The Complexity of the Influence of Growth Substances, Heavy Metals, and Their Combination on the Volume Dynamics of Vacuoles Isolated from Red Beet (Beta vulgaris L.) Taproot Cells
Abstract
:1. Introduction
2. Results
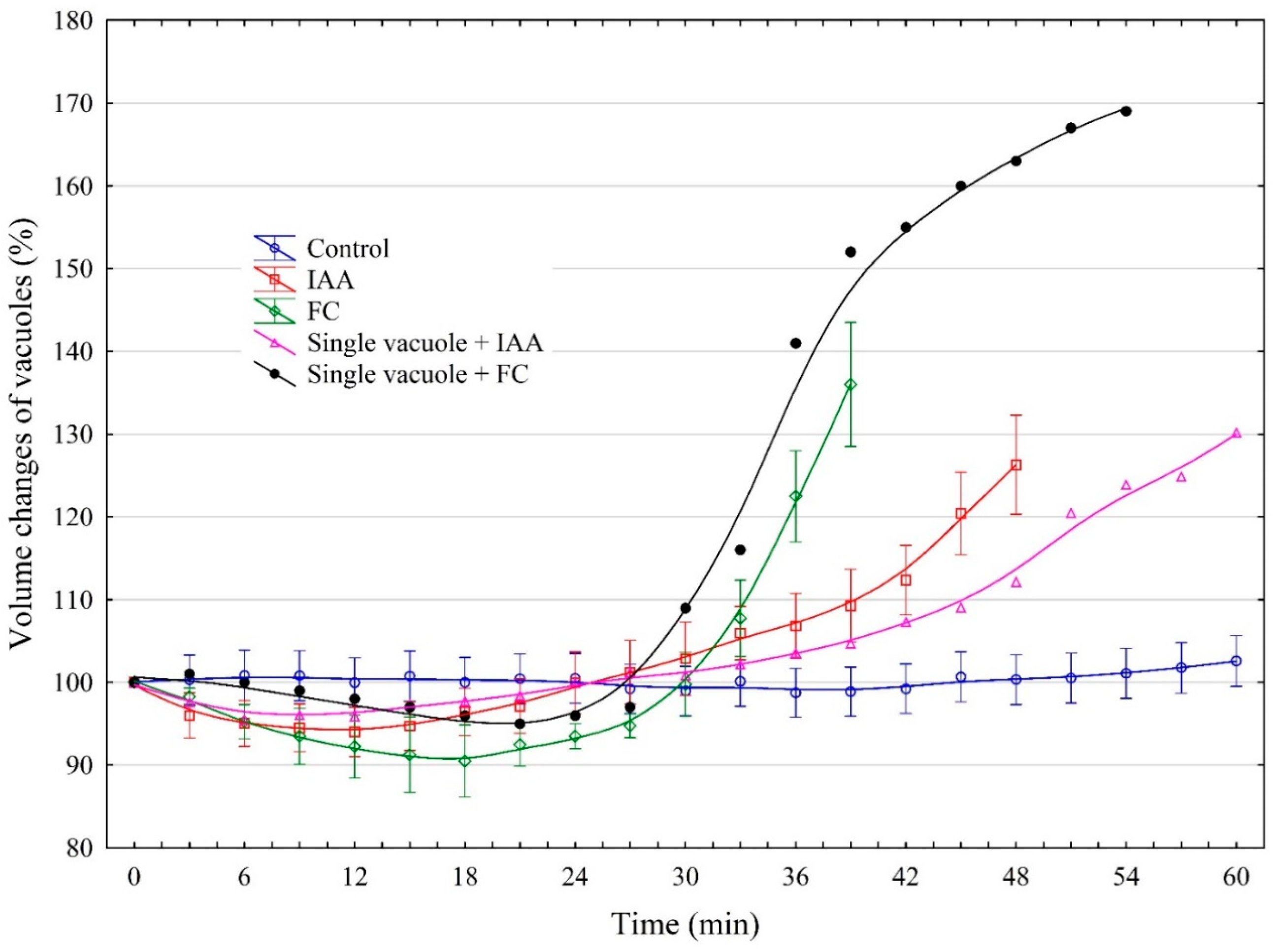
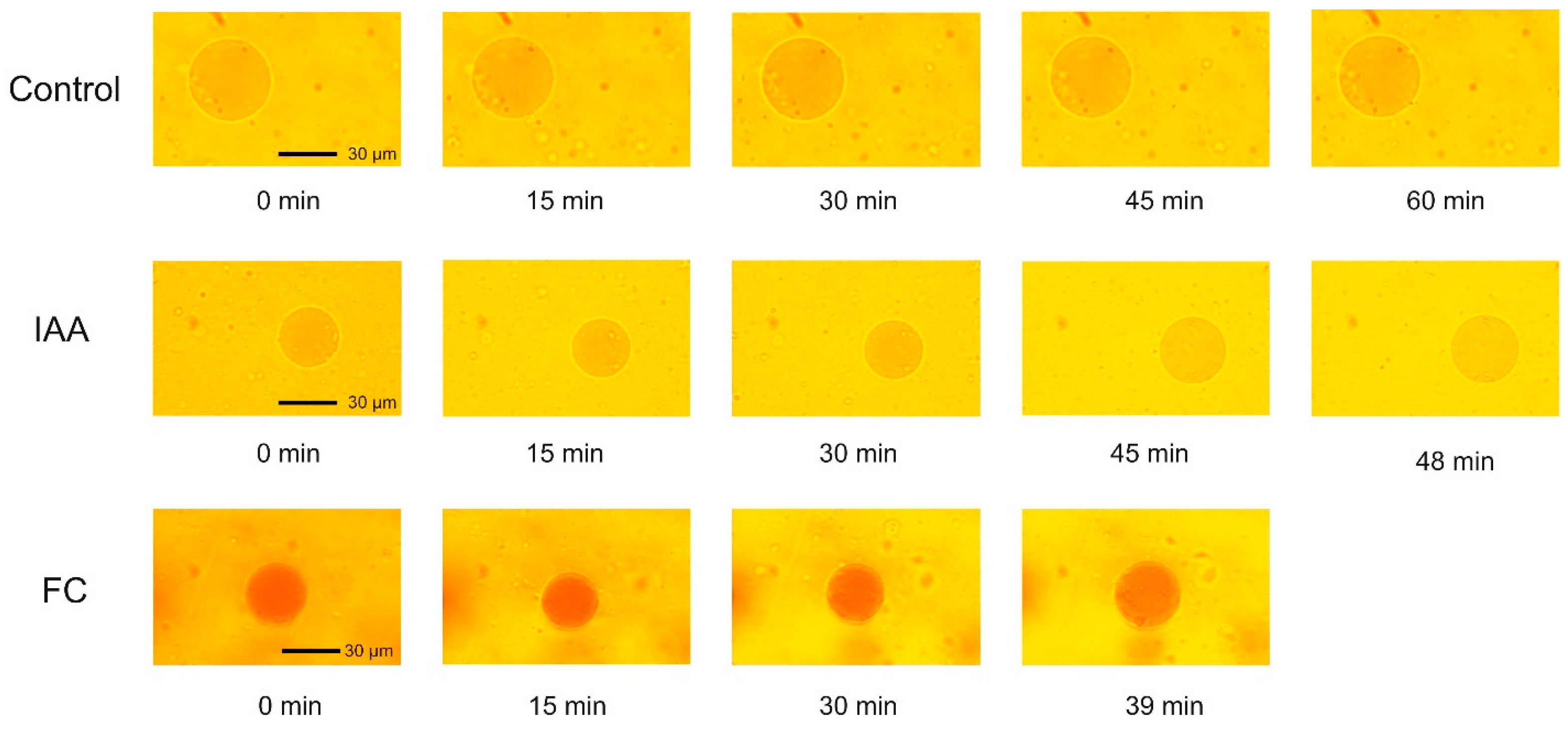
2.1. Effect of IAA and FC on the Volume Changes of Red Beet Taproot Vacuoles
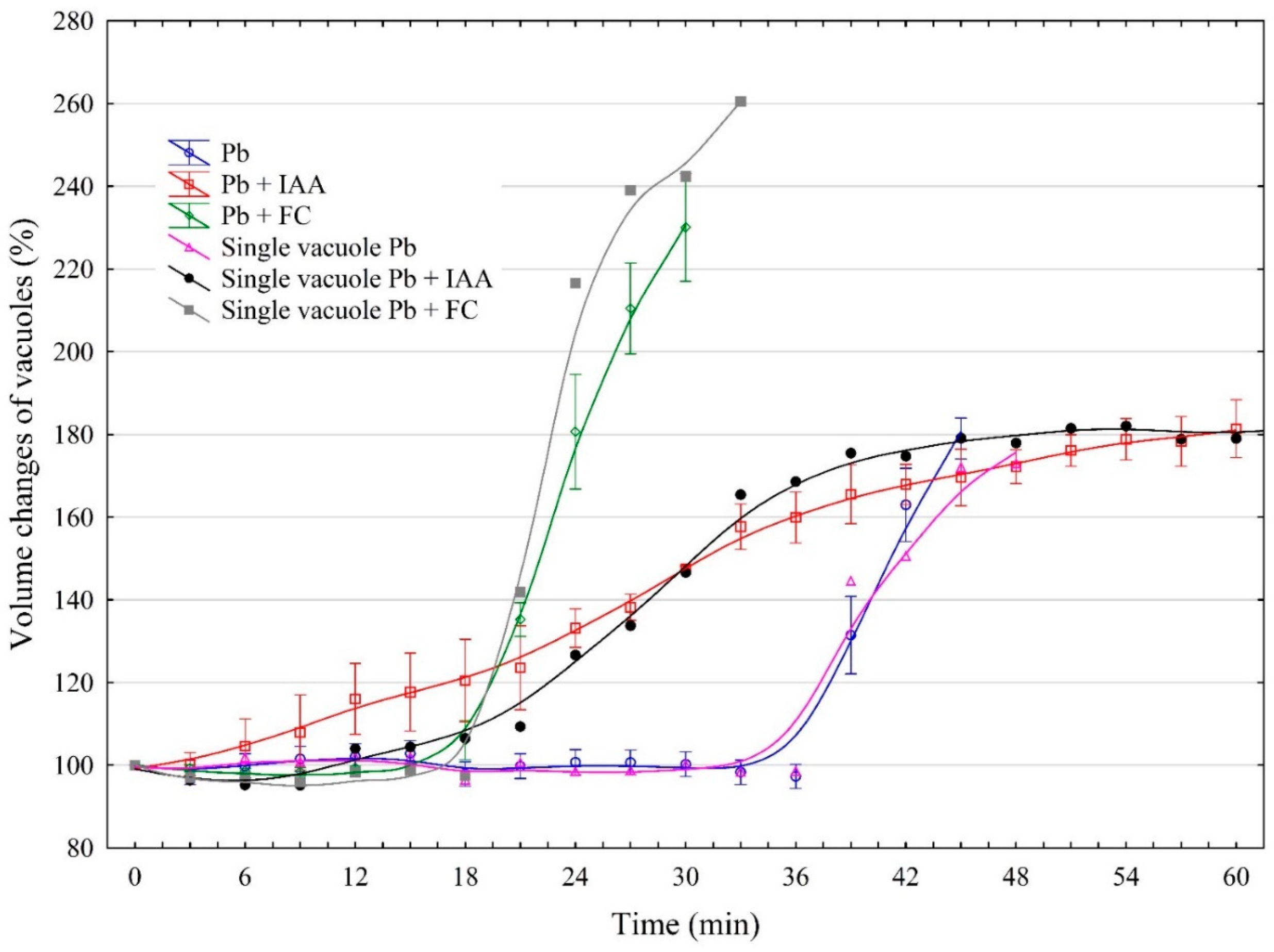
2.2. The Effect of IAA and FC and Their Combination with Pb on the Vacuole Volume Dynamics
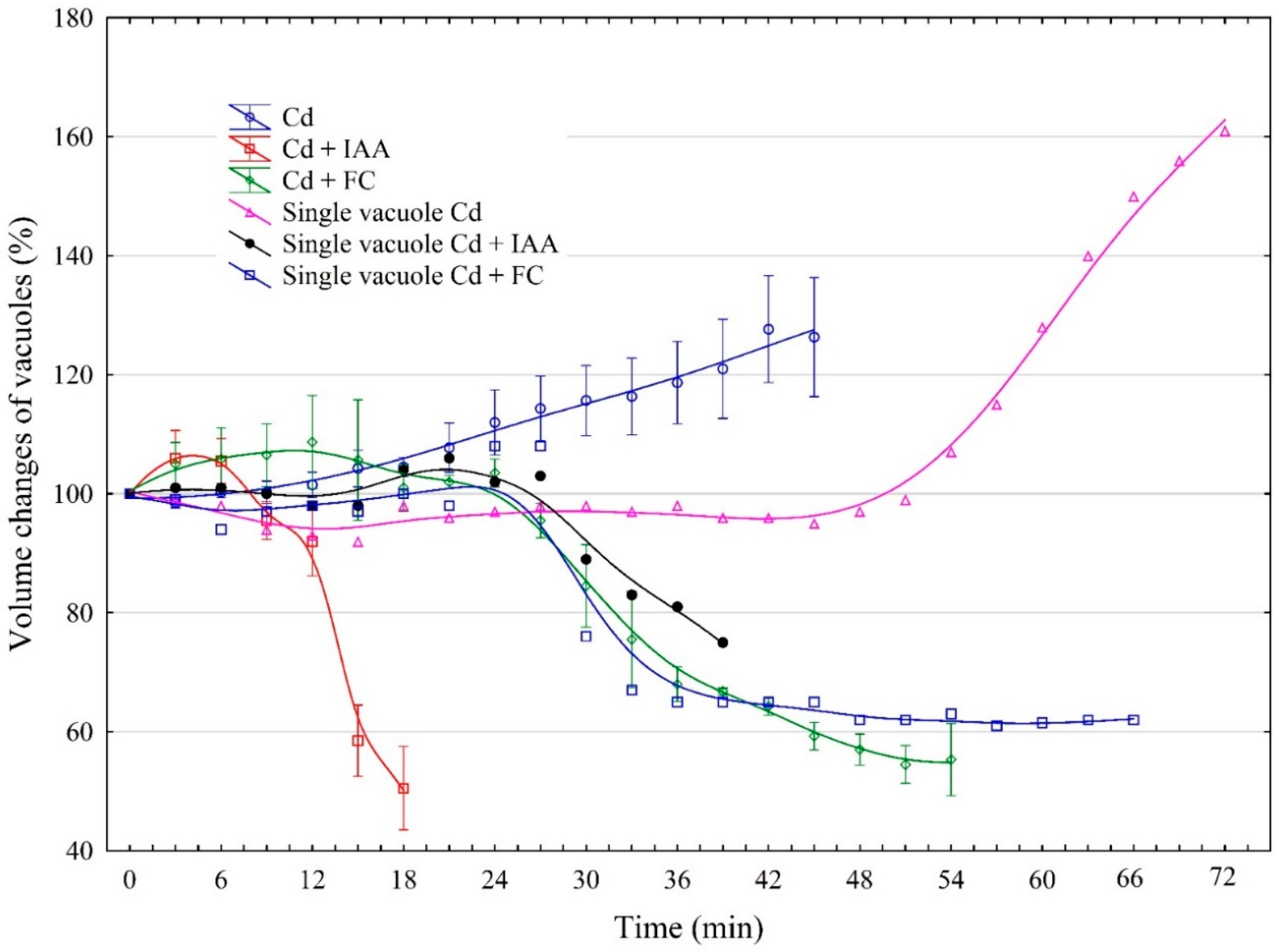
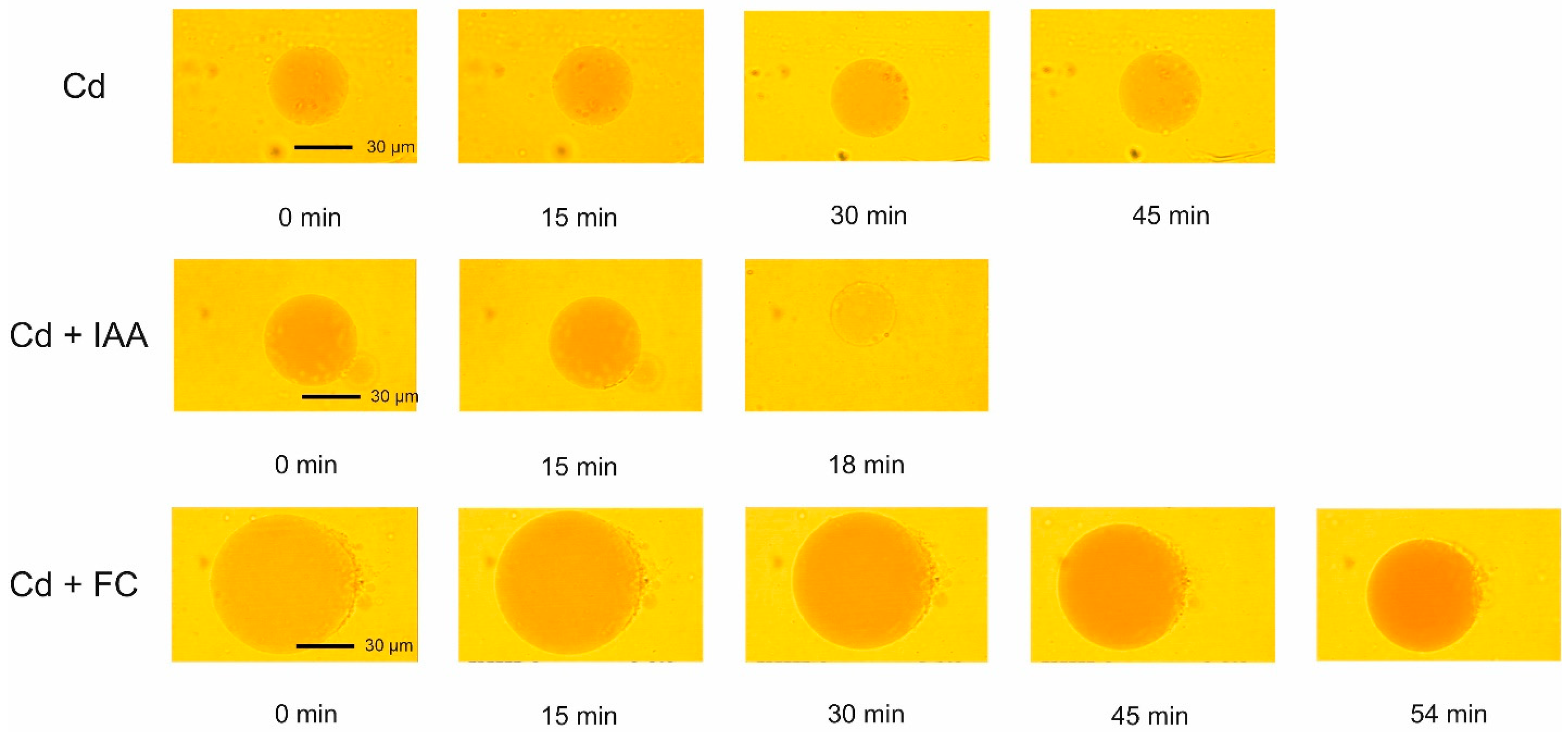
2.3. The Effect of IAA and FC and Their Combination with Cd on the Volume Changes in Vacuoles
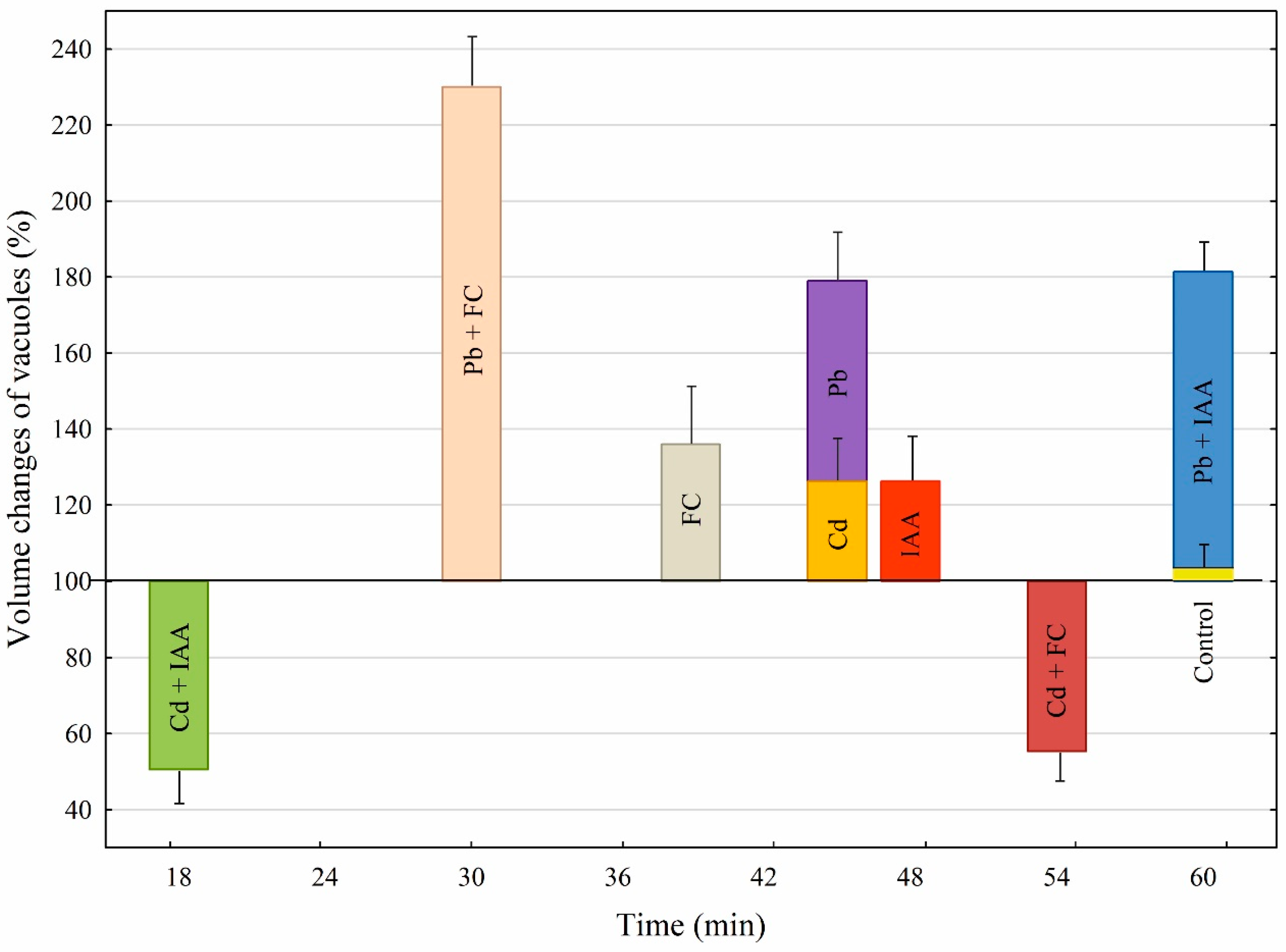
2.4. A Comparison of the Effects of Growth Substances and Heavy Metals as Well as Their Combination on Both the Volume Changes of Vacuoles and the Time after Which the Vacuoles Ruptured
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Chemicals
4.3. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cosgrove, D.J. Diffuse growth of plant cell walls. Plant Physiol. 2018, 176, 16–27. [Google Scholar] [CrossRef]
- Hager, A. Role of the plasma membrane H+-ATPase in auxin-induced elongation growth: Historical and new aspects. J. Plant Res. 2003, 116, 483–505. [Google Scholar] [CrossRef]
