High-Throughput Transcriptomics Identifies Chemoresistance-Associated Gene Expression Signatures in Human Angiosarcoma
Abstract
1. Introduction
2. Results
2.1. Patient Cohort
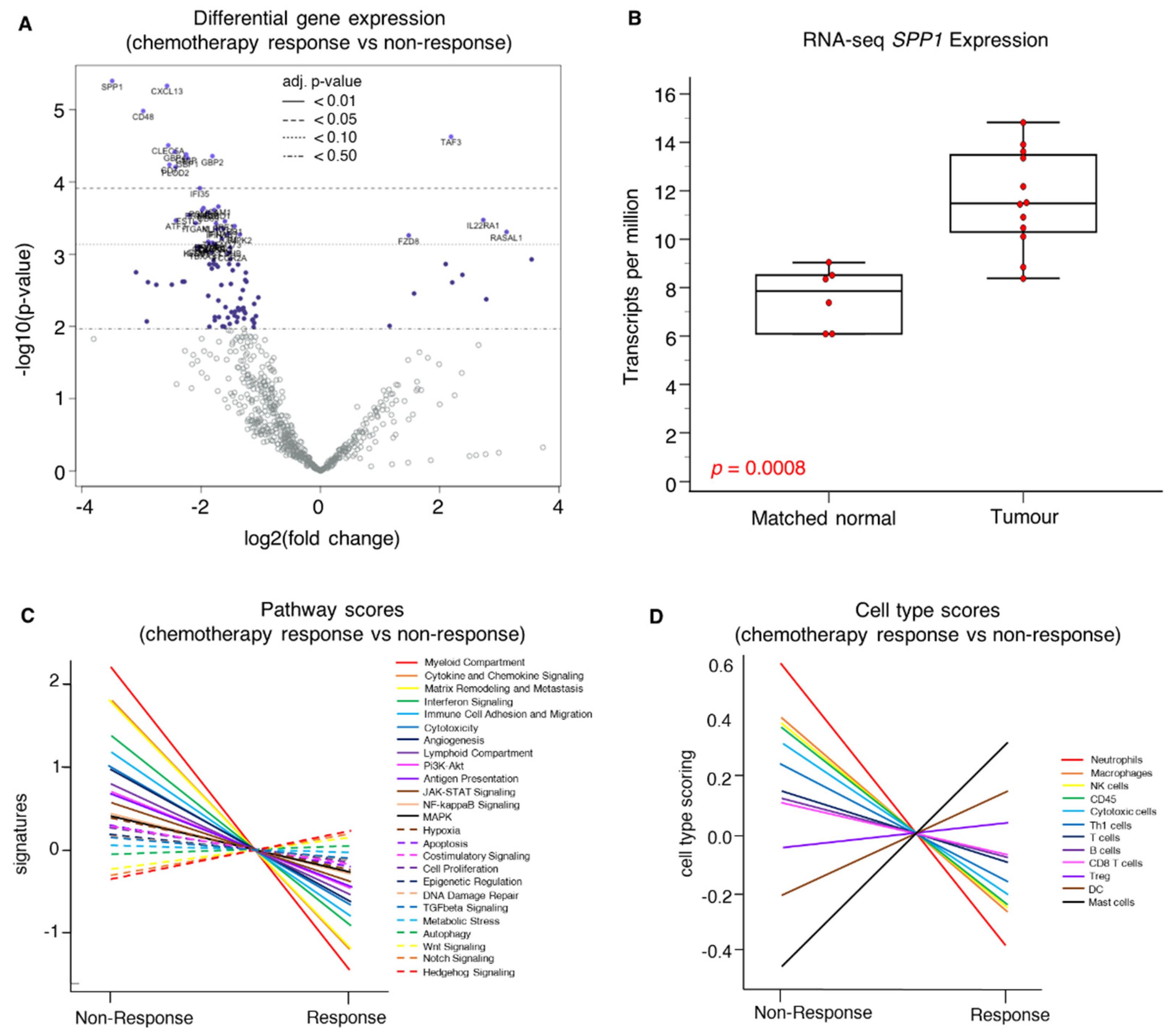
2.2. NanoString Gene Expression Profiling
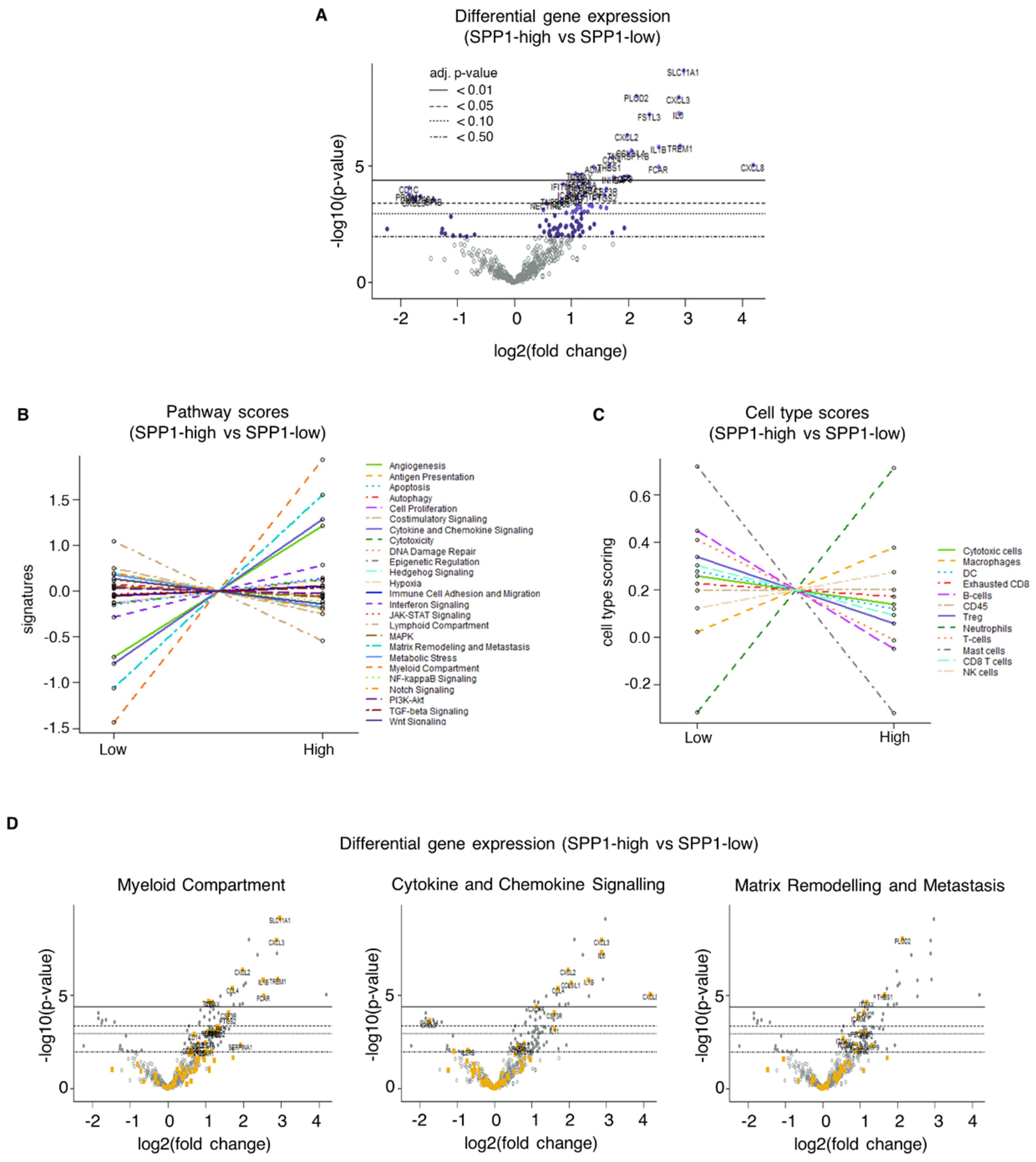
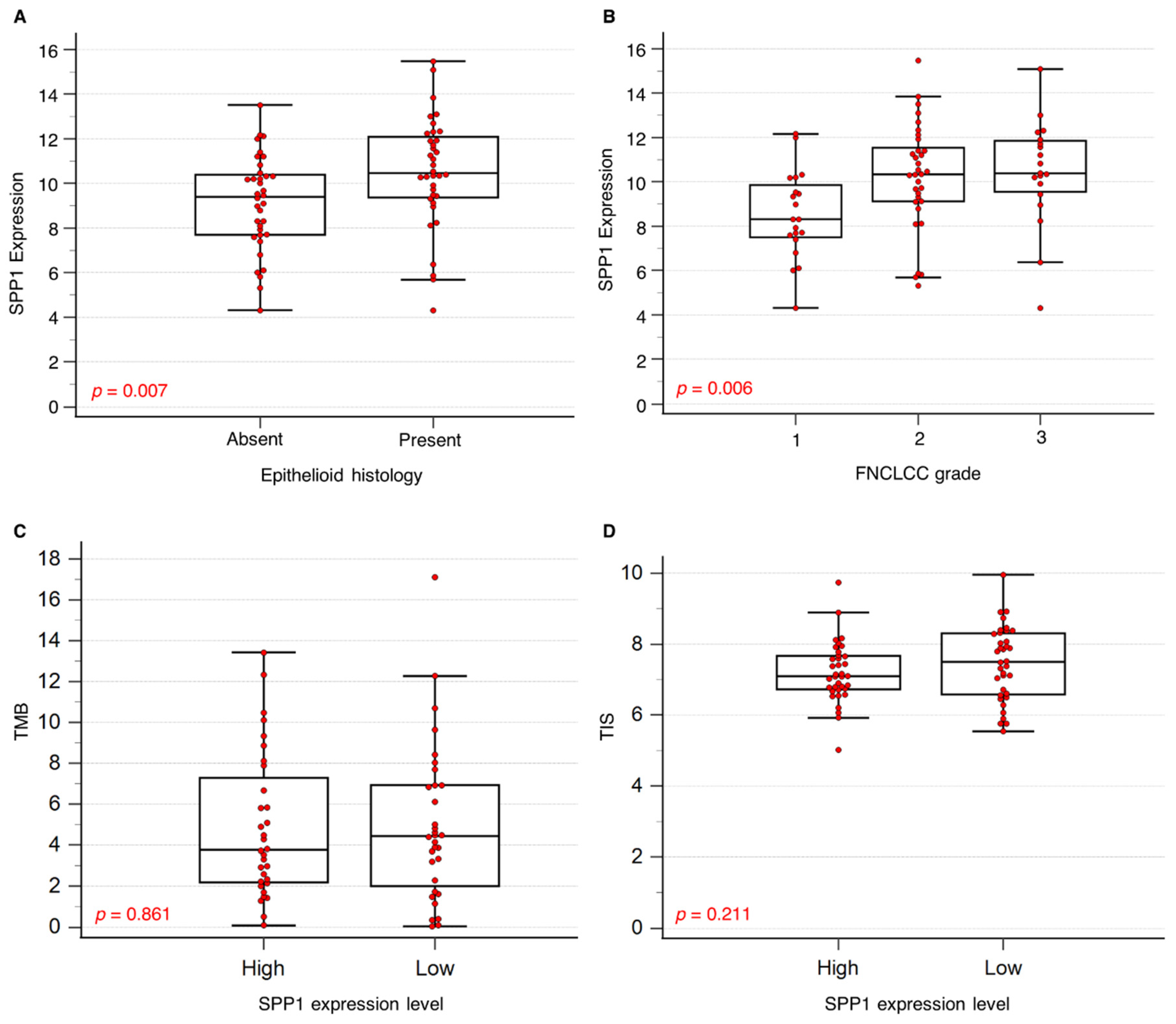
2.2.1. Association of SPP1 mRNA Expression with Clinical Parameters
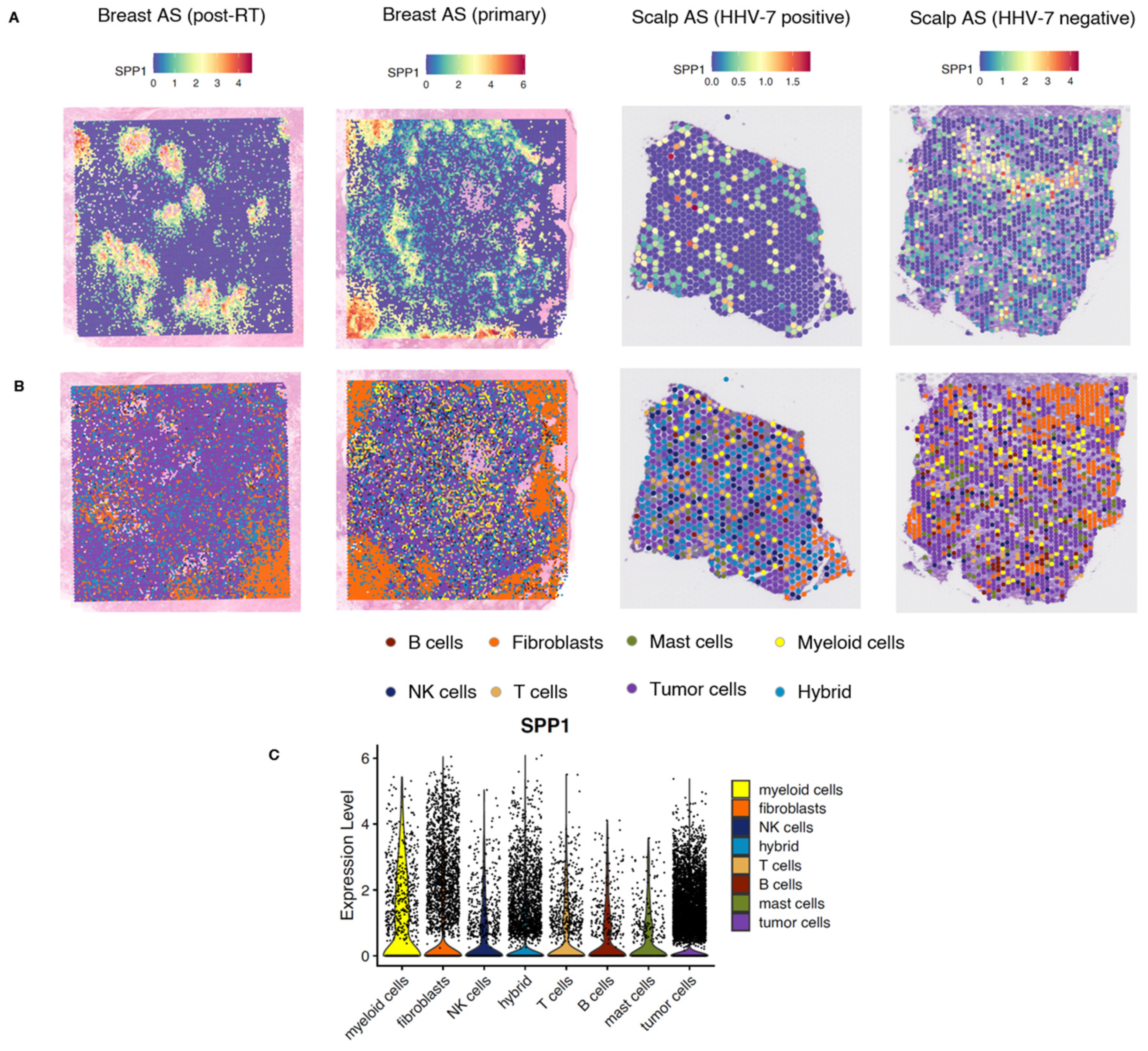
2.2.2. Spatial Analysis of Breast and Scalp Angiosarcoma
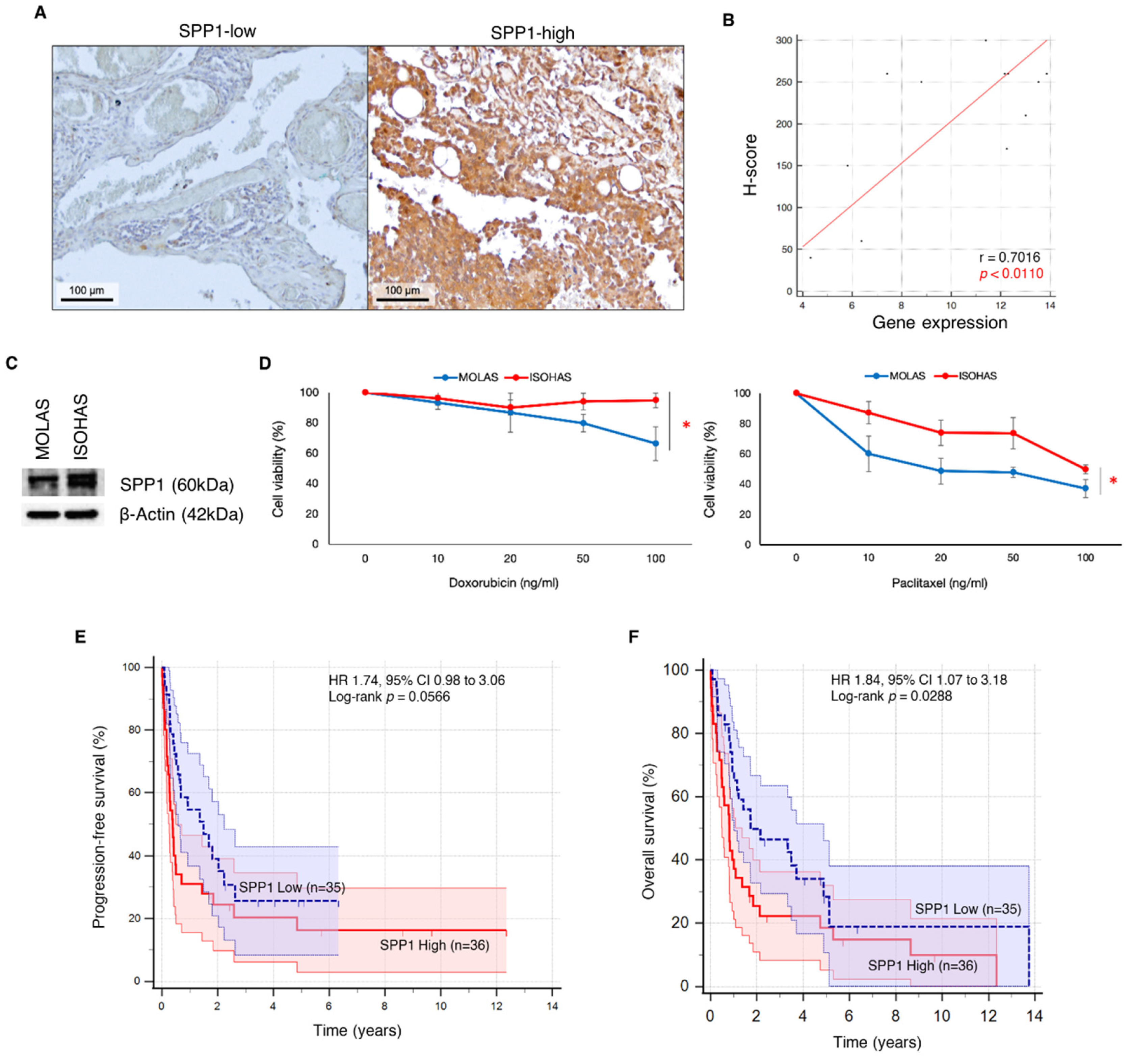
2.2.3. Immunohistochemical Staining for SPP1 Protein Expression
2.2.4. In Vitro Response to Chemotherapeutic Drugs
3. Discussion
4. Materials and Methods
4.1. Patient Cohort
4.1.1. NanoString Gene Expression Profiling
4.1.2. Immunohistochemistry Staining
4.1.3. Western Blotting
4.1.4. Cell Lines and Cell Viability Assays
4.1.5. 10× Genomics Visium Platform
4.1.6. Analysis of Spatial Sequencing Data
4.1.7. Statistics
4.1.8. Study Approval
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Young, R.J.; Brown, N.J.; Reed, M.W.; Hughes, D.; Woll, P.J. Angiosarcoma. Lancet Oncol. 2010, 11, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.Y.; Lim, J.Q.; Yeong, J.; Ravi, V.; Guan, P.; Boot, A.; Tay, T.K.Y.; Selvarajan, S.; Nasir, N.D.M.; Loh, J.H.; et al. Multiomic analysis and immunoprofiling reveal distinct subtypes of human angiosarcoma. J. Clin. Investig. 2020, 130, 5833–5846. [Google Scholar] [CrossRef] [PubMed]
- Lim, R.M.H.; Lee, J.Y.; Kannan, B.; Ko, T.K.; Chan, J.Y. Molecular and immune pathobiology of human angiosarcoma. Biochim. Biophys. Acta Rev. Cancer 2024, 1879, 189159. [Google Scholar] [CrossRef] [PubMed]
- Florou, V.; Wilky, B.A. Current and future directions for angiosarcoma therapy. Curr. Treat. Options Oncol. 2018, 19, 14. [Google Scholar] [CrossRef]
