Maternal Obesity Alters Placental and Umbilical Cord Plasma Oxidative Stress, a Cross-Sectional Study
Abstract
1. Introduction
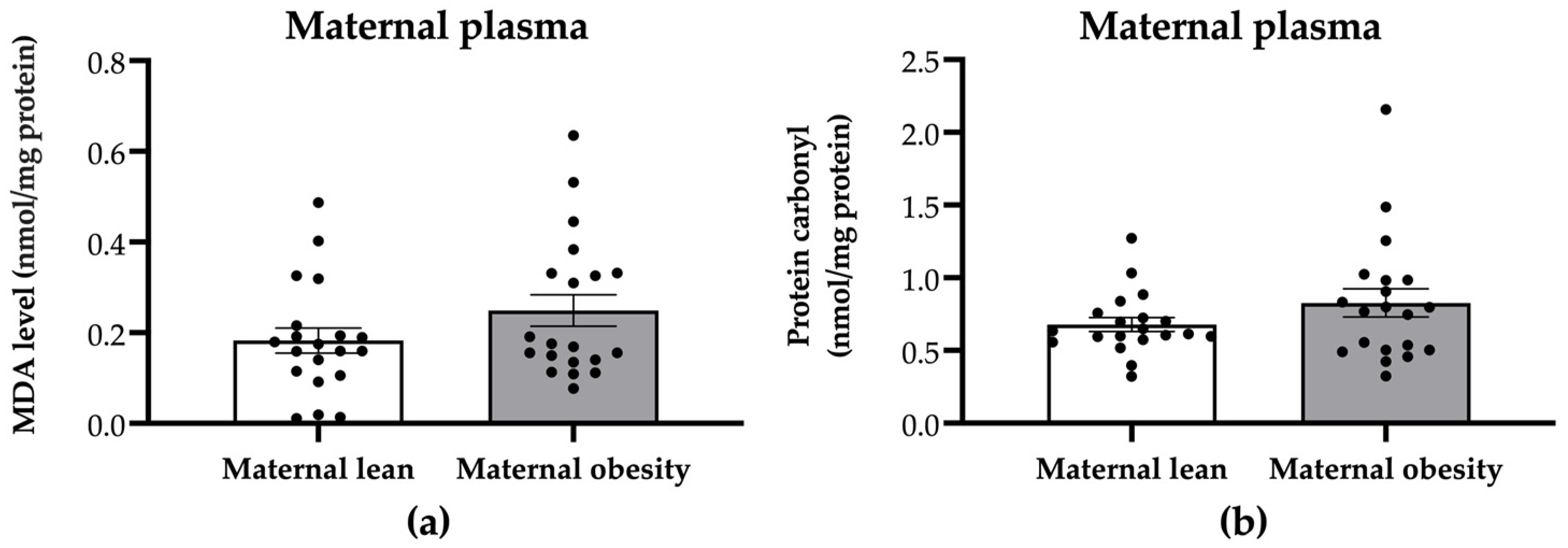
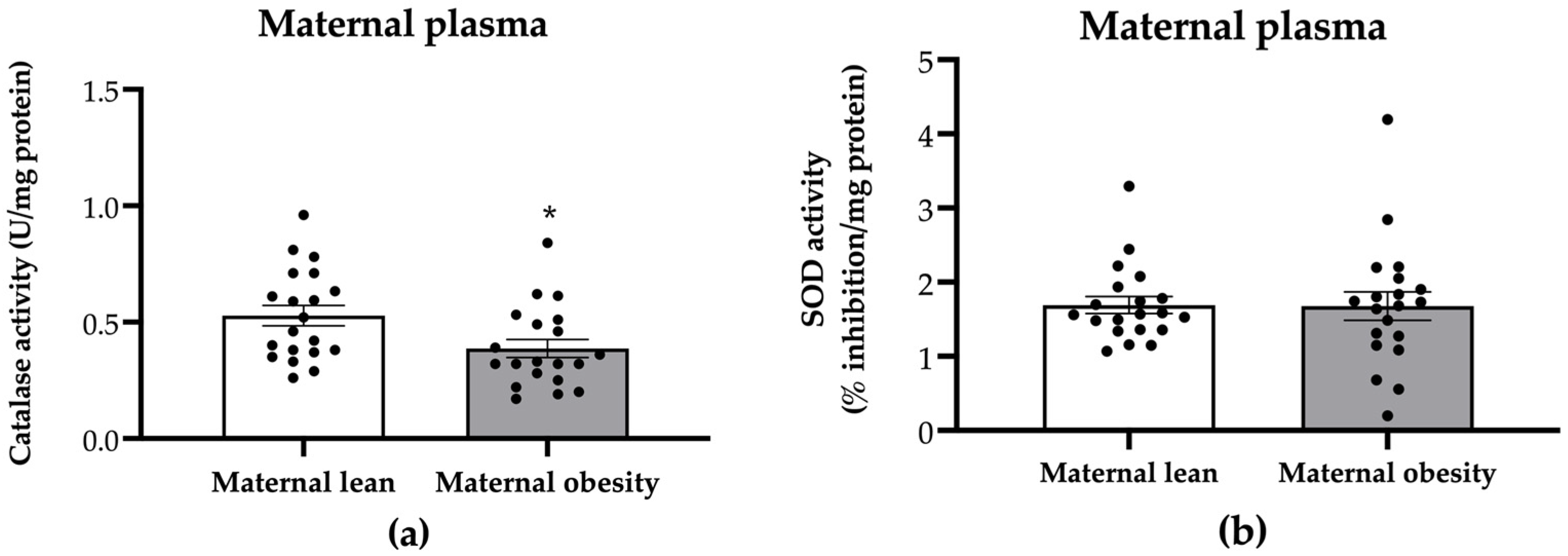
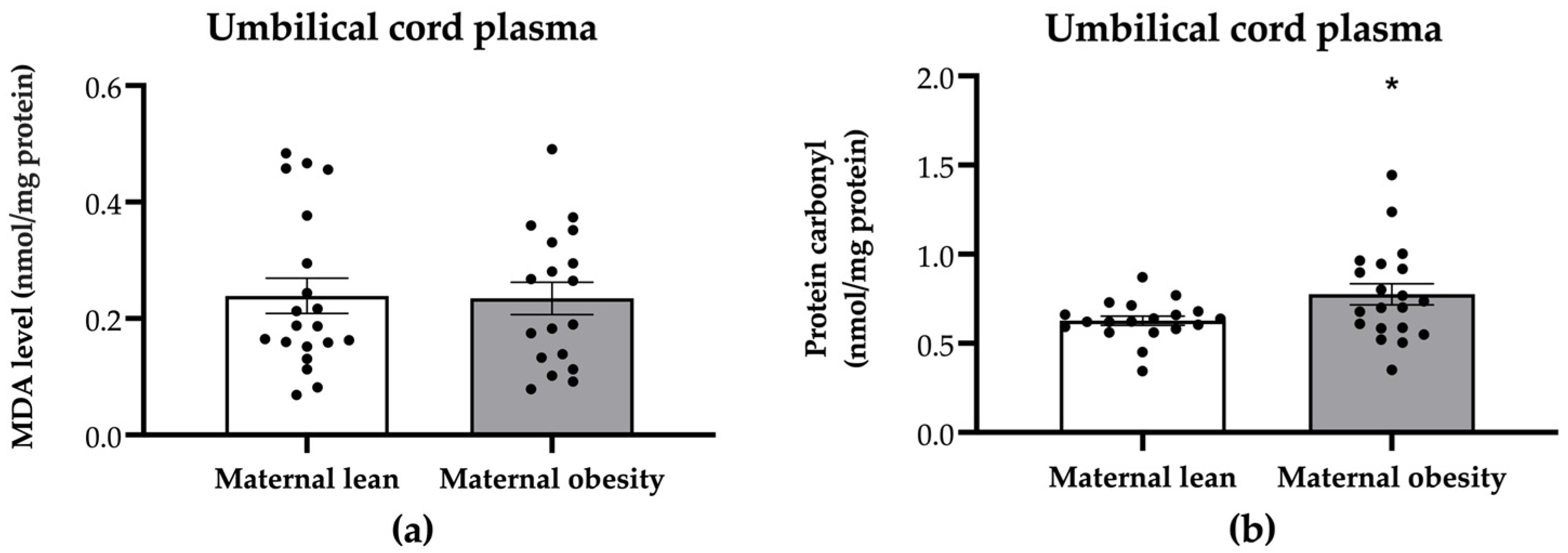
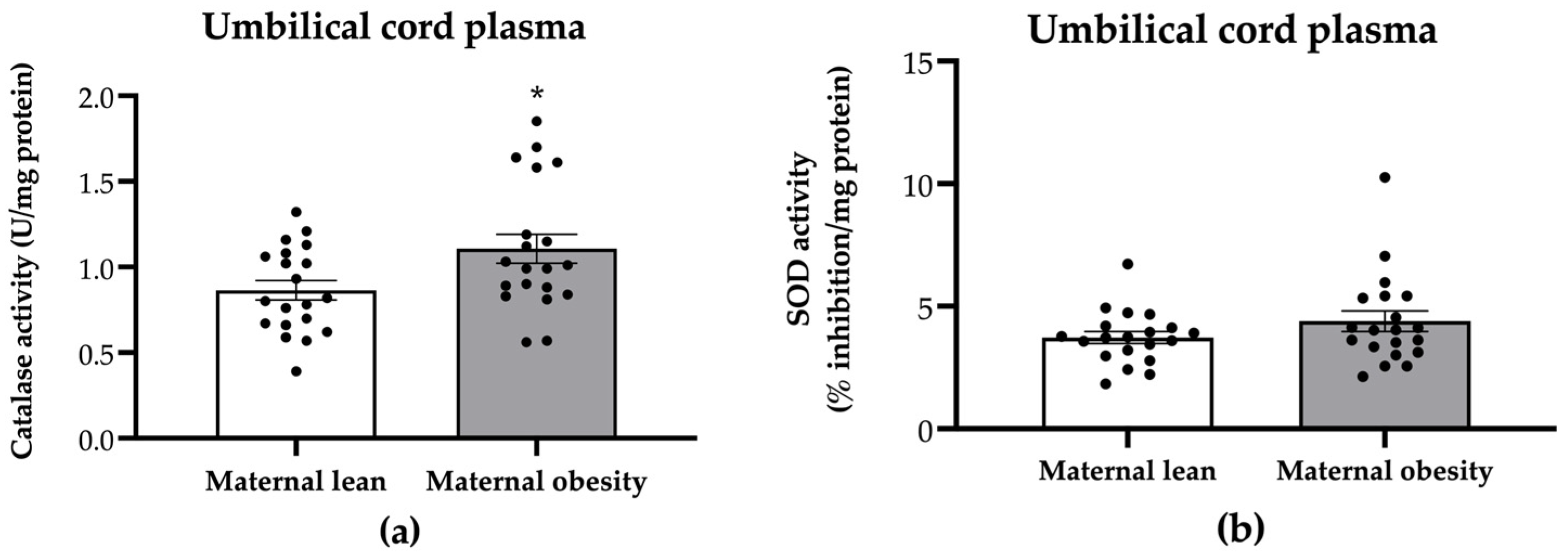
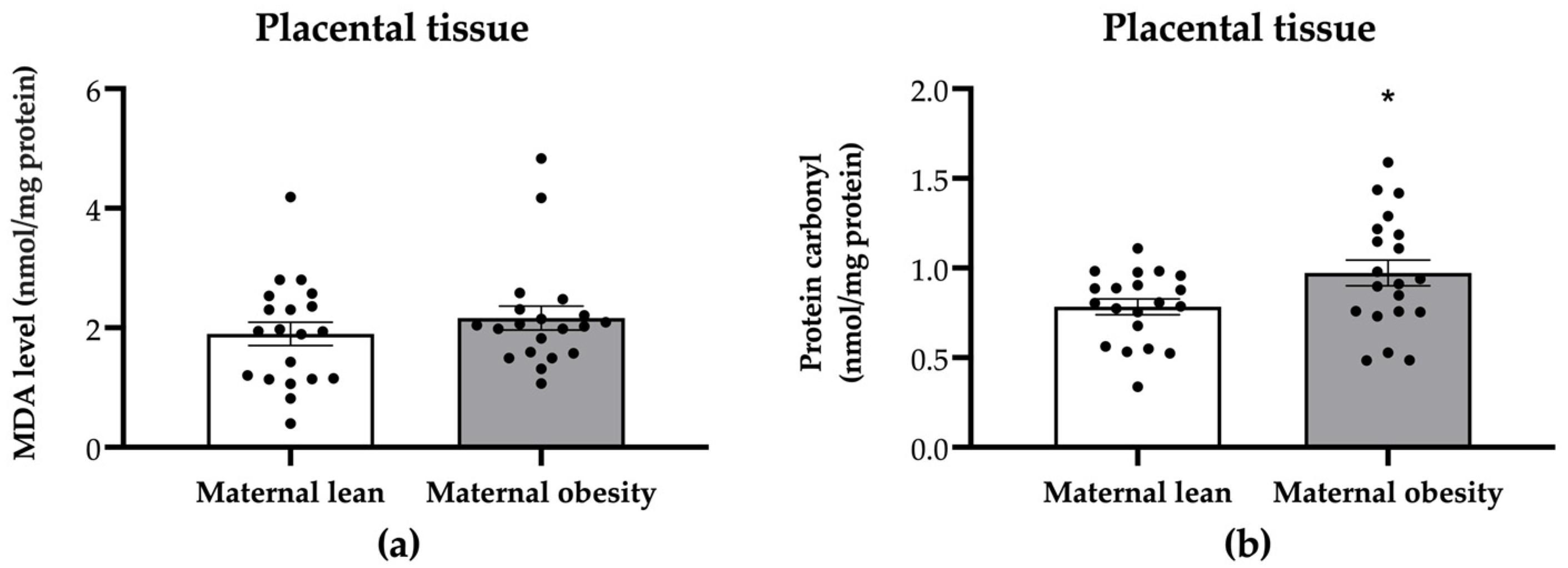