- Becker, D.; Hedrich, R. Channelling auxin action: Modulation of ion transport by indole-3-acetic acid. Plant Mol. Biol. 2002, 49, 349–356. [Google Scholar] [CrossRef]
- Philippar, K.; Fuchs, I.; Lüthen, H.; Hoth, S.; Bauer, C.S.; Haga, K.; Thiel, G.; Ljung, K.; Sandberg, G.; Böttger, M.; et al. Auxin-induced K+ channel expression represents an essential step in coleoptile growth and gravitropism. Proc. Natl. Acad. Sci. USA 1999, 96, 12186–12191. [Google Scholar] [CrossRef]
- D’Amore, A.; Gradogna, A.; Palombi, F.; Minicozzi, V.; Ceccarelli, M.; Carpaneto, A.; Filippini, A. The discovery of naringenin as endolysosomal two -pore channel inhibitor and its emerging role in SARS-CoV-2 infection. Cells 2021, 10, 1130. [Google Scholar] [CrossRef]
- Hedrich, R.; Mueller, T.D.; Becker, D.; Marten, I. Structure and function of TPC1 vacuole SV channel gains shape. Mol. Plant 2018, 11, 764–775. [Google Scholar] [CrossRef]
- Burdach, Z.; Siemieniuk, A.; Trela, Z.; Kurtyka, R.; Karcz, W. Role of auxin (IAA) in the regulation of slow vacuolar (SV) channels and the volume of red beet taproot vacuoles. BMC Plant Biol. 2018, 18, 102. [Google Scholar] [CrossRef]
- Burdach, Z.; Siemieniuk, A.; Karcz, W. Effect of auxin (IAA) on the fast vacuolar (FV) channels in red beet (Beta vulgaris L.) taproot vacuoles. Int. J. Mol. Sci. 2020, 21, 4876. [Google Scholar] [CrossRef]
- Martinoia, E.; Meyer, S.; De Angeli, A.; Nagy, R. Vacuolar transporters in their physiological context. Annu. Rev. Plant Biol. 2012, 63, 183–213. [Google Scholar] [CrossRef]
