Solid-State Nanopore-Based Nanosystem for Registration of Enzymatic Activity of a Single Molecule of Cytochrome P450 BM3
Abstract
1. Introduction
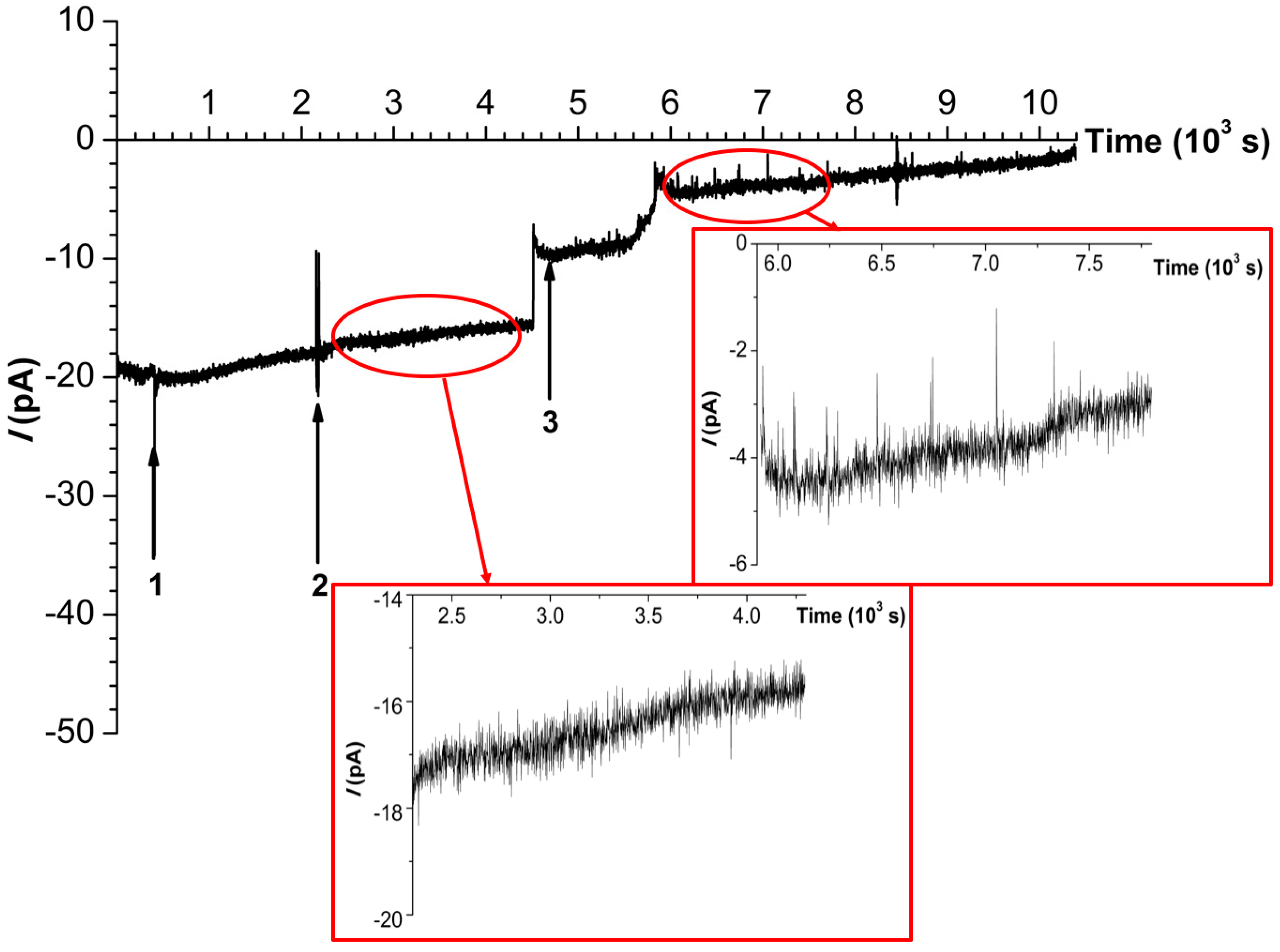
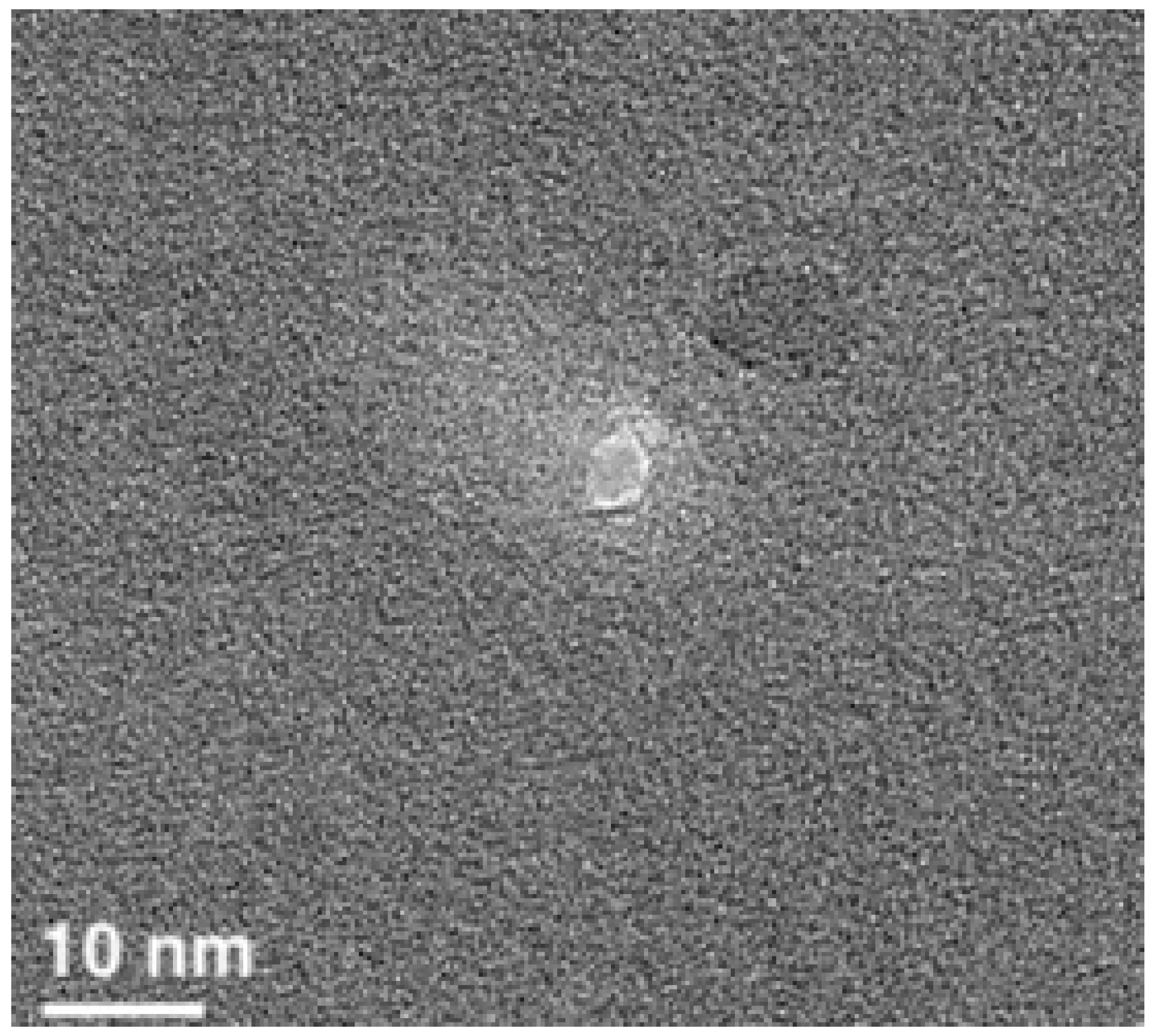
2. Results
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Enzyme Solution Preparation
4.3. Nanopore Fabrication
4.4. Electrical Measurements
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Metzler, D.E. Biochemistry, the Chemical Reactions of Living Cells, 1st ed.; Academic Press: Cambridge, UK, 1977. [Google Scholar]
- Archakov, A.; Ivanov, Y.; Lisitsa, A.; Zgoda, V. Biospecific irreversible fishing coupled with atomic force microscopy for detection of extremely low-abundant proteins. Proteomics 2009, 9, 1326–1343. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, Y.D.; Bukharina, N.S.; Frantsuzov, P.A.; Pleshakova, T.O.; Kanashenko, S.L.; Medvedeva, N.V.; Argentova, V.V.; Zgoda, V.G.; Munro, A.W.; Archakov, A.I. AFM study of cytochrome CYP102A1 oligomeric state. Soft Matter 2012, 8, 4602–4608. [Google Scholar] [CrossRef]
- Pleshakova, T.O.; Shumov, I.D.; Ivanov, Y.D.; Malsagova, K.A.; Kaysheva, A.L.; Archakov, A.I. AFM-based technologies as the way towards the reverse Avogadro number. Biochem. Mosc. Suppl. Ser. B 2015, 9, 244–257. [Google Scholar] [CrossRef]
- Xie, X.S.; Lu, H.P. Single-molecule Enzymology. J. Biol. Chem. 1999, 274, 15967–15970. [Google Scholar] [CrossRef]
- Xie, X.S. Single Molecule Approach to Enzymology. Single Mol. 2001, 4, 229–236. [Google Scholar] [CrossRef]
- Thomson, N.H.; Fritz, M.; Radmacher, M.; Cleveland, J.P.; Schmidt, C.F.; Hansma, P.K. Protein Tracking and Detection of Protein Motion using Atomic Force Microscopy. Biophys. J. 1996, 70, 2421–2431. [Google Scholar] [CrossRef]
- Radmacher, M.; Fritz, M.; Hansma, H.G.; Hansma, P.K. Direct Observation of Enzyme Activity with the Atomic Force Microscope. Science 1994, 265, 1577–1579. [Google Scholar] [CrossRef]
- Hansma, H.G. Atomic force microscopy of biomolecules. J. Vac. Sci. Technol. B 1996, 14, 1390–1394. [Google Scholar] [CrossRef]
