The Versatile Roles of nc886, a Fascinating and Peculiar Regulatory Non-Coding RNA, in Cancer
Abstract
1. Introduction
1.1. Gene Expression, Non-Coding RNAs (ncRNAs), and Cancer
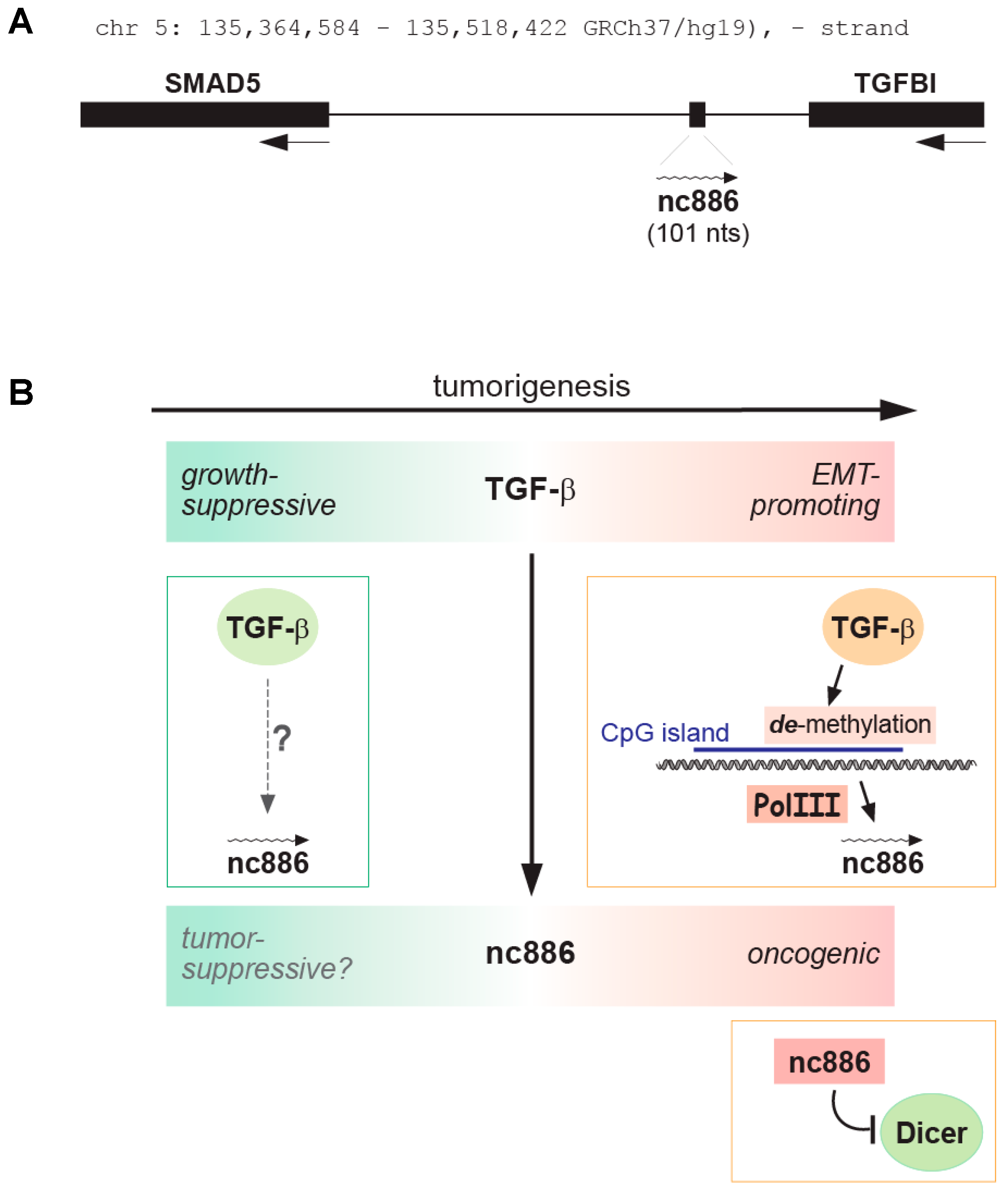
1.2. Introduction of nc886
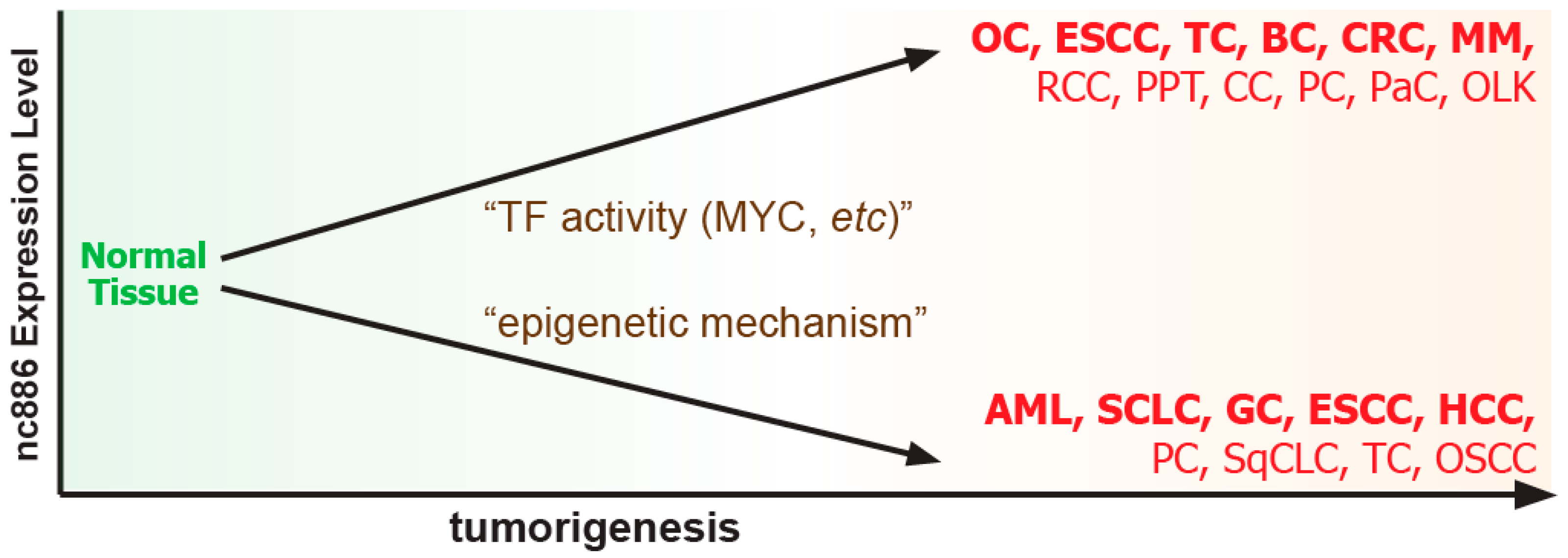
2. The Role of nc886 in Cancer
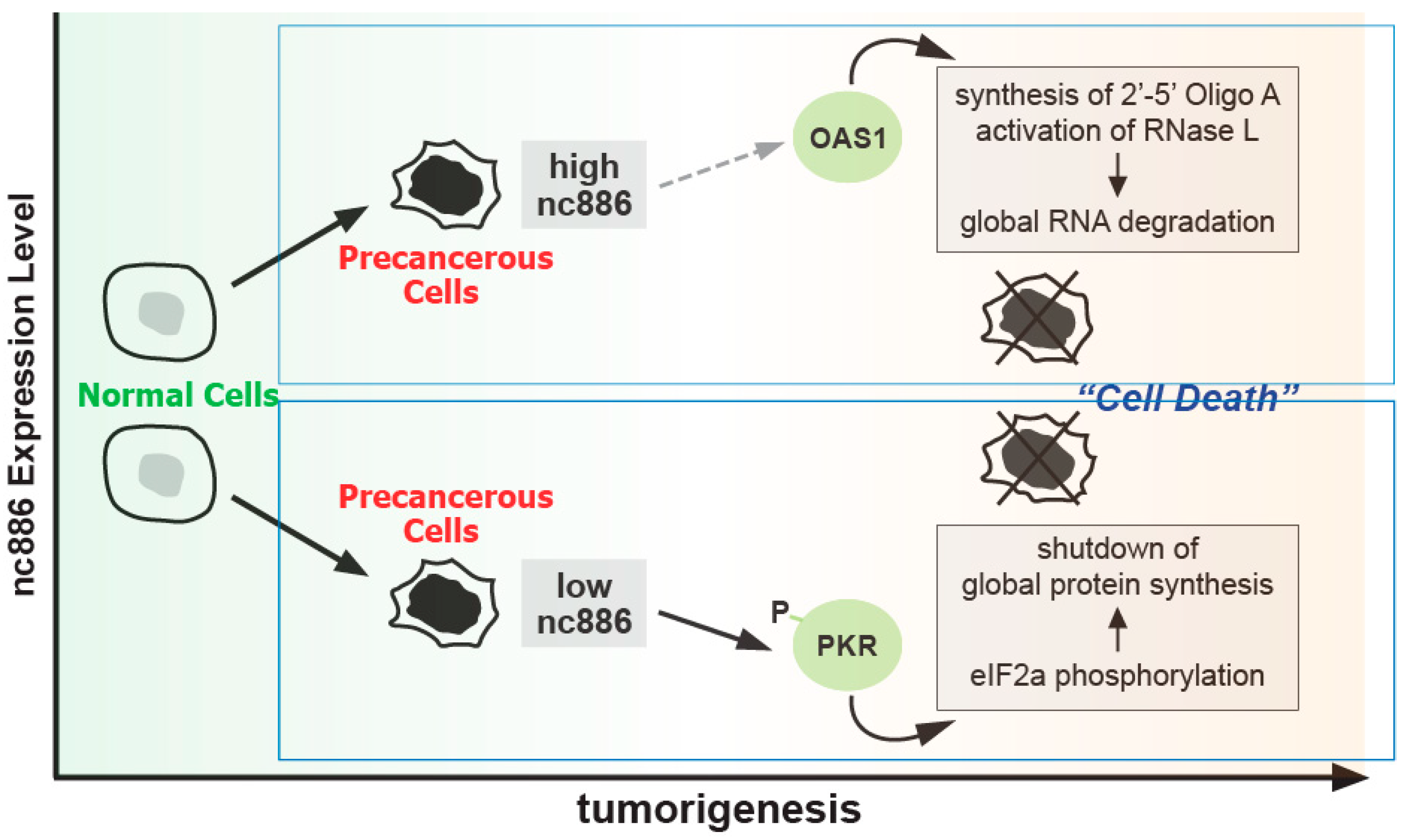
2.1. nc886 as a Tumor Surveillant
2.2. nc886′s Oncogenic Roles
| Cancer Type | Approach | Method | Cellular Phenotype upon Ectopic Expression or KD of nc886 | Ref. |
|---|---|---|---|---|
| ovarian cancer (OC) | ectopic expression | plasmid-based (from Pol III promoters) |
| [14] |
| KD | modified anti-oligo * |
| ||
| renal cell carcinoma (RCC) | ectopic expression | synthetic nc886 mimic RNA ** |
| [45] |
| ||||
| KD | synthetic nc886 inhibitor RNA ** |
| ||
| ||||
| thyroid cancer (TC) | KO | CRISPR-Cas9 *** |
| [31] |
| oropharyngeal squamous cell carcinoma (OPSCC) | KO | CRISPR-Cas9 *** |
| [44] |
2.3. nc886′s Tumor Suppressive Roles
2.4. The Effect of nc886 in the Cancer Therapy
3. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Chakravarthi, B.V.; Nepal, S.; Varambally, S. Genomic and Epigenomic Alterations in Cancer. Am. J. Pathol. 2016, 186, 1724–1735. [Google Scholar] [CrossRef] [PubMed]
- Ratti, M.; Lampis, A.; Ghidini, M.; Salati, M.; Mirchev, M.B.; Valeri, N.; Hahne, J.C. MicroRNAs (miRNAs) and Long Non-Coding RNAs (lncRNAs) as New Tools for Cancer Therapy: First Steps from Bench to Bedside. Target. Oncol. 2020, 15, 261–278. [Google Scholar] [CrossRef] [PubMed]
- White, R.J. RNA polymerases I and III, growth control and cancer. Nat. Rev. Mol. Cell Biol. 2005, 6, 69–78. [Google Scholar] [CrossRef]
- Zhou, S.; Van Bortle, K. The Pol III transcriptome: Basic features, recurrent patterns, and emerging roles in cancer. Wiley Interdiscip. Rev. RNA 2023, 14, e1782. [Google Scholar] [CrossRef]
- Lee, Y.S.; Lee, Y.S. nc886, an RNA Polymerase III-Transcribed Noncoding RNA Whose Expression Is Dynamic and Regulated by Intriguing Mechanisms. Int. J. Mol. Sci. 2023, 24, 8533. [Google Scholar] [CrossRef]
- Lee, Y.S. Are We Studying Non-Coding RNAs Correctly? Lessons from nc886. Int. J. Mol. Sci. 2022, 23, 4251. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuka, M.; Ling, H.; Doki, Y.; Mori, M.; Calin, G.A. MicroRNA Processing and Human Cancer. J. Clin. Med. 2015, 4, 1651–1667. [Google Scholar] [CrossRef]