- Zhang, C.; Hicks, G.R.; Raikhel, N.V. Plant vacuole morphology and vacuolar trafficking. Front. Plant Sci. 2014, 5, 476. [Google Scholar] [CrossRef]
- Löfke, C.; Dünser, K.; Scheuring, D.; Kleine-Vehn, J. Auxin regulates SNARE-dependent vacuolar morphology restricting cell size. eLife 2015, 4, e05868. [Google Scholar] [CrossRef] [PubMed]
- Dünser, K.; Kleine-Vehn, J. Differential growth regulation in plants—The acid growth balloon theory. Curr. Opin. Plant Biol. 2015, 28, 55–59. [Google Scholar] [CrossRef]
- Kaiser, S.; Scheuring, D. To lead or to follow: Contribution of the plant vacuole to cell growth. Front. Plant Sci. 2020, 11, 553. [Google Scholar] [CrossRef]
- Kang, B.H.; Anderson, C.T.; Arimura, S.I.; Bayer, E.; Bezanilla, M.; Botella, M.A.; Brandizzi, F.; Burch-Smith, T.M.; Chapman, K.D.; Dünser, K.; et al. A glossary of plant cell structures: Current insights and future questions. Plant Cell 2022, 34, 10–52. [Google Scholar] [CrossRef]
- Ranocha, P.; Dima, O.; Nagy, R.; Felten, J.; Corratge’-Faillie, C.; Novak, O.; Morreel, K.; Lacombe, B.; Martinez, Y.; Pfrunder, S.; et al. Arabidopsis WAT1 is a vacuolar auxin transport facilitator required for auxin homoeostasis. Nat. Commun. 2013, 4, 2625. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, D.J. Building an extensible cell wall. Plant Physiol. 2022, 189, 1246–1277. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Plant Cell Growth and Cell Wall Enlargement. Encycl. Life Sci. 2022, 2, 11. [Google Scholar]
