Metal-Based Nanoparticles for Cardiovascular Diseases
Abstract
:1. Introduction
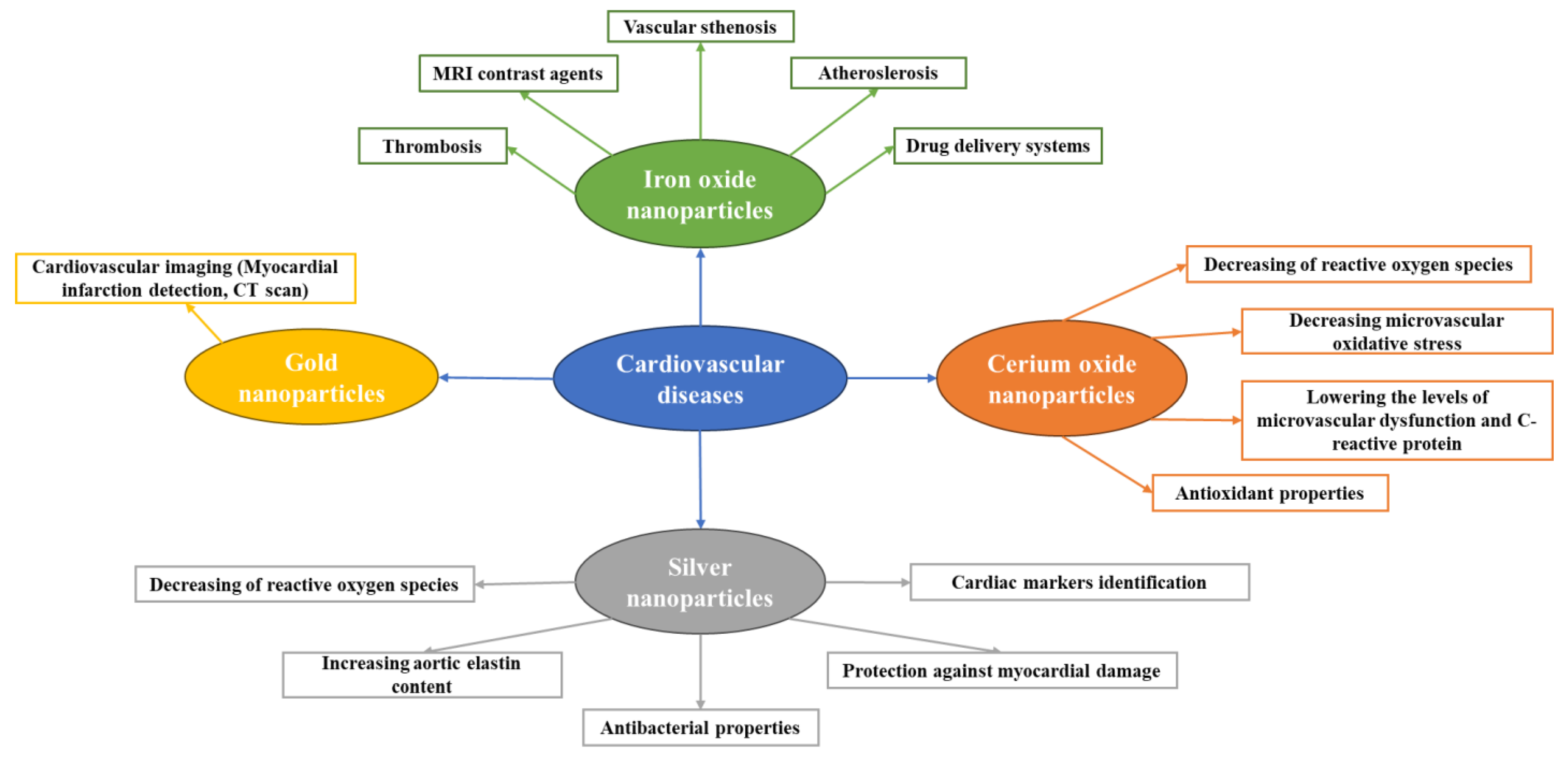
2. Iron Oxide Nanoparticles (IONPs)
2.1. Various Iron Oxide-Based Nanoparticles
2.2. Superparamagnetic Iron Oxide Nanoparticles (SPOIN) and Ultrasmall Superparamagnetic Particles of Iron Oxide (USPIO)
3. Gold Nanoparticles
3.1. Therapeutic Platforms
3.2. Computed Tomography Applications
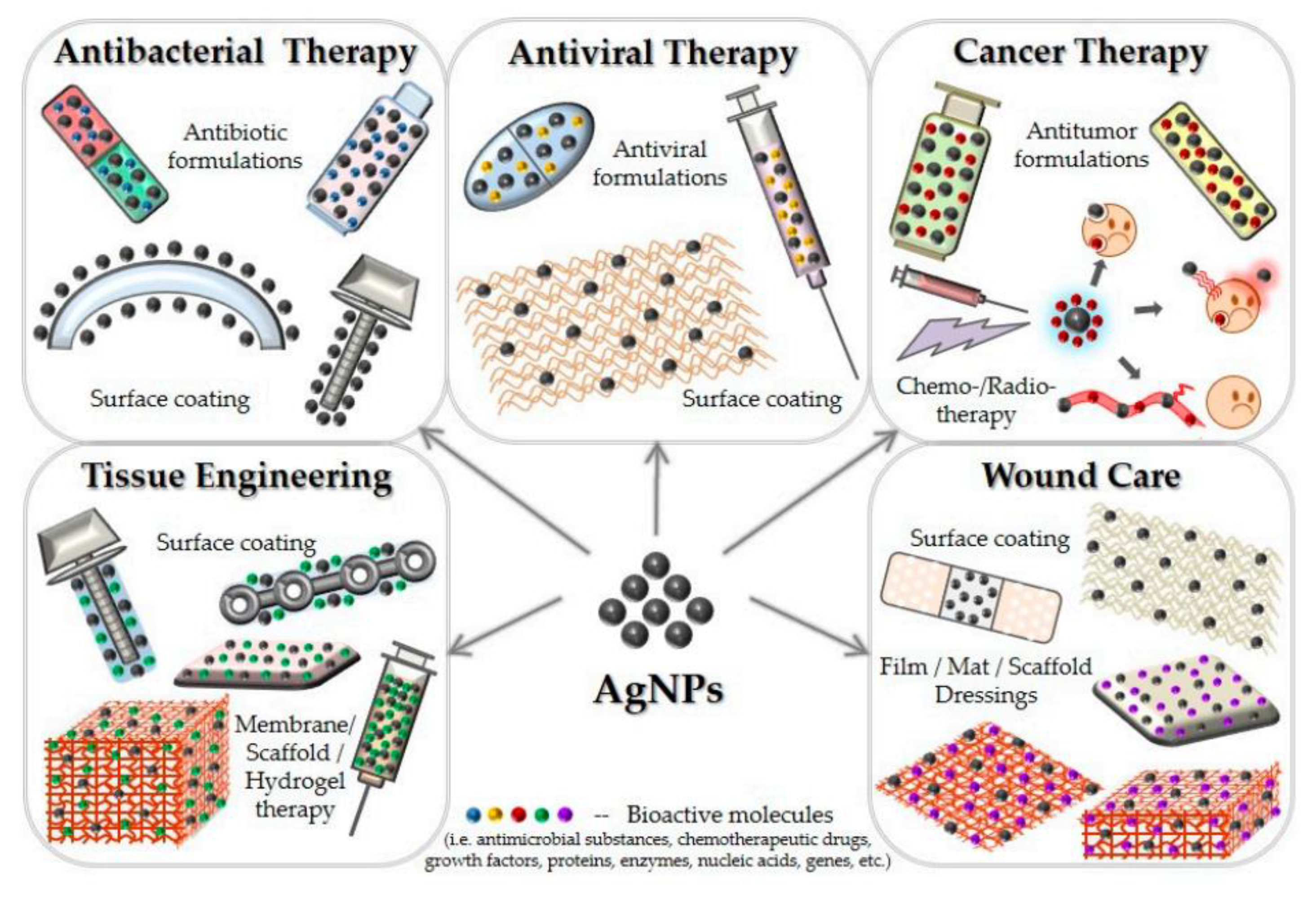
4. Silver Nanoparticles
4.1. Antimicrobial and Anti-Inflammatory Effects
4.2. Antioxidant Effects
5. Cerium Oxide Nanoparticles
6. Toxicity of Metal-Based Nanoparticles
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nedkoff, L.; Briffa, T.; Zemedikun, D.; Herrington, S.; Wright, F.L. Global Trends in Atherosclerotic Cardiovascular Disease. Clin. Ther. 2023, 45, 1087–1091. [Google Scholar] [CrossRef] [PubMed]
- Gaidai, O.; Cao, Y.; Loginov, S. Global Cardiovascular Diseases Death Rate Prediction. Curr. Probl. Cardiol. 2023, 48, 101622. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Fang, Z.; Ge, J.; Li, H. Nanotechnology for cardiovascular diseases. Innov. (Camb. (Mass.)) 2022, 3, 100214. [Google Scholar] [CrossRef] [PubMed]
- Younis, N.K.; Ghoubaira, J.A.; Bassil, E.P.; Tantawi, H.N.; Eid, A.H. Metal-based nanoparticles: Promising tools for the management of cardiovascular diseases. Nanomed. Nanotechnol. Biol. Med. 2021, 36, 102433. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.R.; Edelman, E.R. Nanomedicines for cardiovascular disease. Nat. Cardiovasc. Res. 2023, 2, 351–367. [Google Scholar] [CrossRef]
- Alromi, D.A.; Madani, S.Y.; Seifalian, A. Emerging Application of Magnetic Nanoparticles for Diagnosis and Treatment of Cancer. Polymers 2021, 13, 4146. [Google Scholar] [CrossRef]
- Thapa, R.K.; Kim, J.O. Nanomedicine-based commercial formulations: Current developments and future prospects. J. Pharm. Investig. 2023, 53, 19–33. [Google Scholar] [CrossRef]
- Jagaran, K.; Singh, M. Nanomedicine for COVID-19: Potential of copper nanoparticles. Biointerface Res. Appl. Chem. 2021, 11, 10716–10728. [Google Scholar]
- Sajid, M.; Płotka-Wasylka, J. Nanoparticles: Synthesis, characteristics, and applications in analytical and other sciences. Microchem. J. 2020, 154, 104623. [Google Scholar] [CrossRef]
- Omidian, H.; Babanejad, N.; Cubeddu, L.X. Nanosystems in Cardiovascular Medicine: Advancements, Applications, and Future Perspectives. Pharmaceutics 2023, 15, 1935. [Google Scholar] [CrossRef]
- Păduraru, D.N.; Ion, D.; Niculescu, A.-G.; Mușat, F.; Andronic, O.; Grumezescu, A.M.; Bolocan, A. Recent Developments in Metallic Nanomaterials for Cancer Therapy, Diagnosing and Imaging Applications. Pharmaceutics 2022, 14, 435. [Google Scholar] [CrossRef] [PubMed]