- Penel, N.; Italiano, A.; Ray-Coquard, I.; Chaigneau, L.; Delcambre, C.; Robin, Y.M.; Bui, B.; Bertucci, F.; Isambert, N.; Cupissol, D.; et al. Metastatic angiosarcomas: Doxorubicin-based regimens, weekly paclitaxel and metastasectomy significantly improve the outcome. Ann. Oncol. 2012, 23, 517–523. [Google Scholar] [CrossRef]
- Chan, J.Y.; Tan, G.F.; Yeong, J.; Ong, C.W.; Ng, D.Y.X.; Lee, E.; Koh, J.; Ng, C.C.-Y.; Lee, J.Y.; Liu, W.; et al. Clinical implications of systemic and local immune responses in human angiosarcoma. npj Precis. Oncol. 2021, 5, 11. [Google Scholar] [CrossRef]
- Tai, S.B.; Lee, E.C.Y.; Lim, B.Y.; Kannan, B.; Lee, J.Y.; Guo, Z.; Ko, T.K.; Ng, C.C.-Y.; Teh, B.T.; Chan, J.Y. Tumor-infiltrating mast cells in angiosarcoma correlate with immuno-oncology pathways and adverse clinical outcomes. Lab. Investig. 2024, 104, 100323. [Google Scholar] [CrossRef]
- Tan, G.F.; Goh, S.; Lim, A.H.; Liu, W.; Lee, J.Y.; Rajasegaran, V.; Sam, X.X.; Tay, T.K.Y.; Selvarajan, S.; Ng, C.C.; et al. Bizarre giant cells in human angiosarcoma exhibit chemoresistance and contribute to poor survival outcomes. Cancer Sci. 2021, 112, 397–409. [Google Scholar] [CrossRef]
- Loh, J.W.; Lee, J.Y.; Lim, A.H.; Guang, P.; Lim, B.Y.; Kannan, B.; Lee, E.C.Y.; Gu, N.X.; Ko, T.K.; Ng, C.C.-Y.; et al. Spatial transcriptomics reveal topological immune landscapes of Asian head and neck angiosarcoma. Commun. Biol. 2023, 6, 461. [Google Scholar] [CrossRef]
- Painter, C.A.; Jain, E.; Tomson, B.N.; Dunphy, M.; Stoddard, R.E.; Thomas, B.S.; Damon, A.L.; Shah, S.; Kim, D.; Gómez Tejeda Zañudo, J.; et al. The Angiosarcoma Project: Enabling genomic and clinical discoveries in a rare cancer through patient-partnered research. Nat. Med. 2020, 26, 181–187. [Google Scholar] [CrossRef]