- Majda, M.; Robert, S. The role of auxin in cell wall expansion. Int. J. Mol. Sci. 2018, 19, 951. [Google Scholar] [CrossRef] [PubMed]
- Marra, M.; Camoni, L.; Visconti, S.; Fiorillo, A.; Evidente, A. The surprising story of fusicoccin: A wilt-inducing phytotoxin, a tool in plant physiology and a 14-3-3-targeted drug. Biomolecules 2021, 11, 1393. [Google Scholar] [CrossRef]
- Marrè, E. Fusicoccin: A tool in plant physiology. Annu. Rev. Plant Physiol. 1979, 30, 273–288. [Google Scholar] [CrossRef]
- Polak, M.; Karcz, W. Fusicoccin (FC)-induced rapid growth, proton extrusion and membrane potential changes in maize (Zea mays L.) Coleoptile Cells: Comparison to Auxin Responses. Int. J. Mol. Sci. 2021, 22, 5017. [Google Scholar] [CrossRef]
- Polak, M.; Karcz, W. Some new methodological and conceptual aspects of the “acid growth theory” for the auxin action in maize (Zea mays L.) coleoptile segments: Do acid-and auxin-induced rapid growth differ in their mechanisms? Int. J. Mol. Sci. 2021, 22, 2317. [Google Scholar] [CrossRef]
- Vitali, V.; Sutka, M.; Amodeo, G.; Chara, O.; Ozu, M. The water to solute permeability ratio governs the osmotic volume dynamics in beetroot vacuoles. Front. Plant Sci. 2016, 7, 1388. [Google Scholar] [CrossRef]
