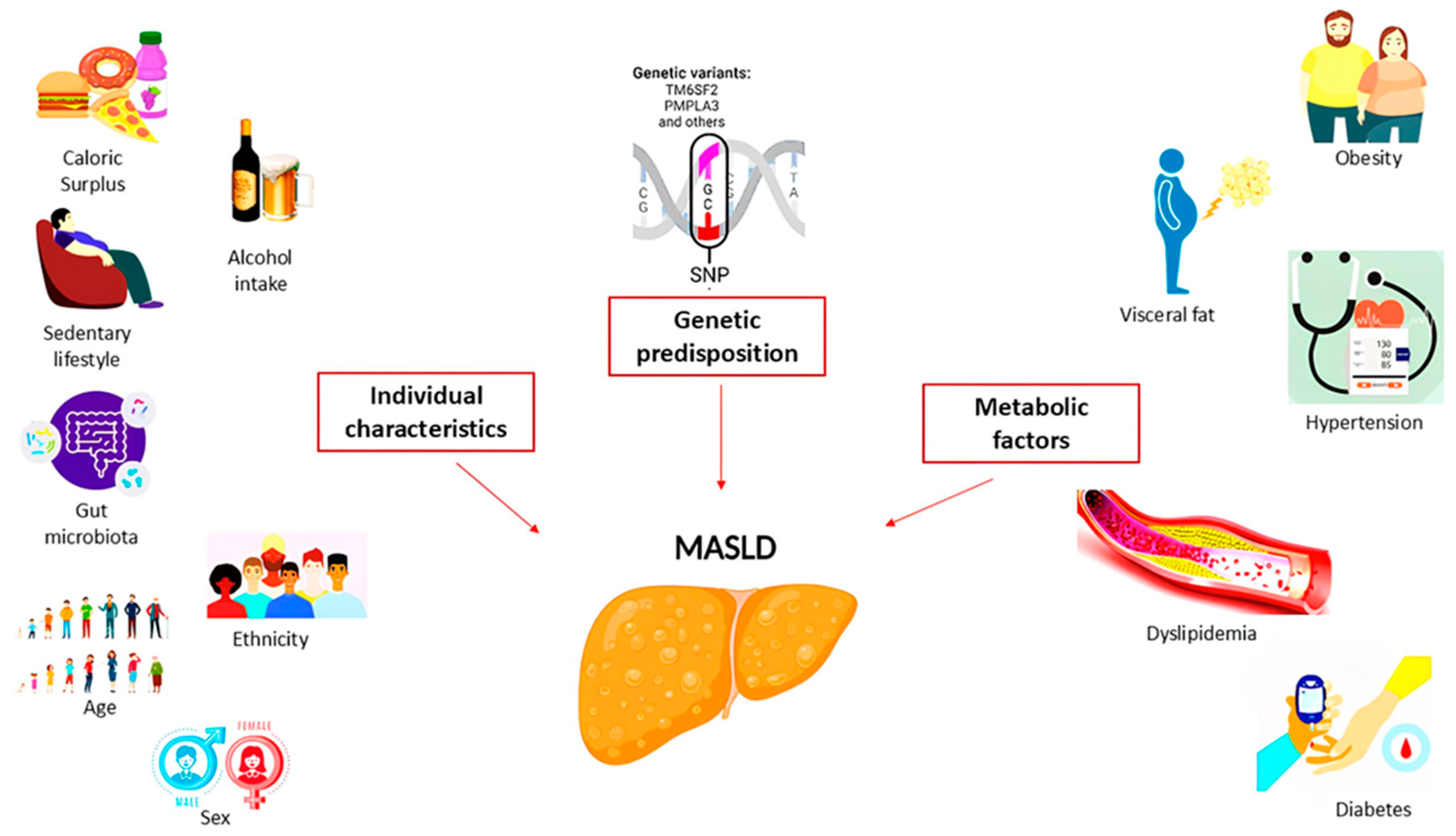
3. MASLD: Risk Factors and Pathogenetic Mechanism
4. MASLD: From Adiposopathy to a Liver-Centered Approach
5. The Analysis of Metabolic Organ-Secreted Factors in MASLD
6. The Role of Hepatokines in the MASLD Metabolic Dysfunction
7. Angiopoietin-like Protein (ANGPTL)
8. Insulin-like Growth Factors (IGFs)
9. Selenoprotein P (SeP)
10. Leukocyte Cell-Derived Chemotaxin 2 (LECT2)
11. Sex Hormone-Binding Globulin (SHBG)
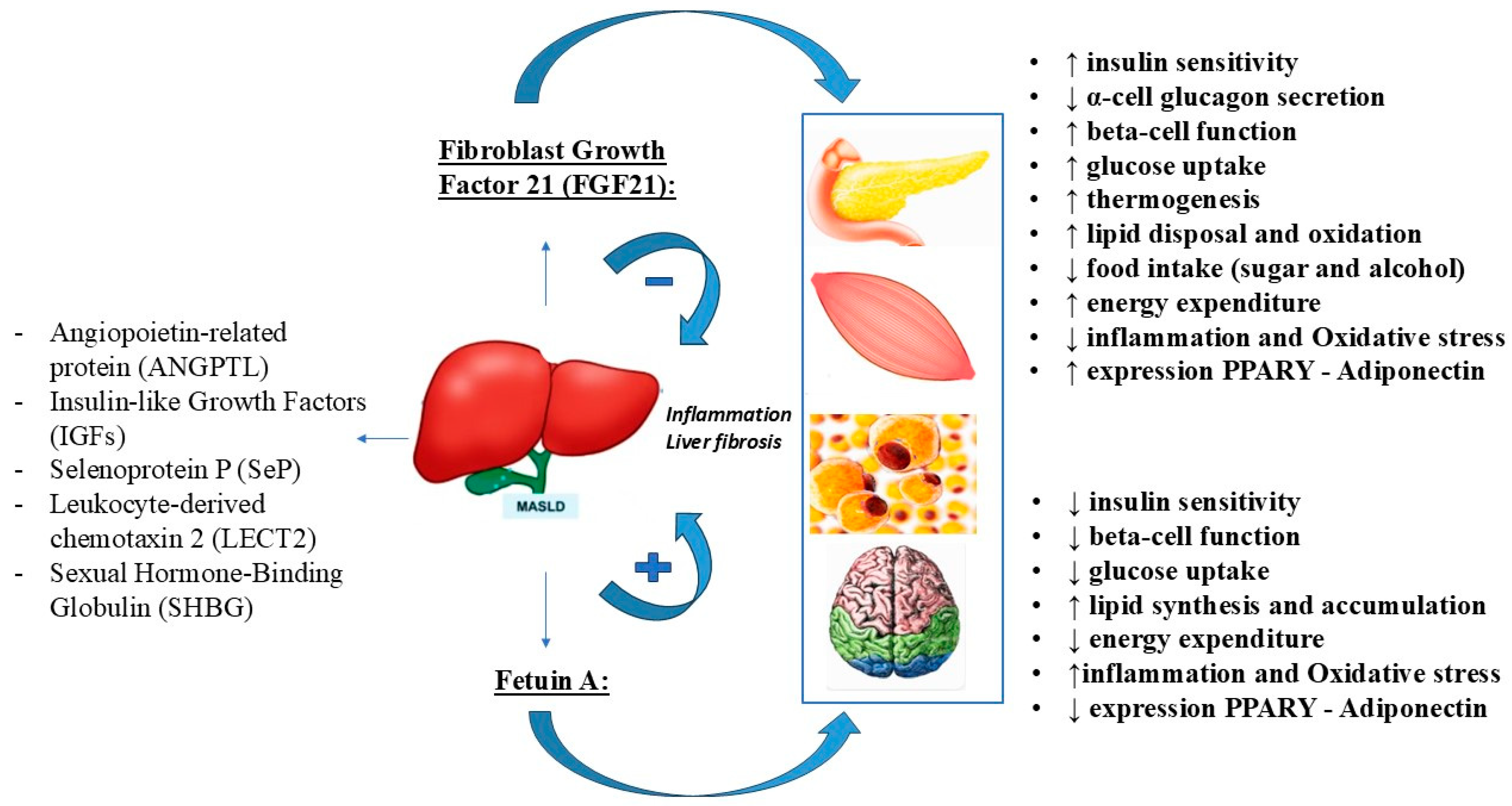
12. Fibroblast Growth Factor 21: Structure and Function
13. MASLD: An Alteration of FGF21 Expression
14. MASLD: Potential Therapeutic Action of FGF21
15. Fetuin-A: Structure and Function
16. Fetuin-A: A Potential Biomarker and Pathogenetic Mechanism for Metabolically Associated Steatotic Liver Disease (MASLD)
17. Therapeutic Strategies That Influence Fetuin-A Levels
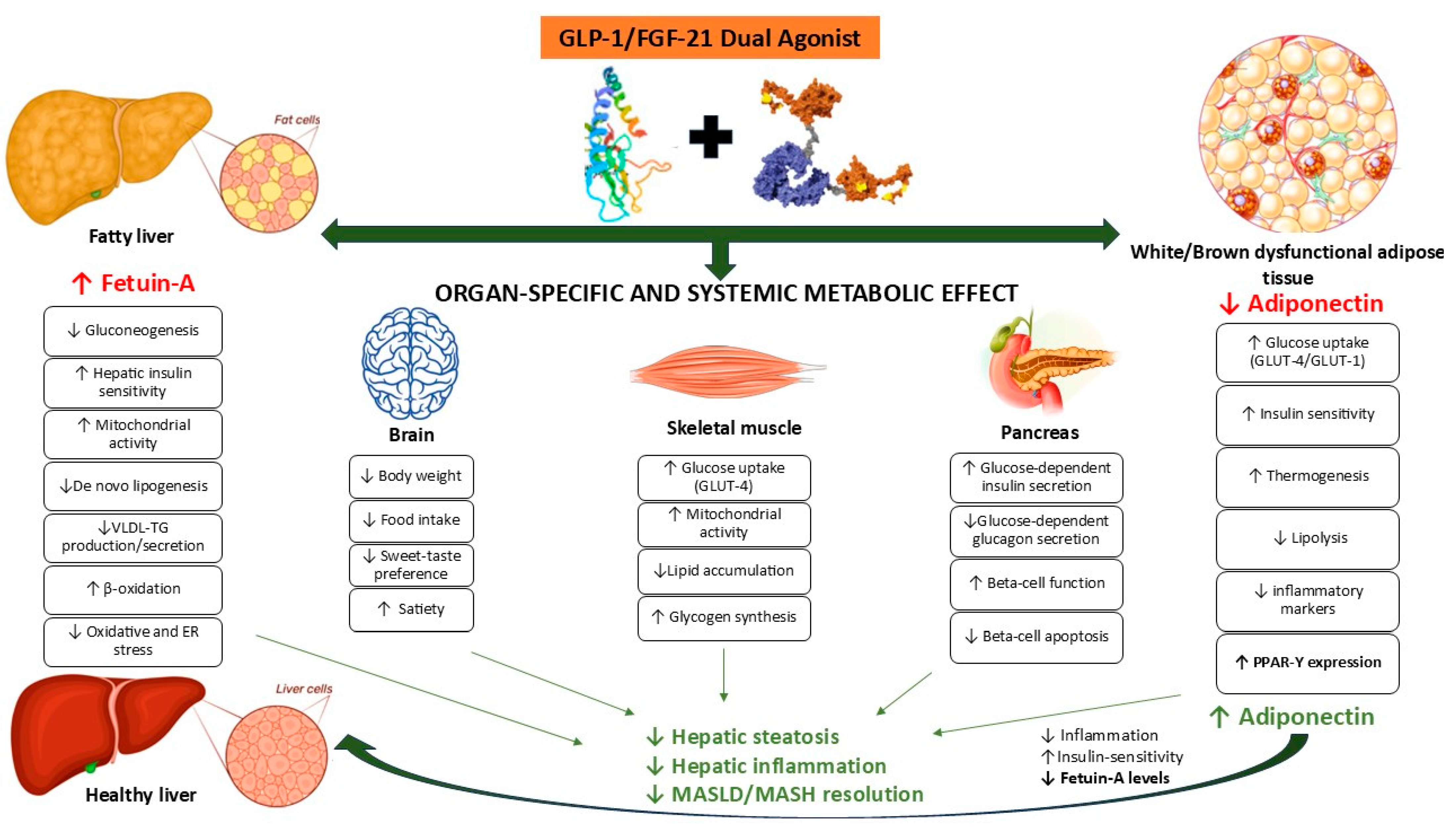
18. GLP-Ras in the Treatment of MASLD: Modulation of FGF21 and Fetuin-A
19. FGF21/GLP-1 Axis in the MASLD: Fetuin-A Can Be the Target of the Dual Agonist?
20. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rinella, M.E.; Sookoian, S. From NAFLD to MASLD: Updated naming and diagnosis criteria for fatty liver disease. J. Lipid Res. 2024, 65, 100485. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.C.Z.; Anand, V.V.; Razavi, A.C.; Alebna, P.L.; Muthiah, M.D.; Siddiqui, M.S.; Chew, N.W.S.; Mehta, A. The Global Epidemic of Metabolic Fatty Liver Disease. Curr. Cardiol. Rep. 2024, 26, 199–210. [Google Scholar] [CrossRef]
- Mantovani, A. MAFLD vs NAFLD: Where are we? Dig. Liver Dis. 2021, 53, 1368–1372. [Google Scholar] [CrossRef]
- Ramírez-Mejía, M.M.; Méndez-Sánchez, N. What Is in a Name: From NAFLD to MAFLD and MASLD—Unraveling the Complexities and Implications. Curr. Hepatol. Rep. 2023, 22, 221–227. [Google Scholar] [CrossRef]
- dos Santos, J.P.M.; de Maio, M.C.; Lemes, M.A.; Laurindo, L.F.; Haber, J.F.d.S.; Bechara, M.D.; Prado, P.S.D.; Rauen, E.C.; Costa, F.; Pereira, B.C.d.A.; et al. Non-Alcoholic Steatohepatitis (NASH) and Organokines: What Is Now and What Will Be in the Future. Int. J. Mol. Sci. 2022, 23, 498. [Google Scholar] [CrossRef]
- Stefan, N.; Schick, F.; Birkenfeld, A.L.; Häring, H.-U.; White, M.F. The role of hepatokines in NAFLD. Cell Metab. 2023, 35, 236–252. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.-K.; Chuah, K.-H.; Rajaram, R.B.; Lim, L.-L.; Ratnasingam, J.; Vethakkan, S.R. Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): A State-of-the-Art Review. J. Obes. Metab. Syndr. 2023, 32, 197–213. [Google Scholar] [CrossRef] [PubMed]
- Eskridge, W.; Cryer, D.R.; Schattenberg, J.M.; Gastaldelli, A.; Malhi, H.; Allen, A.M.; Noureddin, M.; Sanyal, A.J. Metabolic Dysfunction-Associated Steatotic Liver Disease and Metabolic Dysfunction-Associated Steatohepatitis: The Patient and Physician Perspective. J. Clin. Med. 2023, 12, 6216. [Google Scholar] [CrossRef]
- Habib, S. Metabolic dysfunction-associated steatotic liver disease heterogeneity: Need of subtyping. World J. Gastrointest. Pathophysiol. 2024, 15, 92791. [Google Scholar] [CrossRef]
- Chen, L. From metabolic dysfunction-associated fatty liver disease to metabolic dysfunction-associated steatotic liver disease: Controversy and consensus. World J. Hepatol. 2023, 15, 1253–1257. [Google Scholar] [CrossRef]
- Lu, Z.; Shao, W.; Song, J. The transition from NAFLD to MASLD and its impact on clinical practice and outcomes. J. Hepatol. 2024, 81, e155–e156. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wu, L.; Zhu, X.; Bian, H.; Gao, X.; Xia, M. Advances in management of metabolic dysfunction-associated steatotic liver disease: From mechanisms to therapeutics. Lipids Health Dis. 2024, 23, 95. [Google Scholar] [CrossRef] [PubMed]
- Quek, J.; Chan, K.E.; Wong, Z.Y.; Tan, C.; Tan, B.; Lim, W.H.; Tan, D.J.H.; Tang, A.S.P.; Tay, P.; Xiao, J.; et al. Global prevalence of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in the overweight and obese population: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2023, 8, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wang, Q.; Sun, Y.; Zhao, X.; Kong, Y.; Ou, X.; Jia, J.; Wu, S.; You, H. The Prevalence of Lean/Nonobese Nonalcoholic Fatty Liver Disease. J. Clin. Gastroenterol. 2020, 54, 378–387. [Google Scholar] [CrossRef]
- Habibullah, M.; Jemmieh, K.; Ouda, A.; Haider, M.Z.; Malki, M.I.; Elzouki, A.-N. Metabolic-associated fatty liver disease: A selective review of pathogenesis, diagnostic approaches, and therapeutic strategies. Front. Med. 2024, 11, 1291501. [Google Scholar] [CrossRef]
- Bansal, S.; Vachher, M.; Arora, T.; Kumar, B.; Burman, A. Visceral fat: A key mediator of NAFLD development and progression. Hum. Nutr. Metab. 2023, 33, 200210. [Google Scholar] [CrossRef]
- Vesković, M.; Šutulović, N.; Hrnčić, D.; Stanojlović, O.; Macut, D.; Mladenović, D. The Interconnection between Hepatic Insulin Resistance and Metabolic Dysfunction-Associated Steatotic Liver Disease—The Transition from an Adipocentric to Liver-Centric Approach. Curr. Issues Mol. Biol. 2023, 45, 9084–9102. [Google Scholar] [CrossRef]
- Chen, H.; Tan, H.; Wan, J.; Zeng, Y.; Wang, J.; Wang, H.; Lu, X. PPAR-γ signaling in nonalcoholic fatty liver disease: Pathogenesis and therapeutic targets. Pharmacol. Ther. 2023, 245, 108391. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Xiaoli, A.M.; Yang, F. Regulation and Metabolic Significance of De Novo Lipogenesis in Adipose Tissues. Nutrients 2018, 10, 1383. [Google Scholar] [CrossRef]