- Lin, E.Y.H.; Xi, W.; Aggarwal, N.; Shinohara, M.L. Osteopontin (Opn)/SPP1: From its biochemistry to biological functions in the innate immune system and the central nervous system (Cns). Int. Immunol. 2023, 35, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; He, H.; Meng, Q.; Zhu, Y.; Ye, X.; Xu, N.; Yu, J. Osteopontin sequence modified mesoporous calcium silicate scaffolds to promote angiogenesis in bone tissue regeneration. J. Mater. Chem. B 2020, 8, 5849–5861. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.; Zheng, H.; Li, L.; Zhou, C.; Feng, M.; Chen, L.; Li, D.; Chen, X.; Hao, B.; Sun, H.; et al. Secreted phosphoprotein 1 promotes angiogenesis of glioblastoma through upregulating PSMA expression via transcription factor HIF1α. Acta Biochim. Biophys. Sin. 2022, 55, 417–425. [Google Scholar] [PubMed]
- Liaw, L.; Skinner, M.P.; Raines, E.W.; Ross, R.; Cheresh, A.D.; Schwartz, S.M.; Giachelli, C.M. The adhesive and migratory effects of osteopontin are mediated via distinct cell surface integrins. Role of alpha v beta 3 in smooth muscle cell migration to osteopontin in vitro. J. Clin. Investig. 1995, 95, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Castello, L.M.; Raineri, D.; Salmi, L.; Clemente, N.; Vaschetto, R.; Quaglia, M.; Garzaro, M.; Gentilli, S.; Navalesi, P.; Cantaluppi, V.; et al. Osteopontin at the crossroads of inflammation and tumor progression. Mediat. Inflamm. 2017, 2017, 4049098. [Google Scholar] [CrossRef]
- Tu, Y.; Chen, C.; Fan, G. Association between the expression of secreted phosphoprotein—Related genes and prognosis of human cancer. BMC Cancer 2019, 19, 1230. [Google Scholar] [CrossRef]