- Chopra, H.; Bibi, S.; Mishra, A.K.; Tirth, V.; Yerramsetty, S.V.; Murali, S.V.; Ahmad, S.U.; Mohanta, Y.K.; Attia, M.S.; Algahtani, A.; et al. Nanomaterials: A Promising Therapeutic Approach for Cardiovascular Diseases. J. Nanomater. 2022, 2022, 4155729. [Google Scholar] [CrossRef]
- Gonzalez, C.; Rosas-Hernandez, H.; Ramirez-Lee, M.A.; Salazar-García, S.; Ali, S.F. Role of silver nanoparticles (AgNPs) on the cardiovascular system. Arch. Toxicol. 2016, 90, 493–511. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, S.; Liu, Y.S. Nanoparticles in the diagnosis and treatment of vascular aging and related diseases. Signal Transduct. Target. Ther. 2022, 7, 231. [Google Scholar] [CrossRef]
- Li, L.; Zeng, Y.; Liu, G. Metal-based nanoparticles for cardiovascular disease diagnosis and therapy. Particuology 2023, 72, 94–111. [Google Scholar] [CrossRef]
- Haba, M.Ș.; Șerban, D.N.; Șerban, L.; Tudorancea, I.M.; Haba, R.M.; Mitu, O.; Iliescu, R.; Tudorancea, I. Nanomaterial-Based Drug Targeted Therapy for Cardiovascular Diseases: Ischemic Heart Failure and Atherosclerosis. Crystals 2021, 11, 1172. [Google Scholar] [CrossRef]
- Gong, Y.; Liu, H.; Ke, S.; Zhuo, L.; Wang, H. Latest advances in biomimetic nanomaterials for diagnosis and treatment of cardiovascular disease. Front. Cardiovasc. Med. 2023, 9, 1037741. [Google Scholar] [CrossRef] [PubMed]
- Mercan, D.-A.; Niculescu, A.-G.; Grumezescu, A.M. Nanoparticles for Antimicrobial Agents Delivery—An Up-to-Date Review. Int. J. Mol. Sci. 2022, 23, 13862. [Google Scholar] [CrossRef]
- Valenti, G.E.; Alfei, S.; Caviglia, D.; Domenicotti, C.; Marengo, B. Antimicrobial Peptides and Cationic Nanoparticles: A Broad-Spectrum Weapon to Fight Multi-Drug Resistance Not Only in Bacteria. Int. J. Mol. Sci. 2022, 23, 6108. [Google Scholar] [CrossRef]
- Tran, H.M.; Tran, H.; Booth, M.A.; Fox, K.E.; Nguyen, T.H.; Tran, N.; Tran, P.A. Nanomaterials for treating bacterial biofilms on implantable medical devices. Nanomaterials 2020, 10, 2253. [Google Scholar] [CrossRef]
- Larguinho, M.; Baptista, P.V. Gold and silver nanoparticles for clinical diagnostics—From genomics to proteomics. J. Proteom. 2012, 75, 2811–2823. [Google Scholar] [CrossRef]
- Kavitha, M.; Vallakeerthi, N.; Ramesh, P.; Reddy, P.M. Role of Metallic Nanoparticles in Cardiovascular Disease. J. Cardiovasc. Dis. Res. 2020, 11, 162–172. [Google Scholar]
- Zhang, J.; Ma, A.; Shang, L. Conjugating Existing Clinical Drugs With Gold Nanoparticles for Better Treatment of Heart Diseases. Front. Physiol. 2018, 9, 642. [Google Scholar] [CrossRef]