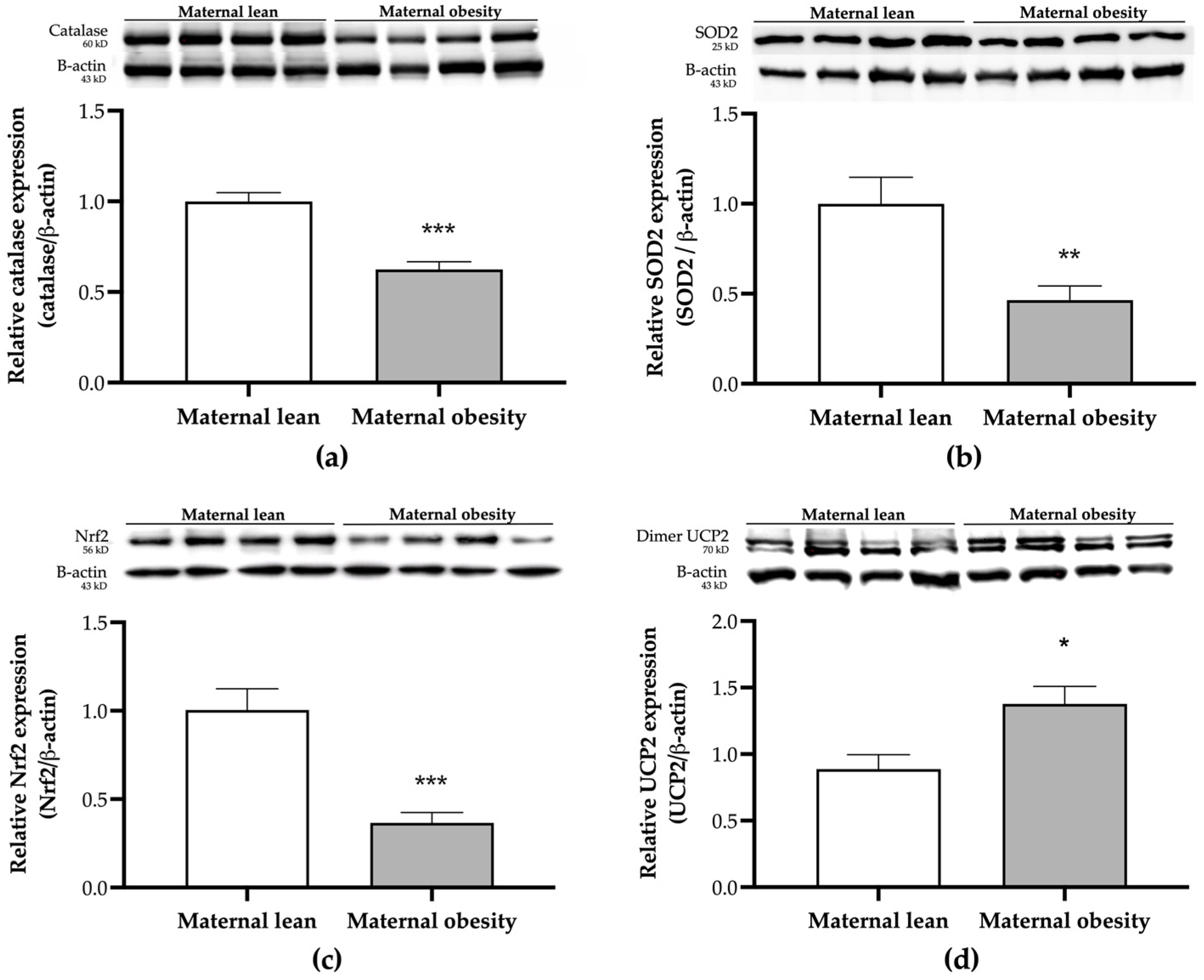
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Subjects
4.2. Characteristics of the Mothers and Their Offspring
4.3. Maternal Plasma Collection
4.4. Umbilical Cord Plasma Collection
4.5. Placental Sample Collection
4.6. Assessment of Plasma and Placental MDA
4.7. Assessment of Plasma and Placental Protein Carbonyl
4.8. Assessment of Plasma Catalase Activity
4.9. Assessment of Plasma SOD Activity
4.10. Assessment of Plasma Protein Content and Lipids
4.11. Placental Protein Extraction and Expression Analysis
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tauqeer, Z.; Gomez, G.; Stanford, F.C. Obesity in Women: Insights for the Clinician. J. Women’s Health 2018, 27, 444–457. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004, 363, 157–163. [Google Scholar] [CrossRef]
- World Health Organization. The Asia-Pacific perspective: Redefining obesity and its treatment. Health Commun. Aust. 2000. Available online: https://apps.who.int/iris/handle/10665/206936 (accessed on 6 October 2024).