- Göthlin Eremo, A.; Lagergren, K.; Othman, L.; Montgomery, S.; Andersson, G.; Tina, E. Evaluation of SPP1/osteopontin expression as predictor of recurrence in tamoxifen treated breast cancer. Sci. Rep. 2020, 10, 1451. [Google Scholar] [CrossRef]
- Pan, H.W.; Ou, Y.H.; Peng, S.Y.; Liu, S.; Lai, P.; Lee, P.; Sheu, J.; Chen, C.; Hsu, H. Overexpression of osteopontin is associated with intrahepatic metastasis, early recurrence, and poorer prognosis of surgically resected hepatocellular carcinoma. Cancer 2003, 98, 119–127. [Google Scholar] [CrossRef]
- Zou, Y.; Tan, X.; Yuan, G.; Tang, Y.; Wang, Y.; Yang, C.; Luo, S.; Wu, Z.; Yao, K. SPP1 is associated with adverse prognosis and predicts immunotherapy efficacy in penile cancer. Hum. Genom. 2023, 17, 116. [Google Scholar] [CrossRef]
- Periyasamy, A.; Gopisetty, G.; Subramanium, M.J.; Velusamy, S.; Rajkumar, T. Identification and validation of differential plasma proteins levels in epithelial ovarian cancer. J. Proteom. 2020, 226, 103893. [Google Scholar] [CrossRef]
- Guo, Z.; Huang, J.; Wang, Y.; Liu, X.-P.; Li, W.; Yao, J.; Li, S.; Hu, W. Analysis of expression and its clinical significance of the secreted phosphoprotein 1 in lung adenocarcinoma. Front. Genet. 2020, 11, 547. [Google Scholar] [CrossRef] [PubMed]
- Zeng, B.; Zhou, M.; Wu, H.; Xiong, Z. SPP1 promotes ovarian cancer progression via Integrin β1/FAK/AKT signaling pathway. OncoTargets Ther. 2018, 11, 1333–1343. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Fan, X.; Tang, M.; Chen, R.; Wang, H.; Jia, R.; Zhou, X.; Jing, W.; Wang, H.; Yang, Y.; et al. Osteopontin induces autophagy to promote chemo-resistance in human hepatocellular carcinoma cells. Cancer Lett. 2016, 383, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Du, W.; Chen, Z.; Xiang, C. Upregulation of PD-L1 by SPP1 mediates macrophage polarization and facilitates immune escape in lung adenocarcinoma. Exp. Cell Res. 2017, 359, 449–457. [Google Scholar] [CrossRef]