- Watanabe, T.; Imamura, T.; Hiasa, Y. Roles of protein kinase R in cancer: Potential as a therapeutic target. Cancer Sci. 2018, 109, 919–925. [Google Scholar] [CrossRef]
- Jiang, S.; Deng, X.; Luo, M.; Zhou, L.; Chai, J.; Tian, C.; Yan, Y.; Luo, Z. Pan-cancer analysis identified OAS1 as a potential prognostic biomarker for multiple tumor types. Front. Oncol. 2023, 13, 1207081. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef]
- Mattick, J.S.; Amaral, P.P.; Carninci, P.; Carpenter, S.; Chang, H.Y.; Chen, L.L.; Chen, R.; Dean, C.; Dinger, M.E.; Fitzgerald, K.A.; et al. Long non-coding RNAs: Definitions, functions, challenges and recommendations. Nat. Rev. Mol. Cell Biol. 2023, 24, 430–447. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Kunkeaw, N.; Jeon, S.H.; Lee, I.; Johnson, B.H.; Kang, G.Y.; Bang, J.Y.; Park, H.S.; Leelayuwat, C.; Lee, Y.S. Precursor miR-886, a novel noncoding RNA repressed in cancer, associates with PKR and modulates its activity. RNA 2011, 17, 1076–1089. [Google Scholar] [CrossRef] [PubMed]
- Im, W.R.; Lee, H.S.; Lee, Y.S.; Lee, J.S.; Jang, H.J.; Kim, S.Y.; Park, J.L.; Lee, Y.; Kim, M.S.; Lee, J.M.; et al. A Regulatory Noncoding RNA, nc886, Suppresses Esophageal Cancer by Inhibiting the AKT Pathway and Cell Cycle Progression. Cells 2020, 9, 801. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Lee, H.S.; Lee, J.S.; Lee, Y.S.; Park, J.L.; Kim, S.Y.; Hwang, J.A.; Kunkeaw, N.; Jung, S.Y.; Kim, T.J.; et al. nc886 is induced by TGF-beta and suppresses the microRNA pathway in ovarian cancer. Nat. Commun. 2018, 9, 1166. [Google Scholar] [CrossRef]
- Yang, R.; Zuo, L.; Ma, H.; Zhou, Y.; Zhou, P.; Wang, L.; Wang, M.; Latif, M.; Kong, L. Downregulation of nc886 contributes to prostate cancer cell invasion and TGFbeta1-induced EMT. Genes. Dis. 2022, 9, 1086–1098. [Google Scholar] [CrossRef]
- Kunkeaw, N.; Lee, Y.S.; Im, W.R.; Jang, J.J.; Song, M.J.; Yang, B.; Park, J.L.; Kim, S.Y.; Ku, Y.; Kim, Y.; et al. Mechanism mediated by a noncoding RNA, nc886, in the cytotoxicity of a DNA-reactive compound. Proc. Natl. Acad. Sci. USA 2019, 116, 8289–8294. [Google Scholar] [CrossRef]
- Calderon, B.M.; Conn, G.L. A human cellular noncoding RNA activates the antiviral protein 2′-5′-oligoadenylate synthetase 1. J. Biol. Chem. 2018, 293, 16115–16124. [Google Scholar] [CrossRef]
- Yoo, B.H.; Bochkareva, E.; Bochkarev, A.; Mou, T.C.; Gray, D.M. 2′-O-methyl-modified phosphorothioate antisense oligonucleotides have reduced non-specific effects in vitro. Nucleic Acids Res. 2004, 32, 2008–2016. [Google Scholar] [CrossRef]
