Functional Relationships between L1CAM, LC3, ATG12, and Aβ
Abstract
:1. Introduction
2. Results
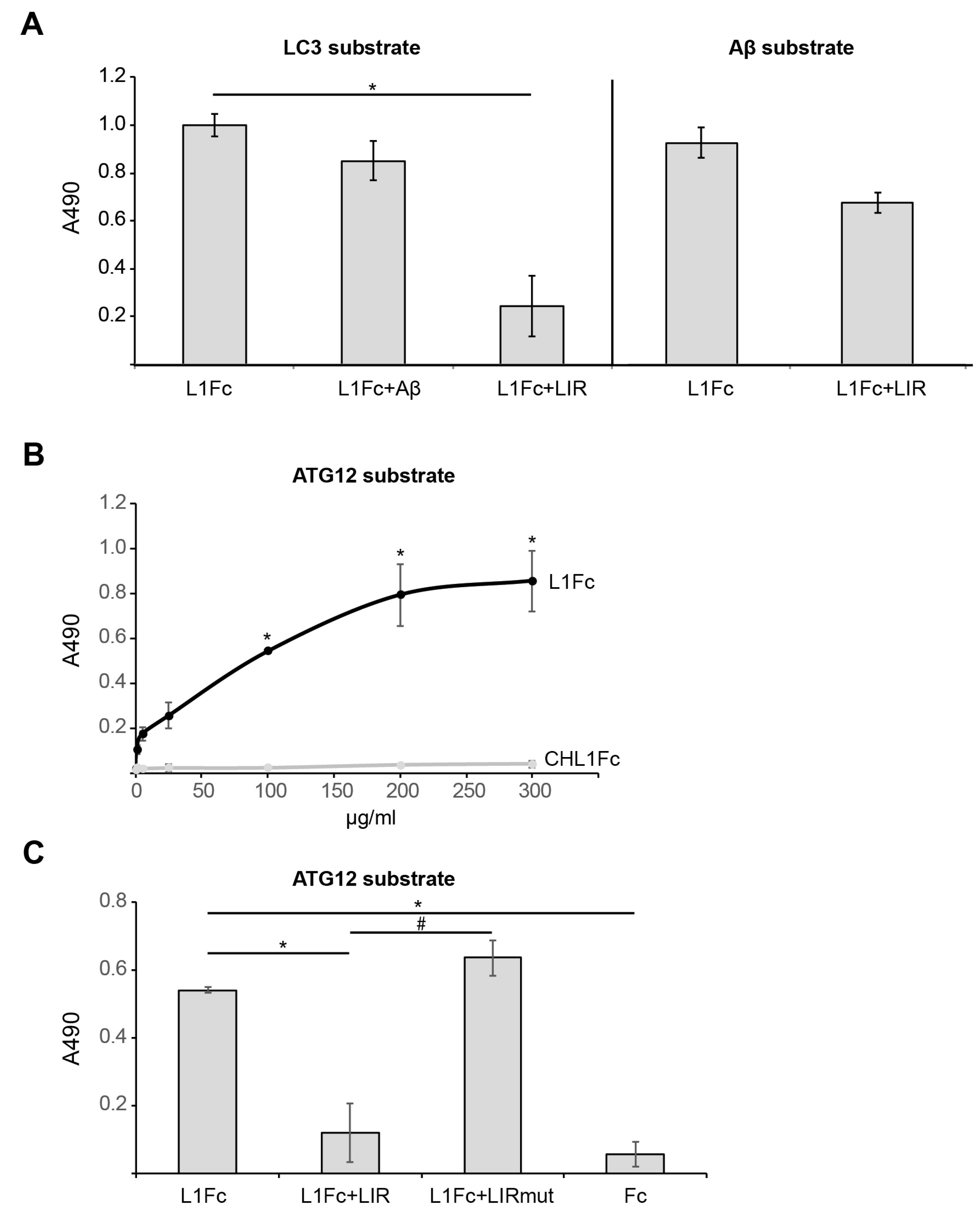
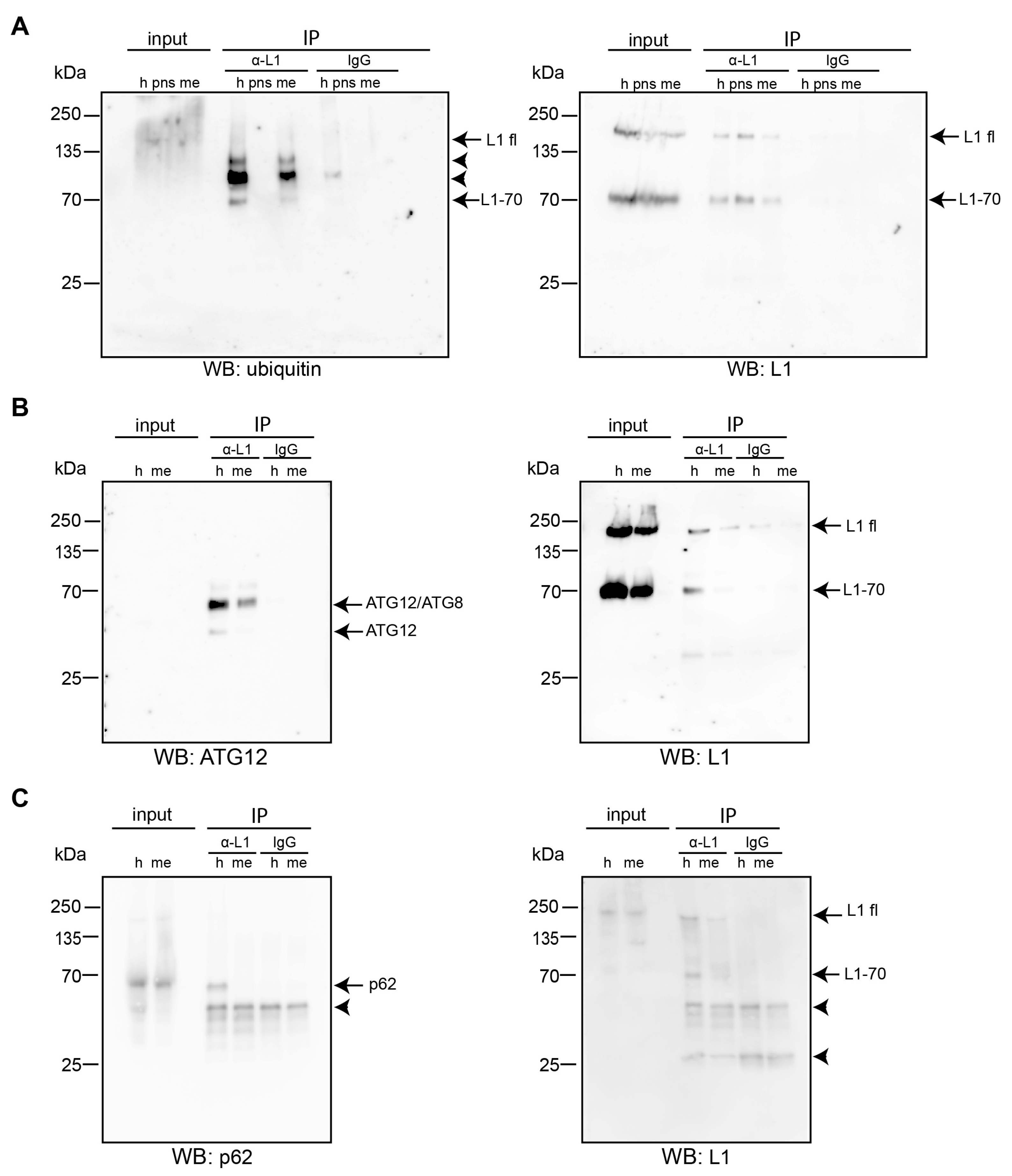
2.1. L1 Binds to LC3, ATG12, and Aβ
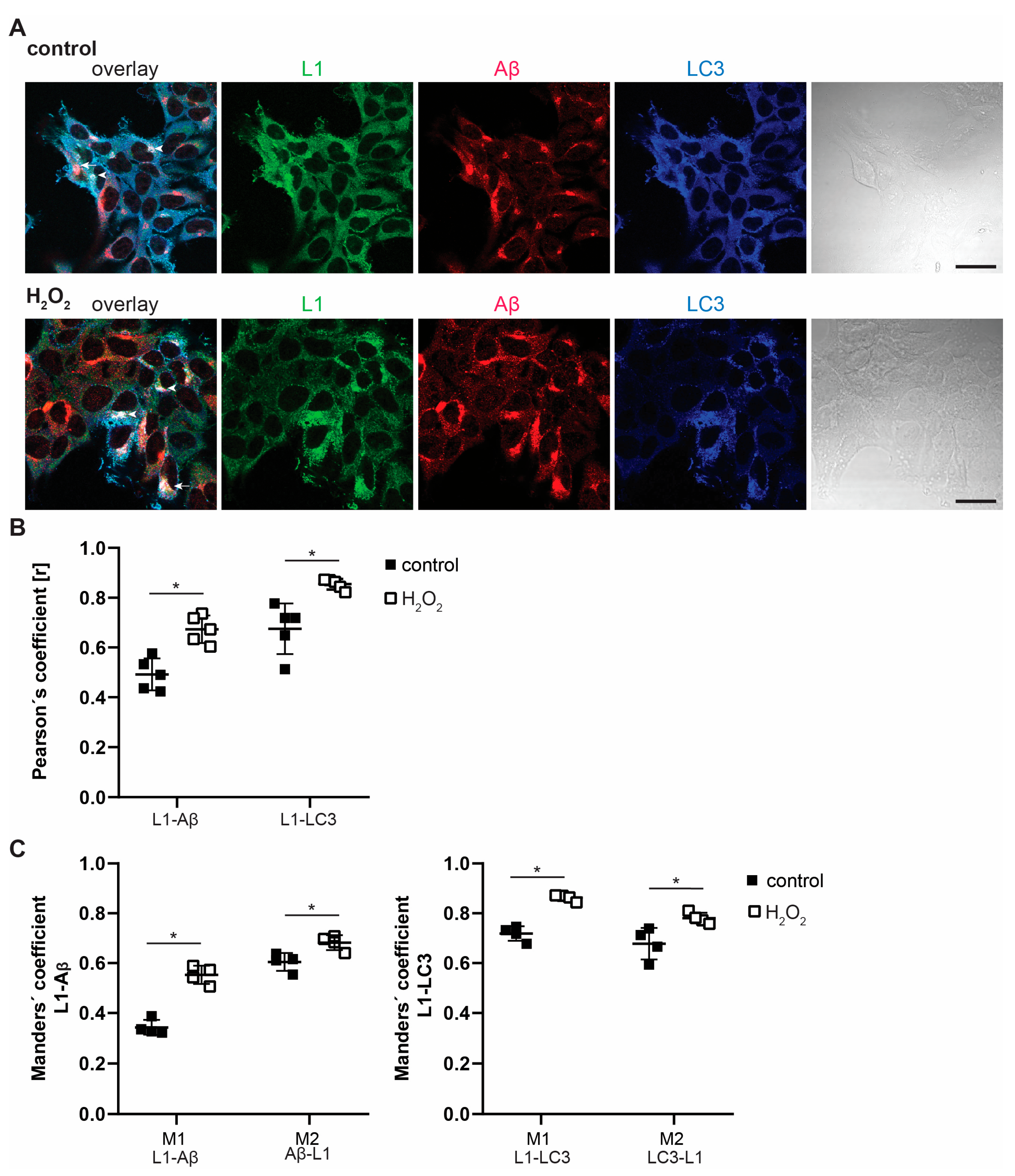
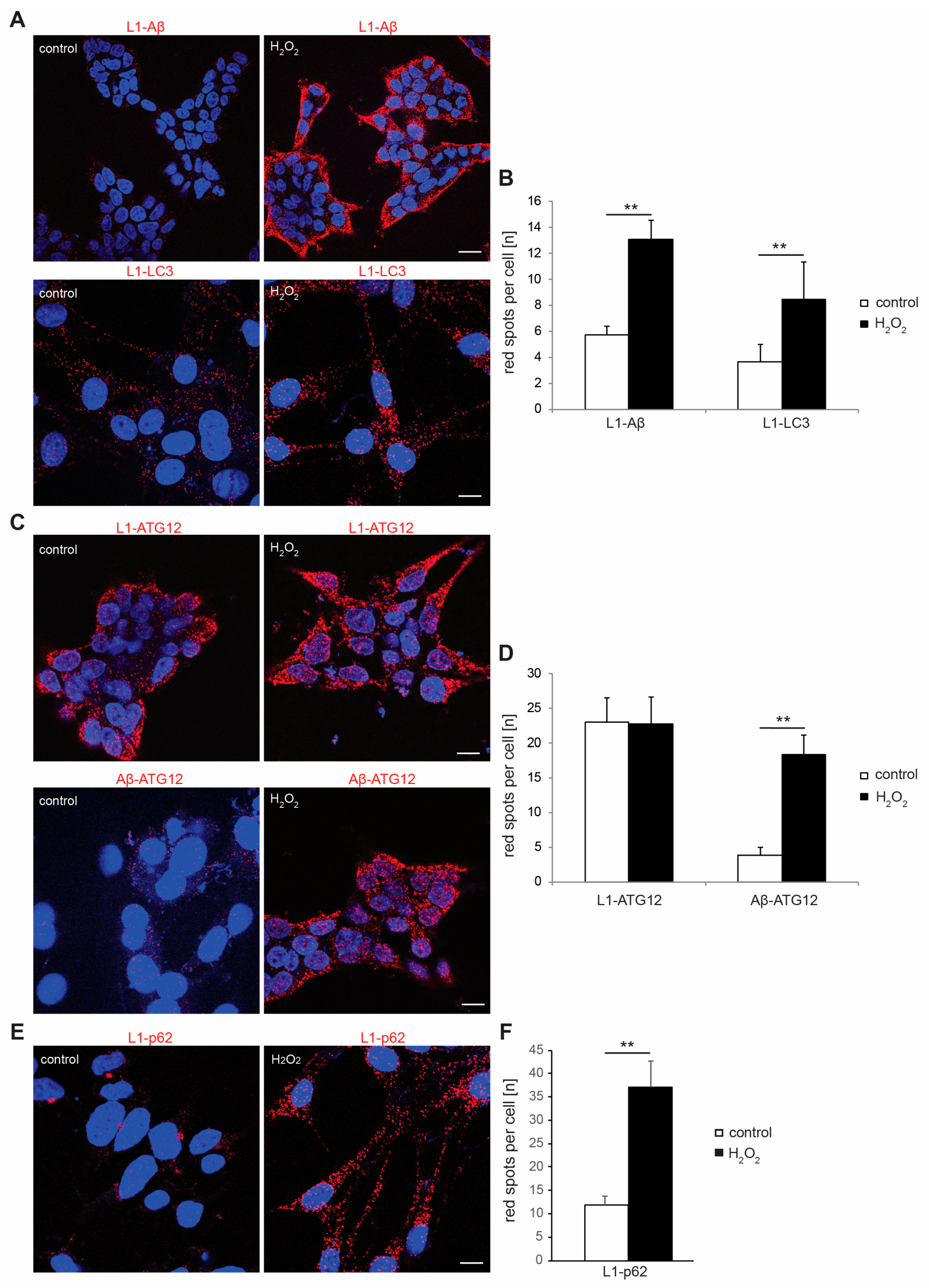
2.2. Interactions of L1 with LC3, p62, and Aβ Are Enhanced under Oxidative Stress
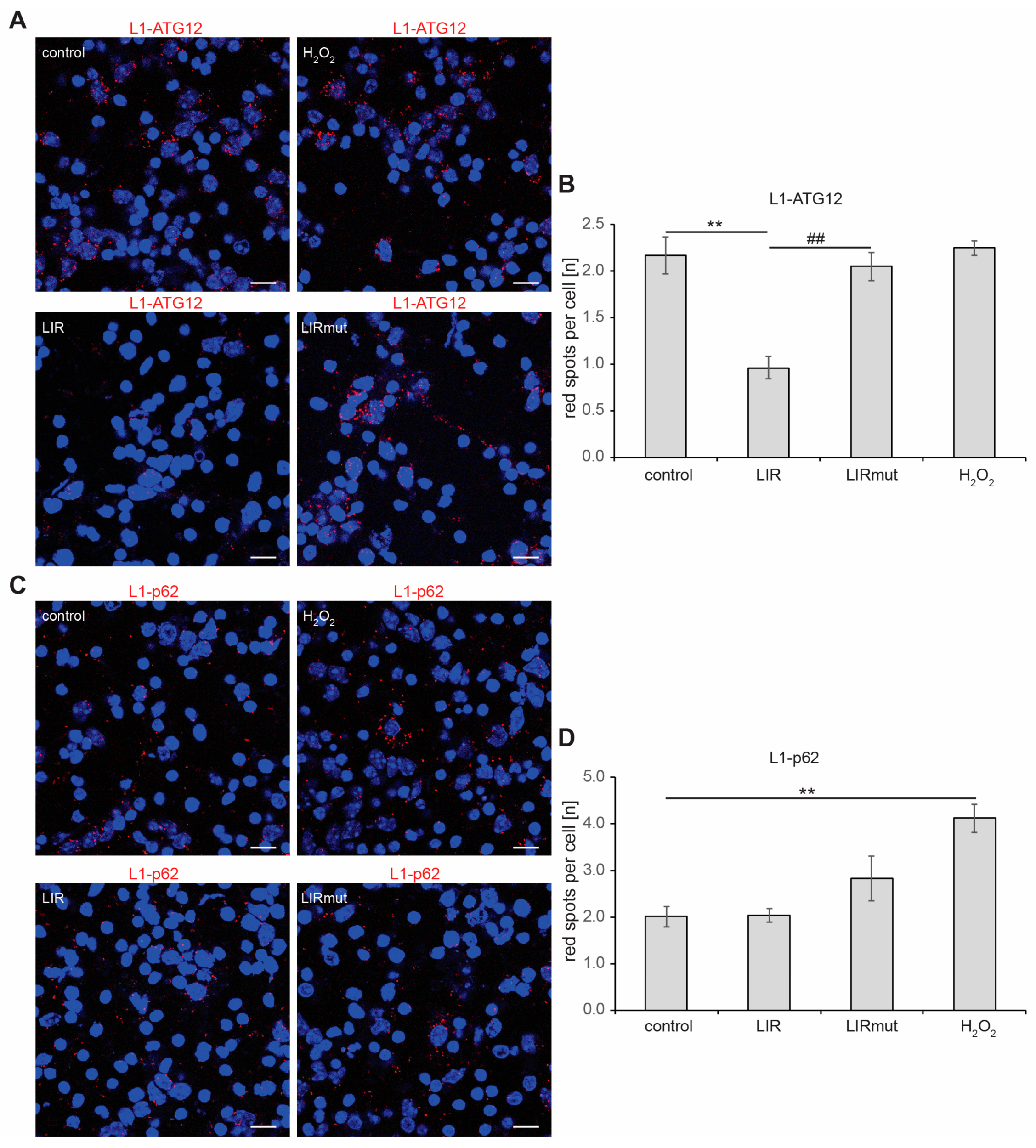
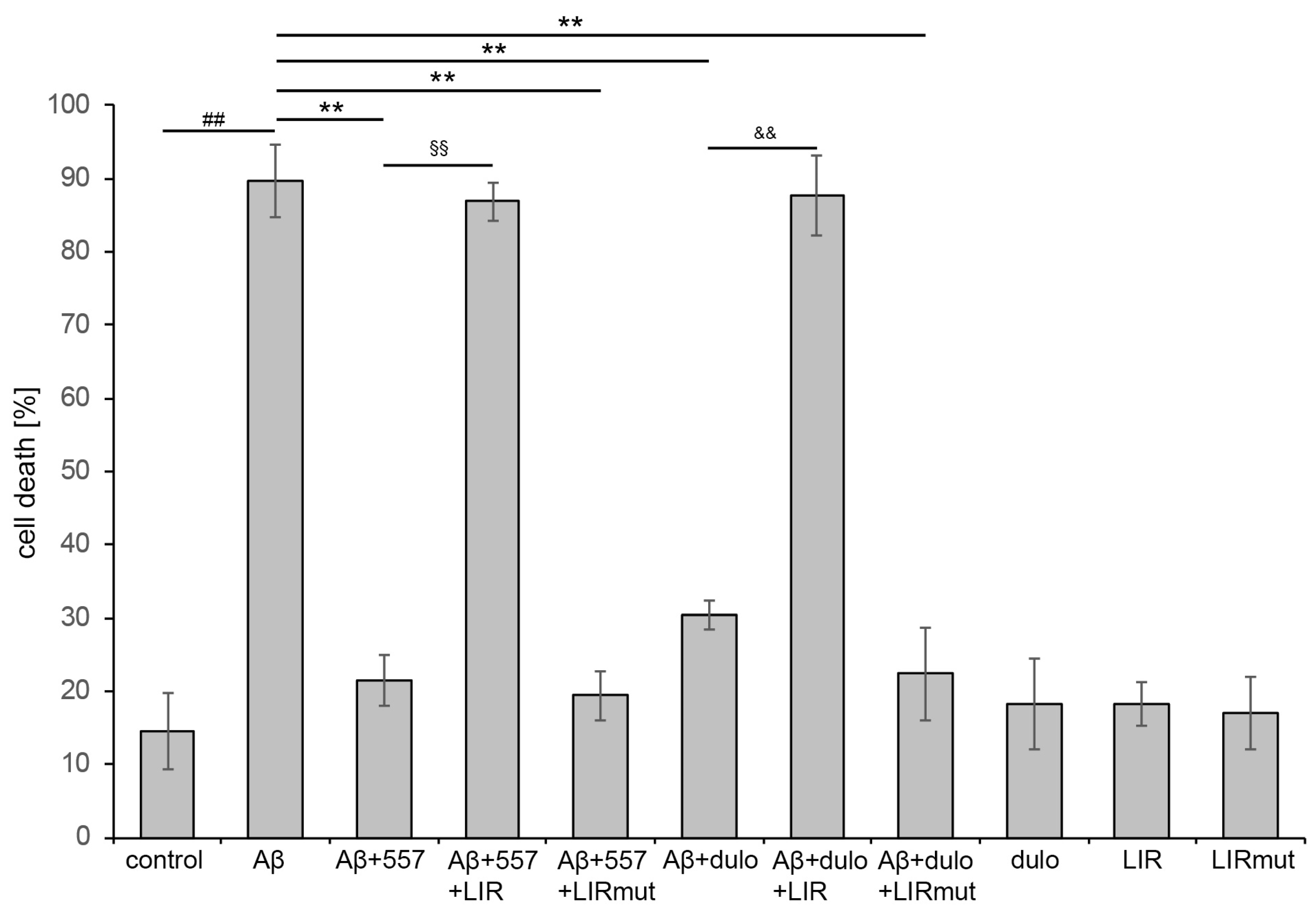
2.3. The Interaction of L1 with ATG Family Proteins Is Required for L1-Mediated Amelioration of Aβ-Induced Toxicity
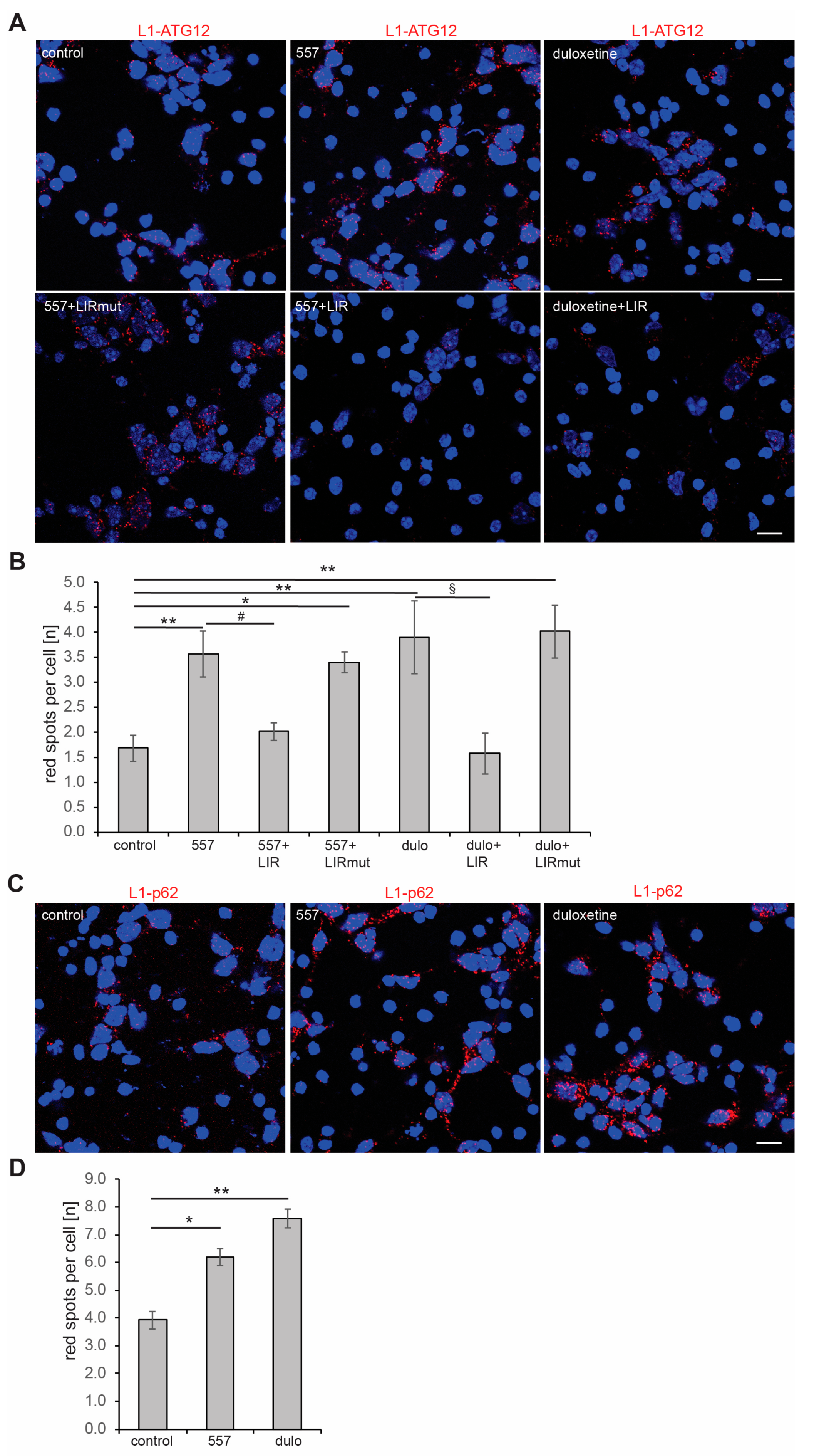
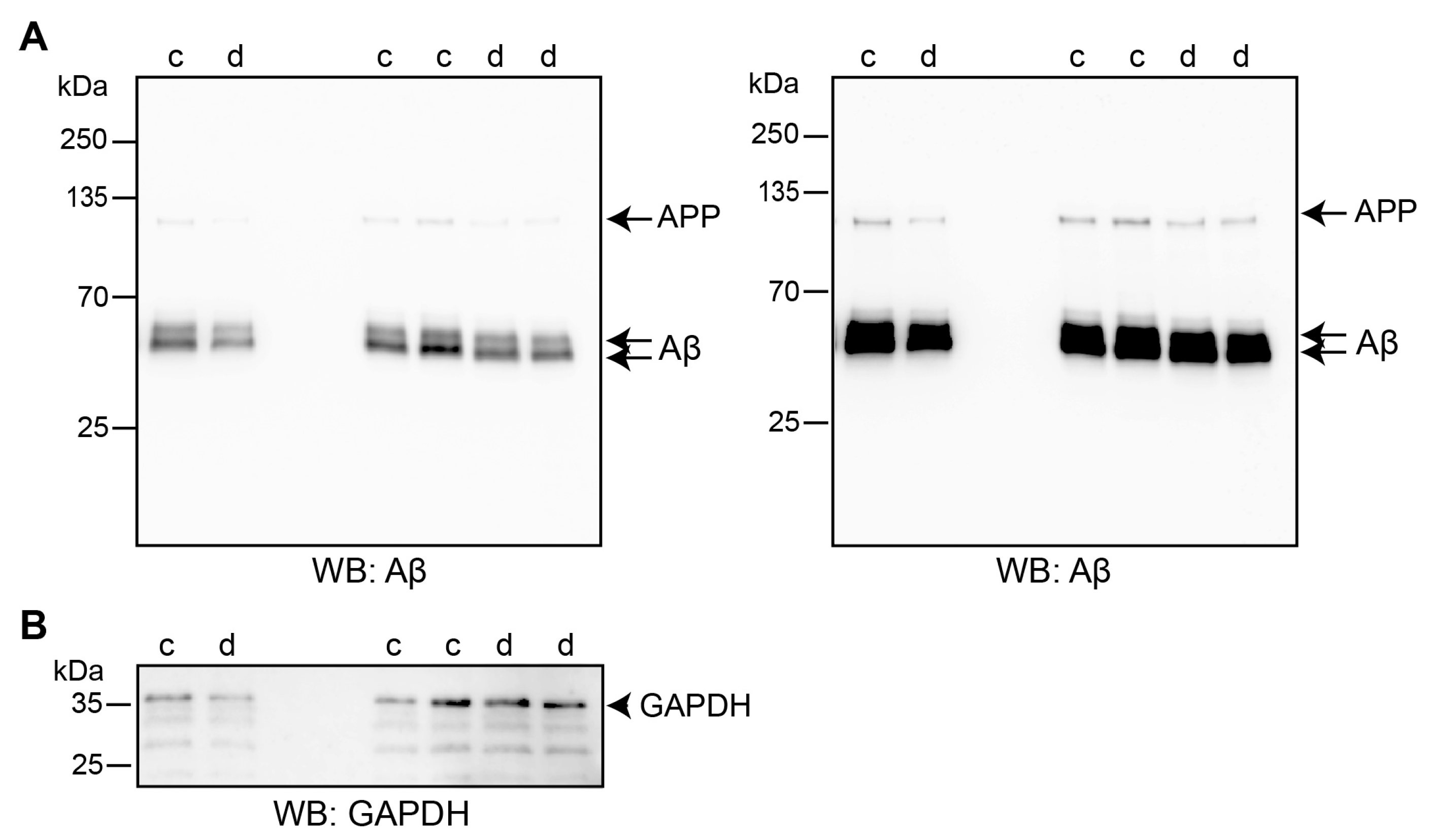
2.4. Triggering L1’s Functions Enhances Autophagy of Aβ
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Antibodies and Reagents
4.3. Isolation and Maintenance of Cortical Neurons and B103 and HEK293 Cells
4.4. Enzyme-Linked Immunosorbent Assay (ELISA)
4.5. Proximity Ligation Assay
4.6. Immunocytochemistry
4.7. Immunoprecipitation and Western Blot Analysis
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kozlova, I.; Sah, S.; Keable, R.; Leshchyns’ka, I.; Janitz, M.; Sytnyk, V. Cell adhesion molecules and protein synthesis regulation in neurons. Front. Mol. Neurosci. 2020, 13, 592126. [Google Scholar] [CrossRef] [PubMed]
- Stoyanova, I.I.; Lutz, D. Functional diversity of neuronal cell adhesion and recognition molecule L1CAM through proteolytic cleavage. Cells 2022, 11, 3085. [Google Scholar] [CrossRef] [PubMed]
- Duncan, B.W.; Murphy, K.E.; Maness, P.F. Molecular mechanisms of L1 and NCAM adhesion molecules in synaptic pruning, plasticity, and stabilization. Front. Cell Dev. Biol. 2021, 9, 625340. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, M.K.E.; Frotscher, M. Role of L1CAM for axon sprouting and branching. Cell Tissue Res. 2012, 349, 39–48. [Google Scholar] [CrossRef]
- Kadmon, G.; Montgomery, A.M.; Altevogt, P. L1 makes immunological progress by expanding its relations. Dev. Immunol. 1998, 6, 205–213. [Google Scholar] [CrossRef]
- Pancook, J.D.; Reisfeld, R.A.; Varki, N.; Vitiello, A.; Fox, R.I.; Mongomery, A.M.P. Expression and regulation of the neural cell adhesion molecule L1 on human cells of myelomonocytic and lymphoid origin. J. Immunol. 1997, 158, 4413–4421. [Google Scholar] [CrossRef]
- Kowitz, A.; Kadmon, G.; Eckert, M.; Schirrmacher, V.; Schachner, M.; Altevogt, P. Expression and function of the neural cell adhesion molecule L1 in mouse leukocytes. Eur. J. Immunol. 1992, 22, 1199–1205. [Google Scholar] [CrossRef]
- Schäfer, M.K.E.; Altevogt, P. L1CAM malfunction in the nervous system and human carcinomas. Cell Mol. Life Sci. 2010, 67, 2425–2437. [Google Scholar] [CrossRef]
- Kiefel, H.; Bondong, S.; Hazin, J.; Ridinger, J.; Schirmer, U.; Riedle, S.; Altevogt, P. L1CAM: A major driver for tumor cell invasion and motility. Cell Adhes. Migr. 2012, 6, 374–384. [Google Scholar] [CrossRef]
- Basu, S.; Gavert, N.; Brabletz, T.; Ben-Ze’ev, A. The intestinal stem cell regulating gene ASCL2 is required for L1-mediated colon cancer progression. Cancer Lett. 2018, 424, 9–18. [Google Scholar] [CrossRef]
- Giordano, M.; Cavallaro, U. Different shades of L1CAM in the pathophysiology of cancer stem cells. J. Clin. Med. 2020, 9, 1502. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Min, J.K.; Liang, Z.L.; Lee, K.; Lee, J.U.; Bae, K.H.; Lee, M.H.; Lee, S.E.; Ryu, M.J.; Kim, S.J.; et al. Aberrant L1 cell adhesion molecule affects tumor behavior and chemosensitivity in anaplastic thyroid carcinoma. Clin. Cancer Res. 2012, 18, 3071–3078. [Google Scholar] [CrossRef] [PubMed]