- Lim, S.; Harrison, C.; Callander, E.; Walker, R.; Teede, H.; Moran, L. Addressing Obesity in Preconception, Pregnancy, and Postpartum: A Review of the Literature. Curr. Obes. Rep. 2022, 11, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Kelly, A.C.; Powell, T.L.; Jansson, T. Placental function in maternal obesity. Clin. Sci. 2020, 134, 961–984. [Google Scholar] [CrossRef]
- Lamothe, J.; Khurana, S.; Tharmalingam, S.; Williamson, C.; Byrne, C.J.; Lees, S.J.; Khaper, N.; Kumar, A.; Tai, T.C. Oxidative Stress Mediates the Fetal Programming of Hypertension by Glucocorticoids. Antioxidants 2021, 10, 531. [Google Scholar] [CrossRef]
- Palaska, E.; Antoniou, E.; Tzitiridou-Chatzopoulou, M.; Eskitzis, P.; Orovou, E. Correlation between Breastfeeding, Maternal Body Mass Index, and Childhood Obesity. Epidemiologia 2024, 5, 411–420. [Google Scholar] [CrossRef]
- Jovandaric, M.Z.; Babic, S.; Raus, M.; Medjo, B. The Importance of Metabolic and Environmental Factors in the Occurrence of Oxidative Stress during Pregnancy. Int. J. Mol. Sci. 2023, 24, 11964. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Y.; Mao, X.; Du, M. Maternal obesity impairs fetal mitochondriogenesis and brown adipose tissue development partially via upregulation of miR-204-5p. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 2706–2715. [Google Scholar] [CrossRef]
- Anam, A.K.; Cooke, K.M.; Dratver, M.B.; O’Bryan, J.V.; Perley, L.E.; Guller, S.M.; Hwang, J.J.; Taylor, H.S.; Goedeke, L.; Kliman, H.J.; et al. Insulin increases placental triglyceride as a potential mechanism for fetal adiposity in maternal obesity. Mol. Metab. 2022, 64, 101574. [Google Scholar] [CrossRef]
- Johns, E.C.; Denison, F.C.; Reynolds, R.M. The impact of maternal obesity in pregnancy on placental glucocorticoid and macronutrient transport and metabolism. Biochim. Biophys. Acta Mol. Basis. Dis. 2020, 1866, 165374. [Google Scholar] [CrossRef] [PubMed]
- Santos-Rosendo, C.; Bugatto, F.; Gonzalez-Dominguez, A.; Lechuga-Sancho, A.M.; Mateos, M.R.; Visiedo, F. Placental Adaptive Changes to Protect Function and Decrease Oxidative Damage in Metabolically Healthy Maternal Obesity. Antioxidants 2020, 9, 794. [Google Scholar] [CrossRef] [PubMed]
- Martino, J.; Sebert, S.; Segura, M.T.; Garcia-Valdes, L.; Florido, J.; Padilla, M.C.; Marcos, A.; Rueda, R.; McArdle, H.J.; Budge, H.; et al. Maternal Body Weight and Gestational Diabetes Differentially Influence Placental and Pregnancy Outcomes. J. Clin. Endocrinol. Metab. 2016, 101, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Phuthong, S.; Reyes-Hernandez, C.G.; Rodriguez-Rodriguez, P.; Ramiro-Cortijo, D.; Gil-Ortega, M.; Gonzalez-Blazquez, R.; Gonzalez, M.C.; Lopez de Pablo, A.L.; Arribas, S.M. Sex Differences in Placental Protein Expression and Efficiency in a Rat Model of Fetal Programming Induced by Maternal Undernutrition. Int. J. Mol. Sci. 2020, 22, 237. [Google Scholar] [CrossRef]
- Jones, M.L.; Mark, P.J.; Lewis, J.L.; Mori, T.A.; Keelan, J.A.; Waddell, B.J. Antioxidant defenses in the rat placenta in late gestation: Increased labyrinthine expression of superoxide dismutases, glutathione peroxidase 3, and uncoupling protein 2. Biol. Reprod. 2010, 83, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Stark, M.J.; Hodyl, N.A.; Butler, M.; Clifton, V.L. Localisation and characterisation of uncoupling protein-2 (UCP2) in the human preterm placenta. Placenta 2012, 33, 1020–1025. [Google Scholar] [CrossRef]
- Gnanalingham, M.G.; Williams, P.; Wilson, V.; Bispham, J.; Hyatt, M.A.; Pellicano, A.; Budge, H.; Stephenson, T.; Symonds, M.E. Nutritional manipulation between early to mid-gestation: Effects on uncoupling protein-2, glucocorticoid sensitivity, IGF-I receptor and cell proliferation but not apoptosis in the ovine placenta. Reproduction 2007, 134, 615–623. [Google Scholar] [CrossRef][Green Version]
- Mando, C.; Castiglioni, S.; Novielli, C.; Anelli, G.M.; Serati, A.; Parisi, F.; Lubrano, C.; Zocchi, M.; Ottria, R.; Giovarelli, M. Placental Bioenergetics and Antioxidant Homeostasis in Maternal Obesity and Gestational Diabetes. Antioxidants 2024, 13, 858. [Google Scholar] [CrossRef]