- Klement, J.D.; Paschall, A.V.; Redd, P.S.; Ibrahim, M.L.; Lu, C.; Yang, D.; Celis, E.; Abrams, S.I.; Ozato, K.; Liu, K. An osteopontin/CD44 immune checkpoint controls CD8+ T cell activation and tumor immune evasion. J. Clin. Investig. 2018, 128, 5549–5560. [Google Scholar] [CrossRef]
- D’Angelo, S.P.; Munhoz, R.R.; Kuk, D.; Landa, J.; Hartley, E.; Bonafede, M.; Dickson, M.A.; Gounder, M.; Keohan, M.L.; Crago, A.M.; et al. Angiosarcoma: Outcomes of systemic therapy for patients with metastatic disease. Oncology 2015, 89, 205–214. [Google Scholar] [CrossRef]
- Venkataramani, V.; Küffer, S.; Cheung, K.C.P.; Jiang, X.; Trümper, L.; Wulf, G.G.; Ströbel, P. Cd31 expression determines redox status and chemoresistance in human angiosarcomas. Clin. Cancer Res. 2018, 24, 460–473. [Google Scholar] [CrossRef]
- Zeng, P.; Zhang, X.; Xiang, T.; Ling, Z.; Lin, C.; Diao, H. Secreted phosphoprotein 1 as a potential prognostic and immunotherapy biomarker in multiple human cancers. Bioengineered 2022, 13, 3221–3239. [Google Scholar] [CrossRef]
- Gao, W.; Liu, D.; Sun, H.; Shao, Z.; Shi, P.; Li, T.; Yin, S.; Zhu, T. SPP1 is a prognostic related biomarker and correlated with tumor-infiltrating immune cells in ovarian cancer. BMC Cancer 2022, 22, 1367. [Google Scholar] [CrossRef]
- Wu, J.; Shen, Y.; Zeng, G.; Liang, Y.; Liao, G. SPP1+ TAM subpopulations in tumor microenvironment promote intravasation and metastasis of head and neck squamous cell carcinoma. Cancer Gene Ther. 2024, 31, 311–321. [Google Scholar] [CrossRef]