- Pleshakova, T.O.; Bukharina, N.S.; Archakov, A.I.; Ivanov, Y.D. Atomic Force Microscopy for Protein Detection and Their Physicochemical Characterization. Int. J. Mol. Sci. 2018, 19, 1142. [Google Scholar] [CrossRef]
- Ivanov, Y.D.; Pleshakova, T.O.; Shumov, I.D.; Kozlov, A.F.; Ivanova, I.A.; Valueva, A.A.; Tatur, V.Y.; Smelov, M.V.; Ivanova, N.D.; Ziborov, V.S. AFM imaging of protein aggregation in studying the impact of knotted electromagnetic field on a peroxidase. Sci. Rep. 2020, 10, 9022. [Google Scholar] [CrossRef]
- Kuznetsov, V.Y.; Ivanov, Y.D.; Archakov, A.I. Atomic force microscopy revelation of molecular complexes in the multiprotein cytochrome P450 2B4-containing system. Proteomics 2004, 4, 2390–2396. [Google Scholar] [CrossRef] [PubMed]
- Kiselyova, O.; Yaminsky, I. Atomic Force Microscopy of Protein Complexes. In Atomic Force Microscopy: Biomedical Methods and Applications; Braga, P.C., Ricci, D., Eds.; Humana Press: Totowa, NJ, USA, 2003; Volume 242, pp. 217–230. [Google Scholar]
- Miura, Y.; Fulco, A.J. ω-1, ω-2 and ω-3 Hydroxylation of Long-Chain Fatty Acids, Amides and Alcohols by a Soluble Enzyme System from Bacillus megatyerium. Biochim. Biophys. Acta (BBA)—Lipids Lipid Metab. 1975, 388, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Neeli, R.; Girvan, H.; Lawrence, A.; Warren, M.; Leys, D.; Scrutton, N.; Munro, A. The dimeric form of flavocytochrome P450 BM3 is catalytically functional as a fatty acid hydroxylase. FEBS Lett. 2005, 579, 5582–5588. [Google Scholar] [CrossRef]
- Girvan, H.M.; Toogood, H.S.; Littleford, R.E.; Seward, H.E.; Smith, W.E.; Ekanem, I.S.; Leys, D.; Cheesman, M.R.; Munro, A.W. Novel heme coordination variants of flavocytochrome P450 BM3. Biochem. J. 2008, 417, 65–76. [Google Scholar] [CrossRef]
- Ruggeri, F.S.; Šneideris, T.; Vendruscolo, M.; Knowles, T.P.J. Atomic force microscopy for single molecule characterisation of protein aggregation. Arch. Biochem. Biophys. 2019, 664, 134–148. [Google Scholar] [CrossRef]