- Qin, Y.; Qiao, Y.; Wang, D.; Tang, C.; Yan, G. Ferritinophagy and ferroptosis in cardiovascular disease: Mechanisms and potential applications. Biomed. Pharmacother. 2021, 141, 111872. [Google Scholar] [CrossRef] [PubMed]
- Niculescu, A.-G.; Grumezescu, A.M. Novel Tumor-Targeting Nanoparticles for Cancer Treatment—A Review. Int. J. Mol. Sci. 2022, 23, 5253. [Google Scholar] [CrossRef]
- Aghebati-Maleki, A.; Dolati, S.; Ahmadi, M.; Baghbanzhadeh, A.; Asadi, M.; Fotouhi, A.; Yousefi, M.; Aghebati-Maleki, L. Nanoparticles and cancer therapy: Perspectives for application of nanoparticles in the treatment of cancers. J. Cell. Physiol. 2020, 235, 1962–1972. [Google Scholar] [CrossRef] [PubMed]
- Gavas, S.; Quazi, S.; Karpiński, T.M. Nanoparticles for cancer therapy: Current progress and challenges. Nanoscale Res. Lett. 2021, 16, 173. [Google Scholar] [CrossRef]
- Anjum, M.M.; Patel, K.K.; Bhattacharya, S.; Arya, D.K.; Pandey, P.; Mr, V.; Singh, S.; Rajinikanth, P.S. Overcoming barriers in cystic fibrosis therapy through inhalational lipid nanoparticles: Challenges and advances. J. Drug Deliv. Sci. Technol. 2023, 89, 105068. [Google Scholar] [CrossRef]
- Kubiatowicz, L.J.; Mohapatra, A.; Krishnan, N.; Fang, R.H.; Zhang, L. mRNA nanomedicine: Design and recent applications. Exploration 2022, 2, 20210217. [Google Scholar] [CrossRef]
- Fang, T.; Cao, X.; Ibnat, M.; Chen, G. Stimuli-responsive nanoformulations for CRISPR-Cas9 genome editing. J. Nanobiotechnology 2022, 20, 354. [Google Scholar] [CrossRef]
- Arshad, I.; Kanwal, A.; Zafar, I.; Unar, A.; Mouada, H.; Razia, I.T.; Arif, S.; Ahsan, M.; Kamal, M.A.; Rashid, S.; et al. Multifunctional role of nanoparticles for the diagnosis and therapeutics of cardiovascular diseases. Environ. Res. 2024, 242, 117795. [Google Scholar] [CrossRef] [PubMed]
- Cicha, I.; Alexiou, C. Cardiovascular applications of magnetic particles. J. Magn. Magn. Mater. 2021, 518, 167428. [Google Scholar] [CrossRef]
- Materón, E.M.; Miyazaki, C.M.; Carr, O.; Joshi, N.; Picciani, P.H.S.; Dalmaschio, C.J.; Davis, F.; Shimizu, F.M. Magnetic nanoparticles in biomedical applications: A review. Appl. Surf. Sci. Adv. 2021, 6, 100163. [Google Scholar] [CrossRef]
- Gad, S.A.; Abomostafa, H.; Selim, M.M.; Moez, A.A. Influence of Fe2O3 dopant on dielectric, optical conductivity and nonlinear optical properties of doped ZnO-polystyrene composites films. Biointerface Res. Appl. Chem 2021, 12, 170–179. [Google Scholar]
- El-Desouky, M.G.; Shahat, A.; El-Bindary, A.A.; El-Bindary, M.A. Description, kinetic and equilibrium studies of the adsorption of carbon dioxide in mesoporous iron oxide nanospheres. Biointerface Res. Appl. Chem 2021, 12, 3034–3054. [Google Scholar]