- Gastaldelli, A.; Cusi, K. From NASH to diabetes and from diabetes to NASH: Mechanisms and treatment options. JHEP Rep. 2019, 1, 312–328. [Google Scholar] [CrossRef]
- Zeng, J.; Fan, J.; Francque, S.M. Therapeutic management of metabolic dysfunction associated steatotic liver disease. United Eur. Gastroenterol. J. 2024, 12, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.Y.; Park, S.H.; Marquez, J.; Kwak, H.-B.; Kim, T.N.; Bae, J.H.; Koh, J.-H.; Han, J. Hepatokines as a Molecular Transducer of Exercise. J. Clin. Med. 2021, 10, 385. [Google Scholar] [CrossRef]
- Meex, R.C.R.; Watt, M.J. Hepatokines: Linking nonalcoholic fatty liver disease and insulin resistance. Nat. Rev. Endocrinol. 2017, 13, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Durán, R.; Ampuero, J.; Maya-Miles, D.; Pastor-Ramírez, H.; Montero-Vallejo, R.; Rivera-Esteban, J.; Álvarez-Amor, L.; Pareja, M.J.; Rico, M.C.; Millán, R.; et al. Fibroblast growth factor 21 is a hepatokine involved in MASLD progression. United Eur. Gastroenterol. J. 2024; online version of record. [Google Scholar] [CrossRef]
- Perakakis, N.; Stefanakis, K.; Mantzoros, C.S. The role of omics in the pathophysiology, diagnosis and treatment of non-alcoholic fatty liver disease. Metabolism 2020, 111, 154320. [Google Scholar] [CrossRef]
- Xiong, X.; Wang, Q.; Wang, S.; Zhang, J.; Liu, T.; Guo, L.; Yu, Y.; Lin, J.D. Mapping the molecular signatures of diet-induced NASH and its regulation by the hepatokine Tsukushi. Mol. Metab. 2019, 20, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Fisher, F.M.; Maratos-Flier, E. Understanding the Physiology of FGF21. Annu. Rev. Physiol. 2016, 78, 223–241. [Google Scholar] [CrossRef]
- Kim, T.H.; Hong, D.-G.; Yang, Y.M. Hepatokines and Non-Alcoholic Fatty Liver Disease: Linking Liver Pathophysiology to Metabolism. Biomedicines 2021, 9, 1903. [Google Scholar] [CrossRef]
- Yuan, X.; Sun, Y.; Cheng, Q.; Hu, K.; Ye, J.; Zhao, Y.; Wu, J.; Shao, X.; Fang, L.; Ding, Y.; et al. Proteomic analysis to identify differentially expressed proteins between subjects with metabolic healthy obesity and non-alcoholic fatty liver disease. J. Proteom. 2020, 221, 103683. [Google Scholar] [CrossRef]
- Baselli, G.A.; Dongiovanni, P.; Rametta, R.; Meroni, M.; Pelusi, S.; Maggioni, M.; Badiali, S.; Pingitore, P.; Maurotti, S.; Montalcini, T.; et al. Liver transcriptomics highlights interleukin-32 as novel NAFLD-related cytokine and candidate biomarker. Gut 2020, 69, 1855–1866. [Google Scholar] [CrossRef]
- Cazanave, S.; Podtelezhnikov, A.; Jensen, K.; Seneshaw, M.; Kumar, D.P.; Min, H.-K.; Santhekadur, P.K.; Banini, B.; Mauro, A.G.; Oseini, A.M.; et al. The Transcriptomic Signature Of Disease Development And Progression Of Nonalcoholic Fatty Liver Disease. Sci. Rep. 2017, 7, 17193. [Google Scholar] [CrossRef]
- Amir, M.; Czaja, M.J. Autophagy in nonalcoholic steatohepatitis. Expert Rev. Gastroenterol. Hepatol. 2011, 5, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.J.; Campbell–Sargent, C.; Mirshahi, F.; Rizzo, W.B.; Contos, M.J.; Sterling, R.K.; Luketic, V.A.; Shiffman, M.L.; Clore, J.N. Nonalcoholic steatohepatitis: Association of insulin resistance and mitochondrial abnormalities. Gastroenterology 2001, 120, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.; Xu, C.; Lin, J.; Li, Y. Role of hepatokines in non-alcoholic fatty liver disease. J. Transl. Intern. Med. 2019, 7, 143–148. [Google Scholar] [CrossRef]
- Watt, M.J.; Miotto, P.M.; De Nardo, W.; Montgomery, M.K. The Liver as an Endocrine Organ—Linking NAFLD and Insulin Resistance. Endocr. Rev. 2019, 40, 1367–1393. [Google Scholar] [CrossRef]
- Qin, L.; Wu, J.; Sun, X.; Huang, X.; Huang, W.; Weng, C.; Cai, J. The regulatory role of metabolic organ-secreted factors in the nonalcoholic fatty liver disease and cardiovascular disease. Front. Cardiovasc. Med. 2023, 10, 1119005. [Google Scholar] [CrossRef]
- Jensen-Cody, S.O.; Potthoff, M.J. Hepatokines and metabolism: Deciphering communication from the liver. Mol. Metab. 2021, 44, 101138. [Google Scholar] [CrossRef]
- Hayashino, Y.; Jackson, J.L.; Hirata, T.; Fukumori, N.; Nakamura, F.; Fukuhara, S.; Tsujii, S.; Ishii, H. Effects of exercise on C-reactive protein, inflammatory cytokine and adipokine in patients with type 2 diabetes: A meta-analysis of randomized controlled trials. Metabolism 2014, 63, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, A.L.; Tavaglione, F.; Romeo, S.; Charlton, M. Endocrine aspects of metabolic dysfunction-associated steatotic liver disease (MASLD): Beyond insulin resistance. J. Hepatol. 2023, 79, 1524–1541. [Google Scholar] [CrossRef]
- Yilmaz, Y.; Ulukaya, E.; Atug, O.; Dolar, E. Serum concentrations of human angiopoietin-like protein 3 in patients with nonalcoholic fatty liver disease: Association with insulin resistance. Eur. J. Gastroenterol. Hepatol. 2009, 21, 1247–1251. [Google Scholar] [CrossRef]
- Ke, Y.; Liu, S.; Zhang, Z.; Hu, J. Circulating angiopoietin-like proteins in metabolic-associated fatty liver disease: A systematic review and meta-analysis. Lipids Health Dis. 2021, 20, 55. [Google Scholar] [CrossRef]