- Ideue, T.; Hino, K.; Kitao, S.; Yokoi, T.; Hirose, T. Efficient oligonucleotide-mediated degradation of nuclear noncoding RNAs in mammalian cultured cells. RNA 2009, 15, 1578–1587. [Google Scholar] [CrossRef][Green Version]
- Wahlestedt, C.; Salmi, P.; Good, L.; Kela, J.; Johnsson, T.; Hokfelt, T.; Broberger, C.; Porreca, F.; Lai, J.; Ren, K.; et al. Potent and nontoxic antisense oligonucleotides containing locked nucleic acids. Proc. Natl. Acad. Sci. USA 2000, 97, 5633–5638. [Google Scholar] [CrossRef]
- Golec, E.; Lind, L.; Qayyum, M.; Blom, A.M.; King, B.C. The Noncoding RNA nc886 Regulates PKR Signaling and Cytokine Production in Human Cells. J. Immunol. 2019, 202, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Sallustio, F.; Serino, G.; Cox, S.N.; Dalla Gassa, A.; Curci, C.; De Palma, G.; Banelli, B.; Zaza, G.; Romani, M.; Schena, F.P. Aberrantly methylated DNA regions lead to low activation of CD4+ T-cells in IgA nephropathy. Clin. Sci. 2016, 130, 733–746. [Google Scholar] [CrossRef] [PubMed]
- Pillai, M.M.; Yang, X.; Balakrishnan, I.; Bemis, L.; Torok-Storb, B. MiR-886-3p down regulates CXCL12 (SDF1) expression in human marrow stromal cells. PLoS ONE 2010, 5, e14304. [Google Scholar] [CrossRef] [PubMed]
- Nordentoft, I.; Birkenkamp-Demtroder, K.; Agerbaek, M.; Theodorescu, D.; Ostenfeld, M.S.; Hartmann, A.; Borre, M.; Orntoft, T.F.; Dyrskjot, L. miRNAs associated with chemo-sensitivity in cell lines and in advanced bladder cancer. BMC Med. Genom. 2012, 5, 40. [Google Scholar] [CrossRef]
- Ma, X.X.; Xiao, L.; Wen, S.J.; Yu, T.X.; Sharma, S.; Chung, H.K.; Warner, B.; Mallard, C.G.; Rao, J.N.; Gorospe, M.; et al. Small noncoding vault RNA2-1 disrupts gut epithelial barrier function via interaction with HuR. EMBO Rep. 2023, 24, e54925. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Song, Y.; Bi, N.; Shen, J.; Liu, W.; Fan, J.; Sun, G.; Tong, T.; He, J.; Shi, Y.; et al. DNA methylation-mediated repression of miR-886-3p predicts poor outcome of human small cell lung cancer. Cancer Res. 2013, 73, 3326–3335. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kunkeaw, N.; Lee, Y.S. Protein kinase R and its cellular regulators in cancer: An active player or a surveillant? Wiley Interdiscip. Rev. RNA 2020, 11, e1558. [Google Scholar] [CrossRef]