- Wei, R.; Wong, J.P.C.; Kwok, H.F. Osteopontin—A promising biomarker for cancer therapy. J. Cancer 2017, 8, 2173–2183. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, E.; Komohara, Y.; Esumi, S.; Shinchi, Y.; Ishizuka, S.; Mito, R.; Pan, C.; Yano, H.; Kobayashi, D.; Fujiwara, Y.; et al. Spp1 derived from macrophages is associated with a worse clinical course and chemo-resistance in lung adenocarcinoma. Cancers 2022, 14, 4374. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; LeSavage, B.L.; Hubka, K.M.; Ma, C.; Natarajan, S.; Eggold, J.T.; Xiao, Y.; Fuh, K.C.; Krishnan, V.; Enejder, A.; et al. Cancer-associated mesothelial cells promote ovarian cancer chemoresistance through paracrine osteopontin signaling. J. Clin. Investig. 2021, 131, e146186. [Google Scholar] [CrossRef] [PubMed]
- Eun, J.W.; Yoon, J.H.; Ahn, H.R.; Kim, S.; Kim, Y.B.; Bin Lim, S.; Park, W.; Kang, T.W.; Baek, G.O.; Yoon, M.G.; et al. Cancer-associated fibroblast-derived secreted phosphoprotein 1 contributes to resistance of hepatocellular carcinoma to sorafenib and lenvatinib. Cancer Commun. 2023, 43, 455–479. [Google Scholar] [CrossRef]
- Nakamura, T.; Shinriki, S.; Jono, H.; Ueda, M.; Nagata, M.; Guo, J.; Hayashi, M.; Yoshida, R.; Ota, T.; Ota, K.; et al. Osteopontin-integrin α(V)β(3) axis is crucial for 5-fluorouracil resistance in oral squamous cell carcinoma. FEBS Lett. 2015, 589, 231–239. [Google Scholar] [CrossRef]
- Song, H.; Yao, X.; Zheng, Y.; Zhou, L. Helicobacter pylori infection induces POU5F1 upregulation and SPP1 activation to promote chemoresistance and T cell inactivation in gastric cancer cells. Biochem. Pharmacol. 2024, 225, 116253. [Google Scholar] [CrossRef]
- Uchibori, T.; Matsuda, K.; Shimodaira, T.; Sugano, M.; Uehara, T.; Honda, T. IL-6 trans-signaling is another pathway to upregulate Osteopontin. Cytokine 2017, 90, 88–95. [Google Scholar] [CrossRef]