- Aryal, B.; Singh, A.K.; Zhang, X.; Varela, L.; Rotllan, N.; Goedeke, L.; Chaube, B.; Camporez, J.-P.; Vatner, D.F.; Horvath, T.L.; et al. Absence of ANGPTL4 in adipose tissue improves glucose tolerance and attenuates atherogenesis. J. Clin. Investig. 2018, 3, e97918. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Chaube, B.; Zhang, X.; Sun, J.; Citrin, K.M.; Canfrán-Duque, A.; Aryal, B.; Rotllan, N.; Varela, L.; Lee, R.G.; et al. Hepatocyte-specific suppression of ANGPTL4 improves obesity-associated diabetes and mitigates atherosclerosis in mice. J. Clin. Investig. 2021, 131, e140989. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Zhang, J.; Lu, Y.; Wu, L. Association of metabolic-dysfunction associated steatotic liver disease with polycystic ovary syndrome. iScience 2024, 27, 108783. [Google Scholar] [CrossRef]
- Misu, H.; Takamura, T.; Takayama, H.; Hayashi, H.; Matsuzawa-Nagata, N.; Kurita, S.; Ishikura, K.; Ando, H.; Takeshita, Y.; Ota, T.; et al. A Liver-Derived Secretory Protein, Selenoprotein P, Causes Insulin Resistance. Cell Metab. 2010, 12, 483–495. [Google Scholar] [CrossRef]
- Xu, M.; Xu, H.-H.; Lin, Y.; Sun, X.; Wang, L.-J.; Fang, Z.-P.; Su, X.-H.; Liang, X.-J.; Hu, Y.; Liu, Z.-M.; et al. LECT2, a Ligand for Tie1, Plays a Crucial Role in Liver Fibrogenesis. Cell 2019, 178, 1478–1492. [Google Scholar] [CrossRef]
- Lan, F.; Misu, H.; Chikamoto, K.; Takayama, H.; Kikuchi, A.; Mohri, K.; Takata, N.; Hayashi, H.; Matsuzawa-Nagata, N.; Takeshita, Y.; et al. LECT2 functions as a hepatokine that links obesity to skeletal muscle insulin resistance. Diabetes 2014, 63, 1649–1664. [Google Scholar] [CrossRef] [PubMed]
- Saez-Lopez, C.; Barbosa-Desongles, A.; Hernandez, C.; Dyer, R.A.; Innis, S.M.; Simó, R.; Selva, D.M. Sex Hormone-Binding Globulin Reduction in Metabolic Disorders May Play a Role in NAFLD Development. Endocrinology 2017, 158, 545–559. [Google Scholar] [CrossRef]
- Martínez-Garza, Ú.; Torres-Oteros, D.; Yarritu-Gallego, A.; Marrero, P.F.; Haro, D.; Relat, J. Fibroblast Growth Factor 21 and the Adaptive Response to Nutritional Challenges. Int. J. Mol. Sci. 2019, 20, 4692. [Google Scholar] [CrossRef] [PubMed]
- Falamarzi, K.; Malekpour, M.; Tafti, M.F.; Azarpira, N.; Behboodi, M.; Zarei, M. The role of FGF21 and its analogs on liver associated diseases. Front. Med. 2022, 9, 967375. [Google Scholar] [CrossRef]
- Lin, W.; Zhang, T.; Zhou, Y.; Zheng, J.; Lin, Z. Advances in Biological Functions and Clinical Studies of FGF21. Diabetes Metab. Syndr. Obes. Targets Ther. 2021, 14, 3281–3290. [Google Scholar] [CrossRef]
- Lin, X.; Liu, Y.B.; Hu, H. Metabolic role of fibroblast growth factor 21 in liver, adipose and nervous system tissues. Biomed. Rep. 2017, 6, 495–502. [Google Scholar] [CrossRef]
- Raptis, D.D.; Mantzoros, C.S.; Polyzos, S.A. Fibroblast Growth Factor-21 as a Potential Therapeutic Target of Nonalcoholic Fatty Liver Disease. Ther. Clin. Risk Manag. 2023, 19, 77–96. [Google Scholar] [CrossRef] [PubMed]
- Kliewer, S.A.; Mangelsdorf, D.J. A Dozen Years of Discovery: Insights into the Physiology and Pharmacology of FGF21. Cell Metab. 2019, 29, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; He, J.; Li, S.; Song, L.; Guo, X.; Yao, W.; Zou, D.; Gao, X.; Liu, Y.; Bai, F.; et al. Fibroblast growth factor 21 (FGF21) inhibits macrophage-mediated inflammation by activating Nrf2 and suppressing the NF-κB signaling pathway. Int. Immunopharmacol. 2016, 38, 144–152. [Google Scholar] [CrossRef]
- Abebe, E.C.; Muche, Z.T.; T/Mariam, A.B.; Ayele, T.M.; Agidew, M.M.; Azezew, M.T.; Zewde, E.A.; Dejenie, T.A.; Mengstie, M.A. The structure, biosynthesis, and biological roles of fetuin-A: A review. Front. Cell Dev. Biol. 2022, 10, 945287. [Google Scholar] [CrossRef]
- Icer, M.A.; Yıldıran, H. Effects of fetuin-A with diverse functions and multiple mechanisms on human health. Clin. Biochem. 2021, 88, 1–10. [Google Scholar] [CrossRef]
- Mathews, S.T.; Singh, G.P.; Ranalletta, M.; Cintron, V.J.; Qiang, X.; Goustin, A.S.; Jen, K.-L.C.; Charron, M.J.; Jahnen-Dechent, W.; Grunberger, G. Improved insulin sensitivity and resistance to weight gain in mice null for the Ahsg gene. Diabetes 2002, 51, 2450–2458. [Google Scholar] [CrossRef]
- Gaudet, D.; Karwatowska-Prokopczuk, E.; Baum, S.J.; Hurh, E.; Kingsbury, J.; Bartlett, V.J.; Figueroa, A.L.; Piscitelli, P.; Singleton, W.; Witztum, J.L.; et al. Vupanorsen, an N-acetyl galactosamine-conjugated antisense drug to ANGPTL3 mRNA, lowers triglycerides and atherogenic lipoproteins in patients with diabetes, hepatic steatosis, and hypertriglyceridaemia. Eur. Heart J. 2020, 41, 3936–3945. [Google Scholar] [CrossRef] [PubMed]
- Stefan, N.; Häring, H.-U. The role of hepatokines in metabolism. Nat. Rev. Endocrinol. 2013, 9, 144–152. [Google Scholar] [CrossRef]
- Yang, S.J.; Hwang, S.Y.; Choi, H.Y.; Yoo, H.J.; Seo, J.A.; Kim, S.G.; Kim, N.H.; Baik, S.H.; Choi, D.S.; Choi, K.M. Serum selenoprotein P levels in patients with type 2 diabetes and prediabetes: Implications for insulin resistance, inflammation, and atherosclerosis. J. Clin. Endocrinol. Metab. 2011, 96, E1325–E1329. [Google Scholar] [CrossRef]
- Jung, T.W.; Chung, Y.H.; Kim, H.-C.; El-Aty, A.M.A.; Jeong, J.H. LECT2 promotes inflammation and insulin resistance in adipocytes via P38 pathways. J. Mol. Endocrinol. 2018, 61, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Tanisawa, K.; Taniguchi, H.; Sun, X.; Ito, T.; Kawakami, R.; Sakamoto, S.; Higuchi, M. Visceral fat area is a strong predictor of leukocyte cell-derived chemotaxin 2, a potential biomarker of dyslipidemia. PLoS ONE 2017, 12, e0173310. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.J.; Hwang, S.Y.; Choi, J.-H.; Lee, H.J.; Chung, H.S.; Seo, J.-A.; Kim, S.G.; Kim, N.H.; Baik, S.H.; Choi, D.S.; et al. Association of leukocyte cell-derived chemotaxin 2 (LECT2) with NAFLD, metabolic syndrome, and atherosclerosis. PLoS ONE 2017, 12, e0174717. [Google Scholar] [CrossRef]