- Vargas-Ortíz, J.R.; Böhnel, H.N.; Gonzalez, C.; Esquivel, K. Magnetic nanoparticle behavior evaluation on cardiac tissue contractility through Langendorff rat heart technique as a nanotoxicology parameter. Appl. Nanosci. 2021, 11, 2383–2396. [Google Scholar] [CrossRef]
- Bao, X.; Mao, Y.; Si, G.; Kang, L.; Xu, B.; Gu, N. Iron oxide nanoparticles: A promising approach for diagnosis and treatment of cardiovascular diseases. Nano Res. 2023, 16, 12453–12470. [Google Scholar] [CrossRef]
- Vazquez-Prada, K.X.; Lam, J.; Kamato, D.; Xu, Z.P.; Little, P.J.; Ta, H.T. Targeted Molecular Imaging of Cardiovascular Diseases by Iron Oxide Nanoparticles. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 601–613. [Google Scholar] [CrossRef]
- Banik, B.; Surnar, B.; Askins, B.W.; Banerjee, M.; Dhar, S. Dual-Targeted Synthetic Nanoparticles for Cardiovascular Diseases. ACS Appl. Mater. Interfaces 2020, 12, 6852–6862. [Google Scholar] [CrossRef]
- Hou, J.; Zhou, J.; Chang, M.; Bao, G.; Xu, J.; Ye, M.; Zhong, Y.; Liu, S.; Wang, J.; Zhang, W.; et al. LIFU-responsive nanomedicine enables acoustic droplet vaporization-induced apoptosis of macrophages for stabilizing vulnerable atherosclerotic plaques. Bioact. Mater. 2022, 16, 120–133. [Google Scholar] [CrossRef]
- Xiong, F.; Wang, H.; Feng, Y.; Li, Y.; Hua, X.; Pang, X.; Zhang, S.; Song, L.; Zhang, Y.; Gu, N. Cardioprotective activity of iron oxide nanoparticles. Sci. Rep. 2015, 5, 8579. [Google Scholar] [CrossRef] [PubMed]
- Colbert, C.M.; Ming, Z.; Pogosyan, A.; Finn, J.P.; Nguyen, K.-L. Comparison of Three Ultrasmall, Superparamagnetic Iron Oxide Nanoparticles for MRI at 3.0 T. J. Magn. Reson. Imaging 2023, 57, 1819–1829. [Google Scholar] [CrossRef] [PubMed]
- Üstün, E.; Önbaş, S.C.; Çelik, S.K.; Ayvaz, M.Ç.; Şahin, N. Green synthesis of iron oxide nanoparticles by using Ficus carica leaf extract and its antioxidant activity. Biointerface Res. Appl. Chem 2022, 12, 2108–2116. [Google Scholar]
- Sosnovik, D.E.; Nahrendorf, M.; Deliolanis, N.; Novikov, M.; Aikawa, E.; Josephson, L.; Rosenzweig, A.; Weissleder, R.; Ntziachristos, V. Fluorescence tomography and magnetic resonance imaging of myocardial macrophage infiltration in infarcted myocardium in vivo. Circulation 2007, 115, 1384–1391. [Google Scholar] [CrossRef] [PubMed]
- Akhmedov, S.; Afanasyev, S.; Trusova, M.; Postnikov, P.; Rogovskaya, Y.; Grakova, E.; Kopeva, K.; Carreon Paz, R.K.; Balakin, S.; Wiesmann, H.P.; et al. Chemically Modified Biomimetic Carbon-Coated Iron Nanoparticles for Stent Coatings: In Vitro Cytocompatibility and In Vivo Structural Changes in Human Atherosclerotic Plaques. Biomedicines 2021, 9, 802. [Google Scholar] [CrossRef] [PubMed]