- Steffens, B.; Lüthen, H. New methods to analyse auxin-induced growth II: The swelling reaction of protoplasts—A model system for the analysis of auxin signal transduction? Plant Growth Regul. 2000, 32, 115–122. [Google Scholar] [CrossRef]
- Cram, W.J. Mannitol transport and suitability as an osmoticum in root cells. Physiol. Plant. 1984, 61, 396–404. [Google Scholar] [CrossRef]
- Greutert, H.; Martinoia, E.; Keller, F. Mannitol transport by vacuoles of storage parenchyma of celery petioles operates by facilitated diffusion. J. Plant Physiol. 1998, 153, 91–96. [Google Scholar] [CrossRef]
- Barbier-Brygoo, H.; Renaudin, J.P.; Manigault, P.; Mathieu, Y.; Kurkdjian, A.; Guern, J. Properties of Vacuoles as a Function of the Isolation Procedure. In NATO ASI Series. Plant Vacuoles; Marin, B., Ed.; Springer: Boston, MA, USA, 1987; Volume 134. [Google Scholar]
- Alexandre, J.; Lassalles, J.P.; Thellier, M. Electrical noise measurements on red beet vacuoles: Another way to detect the ATPase activity. Plant Physiol. 1986, 81, 1147–1150. [Google Scholar] [CrossRef]
- Goldman, R.P.; Jozefkowicz, C.; Canessa Fortuna, A.; Sutka, M.; Alleva, K.; Ozu, M. Tonoplast (Bv TIP 1; 2) and plasma membrane (Bv PIP 2; 1) aquaporins show different mechanosensitive properties. FEBS Lett. 2017, 591, 1555–1565. [Google Scholar] [CrossRef]