- Feng, C.; Jin, Z.; Chi, X.; Zhang, B.; Wang, X.; Sun, L.; Fan, J.; Sun, Q.; Zhang, X. SHBG expression is correlated with PI3K/AKT pathway activity in a cellular model of human insulin resistance. Gynecol. Endocrinol. 2018, 34, 567–573. [Google Scholar] [CrossRef]
- Urbano, F.; Chiarito, M.; Lattanzio, C.; Messa, A.; Ferrante, M.; Francavilla, M.; Mehmeti, I.; Lassandro, G.; Giordano, P.; Faienza, M.F. Sex Hormone-Binding Globulin (SHBG) Reduction: The Alarm Bell for the Risk of Non-Alcoholic Fatty Liver Disease in Adolescents with Polycystic Ovary Syndrome. Children 2022, 9, 1748. [Google Scholar] [CrossRef] [PubMed]
- Sáez-López, C.; Salcedo-Allende, M.T.; Hernandez, C.; Simó-Servat, O.; Simó, R.; Selva, D.M. Sex Hormone–Binding Globulin Expression Correlates With Acetyl-Coenzyme A Carboxylase and Triglyceride Content in Human Liver. J. Clin. Endocrinol. Metab. 2019, 104, 1500–1507. [Google Scholar] [CrossRef]
- Goto, T.; Hirata, M.; Aoki, Y.; Iwase, M.; Takahashi, H.; Kim, M.; Li, Y.; Jheng, H.-F.; Nomura, W.; Takahashi, N.; et al. The hepatokine FGF21 is crucial for peroxisome proliferator-activated receptor-α agonist-induced amelioration of metabolic disorders in obese mice. J. Biol. Chem. 2017, 292, 9175–9190. [Google Scholar] [CrossRef]
- Reinehr, T.; Karges, B.; Meissner, T.; Wiegand, S.; Fritsch, M.; Holl, R.W.; Woelfle, J. Fibroblast Growth Factor 21 And Fetuin-A in Obese Adolescents with and without Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2015, 100, 3004–3010. [Google Scholar] [CrossRef]
- Tillman, E.J.; Rolph, T. FGF21: An Emerging Therapeutic Target for Non-Alcoholic Steatohepatitis and Related Metabolic Diseases. Front. Endocrinol. 2020, 11, 601290. [Google Scholar] [CrossRef]
- Pérez-Martí, A.; Sandoval, V.; Marrero, P.F.; Haro, D.; Relat, J. Nutritional regulation of fibroblast growth factor 21: From macronutrients to bioactive dietary compounds. Horm. Mol. Biol. Clin. Investig. 2017, 30, 20160034. [Google Scholar] [CrossRef]
- De Oliveira Dos Santos, A.; Zanuso, B.D.O.; Miola, V.; Barbalho, S.; Bueno, P.S.; Flato, U.; Detregiachi, C.; Buchaim, D.; Buchaim, R.; Tofano, R.; et al. Adipokines, Myokines, and Hepatokines: Crosstalk and Metabolic Repercussions. Int. J. Mol. Sci. 2021, 22, 2639. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Nie, Y.; Cao, J.; Luo, M.; Yan, M.; Chen, Z.; He, B. The Roles and Pharmacological Effects of FGF21 in Preventing Aging-Associated Metabolic Diseases. Front. Cardiovasc. Med. 2021, 8, 655575. [Google Scholar] [CrossRef] [PubMed]
- Staiger, H.; Keuper, M.; Berti, L.; de Angelis, M.H.; Häring, H.-U. Fibroblast Growth Factor 21—Metabolic Role in Mice and Men. Endocr. Rev. 2017, 38, 468–488. [Google Scholar] [CrossRef] [PubMed]
- Markan, K.R.; Naber, M.C.; Ameka, M.K.; Anderegg, M.D.; Mangelsdorf, D.J.; Kliewer, S.A.; Mohammadi, M.; Potthoff, M.J. Circulating FGF21 is liver derived and enhances glucose uptake during refeeding and overfeeding. Diabetes 2014, 63, 4057–4063. [Google Scholar] [CrossRef]
- Mutanen, A.; Heikkilä, P.; Lohi, J.; Raivio, T.; Jalanko, H.; Pakarinen, M.P. Serum FGF21 increases with hepatic fat accumulation in pediatric onset intestinal failure. J. Hepatol. 2014, 60, 183–190. [Google Scholar] [CrossRef]
- Sanyal, A.; Charles, E.D.; A Neuschwander-Tetri, B.; Loomba, R.; Harrison, S.A.; Abdelmalek, M.F.; Lawitz, E.J.; Halegoua-DeMarzio, D.; Kundu, S.; Noviello, S.; et al. Pegbelfermin (BMS-986036), a PEGylated fibroblast growth factor 21 analogue, in patients with non-alcoholic steatohepatitis: A randomised, double-blind, placebo-controlled, phase 2a trial. Lancet 2018, 392, 2705–2717. [Google Scholar] [CrossRef]
- Targher, G.; Chonchol, M.; Zoppini, G.; Abaterusso, C.; Bonora, E. Risk of chronic kidney disease in patients with non-alcoholic fatty liver disease: Is there a link? J. Hepatol. 2011, 54, 1020–1029. [Google Scholar] [CrossRef]
- Kloock, S.; Ziegler, C.G.; Dischinger, U. Obesity and its comorbidities, current treatment options and future perspectives: Challenging bariatric surgery? Pharmacol Ther. 2023, 251, 108549. [Google Scholar] [CrossRef]
- Elshaer, A.; Chascsa, D.M.H.; Lizaola-Mayo, B.C. Exploring Varied Treatment Strategies for Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Life 2024, 14, 844. [Google Scholar] [CrossRef]
- Talukdar, S.; Zhou, Y.; Li, D.; Rossulek, M.; Dong, J.; Somayaji, V.; Weng, Y.; Clark, R.; Lanba, A.; Owen, B.M.; et al. A Long-Acting FGF21 Molecule, PF-05231023, Decreases Body Weight and Improves Lipid Profile in Non-human Primates and Type 2 Diabetic Subjects. Cell Metab. 2016, 23, 427–440. [Google Scholar] [CrossRef]
- Gaich, G.; Chien, J.Y.; Fu, H.; Glass, L.C.; Deeg, M.A.; Holland, W.L.; Kharitonenkov, A.; Bumol, T.; Schilske, H.K.; Moller, D.E. The effects of LY2405319, an FGF21 analog, in obese human subjects with type 2 diabetes. Cell Metab. 2013, 18, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Schlein, C.; Talukdar, S.; Heine, M.; Fischer, A.W.; Krott, L.M.; Nilsson, S.K.; Brenner, M.B.; Heeren, J.; Scheja, L. FGF21 Lowers Plasma Triglycerides by Accelerating Lipoprotein Catabolism in White and Brown Adipose Tissues. Cell Metab. 2016, 23, 441–453. [Google Scholar] [CrossRef]
- Ciardullo, S.; Muraca, E.; Vergani, M.; Invernizzi, P.; Perseghin, G. Advancements in pharmacological treatment of NAFLD/MASLD: A focus on metabolic and liver-targeted interventions. Gastroenterol. Rep. 2024, 12, goae029. [Google Scholar] [CrossRef]