- Hataminia, F.; Majidi, R.F.; Najafi Tireh Shabankareh, A.; Ghanbari, H. Green synthesis of oxidized starch with a novel catalyst based on Fe3O4 nanoparticles and H2O2 reagent to form thermoplastic as a stable gel coating on the cardiovascular stents. Int. J. Biol. Macromol. 2022, 219, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Zheng, N.; Chen, X.; Zhan, R.; Liao, T.; Shi, Y.; Sun, X.; Chen, S.; Zuo, Z. Ferrous Oxide Nanoparticles Induced Abnormal Cardiac Development in Zebrafish Through Hypoxia and Ferroptosis. Chem. Res. Chin. Univ. 2023, 39, 502–507. [Google Scholar] [CrossRef]
- Lee, J.-R.; Park, B.-W.; Kim, J.; Choo, Y.W.; Kim, H.Y.; Yoon, J.-K.; Kim, H.; Hwang, J.-W.; Kang, M.; Kwon, S.P.; et al. Nanovesicles derived from iron oxide nanoparticles–incorporated mesenchymal stem cells for cardiac repair. Sci. Adv. 2020, 6, eaaz0952. [Google Scholar] [CrossRef]
- Nowak-Jary, J.; Machnicka, B. In vivo biodistribution and clearance of magnetic iron oxide nanoparticles for medical applications. Int. J. Nanomed. 2023, ume 18, 4067–4100. [Google Scholar] [CrossRef]
- Zheng, H.; You, J.; Yao, X.; Lu, Q.; Guo, W.; Shen, Y. Superparamagnetic iron oxide nanoparticles promote ferroptosis of ischemic cardiomyocytes. J. Cell. Mol. Med. 2020, 24, 11030–11033. [Google Scholar] [CrossRef]
- Blanco, E.; Segura-Ibarra, V.; Bawa, D.; Nafiujjaman, M.; Wu, S.; Liu, H.; Ferrari, M.; Lumsden, A.B.; Shah, D.J.; Lin, C.H. Functionalization of endovascular devices with superparamagnetic iron oxide nanoparticles for interventional cardiovascular magnetic resonance imaging. Biomed. Microdevices 2019, 21, 38. [Google Scholar] [CrossRef] [PubMed]
- Tsampasian, V.; Merinopoulos, I.; Cameron, D.; Garg, P.; Vassiliou, V.S. Ultrasmall Superparamagnetic Particles of Iron Oxide and Cardiac Magnetic Resonance: Novel Imaging in Everyday Conditions. Appl. Sci. 2022, 12, 6913. [Google Scholar] [CrossRef]
- Merinopoulos, I.; Gunawardena, T.; Stirrat, C.; Cameron, D.; Eccleshall, S.C.; Dweck, M.R.; Newby, D.E.; Vassiliou, V.S. Diagnostic Applications of Ultrasmall Superparamagnetic Particles of Iron Oxide for Imaging Myocardial and Vascular Inflammation. JACC: Cardiovasc. Imaging 2021, 14, 1249–1264. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wen, W.; Wang, X.; Huang, D.; Cao, J.; Qi, X.; Shen, S. Ultrasmall iron oxide nanoparticles cause significant toxicity by specifically inducing acute oxidative stress to multiple organs. Part Fibre Toxicol. 2022, 19, 24. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Lu, K.; Xiao, L.; Hu, X.; Cai, W.; Liu, L.; Liu, Y.; Li, W.; Zhou, H.; Qian, Z.; et al. Exploring atherosclerosis imaging with contrast-enhanced MRI using PEGylated ultrasmall iron oxide nanoparticles. Front. Bioeng. Biotechnol. 2023, 11, 1279446. [Google Scholar] [CrossRef] [PubMed]