- Kenwrick, S.; Watkins, A.; De Angelis, E. Neural cell recognition molecule L1: Relating biological complexity to human disease mutations. Hum. Mol. Genet. 2000, 9, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, M.K.E.; Nam, Y.C.; Moumen, A.; Keglowich, L.; Bouche, E.; Kuffner, M.; Bock, H.H.; Rathjen, F.G.; Raoul, C.; Frotscher, M. L1 syndrome mutations impair neuronal L1 function at different levels by divergent mechanisms. Neurobiol. Dis. 2010, 40, 222–237. [Google Scholar] [CrossRef]
- Hortsch, M.; Nagaraj, K.; Mualla, R. The L1 family of cell adhesion molecules: A sickening number of mutations and protein functions. Adv. Neurobiol. 2014, 8, 195–229. [Google Scholar] [CrossRef]
- Loers, G.; Appel, D.; Lutz, D.; Congiu, L.; Kleene, R.; Hermans-Borgmeyer, I.; Schafer, M.K.E.; Schachner, M. Amelioration of the abnormal phenotype of a new L1 syndrome mouse mutation with L1 mimetics. FASEB J. 2021, 35, e21329. [Google Scholar] [CrossRef]
- Otter, M.; Wevers, M.; Pisters, M.; Pfundt, R.; Vos, Y.; Nievelstein, R.J.; Stumpel, C. A novel mutation in L1CAM causes a mild form of L1 syndrome: A case report. Clin. Case Rep. 2017, 5, 1213–1217. [Google Scholar] [CrossRef] [PubMed]
- Stumpel, C.; Vos, Y.J. L1 Syndrome. In GeneReviews®; Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Stephens, K., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 2004. [Google Scholar]
- Jouet, M.; Moncla, A.; Paterson, J.; McKeown, C.; Fryer, A.; Carpenter, N.; Holmberg, E.; Wadelius, C.; Kenwrick, S. New domains of neural cell-adhesion molecule L1 implicated in X-linked hydrocephalus and MASA syndrome. Am. J. Hum. Genet. 1995, 56, 1304–1314. [Google Scholar]
- De Angelis, E.; Watkins, A.; Schäfer, M.; Brümmendorf, T.; Kenwrick, S. Disease-associated mutations in L1 CAM interfere with ligand interactions and cell-surface expression. Hum. Mol. Genet. 2002, 11, 1–12. [Google Scholar] [CrossRef]
- Poltorak, M.; Wright, R.; Hemperly, J.J.; Torrey, E.F.; Issa, F.; Wyatt, R.J.; Freed, W.J. Monozygotic twins discordant for schizophrenia are discordant for N-CAM and L1 in CSF. Brain Res. 1997, 751, 152–154. [Google Scholar] [CrossRef]
- Poltorak, M.; Khoja, I.; Hemperly, J.J.; Williams, J.R.; el-Mallakh, R.; Freed, W.J. Disturbances in cell recognition molecules (N-CAM and L1 antigen) in the CSF of patients with schizophrenia. Exp. Neurol. 1995, 131, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, Y.; Uchida, S.; Funato, H.; Matsubara, T.; Watanuki, T.; Otsuki, K.; Fujimoto, M.; Nishida, A.; Watanabe, Y. State-dependent changes in the expression levels of NCAM-140 and L1 in the peripheral blood cells of bipolar disorders, but not in the major depressive disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 2008, 32, 1199–1205. [Google Scholar] [CrossRef] [PubMed]
- Strekalova, H.; Buhmann, C.; Kleene, R.; Eggers, C.; Saffell, J.; Hemperly, J.; Weiller, C.; Muller-Thomsen, T.; Schachner, M. Elevated levels of neural recognition molecule L1 in the cerebrospinal fluid of patients with Alzheimer disease and other dementia syndromes. Neurobiol. Aging 2006, 27, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kurumaji, A.; Nomoto, H.; Okano, T.; Toru, M. An association study between polymorphism of L1CAM gene and schizophrenia in a Japanese sample. Am. J. Med. Genet. 2001, 105, 99–104. [Google Scholar] [CrossRef]