- Harrison, S.; Frias, J.P.; Neff, G.; Abrams, G.A.; Lucas, K.J.; Sanchez, W.; Gogia, S.; Sheikh, M.Y.; Behling, C.; Bedossa, P.; et al. Safety and efficacy of once-weekly efruxifermin versus placebo in non-alcoholic steatohepatitis (HARMONY): A multicentre, randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Gastroenterol. Hepatol. 2023, 8, 1080–1093. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Sanyal, A.J.; Kowdley, K.V.; Bhatt, D.L.; Alkhouri, N.; Frias, J.P.; Bedossa, P.; Harrison, S.A.; Lazas, D.; Barish, R.; et al. Randomized, Controlled Trial of the FGF21 Analogue Pegozafermin in NASH. New Engl. J. Med. 2023, 389, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Bourebaba, L.; Marycz, K. Pathophysiological Implication of Fetuin-A Glycoprotein in the Development of Metabolic Disorders: A Concise Review. J. Clin. Med. 2019, 8, 2033. [Google Scholar] [CrossRef]
- Ix, J.H.; Sharma, K. Mechanisms Linking Obesity, Chronic Kidney Disease, and Fatty Liver Disease. J. Am. Soc. Nephrol. 2010, 21, 406–412. [Google Scholar] [CrossRef]
- Magalhães, P.; Zürbig, P.; Mischak, H.; Schleicher, E. Urinary fetuin-A peptides as a new marker for impaired kidney function in patients with type 2 diabetes. Clin. Kidney J. 2021, 14, 269–276. [Google Scholar] [CrossRef]
- Shen, X.; Yang, L.; Yan, S.; Zheng, H.; Liang, L.; Cai, X.; Liao, M. Fetuin A promotes lipotoxicity in β cells through the TLR4 signaling pathway and the role of pioglitazone in anti-lipotoxicity. Mol. Cell. Endocrinol. 2015, 412, 1–11. [Google Scholar] [CrossRef]
- Sardana, O.; Goyal, R.; Bedi, O. Molecular and pathobiological involvement of fetuin-A in the pathogenesis of NAFLD. Inflammopharmacology 2021, 29, 1061–1074. [Google Scholar] [CrossRef]
- Przybyciński, J.; Dziedziejko, V.; Puchałowicz, K.; Domański, L.; Pawlik, A. Adiponectin in Chronic Kidney Disease. Int. J. Mol. Sci. 2020, 21, 9375. [Google Scholar] [CrossRef]
- Zhang, L.-Y.; Qu, X.-N.; Sun, Z.-Y.; Zhang, Y. Effect of liraglutide therapy on serum fetuin A in patients with type 2 diabetes and non-alcoholic fatty liver disease. Clin. Res. Hepatol. Gastroenterol. 2020, 44, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Shulman, G.I. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N. Engl. J. Med. 2014, 371, 2236–2238. [Google Scholar] [CrossRef] [PubMed]
- Stefan, N.; Sun, Q.; Fritsche, A.; Machann, J.; Schick, F.; Gerst, F.; Jeppesen, C.; Joost, H.-G.; Hu, F.B.; Boeing, H.; et al. Impact of the adipokine adiponectin and the hepatokine fetuin-A on the development of type 2 diabetes: Prospective cohort- and cross-sectional phenotyping studies. PLoS ONE 2014, 9, e92238. [Google Scholar] [CrossRef] [PubMed]
- Haukeland, J.W.; Dahl, T.B.; Yndestad, A.; Gladhaug, I.P.; Løberg, E.M.; Haaland, T.; Konopski, Z.; Wium, C.; Aasheim, E.T.; Johansen, O.E.; et al. Fetuin A in nonalcoholic fatty liver disease: In vivo and in vitro studies. Eur. J. Endocrinol. 2012, 166, 503–510. [Google Scholar] [CrossRef]
- Elhoseeny, M.M.; Abdulaziz, B.A.; Mohamed, M.A.; Elsharaby, R.M.; Rashad, G.M.; Othman, A.A.A. Fetuin-A: A relevant novel serum biomarker for non-invasive diagnosis of metabolic dysfunction-associated steatotic liver disease (MASLD): A retrospective case-control study. BMC Gastroenterol. 2024, 24, 226. [Google Scholar] [CrossRef]
- Filardi, T.; Panimolle, F.; Tiberti, C.; Crescioli, C.; Lenzi, A.; Pallotta, N.; Morano, S. Circulating levels of fetuin-A are associated4839with moderate–severe hepatic steatosis in young adults. J. Endocrinol. Investig. 2021, 44, 105–110. [Google Scholar] [CrossRef]
- Das, S.; Chattopadhyay, D.; Chatterjee, S.K.; Mondal, S.A.; Majumdar, S.S.; Mukhopadhyay, S.; Saha, N.; Velayutham, R.; Bhattacharya, S.; Mukherjee, S. Increase in PPARγ inhibitory phosphorylation by Fetuin—A through the activation of Ras-MEK-ERK pathway causes insulin resistance. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2021, 1867, 166050. [Google Scholar] [CrossRef]
- Nevola, R.; Epifani, R.; Imbriani, S.; Tortorella, G.; Aprea, C.; Galiero, R.; Rinaldi, L.; Marfella, R.; Sasso, F.C. GLP-1 Receptor Agonists in Non-Alcoholic Fatty Liver Disease: Current Evidence and Future Perspectives. Int. J. Mol. Sci. 2023, 24, 1703. [Google Scholar] [CrossRef]
- Khadir, A.; Kavalakatt, S.; Madhu, D.; Hammad, M.; Devarajan, S.; Tuomilehto, J.; Tiss, A. Fetuin-A levels are increased in the adipose tissue of diabetic obese humans but not in circulation. Lipids Health Dis. 2018, 17, 291. [Google Scholar] [CrossRef]
- Brix, J.M.; Stingl, H.; Höllerl, F.; Schernthaner, G.H.; Kopp, H.-P. Elevated fetuin-A concentrations in morbid obesity decrease after dramatic weight Loss. J. Clin. Endocrinol. Metab. 2010, 95, 4877–4881. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.-J.; Ser, K.-H.; Lin, M.-T.; Nien, H.-C.; Chen, C.-N.; Yang, W.-S.; Lee, W.-J. Diabetes Associated Markers After Bariatric Surgery: Fetuin-A, but Not Matrix Metalloproteinase-7, Is Reduced. Obes. Surg. 2015, 25, 2328–2334. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.-H.; Li, W.-Y.; Wu, C.-F.; Lee, Y.-C.; Chen, A.Y.-N.; Tyan, Y.-C.; Chen, Y.-M.A. Reversal of high-fat diet-induced non-alcoholic fatty liver disease by metformin combined with PGG, an inducer of glycine N-methyltransferase. Int. J. Mol. Sci. 2022, 23, 10072. [Google Scholar] [CrossRef]