- Dekker, C. Solid-state nanopores. Nat. Nanotechnol. 2007, 2, 209–215. [Google Scholar] [CrossRef]
- Luo, Y.; Wu, L.; Tu, J.; Lu, Z. Application of Solid-State Nanopore in Protein Detection. Int. J. Mol. Sci. 2020, 21, 2808. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Zhang, S.; Liu, L.; Wu, H.-C. Measuring enzymatic activities with nanopores. ChemBioChem 2020, 21, 2089–2097. [Google Scholar] [CrossRef]
- Pham, B.; Eron, S.J.; Hill, M.E.; Li, X.; Fahie, M.A.; Hardy, J.A.; Chen, M. A nanopore approach for analysis of caspase-7 activity in cell lysates. Biophys. J. 2019, 117, 844–855. [Google Scholar] [CrossRef]
- Chen, H.; Lin, Y.; Long, Y.-T.; Minteer, S.D.; Ying, Y.-L. Nanopore-based measurement of the interaction of P450cam monooxygenase and putidaredoxin at the single-molecule level. Faraday Discuss. 2022, 233, 295–302. [Google Scholar] [CrossRef]
- Wloka, C.; van Meervelt, V.; van Gelder, D.; Danda, N.; Jager, N.; Williams, C.P.; Maglia, G. Label-free and real-time detection of protein ubiquitination with a biological nanopore. ACS Nano 2017, 11, 4387–4394. [Google Scholar] [CrossRef] [PubMed]
- Willems, K.; van Meervelt, V.; Wloka, C.; Maglia, G. Single-molecule nanopore enzymology. Philos. Trans. R. Soc. B 2017, 372, 20160230. [Google Scholar] [CrossRef]
- Iqbal, S.M.; Bashir, R. (Eds.) Nanopores: Sensing and Fundamental Biological Interactions; Springer Science & Business Media: New York, NY, USA, 2011. [Google Scholar]
- Pérez-Mitta, G.; Albesa, A.G.; Trautmann, C.; Toimil-Molares, M.E.; Azzaroni, O. Bioinspired integrated nanosystems based on solid-state nanopores: “Iontronic” transduction of biological, chemical and physical stimuli. Chem. Sci. 2017, 8, 890–913. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.W.; Gu, D.J.; Liu, H.; Liu, Q.J. Detection of a single enzyme molecule based on a solid-state nanopore sensor. Nanotechnology 2016, 27, 155502. [Google Scholar] [CrossRef]
- Wu, M.J.; Krapf, D.; Zandbergen, M.; Zandbergen, H.; Batson, P.E. Formation of nanopores in a SiN∕SiO2 membrane with an electron beam. Appl. Phys. Lett. 2005, 87, 113106. [Google Scholar] [CrossRef]