- Jeon, S.H.; Lee, K.; Lee, K.S.; Kunkeaw, N.; Johnson, B.H.; Holthauzen, L.M.; Gong, B.; Leelayuwat, C.; Lee, Y.S. Characterization of the direct physical interaction of nc886, a cellular non-coding RNA, and PKR. FEBS Lett. 2012, 586, 3477–3484. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, H.; Tang, L.; Lou, M.; Geng, Y. Silencing nc886, a Non-Coding RNA, Induces Apoptosis of Human Endometrial Cancer Cells-1A In Vitro. Med. Sci. Monit. 2017, 23, 1317–1324. [Google Scholar] [CrossRef][Green Version]
- Yu, Z.; Chen, D.; Su, Z.; Li, Y.; Yu, W.; Zhang, Q.; Yang, L.; Li, C.; Yang, S.; Ni, L.; et al. miR-886-3p upregulation in clear cell renal cell carcinoma regulates cell migration, proliferation and apoptosis by targeting PITX1. Int. J. Mol. Med. 2014, 34, 1409–1416. [Google Scholar] [CrossRef]
- Lee, E.K.; Hong, S.H.; Shin, S.; Lee, H.S.; Lee, J.S.; Park, E.J.; Choi, S.S.; Min, J.W.; Park, D.; Hwang, J.A.; et al. nc886, a non-coding RNA and suppressor of PKR, exerts an oncogenic function in thyroid cancer. Oncotarget 2016, 7, 75000–75012. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, K.S.; Park, J.L.; Lee, K.; Richardson, L.E.; Johnson, B.H.; Lee, H.S.; Lee, J.S.; Kim, S.B.; Kwon, O.H.; Song, K.S.; et al. nc886, a non-coding RNA of anti-proliferative role, is suppressed by CpG DNA methylation in human gastric cancer. Oncotarget 2014, 5, 3944–3955. [Google Scholar] [CrossRef] [PubMed]
- Kunkeaw, N.; Jeon, S.H.; Lee, K.; Johnson, B.H.; Tanasanvimon, S.; Javle, M.; Pairojkul, C.; Chamgramol, Y.; Wongfieng, W.; Gong, B.; et al. Cell death/proliferation roles for nc886, a non-coding RNA, in the protein kinase R pathway in cholangiocarcinoma. Oncogene 2013, 32, 3722–3731. [Google Scholar] [CrossRef]
- Kim, Y.; Sim, J.; Jeon, K.; Ryu, D.; Ji, Y.; Kim, Y.; Kim, J.; Jeon, S.; Park, D.; Jung, E. Fermented black ginseng extract prevents UVB-induced inflammation by regulating the nc886-PKR pathway in human keratinocytes. Photodermatol. Photoimmunol. Photomed. 2024, 40. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.H.; Johnson, B.H.; Lee, Y.S. A tumor surveillance model: A non-coding RNA senses neoplastic cells and its protein partner signals cell death. Int. J. Mol. Sci. 2012, 13, 13134–13139. [Google Scholar] [CrossRef] [PubMed]
- Brennan-Laun, S.E.; Ezelle, H.J.; Li, X.L.; Hassel, B.A. RNase-L control of cellular mRNAs: Roles in biologic functions and mechanisms of substrate targeting. J. Interferon Cytokine Res. 2014, 34, 275–288. [Google Scholar] [CrossRef]