- Segers, F.M.E.; Ruder, A.V.; Westra, M.M.; Lammers, T.; Dadfar, S.M.; Roemhild, K.; Lam, T.S.; Kooi, M.E.; Cleutjens, K.B.J.M.; Verheyen, F.K.; et al. Magnetic resonance imaging contrast-enhancement with superparamagnetic iron oxide nanoparticles amplifies macrophage foam cell apoptosis in human and murine atherosclerosis. Cardiovasc. Res. 2022, 118, 3346–3359. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, A.; Dengler, M.A.; van der Kuip, H.; Yildiz, H.; Rösch, S.; Klumpp, S.; Klingel, K.; Kandolf, R.; Helluy, X.; Hiller, K.-H.; et al. Imaging of myocardial infarction using ultrasmall superparamagnetic iron oxide nanoparticles: A human study using a multi-parametric cardiovascular magnetic resonance imaging approach. Eur. Heart J. 2013, 34, 462–475. [Google Scholar] [CrossRef]
- Chen, C.; Ge, J.; Gao, Y.; Chen, L.; Cui, J.; Zeng, J.; Gao, M. Ultrasmall superparamagnetic iron oxide nanoparticles: A next generation contrast agent for magnetic resonance imaging. WIREs Nanomed. Nanobiotech. 2022, 14, e1740. [Google Scholar] [CrossRef]
- Polonschii, C.; Potara, M.; Iancu, M.; David, S.; Banciu, R.M.; Vasilescu, A.; Astilean, S. Progress in the Optical Sensing of Cardiac Biomarkers. Biosensors 2023, 13, 632. [Google Scholar] [CrossRef]
- Tiwari, A.; Elgrably, B.; Saar, G.; Vandoorne, K. Multi-Scale Imaging of Vascular Pathologies in Cardiovascular Disease. Front. Med. 2022, 8, 754369. [Google Scholar] [CrossRef]
- Sun, R.; Wang, X.; Nie, Y.; Hu, A.; Liu, H.; Zhang, K.; Zhang, L.; Wu, Q.; Li, K.; Liu, C.; et al. Targeted trapping of endogenous endothelial progenitor cells for myocardial ischemic injury repair through neutrophil-mediated SPIO nanoparticle-conjugated CD34 antibody delivery and imaging. Acta Biomater. 2022, 146, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Varna, M.; Xuan, H.V.; Fort, E. Gold nanoparticles in cardiovascular imaging. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2018, 10, e1470. [Google Scholar] [CrossRef] [PubMed]
- Selim, A.A.; Sakr, T.M.; Essa, B.M. Gold Nanoparticles: Synthesis, Functionalization and Biomedical Applications Especially in Cardiovascular Therapy. Pharm. Chem. J. 2023, 57, 29–39. [Google Scholar] [CrossRef]
- Luo, D.; Wang, X.; Burda, C.; Basilion, J.P. Recent Development of Gold Nanoparticles as Contrast Agents for Cancer Diagnosis. Cancers 2021, 13, 1825. [Google Scholar] [CrossRef]
- Gonciar, D.; Mocan, T.; Agoston-Coldea, L. Nanoparticles Targeting the Molecular Pathways of Heart Remodeling and Regeneration. Pharmaceutics 2022, 14, 711. [Google Scholar] [CrossRef] [PubMed]