- Djogo, N.; Jakovcevski, I.; Muller, C.; Lee, H.J.; Xu, J.C.; Jakovcevski, M.; Kugler, S.; Loers, G.; Schachner, M. Adhesion molecule L1 binds to amyloid beta and reduces Alzheimer’s disease pathology in mice. Neurobiol. Dis. 2013, 56, 104–115. [Google Scholar] [CrossRef]
- Yoo, M.; Carromeu, C.; Kwon, O.; Muotri, A.; Schachner, M. The L1 adhesion molecule normalizes neuritogenesis in Rett syndrome-derived neural precursor cells. Biochem. Biophys. Res. Commun. 2017, 494, 504–510. [Google Scholar] [CrossRef]
- Kleene, R.; Lutz, D.; Loers, G.; Bork, U.; Borgmeyer, U.; Hermans-Borgmeyer, I.; Schachner, M. Revisiting the proteolytic processing of cell adhesion molecule L1. J. Neurochem. 2021, 157, 1102–1117. [Google Scholar] [CrossRef]
- Lutz, D.; Loers, G.; Kleene, R.; Oezen, I.; Kataria, H.; Katagihallimath, N.; Braren, I.; Harauz, G.; Schachner, M. Myelin basic protein cleaves cell adhesion molecule L1 and promotes neuritogenesis and cell survival. J. Biol. Chem. 2014, 289, 13503–13518. [Google Scholar] [CrossRef]
- Kraus, K.; Kleene, R.; Braren, I.; Loers, G.; Lutz, D.; Schachner, M. A fragment of adhesion molecule L1 is imported into mitochondria, and regulates mitochondrial metabolism and trafficking. J. Cell Sci. 2018, 131, jcs210500. [Google Scholar] [CrossRef]
- Kraus, K.; Kleene, R.; Henis, M.; Braren, I.; Kataria, H.; Sharaf, A.; Loers, G.; Schachner, M.; Lutz, D. A fragment of adhesion molecule L1 binds to nuclear receptors to regulate synaptic plasticity and motor coordination. Mol. Neurobiol. 2018, 55, 7164–7178. [Google Scholar] [CrossRef]
- Gast, D.; Riedle, S.; Kiefel, H.; Muerkoster, S.S.; Schafer, H.; Schafer, M.K.; Altevogt, P. The RGD integrin binding site in human L1-CAM is important for nuclear signaling. Exp. Cell Res. 2008, 314, 2411–2418. [Google Scholar] [CrossRef] [PubMed]
- Gutwein, P.; Oleszewski, M.; Mechtersheimer, S.; Agmon-Levin, N.; Krauss, K.; Altevogt, P. Role of Src kinases in the ADAM-mediated release of L1 adhesion molecule from human tumor cells. J. Biol. Chem. 2000, 275, 15490–15497. [Google Scholar] [CrossRef] [PubMed]
- Riedle, S.; Kiefel, H.; Gast, D.; Bondong, S.; Wolterink, S.; Gutwein, P.; Altevogt, P. Nuclear translocation and signalling of L1-CAM in human carcinoma cells requires ADAM10 and presenilin/gamma-secretase activity. Biochem. J. 2009, 420, 391–402. [Google Scholar] [CrossRef]
- Mechtersheimer, S.; Gutwein, P.; Agmon-Levin, N.; Stoeck, A.; Oleszewski, M.; Riedle, S.; Postina, R.; Fahrenholz, F.; Fogel, M.; Lemmon, V.; et al. Ectodomain shedding of L1 adhesion molecule promotes cell migration by autocrine binding to integrins. J. Cell Biol. 2001, 155, 661–673. [Google Scholar] [CrossRef]
- Yang, M.; Adla, S.; Temburni, M.K.; Patel, V.P.; Lagow, E.L.; Brady, O.A.; Tian, J.; Boulos, M.I.; Galileo, D.S. Stimulation of glioma cell motility by expression, proteolysis, and release of the L1 neural cell recognition molecule. Cancer Cell Int. 2009, 9, 27. [Google Scholar] [CrossRef]
- Loers, G.; Kleene, R.; Bork, U.; Schachner, M. The interactions of the 70 kDa fragment of cell adhesion molecule L1 with topoisomerase 1, peroxisome proliferator-activated receptor gamma and NADH dehydrogenase (ubiquinone) flavoprotein 2 are involved in gene expression and neuronal L1-dependent functions. Int. J. Mol. Sci. 2023, 24, 2097. [Google Scholar] [CrossRef]
- Loers, G.; Kleene, R.; Girbes Minguez, M.; Schachner, M. The cell adhesion molecule L1 interacts with methyl CpG binding protein 2 via its intracellular domain. Int. J. Mol. Sci. 2022, 23, 3554. [Google Scholar] [CrossRef]