- Lopez-Yanez Blanco, A.; Diaz-Lopez, K.M.; Vilchis-Gil, J.; Diaz-Garcia, H.; Gomez-Lopez, J.; Medina-Bravo, P.; Granados-Riveron, J.T.; Gallardo, J.M.; Klunder-Klunder, M.; Sanchez-Urbina, R. Diet and Maternal Obesity Are Associated with Increased Oxidative Stress in Newborns: A Cross-Sectional Study. Nutrients 2022, 14, 746. [Google Scholar] [CrossRef]
- Malti, N.; Merzouk, H.; Merzouk, S.A.; Loukidi, B.; Karaouzene, N.; Malti, A.; Narce, M. Oxidative stress and maternal obesity: Feto-placental unit interaction. Placenta 2014, 35, 411–416. [Google Scholar] [CrossRef]
- Evans, L.; Myatt, L. Sexual dimorphism in the effect of maternal obesity on antioxidant defense mechanisms in the human placenta. Placenta 2017, 51, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Zafaranieh, S.; Stadler, J.T.; Pammer, A.; Marsche, G.; van Poppel, M.N.M.; Desoye, G.; Dali Core Investigator, G. The Association of Physical Activity and Sedentary Behavior with Maternal and Cord Blood Anti-Oxidative Capacity and HDL Functionality: Findings of DALI Study. Antioxidants 2023, 12, 827. [Google Scholar] [CrossRef] [PubMed]
- Mannino, A.; Sarapis, K.; Moschonis, G. The Effect of Maternal Overweight and Obesity Pre-Pregnancy and During Childhood in the Development of Obesity in Children and Adolescents: A Systematic Literature Review. Nutrients 2022, 14, 5125. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Chen, Y.; Wu, X.; Yin, A.; Tian, F.; Zhang, H.; Huang, X.; Wu, L.; Niu, J. Mediation effect of maternal triglyceride and fasting glucose level on the relationship between maternal overweight/ obesity and fetal growth: A prospective cohort study. BMC Pregnancy Childbirth 2023, 23, 449. [Google Scholar] [CrossRef] [PubMed]
- Francis, E.C.; Kechris, K.; Johnson, R.K.; Rawal, S.; Pathmasiri, W.; Rushing, B.R.; Du, X.; Jansson, T.; Dabelea, D.; Sumner, S.J.; et al. Maternal Serum Metabolomics in Mid-Pregnancy Identifies Lipid Pathways as a Key Link to Offspring Obesity in Early Childhood. Int. J. Mol. Sci. 2024, 25, 7620. [Google Scholar] [CrossRef]
- Corrales, P.; Vidal-Puig, A.; Medina-Gomez, G. Obesity and pregnancy, the perfect metabolic storm. Eur. J. Clin. Nutr. 2021, 75, 1723–1734. [Google Scholar] [CrossRef]
- Song, X.; Chen, L.; Zhang, S.; Liu, Y.; Wei, J.; Sun, M.; Shu, J.; Wang, T.; Qin, J. High Maternal Triglyceride Levels Mediate the Association between Pre-Pregnancy Overweight/Obesity and Macrosomia among Singleton Term Non-Diabetic Pregnancies: A Prospective Cohort Study in Central China. Nutrients 2022, 14, 2075. [Google Scholar] [CrossRef]
- Abbassi-Ghanavati, M.; Greer, L.G.; Cunningham, F.G. Pregnancy and laboratory studies: A reference table for clinicians. Obstet. Gynecol. 2009, 114, 1326–1331. [Google Scholar] [CrossRef]
- Go, H.; Hashimoto, K.; Maeda, H.; Ogasawara, K.; Kume, Y.; Murata, T.; Sato, A.; Ogata, Y.; Shinoki, K.; Nishigori, H.; et al. Cord blood triglyceride and total cholesterol in preterm and term neonates: Reference values and associated factors from the Japan Environment and Children’s Study. Eur. J. Pediatr. 2023, 182, 4547–4556. [Google Scholar] [CrossRef]
- Stein, R.; Ferrari, F.; Scolari, F. Genetics, Dyslipidemia, and Cardiovascular Disease: New Insights. Curr. Cardiol. Rep. 2019, 21, 68. [Google Scholar] [CrossRef]
- Zhou, J.; Bai, J.; Guo, Y.; Fu, L.; Xing, J. Higher Levels of Triglyceride, Fatty Acid Translocase, and Toll-Like Receptor 4 and Lower Level of HDL-C in Pregnant Women with GDM and Their Close Correlation with Neonatal Weight. Gynecol. Obstet. Investig. 2021, 86, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Keleher, M.R.; Erickson, K.; Kechris, K.; Yang, I.V.; Dabelea, D.; Friedman, J.E.; Boyle, K.E.; Jansson, T. Associations between the activity of placental nutrient-sensing pathways and neonatal and postnatal metabolic health: The ECHO Healthy Start cohort. Int. J. Obes. 2020, 44, 2203–2212. [Google Scholar] [CrossRef] [PubMed]
- Leonard, S.A.; Rasmussen, K.M.; King, J.C.; Abrams, B. Trajectories of maternal weight from before pregnancy through postpartum and associations with childhood obesity. Am. J. Clin. Nutr. 2017, 106, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Balachandiran, M.; Bobby, Z.; Dorairajan, G.; Jacob, S.E.; Gladwin, V.; Vinayagam, V.; Packirisamy, R.M. Placental Accumulation of Triacylglycerols in Gestational Diabetes Mellitus and Its Association with Altered Fetal Growth are Related to the Differential Expressions of Proteins of Lipid Metabolism. Exp. Clin. Endocrinol. Diabetes 2021, 129, 803–812. [Google Scholar] [CrossRef]