- Leitão, L.; Prista, C.; Loureiro-Dias, M.C.; Moura, T.F.; Soveral, G. The grapevine tonoplast aquaporin TIP2; 1 is a pressure gated water channel. Biochem. Biophys. Res. Commun. 2014, 450, 289–294. [Google Scholar] [CrossRef]
- Hąc-Wydro, K.; Flasiński, M. The studies on the toxicity mechanism of environmentally hazardous natural (IAA) and synthetic (NAA) auxin-the experiments on model Arabidopsis thaliana and rat liver plasma membranes. Colloids Surf. B 2015, 130, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Angulo-Bejarano, P.I.; Puente-Rivera, J.; Cruz-Ortega, R. Metal and metalloid toxicity in plants: An overview on molecular aspects. Plants 2021, 10, 635. [Google Scholar] [CrossRef] [PubMed]
- Przedpelska-Wasowicz, E.M.; Wierzbicka, M. Gating of aquaporins by heavy metals in Allium cepa L. epidermal cells. Protoplasma 2011, 248, 663–671. [Google Scholar] [CrossRef]
- Hąc-Wydro, K.; Sroka, A.; Jabłońska, K. The impact of auxins used in assisted phytoextraction of metals from the contaminated environment on the alterations caused by lead (II) ions in the organization of model lipid membranes. Colloids Surf. B 2016, 143, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Hąc-Wydro, K.; Mach, M.; Węder, K.; Pająk, K.; Wydro, P. Effect of Cd2+ and Cd2+/auxin mixtures on lipid monolayers-Model membrane studies on the role of auxins in phytoremediation of metal ions from contaminated environment. Biochim. Biophys. Acta Biomembr. 2017, 1859, 1164–1171. [Google Scholar] [CrossRef] [PubMed]