- Congiu, L.; Granato, V.; Loers, G.; Kleene, R.; Schachner, M. Mitochondrial and neuronal dysfunctions in L1 mutant mice. Int. J. Mol. Sci. 2022, 23, 4337. [Google Scholar] [CrossRef]
- Kleene, R.; Loers, G.; Schachner, M. The KDET motif in the intracellular domain of the cell adhesion molecule L1 interacts with several nuclear, cytoplasmic, and mitochondrial proteins essential for neuronal functions. Int. J. Mol. Sci. 2023, 24, 932. [Google Scholar] [CrossRef]
- Hu, J.; Lin, S.L.; Schachner, M. A fragment of cell adhesion molecule L1 reduces amyloid-beta plaques in a mouse model of Alzheimer’s disease. Cell Death Dis. 2022, 13, 48. [Google Scholar] [CrossRef]
- Fogel, M.; Gutwein, P.; Mechtersheimer, S.; Riedle, S.; Stoeck, A.; Smirnov, A.; Edler, L.; Ben-Arie, A.; Huszar, M.; Altevogt, P. L1 expression as a predictor of progression and survival in patients with uterine and ovarian carcinomas. Lancet 2003, 362, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Gutwein, P.; Stoeck, A.; Riedle, S.; Gast, D.; Runz, S.; Condon, T.P.; Marme, A.; Phong, M.C.; Linderkamp, O.; Skorokhod, A.; et al. Cleavage of L1 in exosomes and apoptotic membrane vesicles released from ovarian carcinoma cells. Clin. Cancer Res. 2005, 11, 2492–2501. [Google Scholar] [CrossRef] [PubMed]
- Mishra, E.; Thakur, M.K. Mitophagy: A promising therapeutic target for neuroprotection during ageing and age-related diseases. Br. J. Pharmacol. 2023, 180, 1542–1561. [Google Scholar] [CrossRef] [PubMed]
- Tecalco-Cruz, A.C.; Pedraza-Chaverri, J.; Briones-Herrera, A.; Cruz-Ramos, E.; Lopez-Canovas, L.; Zepeda-Cervantes, J. Protein degradation-associated mechanisms that are affected in Alzheimer s disease. Mol. Cell Biochem. 2022, 477, 915–925. [Google Scholar] [CrossRef]
- Caponio, D.; Veverova, K.; Zhang, S.Q.; Shi, L.; Wong, G.; Vyhnalek, M.; Fang, E.F. Compromised autophagy and mitophagy in brain ageing and Alzheimer’s diseases. Aging Brain 2022, 2, 100056. [Google Scholar] [CrossRef]
- Reiss, A.B.; Gulkarov, S.; Jacob, B.; Srivastava, A.; Pinkhasov, A.; Gomolin, I.H.; Stecker, M.M.; Wisniewski, T.; De Leon, J. Mitochondria in Alzheimer’s disease pathogenesis. Life 2024, 14, 196. [Google Scholar] [CrossRef]
- Brooks, C.D.; Kodati, B.; Stankowska, D.L.; Krishnamoorthy, R.R. Role of mitophagy in ocular neurodegeneration. Front. Neurosci. 2023, 17, 1299552. [Google Scholar] [CrossRef]
- Panwar, S.; Uniyal, P.; Kukreti, N.; Hashmi, A.; Verma, S.; Arya, A.; Joshi, G. Role of autophagy and proteostasis in neurodegenerative diseases: Exploring the therapeutic interventions. Chem. Biol. Drug Des. 2024, 103, e14515. [Google Scholar] [CrossRef] [PubMed]
- Ragupathy, H.; Vukku, M.; Barodia, S.K. Cell-type-specific mitochondrial quality control in the brain: A plausible mechanism of neurodegeneration. Int. J. Mol. Sci. 2023, 24, 14421. [Google Scholar] [CrossRef]
- Verma, H.; Gangwar, P.; Yadav, A.; Yadav, B.; Rao, R.; Kaur, S.; Kumar, P.; Dhiman, M.; Taglialatela, G.; Mantha, A.K. Understanding the neuronal synapse and challenges associated with the mitochondrial dysfunction in mild cognitive impairment and Alzheimer’s disease. Mitochondrion 2023, 73, 19–29. [Google Scholar] [CrossRef]
- Loers, G.; Kleene, R.; Granato, V.; Bork, U.; Schachner, M. Interaction of L1CAM with LC3 is required for L1-dependent neurite outgrowth and neuronal survival. Int. J. Mol. Sci. 2023, 24, 12531. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.V.; Mills, J. Non-canonical autophagy in aging and age-related diseases. Front. Cell Dev. Biol. 2023, 11, 1137870. [Google Scholar] [CrossRef] [PubMed]