- Samsulkahar, N.F.; Hadi, A.A.; Shamsuddin, M.; Nik, N.A.N. Biosynthesis of Gold Nanoparticles Using Strobilanthes crispa Aqueous Leaves Extract and Evaluation of Its Antibacterial Activity. Biointerface Res. Appl. Chem 2023, 13, 63. [Google Scholar]
- Flora, G.D.; Nayak, M.K. A Brief Review of Cardiovascular Diseases, Associated Risk Factors and Current Treatment Regimes. Curr. Pharm. Des. 2019, 25, 4063–4084. [Google Scholar] [CrossRef]
- Si, P.; Razmi, N.; Nur, O.; Solanki, S.; Pandey, C.M.; Gupta, R.K.; Malhotra, B.D.; Willander, M.; de la Zerda, A. Gold nanomaterials for optical biosensing and bioimaging. Nanoscale Adv. 2021, 3, 2679–2698. [Google Scholar] [CrossRef]
- Milan, J.; Niemczyk, K.; Kus-Liśkiewicz, M. Treasure on the Earth—Gold Nanoparticles and Their Biomedical Applications. Materials 2022, 15, 3355. [Google Scholar] [CrossRef]
- Anik, M.I.; Mahmud, N.; Al Masud, A.; Hasan, M. Gold nanoparticles (GNPs) in biomedical and clinical applications: A review. Nano Sel. 2022, 3, 792–828. [Google Scholar] [CrossRef]
- Shevach, M.; Fleischer, S.; Shapira, A.; Dvir, T. Gold nanoparticle-decellularized matrix hybrids for cardiac tissue engineering. Nano Lett. 2014, 14, 5792–5796. [Google Scholar] [CrossRef]
- Bakir, E.M.; Younis, N.S.; Mohamed, M.E.; El Semary, N.A. Cyanobacteria as Nanogold Factories: Chemical and Anti-Myocardial Infarction Properties of Gold Nanoparticles Synthesized by Lyngbya majuscula. Mar. Drugs 2018, 16, 217. [Google Scholar] [CrossRef] [PubMed]
- Mahan, M.M.; Doiron, A.L. Gold Nanoparticles as X-Ray, CT, and Multimodal Imaging Contrast Agents: Formulation, Targeting, and Methodology. J. Nanomater. 2018, 2018, 5837276. [Google Scholar] [CrossRef]
- Daems, N.; Michiels, C.; Lucas, S.; Baatout, S.; Aerts, A. Gold nanoparticles meet medical radionuclides. Nucl. Med. Biol. 2021, 100–101, 61–90. [Google Scholar] [CrossRef] [PubMed]
- Danila, D.; Johnson, E.; Kee, P. CT imaging of myocardial scars with collagen-targeting gold nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-C.; Perevedentseva, E.; Lin, Z.-R.; Chang, C.-C.; Chen, H.-H.; Yang, S.-M.; Lin, M.-D.; Karmenyan, A.; Speranza, G.; Minati, L.; et al. Multimodal bioimaging using nanodiamond and gold hybrid nanoparticles. Sci. Rep. 2022, 12, 5331. [Google Scholar] [CrossRef] [PubMed]
- Shevach, M.; Maoz, B.M.; Feiner, R.; Shapira, A.; Dvir, T. Nanoengineering gold particle composite fibers for cardiac tissue engineering. Journal of Materials Chemistry B 2013, 1, 5210–5217. [Google Scholar] [CrossRef] [PubMed]