- Lee, H.S.; Lee, K.; Jang, H.J.; Lee, G.K.; Park, J.L.; Kim, S.Y.; Kim, S.B.; Johnson, B.H.; Zo, J.I.; Lee, J.S.; et al. Epigenetic silencing of the non-coding RNA nc886 provokes oncogenes during human esophageal tumorigenesis. Oncotarget 2014, 5, 3472–3481. [Google Scholar] [CrossRef]
- Stadler, P.F.; Chen, J.J.; Hackermuller, J.; Hoffmann, S.; Horn, F.; Khaitovich, P.; Kretzschmar, A.K.; Mosig, A.; Prohaska, S.J.; Qi, X.; et al. Evolution of vault RNAs. Mol. Biol. Evol. 2009, 26, 1975–1991. [Google Scholar] [CrossRef]
- Saruuldalai, E.; Park, J.; Kang, D.; Shin, S.P.; Im, W.R.; Lee, H.H.; Jang, J.J.; Park, J.L.; Kim, S.Y.; Hwang, J.A.; et al. A host non-coding RNA, nc886, plays a pro-viral role by promoting virus trafficking to the nucleus. Mol. Ther. Oncolytics 2022, 24, 683–694. [Google Scholar] [CrossRef]
- Park, J.L.; Lee, Y.S.; Song, M.J.; Hong, S.H.; Ahn, J.H.; Seo, E.H.; Shin, S.P.; Lee, S.J.; Johnson, B.H.; Stampfer, M.R.; et al. Epigenetic regulation of RNA polymerase III transcription in early breast tumorigenesis. Oncogene 2017, 36, 6793–6804. [Google Scholar] [CrossRef]
- Cole, M.D. The myc oncogene: Its role in transformation and differentiation. Annu. Rev. Genet. 1986, 20, 361–384. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Roman, N.; Grandori, C.; Eisenman, R.N.; White, R.J. Direct activation of RNA polymerase III transcription by c-Myc. Nature 2003, 421, 290–294. [Google Scholar] [CrossRef]
- Roberts, A.B.; Wakefield, L.M. The two faces of transforming growth factor beta in carcinogenesis. Proc. Natl. Acad. Sci. USA 2003, 100, 8621–8623. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Z.; Wei, P.; Gairola, R.; Kelsey, K.T.; Sikora, A.G.; Li, G.; Gu, J. Hypermethylation of nc886 in HPV-positive oropharyngeal cancer and its clinical implications: An epigenome-wide association study. Mol. Ther. Nucleic Acids 2022, 30, 596–605. [Google Scholar] [CrossRef]
- Lei, J.; Xiao, J.H.; Zhang, S.H.; Liu, Z.Q.; Huang, K.; Luo, Z.P.; Xiao, X.L.; Hong, Z.D. Non-coding RNA 886 promotes renal cell carcinoma growth and metastasis through the Janus kinase 2/signal transducer and activator of transcription 3 signaling pathway. Mol. Med. Rep. 2017, 16, 4273–4278. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, N.R.; Mayall, E.S.; Jones, T.; Sheer, D.; McDermid, S.; Kendall-Taylor, P.; Wynford-Thomas, D. Characterisation of human thyroid epithelial cells immortalised in vitro by simian virus 40 DNA transfection. Br. J. Cancer 1989, 60, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Fogh, J.; Fogh, J.M.; Orfeo, T. One hundred and twenty-seven cultured human tumor cell lines producing tumors in nude mice. J. Natl. Cancer Inst. 1977, 59, 221–226. [Google Scholar] [CrossRef]