- Phengpol, N.; Thongnak, L.; Lungkaphin, A. The programming of kidney injury in offspring affected by maternal overweight and obesity: Role of lipid accumulation, inflammation, oxidative stress, and fibrosis in the kidneys of offspring. J. Physiol. Biochem. 2023, 79, 1–17. [Google Scholar] [CrossRef]
- Wang, L.; O’Kane, A.M.; Zhang, Y.; Ren, J. Maternal obesity and offspring health: Adapting metabolic changes through autophagy and mitophagy. Obes. Rev. 2023, 24, e13567. [Google Scholar] [CrossRef]
- Hernandez-Trejo, M.; Montoya-Estrada, A.; Torres-Ramos, Y.; Espejel-Nunez, A.; Guzman-Grenfell, A.; Morales-Hernandez, R.; Tolentino-Dolores, M.; Laresgoiti-Servitje, E. Oxidative stress biomarkers and their relationship with cytokine concentrations in overweight/obese pregnant women and their neonates. BMC Immunol. 2017, 18, 3. [Google Scholar] [CrossRef]
- Hussain, T.; Murtaza, G.; Metwally, E.; Kalhoro, D.H.; Kalhoro, M.S.; Rahu, B.A.; Sahito, R.G.A.; Yin, Y.; Yang, H.; Chughtai, M.I.; et al. The Role of Oxidative Stress and Antioxidant Balance in Pregnancy. Mediat. Inflamm. 2021, 2021, 9962860. [Google Scholar] [CrossRef]
- Ballesteros-Guzman, A.K.; Carrasco-Legleu, C.E.; Levario-Carrillo, M.; Chavez-Corral, D.V.; Sanchez-Ramirez, B.; Marinelarena-Carrillo, E.O.; Guerrero-Salgado, F.; Reza-Lopez, S.A. Prepregnancy Obesity, Maternal Dietary Intake, and Oxidative Stress Biomarkers in the Fetomaternal Unit. Biomed. Res. Int. 2019, 2019, 5070453. [Google Scholar] [CrossRef]
- Alfonso-Prieto, M.; Biarnes, X.; Vidossich, P.; Rovira, C. The molecular mechanism of the catalase reaction. J. Am. Chem. Soc. 2009, 131, 11751–11761. [Google Scholar] [CrossRef]
- Biri, A.; Onan, A.; Devrim, E.; Babacan, F.; Kavutcu, M.; Durak, I. Oxidant status in maternal and cord plasma and placental tissue in gestational diabetes. Placenta 2006, 27, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Psefteli, P.M.; Morris, J.K.; Ehler, E.; Smith, L.; Bowe, J.; Mann, G.E.; Taylor, P.D.; Chapple, S.J. Sulforaphane induced NRF2 activation in obese pregnancy attenuates developmental redox imbalance and improves early-life cardiovascular function in offspring. Redox Biol. 2023, 67, 102883. [Google Scholar] [CrossRef] [PubMed]
- Ruano, C.S.M.; Miralles, F.; Mehats, C.; Vaiman, D. The Impact of Oxidative Stress of Environmental Origin on the Onset of Placental Diseases. Antioxidants 2022, 11, 106. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Zhai, X.; Qiu, Y.; Lu, X.; Jiao, Y. The Nrf2 in Obesity: A Friend or Foe? Antioxidants 2022, 11, 2067. [Google Scholar] [CrossRef]
- Cindrova-Davies, T.; Yung, H.W.; Johns, J.; Spasic-Boskovic, O.; Korolchuk, S.; Jauniaux, E.; Burton, G.J.; Charnock-Jones, D.S. Oxidative stress, gene expression, and protein changes induced in the human placenta during labor. Am. J. Pathol. 2007, 171, 1168–1179. [Google Scholar] [CrossRef]
- Song, Y.R.; Kim, J.K.; Lee, H.S.; Kim, S.G.; Choi, E.K. Serum levels of protein carbonyl, a marker of oxidative stress, are associated with overhydration, sarcopenia and mortality in hemodialysis patients. BMC Nephrol. 2020, 21, 281. [Google Scholar] [CrossRef]
- Negro, S.; Boutsikou, T.; Briana, D.D.; Tataranno, M.L.; Longini, M.; Proietti, F.; Bazzini, F.; Dani, C.; Malamitsi-Puchner, A.; Buonocore, G.; et al. Maternal obesity and perinatal oxidative stress: The strength of the association. J. Biol. Regul. Homeost. Agents 2017, 31, 221–227. [Google Scholar]
- Rocha, S.; Gomes, D.; Lima, M.; Bronze-da-Rocha, E.; Santos-Silva, A. Peroxiredoxin 2, glutathione peroxidase, and catalase in the cytosol and membrane of erythrocytes under H2O2-induced oxidative stress. Free Radic. Res. 2015, 49, 990–1003. [Google Scholar] [CrossRef]
- Fukai, T.; Ushio-Fukai, M. Superoxide dismutases: Role in redox signaling, vascular function, and diseases. Antioxid. Redox Signal. 2011, 15, 1583–1606. [Google Scholar] [CrossRef]
- Arogbokun, O.; Rosen, E.; Keil, A.P.; Milne, G.L.; Barrett, E.; Nguyen, R.; Bush, N.R.; Swan, S.H.; Sathyanarayana, S.; Ferguson, K.K. Maternal Oxidative Stress Biomarkers in Pregnancy and Child Growth from Birth to Age 6. J. Clin. Endocrinol. Metab. 2021, 106, 1427–1436. [Google Scholar] [CrossRef]