- Serlin, D.M.; Kuang, P.P.; Subramanian, M.; O’Regan, A.; Li, X.; Berman, J.S.; Goldstein, R.H. Interleukin-1beta induces osteopontin expression in pulmonary fibroblasts. J. Cell. Biochem. 2006, 97, 519–529. [Google Scholar] [CrossRef]
- Ko, T.K.; Guo, Z.; Kannan, B.; Lim, B.Y.; Lee, J.Y.; Lim, A.H.; Li, Z.; Lee, E.C.Y.; Ng, C.C.-Y.; Teh, B.T.; et al. Abstract 7048: Multiomics characterization of breast angiosarcoma from an Asian cohort reveals enrichment for angiogenesis signaling pathway and tumor-infiltrating macrophages. Cancer Res. 2024, 84 (Suppl. 6), 7048. [Google Scholar] [CrossRef]

| SPP1 Levels | Total | ||||
|---|---|---|---|---|---|
| High | Low | p | |||
| n (%) | 72 (100%) | ||||
| Sex | |||||
| Male | 21 (58.3%) | 26 (72.2%) | 0.219 | 47 (65.3%) | |
| Female | 15 (41.7%) | 10 (27.8%) | 25 (34.7%) | ||
| Age at diagnosis (years) | |||||
| <65 | 21 (58.3%) | 15 (41.7%) | 0.16 | 36 (50.0%) | |
| ≥65 | 15 (41.7%) | 21 (58.3%) | 36 (50.0%) | ||
| Ethnicity | |||||
| Chinese | 27 (75.0%) | 31 (86.1%) | 0.237 | 58 (80.6%) | |
| Other | 9 (25.0%) | 5 (13.9%) | 14 (19.4%) | ||
| FNCLCC tumor grade | |||||
| 3 | 13 (36.1%) | 6 (16.7%) | 0.005 | 19 (26.4%) | |
| 2 | 19 (52.8%) | 14 (38.9%) | 33 (45.8%) | ||
| 1 | 4 (11.1%) | 16 (44.4%) | 20 (27.8%) | ||
| Site of primary tumor | |||||
| Head and neck | 17 (47.2%) | 25 (69.4%) | 0.058 | 42 (58.3%) | |
| Others a | 19 (52.8%) | 11 (30.6%) | 30 (41.7%) | ||
| Human herpesvirus-7 | |||||
| Positive | 25 (69.4%) | 22 (61.1%) | 0.461 | 25 (34.7%) | |
| Negative | 11 (30.6%) | 14 (38.9%) | 47 (65.3%) | ||
| UV signature | |||||
| Present | 11 (30.6%) | 10 (27.8%) | 0.797 | 51 (70.8%) | |
| Absent | 25 (69.4%) | 26 (72.2%) | 21 (29.2%) | ||