- Rangan, S.R. A new human cell line (FaDu) from a hypopharyngeal carcinoma. Cancer 1972, 29, 117–121. [Google Scholar] [CrossRef]
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT signaling pathway: From bench to clinic. Signal Transduct. Target. Ther. 2021, 6, 402. [Google Scholar] [CrossRef]
- Esteller, M. CpG island hypermethylation and tumor suppressor genes: A booming present, a brighter future. Oncogene 2002, 21, 5427–5440. [Google Scholar] [CrossRef]
- Shao, G.; Berenguer, J.; Borczuk, A.C.; Powell, C.A.; Hei, T.K.; Zhao, Y. Epigenetic inactivation of Betaig-h3 gene in human cancer cells. Cancer Res. 2006, 66, 4566–4573. [Google Scholar] [CrossRef]
- Fort, R.S.; Matho, C.; Geraldo, M.V.; Ottati, M.C.; Yamashita, A.S.; Saito, K.C.; Leite, K.R.M.; Mendez, M.; Maedo, N.; Mendez, L.; et al. Nc886 is epigenetically repressed in prostate cancer and acts as a tumor suppressor through the inhibition of cell growth. BMC Cancer 2018, 18, 127. [Google Scholar] [CrossRef] [PubMed]
- Treppendahl, M.B.; Qiu, X.; Sogaard, A.; Yang, X.; Nandrup-Bus, C.; Hother, C.; Andersen, M.K.; Kjeldsen, L.; Mollgard, L.; Hellstrom-Lindberg, E.; et al. Allelic methylation levels of the noncoding VTRNA2-1 located on chromosome 5q31.1 predict outcome in AML. Blood 2012, 119, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Bao, X.; Lee, H.H.; Jang, J.J.; Saruuldalai, E.; Park, G.; Im, W.R.; Park, J.L.; Kim, S.Y.; Shin, S.; et al. Nc886, a Novel Suppressor of the Type I Interferon Response Upon Pathogen Intrusion. Int. J. Mol. Sci. 2021, 22, 2003. [Google Scholar] [CrossRef]
- Williams, B.R. PKR; a sentinel kinase for cellular stress. Oncogene 1999, 18, 6112–6120. [Google Scholar] [CrossRef]
- Liang, J.; Slingerland, J.M. Multiple roles of the PI3K/PKB (Akt) pathway in cell cycle progression. Cell Cycle 2003, 2, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Garcia, M.B.; Mesa, A.; Moya, E.L.J.; Rueda, B.; Lopez-Ordono, G.; Garcia, J.A.; Conde, V.; Redondo-Cerezo, E.; Lopez-Hidalgo, J.L.; Jimenez, G.; et al. Uncovering Tumour Heterogeneity through PKR and nc886 Analysis in Metastatic Colon Cancer Patients Treated with 5-FU-Based Chemotherapy. Cancers 2020, 12, 379. [Google Scholar] [CrossRef]