- Huang, J.-W.; Chen, C.-J.; Yen, C.-H.; Chen, Y.-M.A.; Liu, Y.-P. Loss of Glycine N-methyltransferase associates with angiopoietin-like protein 8 expression in high fat-diet-fed mice. Int. J. Mol. Sci. 2019, 20, 4223. [Google Scholar] [CrossRef] [PubMed]
- Esteghamati, A.; Afarideh, M.; Feyzi, S.; Noshad, S.; Nakhjavani, M. Comparative effects of metformin and pioglitazone on fetuin-A and osteoprotegerin concentrations in patients with newly diagnosed diabetes: A randomized clinical trial. Diabetes Metab. Syndr. Clin. Res. Rev. 2015, 9, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Abushamat, L.A.; Shah, P.A.; Eckel, R.H.; Harrison, S.A.; Barb, D. The Emerging Role of Glucagon-Like Peptide-1 Receptor Agonists for the Treatment of Metabolic Dysfunction-Associated Steatohepatitis. Clin. Gastroenterol. Hepatol. 2024, 22, 1565–1574. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Houlihan, D.D.; Rowe, I.A.; Clausen, W.H.O.; Elbrønd, B.; Gough, S.C.L.; Tomlinson, J.W.; Newsome, P.N. Safety and efficacy of liraglutide in patients with type 2 diabetes and elevated liver enzymes: Individual patient data meta-analysis of the LEAD program. Aliment. Pharmacol. Ther. 2013, 37, 234–242. [Google Scholar] [CrossRef]
- Yan, J.; Yao, B.; Kuang, H.; Yang, X.; Huang, Q.; Hong, T.; Li, Y.; Dou, J.; Yang, W.; Qin, G.; et al. Liraglutide, sitagliptin and insulin glargine added to metformin: The effect on body weight and intrahepatic lipid in patients with type 2 diabetes mellitus and NAFLD. Hepatology 2019, 69, 2414–2426. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Gaunt, P.; Aithal, G.P.; Barton, D.; Hull, D.; Parker, R.; Hazlehurst, J.M.; Guo, K.; Abouda, G.; Aldersley, M.A.; et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): A multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet 2016, 387, 679–690. [Google Scholar] [CrossRef]
- Chavez, C.P.; Cusi, K.; Kadiyala, S. The Emerging Role of Glucagon-like Peptide-1 Receptor Agonists for the Management of NAFLD. J. Clin. Endocrinol. Metab. 2022, 107, 29–38. [Google Scholar] [CrossRef]
- Yabut, J.M.; Drucker, D.J. Glucagon-like Peptide-1 Receptor-based Therapeutics for Metabolic Liver Disease. Endocr. Rev. 2023, 44, 14–32. [Google Scholar] [CrossRef] [PubMed]
- Perakakis, N.; Stefanakis, K.; Feigh, M.; Veidal, S.S.; Mantzoros, C.S. Elafibranor and liraglutide improve differentially liver health and metabolism in a mouse model of non-alcoholic steatohepatitis. Liver Int. 2021, 41, 1853–1866. [Google Scholar] [CrossRef]
- Newsome, P.N.; Buchholtz, K.; Cusi, K.; Linder, M.; Okanoue, T.; Ratziu, V.; Sanyal, A.J.; Sejling, A.-S.; Harrison, S.A. A Placebo-Controlled Trial of Subcutaneous Semaglutide in Nonalcoholic Steatohepatitis. N. Engl. J. Med. 2021, 384, 1113–1124. [Google Scholar] [CrossRef]
- Pyke, C.; Heller, R.S.; Kirk, R.K.; Ørskov, C.; Reedtz-Runge, S.; Kaastrup, P.; Hvelplund, A.; Bardram, L.; Calatayud, D.; Knudsen, L.B. GLP-1 Receptor localization in monkey and human tissue: Novel distribution revealed with extensively validated monoclonal antibody. Endocrinology 2014, 155, 1280–1290. [Google Scholar] [CrossRef]
- Panjwani, N.; Mulvihill, E.E.; Longuet, C.; Yusta, B.; Campbell, J.E.; Brown, T.J.; Streutker, C.; Holland, D.; Cao, X.; Baggio, L.L.; et al. GLP-1 receptor activation indirectly reduces hepatic lipid accumulation but does not attenuate development of atherosclerosis in diabetic male apoe−/− mice. Endocrinology 2013, 154, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.A.; Mells, J.; Dunham, R.M.; Grakoui, A.; Handy, J.; Saxena, N.K.; Anania, F.A. Glucagon-like peptide-1 receptor is present on human hepatocytes and has a direct role in decreasing hepatic steatosis in vitro by modulating elements of the insulin signaling pathway. Hepatology 2010, 51, 1584–1592. [Google Scholar] [CrossRef]
- Fang, Y.; Ji, L.; Zhu, C.; Xiao, Y.; Zhang, J.; Lu, J.; Yin, J.; Wei, L. Liraglutide Alleviates Hepatic Steatosis by Activating the TFEB-Regulated Autophagy-Lysosomal Pathway. Front. Cell Dev. Biol. 2020, 8, 602574. [Google Scholar] [CrossRef] [PubMed]
- Yokomori, H.; Ando, W. Spatial expression of glucagon-like peptide 1 receptor and caveolin-1 in hepatocytes with macrovesicular steatosis in non-alcoholic steatohepatitis. BMJ Open Gastroenterol. 2020, 7, e000370. [Google Scholar] [CrossRef]
- Svegliati-Baroni, G.; Saccomanno, S.; Rychlicki, C.; Agostinelli, L.; De Minicis, S.; Candelaresi, C.; Faraci, G.; Pacetti, D.; Vivarelli, M.; Nicolini, D.; et al. Glucagon-like peptide-1 receptor activation stimulates hepatic lipid oxidation and restores hepatic signalling alteration induced by a high-fat diet in nonalcoholic steatohepatitis. Liver Int. 2011, 31, 1285–1297. [Google Scholar] [CrossRef]
- Astapova, O.; Leff, T. Adiponectin and PPARgamma: Cooperative and interdependent actions of two key regulators of metabolism. Vitam. Horm. 2012, 90, 143–162. [Google Scholar] [CrossRef]
- Simental-Mendía, L.E.; Sánchez-García, A.; Linden-Torres, E.; Simental-Mendía, M. Impact of glucagon-like peptide-1 receptor agonists on adiponectin concentrations: A meta-analysis of randomized controlled trials. Br. J. Clin. Pharmacol. 2021, 87, 4140–4149. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, M.M.I. Therapeutic potential of adiponectin in prediabetes: Strategies, challenges, and future directions. Ther. Adv. Endocrinol. Metab. 2024, 15, 20420188231222371. [Google Scholar] [CrossRef] [PubMed]