- Storm, A.J.; Chen, G.H.; Ling, X.S.; Zandbergen, H.W.; Dekker, C. Fabrication of solid-state nanopores with single-nanometre precision. Nat. Mater. 2003, 2, 537–540. [Google Scholar] [CrossRef]
- Chen, C.H.; Chang, X.; Wu, C.S. A novel shaped-controlled fabrication of nanopore and its applications in quantum electronics. Sci. Rep. 2019, 9, 18663. [Google Scholar] [CrossRef]
- Chen, S.J.; Howitt, D.G.; Gierhart, B.C.; Smith, R.L.; Collins, S.D. Electron Beam Drilling of Nanopores on Silicon Nitride Membranes Using a Transmission Electron Microscope. Microsc. Microanal. 2007, 13 (Suppl. S2), 534CD. [Google Scholar] [CrossRef]
- Sajeer, M.; Simran, P.; Nukala, P.; Varma, M.M. TEM based applications in solid state nanopores: From fabrication to liquid in-situ bio-imaging. Micron 2022, 162, 103347. [Google Scholar] [CrossRef]
- Ivanov, Y.D.; Ableev, A.N.; Shumov, I.D.; Ivanova, I.A.; Vaulin, N.V.; Lebedev, D.V.; Bukatin, A.S.; Mukhin, I.S.; Archakov, A.I. Registration of Functioning of a Single Horseradish Peroxidase Macromolecule with a Solid-State Nanopore. Int. J. Mol. Sci. 2023, 24, 15636. [Google Scholar] [CrossRef]
- Narhi, L.O.; Fulco, A.J. Characterization of a catalytically self-sufficient 119,000-dalton cytochrome P-450 monooxygenase induced by barbiturates in Bacillus megaterium. J. Biol. Chem. 1986, 261, 7160–7169. [Google Scholar] [CrossRef] [PubMed]
- Sevrioukova, I.; Li, H.; Zhang, H.; Peterson, J.; Poulos, T. Structure of a cytochrome P450-redox partner electron-transfer complex. Proc. Natl. Acad. Sci. USA 1999, 96, 1863–1868. [Google Scholar] [CrossRef] [PubMed]
- Boddupalli, S.S.; Pramanik, B.C.; Slaughter, C.A.; Estabrook, R.W.; Peerson, J.A. Fatty acid monooxygenation by P450BM-3: Product identification and proposed mechanisms for the sequential hydroxylation reactions. Arch. Biochem. Biophys. 1992, 292, 20–28. [Google Scholar] [CrossRef] [PubMed]
- CYP102A1 in Open Conformation. Available online: https://www.rcsb.org/structure/8DME (accessed on 4 July 2024).