- Li, J.H.; Wang, M.; Zhang, R.; Gao, W.L.; Meng, S.H.; Ma, X.L.; Hou, X.H.; Feng, L.M. E2F1-directed activation of nc886 mediates drug resistance in cervical cancer cells via regulation of major vault protein. Int. J. Clin. Exp. Pathol. 2017, 10, 9233–9242. [Google Scholar]
- Gao, W.; Zhang, S.; Guorong, L.; Liu, Q.; Zhu, A.; Gui, F.; Zou, Y.; Wu, Y.; Luo, Y.; Hong, Z. Nc886 promotes renal cancer cell drug-resistance by enhancing EMT through Rock2 phosphorylation-mediated beta-catenin nuclear translocation. Cell Cycle 2022, 21, 340–351. [Google Scholar] [CrossRef]
- Kang, M.; Kharbash, R.; Byun, J.M.; Jeon, J.; Ali, A.A.; Ku, D.; Yoon, J.; Ku, Y.; Sohn, J.; Lee, S.V.; et al. Double-stranded RNA induction asa potential dynamic biomarkerfor DNA-demethylating agents. Mol. Ther. Nucleic Acids 2022, 29, 370–383. [Google Scholar] [CrossRef]
- McMahon, S.B. MYC and the control of apoptosis. Cold Spring Harb. Perspect. Med. 2014, 4, a014407. [Google Scholar] [CrossRef] [PubMed]
- Perkins, N.D. NF-kappaB: Tumor promoter or suppressor? Trends Cell Biol. 2004, 14, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Derynck, R.; Akhurst, R.J.; Balmain, A. TGF-beta signaling in tumor suppression and cancer progression. Nat. Genet. 2001, 29, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Lobry, C.; Oh, P.; Mansour, M.R.; Look, A.T.; Aifantis, I. Notch signaling: Switching an oncogene to a tumor suppressor. Blood 2014, 123, 2451–2459. [Google Scholar] [CrossRef]

| Organ or Cell Type | Methods for nc886 KD | Results (Events of Cells upon nc886 KD) | Ref. |
|---|---|---|---|
| endometrium | siRNA |
| [29] |
| renal cell | synthesized miR-886-3p inhibitor (GenePharma) |
| [30] |
| |||
| thyroid | modified anti-oligo * |
| [31] |
| stomach | modified anti-oligo * |
| [32] |
| |||
| |||
| bile duct | modified anti-oligo * |
| [33] |
| |||
| various | modified anti-oligo * |
| [16] |
| |||
| |||
| various | modified anti-oligo * |
| [12] |
| |||
| CD4+ T cell | pre-miR-886 inhibitor (Qiagen) ** |
| [22] |
| keratinocyte | modified anti-oligo * |
| [34] |
| Cancer Type | Approach | Method | Cellular Phenotype upon Ectopic Expression or KD of nc886 | Ref. |
|---|---|---|---|---|
| prostate cancer (PC) | ectopic expression | plasmid-based (from the CMV promoter) |
| [52] |
| ||||
| ||||
| ectopic expression | plasmid-based (from the H1 promoter) |
| [15] | |
| ||||
| ||||