- Gastaldelli, A.; Sabatini, S.; Carli, F.; Gaggini, M.; Bril, F.; Belfort-DeAguiar, R.; Positano, V.; Barb, D.; Kadiyala, S.; Harrison, S.; et al. PPAR-γ-induced changes in visceral fat and adiponectin levels are associated with improvement of steatohepatitis in patients with NASH. Liver Int. 2021, 41, 2659–2670. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, K.; Yang, J.; Xiao, W.; Le, Y.; Yu, F.; Gu, L.; Lang, S.; Tian, Q.; Jin, T.; et al. Liver-derived fibroblast growth factor 21 mediates effects of glucagon-like peptide-1 in attenuating hepatic glucose output. EBioMedicine 2019, 41, 73–84. [Google Scholar] [CrossRef]
- Nonogaki, K.; Hazama, M.; Satoh, N. Liraglutide Suppresses Obesity and Hyperglycemia Associated with Increases in Hepatic Fibroblast Growth Factor 21 Production in KKAyMice. BioMed Res. Int. 2014, 2014, 751930. [Google Scholar] [CrossRef]
- Li, X.; Wu, X.; Jia, Y.; Fu, J.; Zhang, L.; Jiang, T.; Liu, J.; Wang, G. Liraglutide Decreases Liver Fat Content and Serum Fibroblast Growth Factor 21 Levels in Newly Diagnosed Overweight Patients with Type 2 Diabetes and Nonalcoholic Fatty Liver Disease. J. Diabetes Res. 2021, 2021, 3715026. [Google Scholar] [CrossRef]
- Li, D.; Xu, X.; Zhang, Y.; Zhu, J.; Ye, L.; Lee, K.O.; Ma, J. Liraglutide treatment causes upregulation of adiponectin and downregulation of resistin in Chinese type 2 diabetes. Diabetes Res. Clin. Pract. 2015, 110, 224–228. [Google Scholar] [CrossRef]
- Simental-Mendía, L.E.; Simental-Mendía, M.; Barragán-Zúñiga, L.J.; Navarro-Tinoco, L. Effect of tirzepatide on leptin and adiponectin levels. Eur. J. Intern. Med. 2024; online ahead of print. [Google Scholar] [CrossRef]
- Savvidou, S.; Karatzidou, K.; Tsakiri, K.; Gagalis, A.; Hytiroglou, P.; Goulis, J. Circulating adiponectin levels in type 2 diabetes mellitus patients with or without non-alcoholic fatty liver disease: Results of a small, open-label, randomized controlled intervention trial in a subgroup receiving short-term exenatide. Diabetes Res. Clin. Pract. 2016, 113, 125–134. [Google Scholar] [CrossRef]
- Chung, L.T.K.; Hosaka, T.; Yoshida, M.; Harada, N.; Sakaue, H.; Sakai, T.; Nakaya, Y. Exendin-4, a GLP-1 receptor agonist, directly induces adiponectin expression through protein kinase A pathway and prevents inflammatory adipokine expression. Biochem. Biophys. Res. Commun. 2009, 390, 613–618. [Google Scholar] [CrossRef]
- Onuma, H.; Inukai, K.; Kitahara, A.; Moriya, R.; Nishida, S.; Tanaka, T.; Katsuta, H.; Takahashi, K.; Sumitani, Y.; Hosaka, T.; et al. The glucagon-like peptide 1 receptor agonist enhances intrinsic peroxisome proliferator-activated receptor γ activity in endothelial cells. Biochem. Biophys. Res. Commun. 2014, 451, 339–344. [Google Scholar] [CrossRef]
- Zhou, J.Y.; Poudel, A.; Welchko, R.; Mekala, N.; Chandramani-Shivalingappa, P.; Rosca, M.G.; Li, L. Liraglutide improves insulin sensitivity in high fat diet induced diabetic mice through multiple pathways. Eur. J. Pharmacol. 2019, 861, 172594. [Google Scholar] [CrossRef] [PubMed]
- Challa, T.D.; Beaton, N.; Arnold, M.; Rudofsky, G.; Langhans, W.; Wolfrum, C. Regulation of adipocyte formation by GLP-1/GLP-1R signaling. J. Biol. Chem. 2012, 287, 6421–6430. [Google Scholar] [CrossRef] [PubMed]
- Ochi, A.; Mori, K.; Emoto, M.; Nakatani, S.; Morioka, T.; Motoyama, K.; Fukumoto, S.; Imanishi, Y.; Koyama, H.; Ishimura, E.; et al. Direct inhibitory effects of pioglitazone on hepatic fetuin-A expression. PLoS ONE 2014, 9, e88704. [Google Scholar] [CrossRef]
- Zhou, Z.; Chen, H.; Sun, M.; Jin, H.; Ju, H. Fetuin-A to adiponectin ratio is an independent indicator of subclinical atherosclerosis in patients with newly diagnosed type 2 diabetes mellitus. J. Diabetes its Complicat. 2022, 36, 108102. [Google Scholar] [CrossRef]
- Jung, T.W.; Youn, B.-S.; Choi, H.Y.; Lee, S.Y.; Hong, H.C.; Yang, S.J.; Yoo, H.J.; Kim, B.-H.; Baik, S.H.; Choi, K.M. Salsalate and adiponectin ameliorate hepatic steatosis by inhibition of the hepatokine fetuin-A. Biochem. Pharmacol. 2013, 86, 960–969. [Google Scholar] [CrossRef]
- Zhang, L.-Y.; Liu, T.; Teng, Y.-Q.; Yao, X.-Y.; Zhao, T.-T.; Lin, L.-Y.; Jin, Q.-S.; Jin, Y.-J. Effect of a 12-Week Aerobic Exercise Training on Serum Fetuin-A and Adipocytokine Levels in Type 2 Diabetes. Exp. Clin. Endocrinol. Diabetes 2017, 126, 487–492. [Google Scholar] [CrossRef]
- Harrison, S.A.; Rolph, T.; Knott, M.; Dubourg, J. FGF21 agonists: An emerging therapeutic for metabolic dysfunction-associated steatohepatitis and beyond. J. Hepatol. 2024, 81, 562–576. [Google Scholar] [CrossRef]
- Tacke, F.; Horn, P.; Wong, V.W.-S.; Ratziu, V.; Bugianesi, E.; Francque, S.; Zelber-Sagi, S.; Valenti, L.; Roden, M.; Schick, F.; et al. EASL–EASD–EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J. Hepatol. 2024, 81, 492–542. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Choi, H.; Kim, D.; Kim, T.; Tølbøl, K.; Feigh, M.; Illemann, M.; Rigbolt, K.; Vrang, N.; Kim, J. YH25724, a novel long-acting GLP-1/FGF21 dual agonist lowers both non-alcoholic fatty liver disease activity score and fibrosis stage in a diet-induced obese mouse model of biopsy-confirmed non-alcoholic steatohepatitis. J. Hepatol. 2017, 66, S16–S17. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, G.Y.; Maeng, H.J.; Kim, H.; Bae, J.H.; Kim, K.M.; Lim, S. Effects of Glucagon-Like Peptide-1 Analogue and Fibroblast Growth Factor 21 Combination on the Atherosclerosis-Related Process in a Type 2 Diabetes Mouse Model. Endocrinol. Metab. 2021, 36, 157–170. [Google Scholar] [CrossRef]