- Birgisdottir, A.B.; Lamark, T.; Johansen, T. The LIR motif—crucial for selective autophagy. J. Cell Sci. 2013, 126, 3237–3247. [Google Scholar] [CrossRef] [PubMed]
- Johansen, T.; Lamark, T. Selective autophagy: ATG8 family proteins, LIR motifs and cargo receptors. J. Mol. Biol. 2020, 432, 80–103. [Google Scholar] [CrossRef]
- Lamark, T.; Johansen, T. Mechanisms of selective autophagy. Annu. Rev. Cell Dev. Biol. 2021, 37, 143–169. [Google Scholar] [CrossRef]
- Cui, M.; Yoshimori, T.; Nakamura, S. Autophagy system as a potential therapeutic target for neurodegenerative diseases. Neurochem. Int. 2022, 155, 105308. [Google Scholar] [CrossRef]
- Zhang, L.; Dai, L.; Li, D. Mitophagy in neurological disorders. J. Neuroinflammation 2021, 18, 297. [Google Scholar] [CrossRef]
- Ochneva, A.; Zorkina, Y.; Abramova, O.; Pavlova, O.; Ushakova, V.; Morozova, A.; Zubkov, E.; Pavlov, K.; Gurina, O.; Chekhonin, V. Protein misfolding and aggregation in the brain: Common pathogenetic pathways in neurodegenerative and mental disorders. Int. J. Mol. Sci. 2022, 23, 14498. [Google Scholar] [CrossRef]
- Klemmensen, M.M.; Borrowman, S.H.; Pearce, C.; Pyles, B.; Chandra, B. Mitochondrial dysfunction in neurodegenerative disorders. Neurotherapeutics 2023, 21, e00292. [Google Scholar] [CrossRef]
- Soto, C.; Pritzkow, S. Protein misfolding, aggregation, and conformational strains in neurodegenerative diseases. Nat. Neurosci. 2018, 21, 1332–1340. [Google Scholar] [CrossRef]
- Chu, C.T. Mechanisms of selective autophagy and mitophagy: Implications for neurodegenerative diseases. Neurobiol. Dis. 2019, 122, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yin, J.; Zheng, X. Artemisinin upregulates neural cell adhesion molecule L1 to attenuate neurological deficits after intracerebral hemorrhage in mice. Brain Behav. 2022, 12, e2558. [Google Scholar] [CrossRef] [PubMed]
- Keulers, T.G.; Koch, A.; van Gisbergen, M.W.; Barbeau, L.M.O.; Zonneveld, M.I.; de Jong, M.C.; Savelkouls, K.G.M.; Wanders, R.G.; Bussink, J.; Melotte, V.; et al. ATG12 deficiency results in intracellular glutamine depletion, abrogation of tumor hypoxia and a favorable prognosis in cancer. Autophagy 2022, 18, 1898–1914. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, A.D.; Eisenstein, M.; Ber, Y.; Bialik, S.; Kimchi, A. The autophagy protein Atg12 associates with antiapoptotic Bcl-2 family members to promote mitochondrial apoptosis. Mol. Cell 2011, 44, 698–709. [Google Scholar] [CrossRef]
- Saleem, S. Apoptosis, autophagy, necrosis and their multi galore crosstalk in neurodegeneration. Neuroscience 2021, 469, 162–174. [Google Scholar] [CrossRef]
- Liu, W.J.; Ye, L.; Huang, W.F.; Guo, L.J.; Xu, Z.G.; Wu, H.L.; Yang, C.; Liu, H.F. p62 links the autophagy pathway and the ubiqutin-proteasome system upon ubiquitinated protein degradation. Cell Mol. Biol. Lett. 2016, 21, 29. [Google Scholar] [CrossRef]
- Thal, D.R.; Gawor, K.; Moonen, S. Regulated cell death and its role in Alzheimer’s disease and amyotrophic lateral sclerosis. Acta Neuropathol. 2024, 147, 69. [Google Scholar] [CrossRef]
- Schäfer, M.K.; Schmitz, B.; Diestel, S. L1CAM ubiquitination facilitates its lysosomal degradation. FEBS Lett. 2010, 584, 4475–4480. [Google Scholar] [CrossRef]
- Aikawa, Y. Ubiquitination within the membrane-proximal ezrin-radixin-moesin (ERM)-binding region of the L1 cell adhesion molecule. Commun. Integr. Biol. 2013, 6, e24750. [Google Scholar] [CrossRef] [PubMed]