| gastric cancer (GC) | ectopic expression | plasmid-based (from Pol III promoters) |
| [32] |
| synthetic nc886 RNA (in vitro transcripts) |
| |||
| KD | modified anti-oligo * |
| ||
| esophageal squamous cell carcinoma (ESCC) | ectopic expression | plasmid-based (from Pol III promoters) |
| [13] |
| ||||
| ectopic expression | synthetic nc886 RNA (in vitro transcripts) |
| [37] | |
| ||||
| KD | modified anti-oligo * |
| ||
| small cell lung cancer (SCLC) | ectopic expression | plasmid-based *** |
| [26] |
|
| Cancer Type | Therapeutic Drug | nc886 Expression upon Drug Treatment | Approach and Method | Change in Drug Sensitivity upon Ectopic Expression or KD of nc886 | Ref. |
|---|---|---|---|---|---|
| cervical cancer (CC) | paclitaxel and VP16 | increase | nc886 KD by anti-oligo * |
| [58] |
| not specific | 2′-deoxy-5-azacytidine (DAC) | increase | nc886 KD by siRNA |
| [60] |
| renal cell carcinoma (RCC) | sunitinib and everolimus | decrease | ectopic expression by transfecting a plasmid that expresses nc886 |
| [59] |
| |||||
| nc886 KD by siRNA |
| ||||
| |||||
| not specific | doxorubicin | decrease | ectopic expression by transfecting the in vitro transcript of nc886 |
| [16] |
| esophageal squamous cell carcinoma (ESCC) | palbociclib | nc886 KD by anti-oligo * |
| [13] | |
| ovarian cancer (OC) | paclitaxel | ectopic expression (by generating a cell line stably expressing nc886) |
| [14] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, J.J.; Kang, D.; Lee, Y.-S.; Lee, Y.S. The Versatile Roles of nc886, a Fascinating and Peculiar Regulatory Non-Coding RNA, in Cancer. Int. J. Mol. Sci. 2024, 25, 10825. https://doi.org/10.3390/ijms251910825
Jang JJ, Kang D, Lee Y-S, Lee YS. The Versatile Roles of nc886, a Fascinating and Peculiar Regulatory Non-Coding RNA, in Cancer. International Journal of Molecular Sciences. 2024; 25(19):10825. https://doi.org/10.3390/ijms251910825
Chicago/Turabian StyleJang, Jiyoung Joan, Dongmin Kang, Yeon-Su Lee, and Yong Sun Lee. 2024. "The Versatile Roles of nc886, a Fascinating and Peculiar Regulatory Non-Coding RNA, in Cancer" International Journal of Molecular Sciences 25, no. 19: 10825. https://doi.org/10.3390/ijms251910825
APA StyleJang, J. J., Kang, D., Lee, Y.-S., & Lee, Y. S. (2024). The Versatile Roles of nc886, a Fascinating and Peculiar Regulatory Non-Coding RNA, in Cancer. International Journal of Molecular Sciences, 25(19), 10825. https://doi.org/10.3390/ijms251910825