- Kurtyka, R.; Kita, A.; Karcz, W. Fusicoccin counteracts the toxic effect of cadmium on the growth of maize coleoptile segments. Arch. Environ. Cont. Tox. 2011, 61, 568–577. [Google Scholar] [CrossRef]
- Kurtyka, R.; Burdach, Z.; Siemieniuk, A.; Karcz, W. Single and combined effects of Cd and Pb on the growth, medium pH, membrane potential and metal contents in maize (Zea mays L.) coleoptile segments. Ecotox. Environ. Safe. 2018, 161, 8–16. [Google Scholar] [CrossRef]
- Coyaud, L.; Kurkdjian, A.; Kado, R.; Hedrich, R. Ion channels and ATP-driven pumps involved in ion transport across the tonoplast of sugarbeet vacuoles. Biochim. Biophys. Acta 1987, 902, 263–268. [Google Scholar] [CrossRef]
- Karcz, W.; Kurtyka, R. Effect of cadmium on growth, proton extrusion and membrane potential in maize coleoptile segments. Biol. Plant. 2007, 51, 713–719. [Google Scholar] [CrossRef]
- Siemieniuk, A.; Burdach, Z.; Karcz, W. A comparison of the effect of lead (Pb) on the slow vacuolar (SV) and fast vacuolar (FV) channels in red beet (Beta vulgaris L.) taproot vacuoles. Int. J. Mol. Sci. 2021, 22, 12621. [Google Scholar] [CrossRef]
- Llamas, A.; Ullrich, C.I.; Sanz, A. Cd2+ effects on transmembrane electrical potential difference, respiration and membrane permeability of rice (Oryza sativa L) roots. Plant Soil 2000, 219, 21–28. [Google Scholar] [CrossRef]