- Höhn, A.; Tramutola, A.; Cascella, R. Proteostasis failure in neurodegenerative diseases: Focus on oxidative stress. Oxid. Med. Cell. Longev. 2020, 2020, 5497046. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Boyd-Kimball, D. Mitochondrial oxidative and nitrosative stress and Alzheimer disease. Antioxidants 2020, 9, 818. [Google Scholar] [CrossRef] [PubMed]
- Kawamata, H.; Manfredi, G. Proteinopathies and OXPHOS dysfunction in neurodegenerative diseases. J. Cell Biol. 2017, 216, 3917–3929. [Google Scholar] [CrossRef]
- Yu, N.; Pasha, M.; Chua, J.J.E. Redox changes and cellular senescence in Alzheimer’s disease. Redox Biol. 2024, 70, 103048. [Google Scholar] [CrossRef] [PubMed]
- Kaarniranta, K.; Blasiak, J.; Liton, P.; Boulton, M.; Klionsky, D.J.; Sinha, D. Autophagy in age-related macular degeneration. Autophagy 2023, 19, 388–400. [Google Scholar] [CrossRef]
- Opazo, C.; Huang, X.; Cherny, R.A.; Moir, R.D.; Roher, A.E.; White, A.R.; Cappai, R.; Masters, C.L.; Tanzi, R.E.; Inestrosa, N.C.; et al. Metalloenzyme-like activity of Alzheimer’s disease beta-amyloid. Cu-dependent catalytic conversion of dopamine, cholesterol, and biological reducing agents to neurotoxic H(2)O(2). J. Biol. Chem. 2002, 277, 40302–40308. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.T.; Lu, M.H.; Zhang, Y.; Ji, W.L.; Lei, L.; Wang, W.; Fang, L.P.; Wang, L.W.; Yu, F.; Wang, J.; et al. Disrupted-in-schizophrenia-1 protects synaptic plasticity in a transgenic mouse model of Alzheimer’s disease as a mitophagy receptor. Aging Cell 2019, 18, e12860. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, S.; Zheng, H. The cargo receptor SQSTM1 ameliorates neurofibrillary tangle pathology and spreading through selective targeting of pathological MAPT (microtubule associated protein tau). Autophagy 2019, 15, 583–598. [Google Scholar] [CrossRef]
- Pankiv, S.; Clausen, T.H.; Lamark, T.; Brech, A.; Bruun, J.A.; Outzen, H.; Overvatn, A.; Bjorkoy, G.; Johansen, T. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J. Biol. Chem. 2007, 282, 24131–24145. [Google Scholar] [CrossRef]
- Ren, X.; Yao, L.; Wang, Y.; Mei, L.; Xiong, W.C. Microglial VPS35 deficiency impairs Abeta phagocytosis and Abeta-induced disease-associated microglia, and enhances Abeta associated pathology. J. Neuroinflammation 2022, 19, 61. [Google Scholar] [CrossRef]
- Syed, R.A.; Hayat, M.; Qaiser, H.; Uzair, M.; Al-Regaiey, K.; Khallaf, R.; Kaleem, I.; Bashir, S. Aging-related protein alterations in the brain. J. Alzheimers Dis. 2024, 99, S5–S22. [Google Scholar] [CrossRef]
- Wei, D.; Qu, C.; Zhao, N.; Li, S.; Pu, N.; Song, Z.; Tao, Y. The significance of precisely regulating heme oxygenase-1 expression: Another avenue for treating age-related ocular disease? Ageing Res. Rev. 2024, 97, 102308. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, M.; Pezone, A.; Quadrini, G.I.; Abbadessa, G.; Laezza, M.P.; Passaro, M.L.; Porcellini, A.; Costagliola, C. Targeting shared pathways in tauopathies and age-related macular degeneration: Implications for novel therapies. Front. Aging Neurosci. 2024, 16, 1371745. [Google Scholar] [CrossRef]