- Redmer, D.A.; Milne, J.S.; Aitken, R.P.; Johnson, M.L.; Borowicz, P.P.; Reynolds, L.P.; Caton, J.S.; Wallace, J.M. Decreasing maternal nutrient intake during the final third of pregnancy in previously overnourished adolescent sheep: Effects on maternal nutrient partitioning and feto-placental development. Placenta 2012, 33, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Louwen, F.; Kreis, N.N.; Ritter, A.; Yuan, J. Maternal obesity and placental function: Impaired maternal-fetal axis. Arch. Gynecol. Obstet. 2024, 309, 2279–2288. [Google Scholar] [CrossRef] [PubMed]
- Correia-Branco, A.; Keating, E.; Martel, F. Maternal undernutrition and fetal developmental programming of obesity: The glucocorticoid connection. Reprod. Sci. 2015, 22, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.D.; Hernandez, M.H.; Serazin, V.; Vialard, F.; Dieudonne, M.N. Human Placental Adaptive Changes in Response to Maternal Obesity: Sex Specificities. Int. J. Mol. Sci. 2023, 24, 9770. [Google Scholar] [CrossRef]
- Poasakate, A.; Maneesai, P.; Rattanakanokchai, S.; Bunbupha, S.; Tong-Un, T.; Pakdeechote, P. Genistein Prevents Nitric Oxide Deficiency-Induced Cardiac Dysfunction and Remodeling in Rats. Antioxidants 2021, 10, 237. [Google Scholar] [CrossRef]

| Parameter | Maternal Lean | Maternal Obesity | p-Value |
|---|---|---|---|
| Maternal age at delivery (year) | 27.65 ± 0.85 | 30.30 ± 1.20 | 0.0779 |
| Gestational age at delivery (weeks) | 38.71 ± 0.21 | 38.81 ± 0.28 | 0.7744 |
| Pre-pregnancy weight (kg) | 52.56 ± 1.29 | 75.32 ± 1.79 *** | <0.0001 |
| Pre-pregnancy BMI (kg/m2) | 20.26 ± 0.45 | 29.40 ± 0.58 *** | <0.0001 |
| Weight at delivery (kg) | 66.90 ± 2.10 | 90.64 ± 2.22 *** | <0.0001 |
| Vaginal delivery n (%)/ Cesarean delivery n (%) | 13 (65)/7 (35) | 8 (40)/12 (60) | 0.1134 |
| Neonatal sex, male n (%) | 10 (50) | 11 (55) | 0.7575 |
| Neonatal birth weight (g) | 3083 ± 101 | 3327 ± 110 | 0.1103 |
| Neonatal length (cm) | 49.20 ± 0.57 | 49.21 ± 0.59 | 0.9898 |
| Placental weight (g) | 581.50 ± 28.02 | 701.50 ± 41.89 * | 0.0224 |
| Feto-placental ratio | 5.43 ± 0.19 | 4.90 ± 0.16 * | 0.0374 |
| Umbilical Cord Protein Carbonyl | r | p-Value |
|---|---|---|
| Placental protein carbonyl | 0.7405 | <0.0001 *** |
| Placental catalase expression | −0.3942 | 0.0566 |
| Placental SOD2 expression | −0.1674 | 0.4343 |
| Placental UCP2 expression | 0.0654 | 0.8099 |
| Placental Nrf2 expression | −0.3546 | 0.0891 |
| Maternal protein carbonyl | −0.09101 | 0.5765 |
| Maternal catalase activity | −0.4332 | 0.0052 ** |
| Maternal SOD activity | −0.2246 | 0.1635 |
| Maternal pre-pregnancy weight | 0.3621 | 0.0217 * |
| Maternal pre-pregnancy BMI | 0.3832 | 0.0147 * |
| Maternal weight at delivery | 0.3168 | 0.0464 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jantape, T.; Kongwattanakul, K.; Arribas, S.M.; Rodríguez-Rodríguez, P.; Iampanichakul, M.; Settheetham-Ishida, W.; Phuthong, S. Maternal Obesity Alters Placental and Umbilical Cord Plasma Oxidative Stress, a Cross-Sectional Study. Int. J. Mol. Sci. 2024, 25, 10866. https://doi.org/10.3390/ijms251910866
Jantape T, Kongwattanakul K, Arribas SM, Rodríguez-Rodríguez P, Iampanichakul M, Settheetham-Ishida W, Phuthong S. Maternal Obesity Alters Placental and Umbilical Cord Plasma Oxidative Stress, a Cross-Sectional Study. International Journal of Molecular Sciences. 2024; 25(19):10866. https://doi.org/10.3390/ijms251910866
Chicago/Turabian StyleJantape, Thanyawan, Kiattisak Kongwattanakul, Silvia M. Arribas, Pilar Rodríguez-Rodríguez, Metee Iampanichakul, Wannapa Settheetham-Ishida, and Sophida Phuthong. 2024. "Maternal Obesity Alters Placental and Umbilical Cord Plasma Oxidative Stress, a Cross-Sectional Study" International Journal of Molecular Sciences 25, no. 19: 10866. https://doi.org/10.3390/ijms251910866
APA StyleJantape, T., Kongwattanakul, K., Arribas, S. M., Rodríguez-Rodríguez, P., Iampanichakul, M., Settheetham-Ishida, W., & Phuthong, S. (2024). Maternal Obesity Alters Placental and Umbilical Cord Plasma Oxidative Stress, a Cross-Sectional Study. International Journal of Molecular Sciences, 25(19), 10866. https://doi.org/10.3390/ijms251910866