- Ignatenko, O.V.; Sjölander, A.; Hushpulian, D.M.; Kazakov, S.V.; Ouporov, I.V.; Chubar, T.A.; Poloznikov, A.A.; Ruzgas, T.; Tishkov, V.I.; Gorton, L.; et al. Electrochemistry of chemically trapped dimeric and monomeric recombinant horseradish peroxidase. Adv. Biosens. Bioelectron. 2013, 2, 25–34. [Google Scholar]
- Bukharina, N.S. Visualization and Monitoring of Activity of Cytochrome P450 BM3 with the Use of Atomic Force Microscopy. Ph.D. Thesis, Institute of Biomedical Chemistry, Moscow, Russia, 25 September 2014. [Google Scholar]
- Omura, T.; Sato, R. The carbon monoxide-binding pigment of liver microsomes. J. Biol. Chem. 1964, 239, 2370–2378. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanov, Y.D.; Vinogradova, A.V.; Nevedrova, E.D.; Ableev, A.N.; Kozlov, A.F.; Shumov, I.D.; Ziborov, V.S.; Afonin, O.N.; Vaulin, N.V.; Lebedev, D.V.; et al. Solid-State Nanopore-Based Nanosystem for Registration of Enzymatic Activity of a Single Molecule of Cytochrome P450 BM3. Int. J. Mol. Sci. 2024, 25, 10864. https://doi.org/10.3390/ijms251910864
Ivanov YD, Vinogradova AV, Nevedrova ED, Ableev AN, Kozlov AF, Shumov ID, Ziborov VS, Afonin ON, Vaulin NV, Lebedev DV, et al. Solid-State Nanopore-Based Nanosystem for Registration of Enzymatic Activity of a Single Molecule of Cytochrome P450 BM3. International Journal of Molecular Sciences. 2024; 25(19):10864. https://doi.org/10.3390/ijms251910864
Chicago/Turabian StyleIvanov, Yuri D., Angelina V. Vinogradova, Ekaterina D. Nevedrova, Alexander N. Ableev, Andrey F. Kozlov, Ivan D. Shumov, Vadim S. Ziborov, Oleg N. Afonin, Nikita V. Vaulin, Denis V. Lebedev, and et al. 2024. "Solid-State Nanopore-Based Nanosystem for Registration of Enzymatic Activity of a Single Molecule of Cytochrome P450 BM3" International Journal of Molecular Sciences 25, no. 19: 10864. https://doi.org/10.3390/ijms251910864
APA StyleIvanov, Y. D., Vinogradova, A. V., Nevedrova, E. D., Ableev, A. N., Kozlov, A. F., Shumov, I. D., Ziborov, V. S., Afonin, O. N., Vaulin, N. V., Lebedev, D. V., Bukatin, A. S., Afonicheva, P. K., Mukhin, I. S., Usanov, S. A., & Archakov, A. I. (2024). Solid-State Nanopore-Based Nanosystem for Registration of Enzymatic Activity of a Single Molecule of Cytochrome P450 BM3. International Journal of Molecular Sciences, 25(19), 10864. https://doi.org/10.3390/ijms251910864