- Gilroy, C.A.; Capozzi, M.E.; Varanko, A.K.; Tong, J.; D’Alessio, D.A.; Campbell, J.E.; Chilkoti, A. Sustained release of a GLP-1 and FGF21 dual agonist from an injectable depot protects mice from obesity and hyperglycemia. Sci. Adv. 2020, 6, eaaz9890. [Google Scholar] [CrossRef] [PubMed]
- Amatya, R.; Lee, D.; Min, K.A.; Shin, M.C. Pharmaceutical Strategies to Improve Druggability of Potential Drug Candidates in Nonalcoholic Fatty Liver Disease Therapy. Pharmaceutics 2023, 15, 1963. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y.; Zhao, C.; Lin, S.; Guo, W.; Wu, B. 297-OR: A Novel GLP-1/FGF21 Dual Agonist ZT003 Has Therapeutic Potential for Obesity, Diabetes, and Nonalcoholic Steatohepatitis. Diabetes 2024, 73, 297. [Google Scholar] [CrossRef]
- Pan, Q.; Lin, S.; Li, Y.; Liu, L.; Li, X.; Gao, X.; Yan, J.; Gu, B.; Chen, X.; Li, W.; et al. A novel GLP-1 and FGF21 dual agonist has therapeutic potential for diabetes and non-alcoholic steatohepatitis. EBioMedicine 2021, 63, 103202. [Google Scholar] [CrossRef] [PubMed]
- Sonoda, J.; Chen, M.Z.; Baruch, A. FGF21-receptor agonists: An emerging therapeutic class for obesity-related diseases. Horm. Mol. Biol. Clin. Investig. 2017, 30, 20170002. [Google Scholar] [CrossRef]
- Shao, W.; Jin, T. Hepatic hormone FGF21 and its analogues in clinical trials. Chronic Dis. Transl. Med. 2022, 8, 19–25. [Google Scholar] [CrossRef]
- Agarwal, S.; Chattopadhyay, M.; Mukherjee, S.; Dasgupta, S.; Mukhopadhyay, S.; Bhattacharya, S. Fetuin-A downregulates adiponectin through Wnt-PPARγ pathway in lipid induced inflamed adipocyte. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2017, 1863, 174–181. [Google Scholar] [CrossRef]
- Feng, J.N.; Shao, W.; Jin, T. Short-term semaglutide treatment improves FGF21 responsiveness in primary hepatocytes isolated from high fat diet challenged mice. Physiol. Rep. 2023, 11, e15620. [Google Scholar] [CrossRef]
- Hartman, M.L.; Sanyal, A.J.; Loomba, R.; Wilson, J.M.; Nikooienejad, A.; Bray, R.; Karanikas, C.A.; Duffin, K.L.; Robins, D.A.; Haupt, A. Effects of Novel Dual GIP and GLP-1 Receptor Agonist Tirzepatide on Biomarkers of Nonalcoholic Steatohepatitis in Patients With Type 2 Diabetes. Diabetes Care 2020, 43, 1352–1355. [Google Scholar] [CrossRef]

| Expression | Change with Steatosis | Metabolic Functions | Reference | |
|---|---|---|---|---|
| ANGPTL3 | Liver | Increased | Inhibits LPL activity, increases plasma triglycerides TG and FFA, and increases TG-VLDL uptake. Decreases glucose uptake and promotes insulin resistance. Increases cardiovascular risk. | [6,28,37,40,41] |
| ANGPTL4 | Liver Skeletal muscle Heart | Debated | Inhibits LPL activity, increases lipolysis, downregulates blood TG clearance, and causes hepatic steatosis. Role in regulation of glucose metabolism is unclear. | [23,37,42,43] |
| ANGPTL6 | Liver | Increased with impairment signaling (resistance) | Increases insulin sensitivity and energy expenditure (muscle). Decreases gluconeogenesis (liver). Increases AMPK. | [6,34,37] |
| IGF1 | Several tissues (especially liver) | Decreased | Improves insulin sensitivity (muscle). | [6,37] |
| SeP | Liver | Increased | Interferes with insulin signaling and glucose metabolism, increases insulin resistance, impairs β-cell function, and decreases glucose uptake (muscle). Causes carotid intima media thickness. | [6,23,34,37,44,45] |
| LECT2 | Liver (mainly) WAT, neurons, and white blood cells | Increased | Interferes with insulin signaling. Promotes lipid accumulation (liver). Increases liver inflammation and fibrosis (muscle). | [6,28,46,47] |
| SHBG | Liver | Decreased | ER protection. Downregulate lipogenesis Reduced hepatic steatosis, inflammation | [31,44,48] |
| FGF21 | Liver (mainly) Intestine, heart, kidney, pancreas, WAT, and BAT | Increased | Promotes glucose uptake (WAT and muscle). Stimulates thermogenesis (BAT). Increases FFA oxidation and insulin sensitivity (liver, muscle). Decreases steatosis, VLDL uptake, and lipogenesis (liver). Decreases alcohol and sugar intake. Increases energy expenditure and decreases body weight (CNS). Increases insulin secretion (beta cells). | [24,49,50,51,52,53,54,55] |
| Fetuin-A | Liver (mainly) WAT | Increased | Promotes inflammation (monocytes and adipocytes). Inhibits adiponectin production. Interferes with insulin receptor phosphorylation (liver, WAT, and skeletal muscle) and causes insulin resistance. | [28,37,56,57,58] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milani, I.; Codini, M.; Guarisco, G.; Chinucci, M.; Gaita, C.; Leonetti, F.; Capoccia, D. Hepatokines and MASLD: The GLP1-Ras-FGF21-Fetuin-A Crosstalk as a Therapeutic Target. Int. J. Mol. Sci. 2024, 25, 10795. https://doi.org/10.3390/ijms251910795
Milani I, Codini M, Guarisco G, Chinucci M, Gaita C, Leonetti F, Capoccia D. Hepatokines and MASLD: The GLP1-Ras-FGF21-Fetuin-A Crosstalk as a Therapeutic Target. International Journal of Molecular Sciences. 2024; 25(19):10795. https://doi.org/10.3390/ijms251910795
Chicago/Turabian StyleMilani, Ilaria, Michela Codini, Gloria Guarisco, Marianna Chinucci, Chiara Gaita, Frida Leonetti, and Danila Capoccia. 2024. "Hepatokines and MASLD: The GLP1-Ras-FGF21-Fetuin-A Crosstalk as a Therapeutic Target" International Journal of Molecular Sciences 25, no. 19: 10795. https://doi.org/10.3390/ijms251910795
APA StyleMilani, I., Codini, M., Guarisco, G., Chinucci, M., Gaita, C., Leonetti, F., & Capoccia, D. (2024). Hepatokines and MASLD: The GLP1-Ras-FGF21-Fetuin-A Crosstalk as a Therapeutic Target. International Journal of Molecular Sciences, 25(19), 10795. https://doi.org/10.3390/ijms251910795