- Rahman, M.A.; Rahman, M.S.; Rahman, M.D.H.; Rasheduzzaman, M.; Mamun-Or-Rashid, A.; Uddin, M.J.; Rahman, M.R.; Hwang, H.; Pang, M.G.; Rhim, H. Modulatory effects of autophagy on APP processing as a potential treatment target for Alzheimer’s disease. Biomedicines 2020, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Kang, S.; Zhao, Z.; Jin, L.; Cui, X.; Chen, L.; Schachner, M.; Li, S.; Guo, Y.; Zhao, J. Chicoric acid ameliorated beta-amyloid pathology and enhanced expression of synaptic-function-related markers via L1CAM in Alzheimer’s disease models. Int. J. Mol. Sci. 2024, 25, 3408. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; He, Z.; Germic, N.; Ademi, H.; Frangez, Z.; Felser, A.; Peng, S.; Riether, C.; Djonov, V.; Nuoffer, J.M.; et al. ATG12 deficiency leads to tumor cell oncosis owing to diminished mitochondrial biogenesis and reduced cellular bioenergetics. Cell Death Differ. 2020, 27, 1965–1980. [Google Scholar] [CrossRef] [PubMed]
- Radoshevich, L.; Murrow, L.; Chen, N.; Fernandez, E.; Roy, S.; Fung, C.; Debnath, J. ATG12 conjugation to ATG3 regulates mitochondrial homeostasis and cell death. Cell 2010, 142, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Huangfu, W.C.; Fuchs, S.Y. Ubiquitination-dependent regulation of signaling receptors in cancer. Genes Cancer 2010, 1, 725–734. [Google Scholar] [CrossRef]
- Kumar, A.V.; Mills, J.; Lapierre, L.R. Selective autophagy receptor p62/SQSTM1, a pivotal player in stress and aging. Front. Cell Dev. Biol. 2022, 10, 793328. [Google Scholar] [CrossRef]
- Maniv, I.; Sarji, M.; Bdarneh, A.; Feldman, A.; Ankawa, R.; Koren, E.; Magid-Gold, I.; Reis, N.; Soteriou, D.; Salomon-Zimri, S.; et al. Altered ubiquitin signaling induces Alzheimer’s disease-like hallmarks in a three-dimensional human neural cell culture model. Nat. Commun. 2023, 14, 5922. [Google Scholar] [CrossRef]
- Nixon, R.A. Autophagy, amyloidogenesis and Alzheimer disease. J. Cell Sci. 2007, 120, 4081–4091. [Google Scholar] [CrossRef]
- Lippai, M.; Low, P. The role of the selective adaptor p62 and ubiquitin-like proteins in autophagy. Biomed. Res. Int. 2014, 2014, 832704. [Google Scholar] [CrossRef] [PubMed]
- Hennig, P.; Fenini, G.; Di Filippo, M.; Karakaya, T.; Beer, H.D. The pathways underlying the multiple roles of p62 in inflammation and cancer. Biomedicines 2021, 9, 707. [Google Scholar] [CrossRef] [PubMed]
- Altevogt, P.; Ben-Ze’Ev, A.; Gavert, N.; Schumacher, U.; Schäfer, H.; Sebens, S. Recent insights into the role of L1CAM in cancer initiation and progression. Int. J. Cancer 2020, 147, 3292–3296. [Google Scholar] [CrossRef]
- Barman, B.; Thakur, M.K. Neuropsin promotes hippocampal synaptogenesis by regulating the expression and cleavage of L1CAM. J. Cell Sci. 2024, 137, jcs261422. [Google Scholar] [CrossRef]
- Nixon, R.A.; Wegiel, J.; Kumar, A.; Yu, W.H.; Peterhoff, C.; Cataldo, A.; Cuervo, A.M. Extensive involvement of autophagy in Alzheimer disease: An immuno-electron microscopy study. J. Neuropathol. Exp. Neurol. 2005, 64, 113–122. [Google Scholar] [CrossRef]
- Tammineni, P.; Ye, X.; Feng, T.; Aikal, D.; Cai, Q. Impaired retrograde transport of axonal autophagosomes contributes to autophagic stress in Alzheimer’s disease neurons. Elife 2017, 6, e21776. [Google Scholar] [CrossRef] [PubMed]
- Manczak, M.; Kandimalla, R.; Yin, X.; Reddy, P.H. Hippocampal mutant APP and amyloid beta-induced cognitive decline, dendritic spine loss, defective autophagy, mitophagy and mitochondrial abnormalities in a mouse model of Alzheimer’s disease. Hum. Mol. Genet. 2018, 27, 1332–1342. [Google Scholar] [CrossRef]
- Lauritzen, I.; Pardossi-Piquard, R.; Bourgeois, A.; Pagnotta, S.; Biferi, M.G.; Barkats, M.; Lacor, P.; Klein, W.; Bauer, C.; Checler, F. Intraneuronal aggregation of the beta-CTF fragment of APP (C99) induces Abeta-independent lysosomal-autophagic pathology. Acta Neuropathol. 2016, 132, 257–276. [Google Scholar] [CrossRef]