| Epithelioid histology | |||||
| Present | 23 (63.9%) | 13 (36.1%) | 0.019 | 36 (50.0%) | |
| Absent | 13 (36.1%) | 23 (63.9%) | 36 (50.0%) | ||
| Disease state at diagnosis b | |||||
| Metastatic | 14 (38.9%) | 8 (22.9%) | 0.147 | 22 (31.0%) | |
| Non-metastatic | 22 (61.1%) | 27 (77.1%) | 49 (69.0%) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khor, G.M.S.; Haghani, S.; Tan, T.R.E.; Lee, E.C.Y.; Kannan, B.; Lim, B.Y.; Lee, J.Y.; Guo, Z.; Ko, T.K.; Chan, J.Y. High-Throughput Transcriptomics Identifies Chemoresistance-Associated Gene Expression Signatures in Human Angiosarcoma. Int. J. Mol. Sci. 2024, 25, 10863. https://doi.org/10.3390/ijms251910863
Khor GMS, Haghani S, Tan TRE, Lee ECY, Kannan B, Lim BY, Lee JY, Guo Z, Ko TK, Chan JY. High-Throughput Transcriptomics Identifies Chemoresistance-Associated Gene Expression Signatures in Human Angiosarcoma. International Journal of Molecular Sciences. 2024; 25(19):10863. https://doi.org/10.3390/ijms251910863
Chicago/Turabian StyleKhor, Glenys Mai Shia, Sara Haghani, Tiffany Rui En Tan, Elizabeth Chun Yong Lee, Bavani Kannan, Boon Yee Lim, Jing Yi Lee, Zexi Guo, Tun Kiat Ko, and Jason Yongsheng Chan. 2024. "High-Throughput Transcriptomics Identifies Chemoresistance-Associated Gene Expression Signatures in Human Angiosarcoma" International Journal of Molecular Sciences 25, no. 19: 10863. https://doi.org/10.3390/ijms251910863
APA StyleKhor, G. M. S., Haghani, S., Tan, T. R. E., Lee, E. C. Y., Kannan, B., Lim, B. Y., Lee, J. Y., Guo, Z., Ko, T. K., & Chan, J. Y. (2024). High-Throughput Transcriptomics Identifies Chemoresistance-Associated Gene Expression Signatures in Human Angiosarcoma. International Journal of Molecular Sciences, 25(19), 10863. https://doi.org/10.3390/ijms251910863