- Puertas-Mejía, M.A.; Ruiz-Díez, B.; Fernández-Pascual, M. Effect of cadmium ion excess over cell structure and functioning of Zea mays and Hordeum vulgare. Biochem. Syst. Ecol. 2010, 38, 285–291. [Google Scholar] [CrossRef]
- Sanz, A.; Llamas, A.; Ullrich, C.I. Distinctive phytotoxic effects of Cd and Ni on membrane functionality. Plant Signal. Behav. 2009, 4, 980–982. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karcz, W.; Burdach, Z. The Complexity of the Influence of Growth Substances, Heavy Metals, and Their Combination on the Volume Dynamics of Vacuoles Isolated from Red Beet (Beta vulgaris L.) Taproot Cells. Int. J. Mol. Sci. 2024, 25, 10842. https://doi.org/10.3390/ijms251910842
Karcz W, Burdach Z. The Complexity of the Influence of Growth Substances, Heavy Metals, and Their Combination on the Volume Dynamics of Vacuoles Isolated from Red Beet (Beta vulgaris L.) Taproot Cells. International Journal of Molecular Sciences. 2024; 25(19):10842. https://doi.org/10.3390/ijms251910842
Chicago/Turabian StyleKarcz, Waldemar, and Zbigniew Burdach. 2024. "The Complexity of the Influence of Growth Substances, Heavy Metals, and Their Combination on the Volume Dynamics of Vacuoles Isolated from Red Beet (Beta vulgaris L.) Taproot Cells" International Journal of Molecular Sciences 25, no. 19: 10842. https://doi.org/10.3390/ijms251910842