- Feng, Q.; Luo, Y.; Zhang, X.N.; Yang, X.F.; Hong, X.Y.; Sun, D.S.; Li, X.C.; Hu, Y.; Li, X.G.; Zhang, J.F.; et al. MAPT/Tau accumulation represses autophagy flux by disrupting IST1-regulated ESCRT-III complex formation: A vicious cycle in Alzheimer neurodegeneration. Autophagy 2020, 16, 641–658. [Google Scholar] [CrossRef]
- Jaqua, E.E.; Tran, M.N.; Hanna, M. Alzheimer disease: Treatment of cognitive and functional symptoms. Am. Fam. Physician 2024, 110, 281–293. [Google Scholar]
- Goncalves, P.B.; Sodero, A.C.R.; Cordeiro, Y. Natural products targeting amyloid-beta oligomer neurotoxicity in Alzheimer’s disease. Eur. J. Med. Chem. 2024, 276, 116684. [Google Scholar] [CrossRef] [PubMed]
- Bhadane, P.; Roul, K.; Belemkar, S.; Kumar, D. Immunotherapeutic approaches for Alzheimer’s disease: Exploring active and passive vaccine progress. Brain Res. 2024, 1840, 149018. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Simpson, C.; Patel, K. Lecanemab: A humanized monoclonal antibody for the treatment of early Alzheimer disease. Ann. Pharmacother. 2024, 58, 1045–1053. [Google Scholar] [CrossRef]
- Mahboob, A.; Ali, H.; AlNaimi, A.; Yousef, M.; Rob, M.; Al-Muhannadi, N.A.; Senevirathne, D.K.L.; Chaari, A. Immunotherapy for Parkinson’s disease and Alzheimer’s disease: A promising disease-modifying therapy. Cells 2024, 13, 1527. [Google Scholar] [CrossRef] [PubMed]
- Lutz, D.; Kataria, H.; Kleene, R.; Loers, G.; Chaudhary, H.; Guseva, D.; Wu, B.; Jakovcevski, I.; Schachner, M. Myelin basic protein cleaves cell adhesion molecule L1 and improves regeneration after injury. Mol. Neurobiol. 2016, 53, 3360–3376. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef]
- Appel, F.; Holm, J.; Conscience, J.F.; von Bohlen und Halbach, F.; Faissner, A.; James, P.; Schachner, M. Identification of the border between fibronectin type III homologous repeats 2 and 3 of the neural cell adhesion molecule L1 as a neurite outgrowth promoting and signal transducing domain. J. Neurobiol. 1995, 28, 297–312. [Google Scholar] [CrossRef]
- Makhina, T.; Loers, G.; Schulze, C.; Ueberle, B.; Schachner, M.; Kleene, R. Extracellular GAPDH binds to L1 and enhances neurite outgrowth. Mol. Cell Neurosci. 2009, 41, 206–218. [Google Scholar] [CrossRef]
- Schwarze, S.R.; Dowdy, S.F. In vivo protein transduction: Intracellular delivery of biologically active proteins, compounds and DNA. Trends Pharmacol. Sci. 2000, 21, 45–48. [Google Scholar] [CrossRef]
- Chen, S.; Mantei, N.; Dong, L.; Schachner, M. Prevention of neuronal cell death by neural adhesion molecules L1 and CHL1. J. Neurobiol. 1999, 38, 428–439. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Doherty, P.; Williams, E.; Walsh, F.S. A soluble chimeric form of the L1 glycoprotein stimulates neurite outgrowth. Neuron 1995, 14, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Bolte, S.; Cordelieres, F.P. A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 2006, 224, 213–232. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loers, G.; Bork, U.; Schachner, M. Functional Relationships between L1CAM, LC3, ATG12, and Aβ. Int. J. Mol. Sci. 2024, 25, 10829. https://doi.org/10.3390/ijms251910829
Loers G, Bork U, Schachner M. Functional Relationships between L1CAM, LC3, ATG12, and Aβ. International Journal of Molecular Sciences. 2024; 25(19):10829. https://doi.org/10.3390/ijms251910829
Chicago/Turabian StyleLoers, Gabriele, Ute Bork, and Melitta Schachner. 2024. "Functional Relationships between L1CAM, LC3, ATG12, and Aβ" International Journal of Molecular Sciences 25, no. 19: 10829. https://doi.org/10.3390/ijms251910829