- Cheheltani, R.; Ezzibdeh, R.M.; Chhour, P.; Pulaparthi, K.; Kim, J.; Jurcova, M.; Hsu, J.C.; Blundell, C.; Litt, H.I.; Ferrari, V.A.; et al. Tunable, biodegradable gold nanoparticles as contrast agents for computed tomography and photoacoustic imaging. Biomaterials 2016, 102, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.C.; Hajfathalian, M.; Maidment, P.S.N.; Hsu, J.C.; Naha, P.C.; Si-Mohamed, S.; Breuilly, M.; Kim, J.; Chhour, P.; Douek, P.; et al. Effect of Gold Nanoparticle Size on Their Properties as Contrast Agents for Computed Tomography. Sci. Rep. 2019, 9, 14912. [Google Scholar] [CrossRef]
- Chhour, P.; Naha, P.C.; O’Neill, S.M.; Litt, H.I.; Reilly, M.P.; Ferrari, V.A.; Cormode, D.P. Labeling monocytes with gold nanoparticles to track their recruitment in atherosclerosis with computed tomography. Biomaterials 2016, 87, 93–103. [Google Scholar] [CrossRef]
- Karademir, F.; Ayhan, F. Antimicrobial Surface Functionality of PEG Coated and AgNPs Immobilized Extracorporeal Biomaterials. Biointerface Res. Appl. Chem. 2021, 12, 1039–1052. [Google Scholar]
- Ferdous, Z.; Beegam, S.; Zaaba, N.E.; Elzaki, O.; Tariq, S.; Greish, Y.E.; Ali, B.H.; Nemmar, A. Exacerbation of Thrombotic Responses to Silver Nanoparticles in Hypertensive Mouse Model. Oxidative Med. Cell. Longev. 2022, 2022, 2079630. [Google Scholar] [CrossRef] [PubMed]
- Dixon, K.; Bonon, R.; Ivander, F.; Ale Ebrahim, S.; Namdar, K.; Shayegannia, M.; Khalvati, F.; Kherani, N.P.; Zavodni, A.; Matsuura, N. Using Machine Learning and Silver Nanoparticle-Based Surface-Enhanced Raman Spectroscopy for Classification of Cardiovascular Disease Biomarkers. ACS Appl. Nano Mater. 2023, 6, 15385–15396. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef] [PubMed]
- Gherasim, O.; Puiu, R.A.; Bîrcă, A.C.; Burdușel, A.-C.; Grumezescu, A.M. An Updated Review on Silver Nanoparticles in Biomedicine. Nanomaterials 2020, 10, 2318. [Google Scholar] [CrossRef] [PubMed]
- Kumawat, M.; Madhyastha, H.; Singh, M.; Revaprasadu, N.; Srinivas, S.P.; Daima, H.K. Double functionalized haemocompatible silver nanoparticles control cell inflammatory homeostasis. PLoS ONE 2022, 17, e0276296. [Google Scholar] [CrossRef] [PubMed]
- Almatroudi, A. Silver nanoparticles: Synthesis, characterisation and biomedical applications. Open Life Sci. 2020, 15, 819–839. [Google Scholar] [CrossRef] [PubMed]
- Nemmar, A.; Al-Salam, S.; Greish, Y.E.; Beegam, S.; Zaaba, N.E.; Ali, B.H. Impact of Intratracheal Administration of Polyethylene Glycol-Coated Silver Nanoparticles on the Heart of Normotensive and Hypertensive Mice. Int. J. Mol. Sci. 2023, 24, 8890. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, D.; Muzakkar, M.Z.; Maulidiyah, M.; Nurdin, M.; Saad, S.K.M.; Umar, A.A. Morphological analysis of Ag doped on TiO2/Ti prepared via anodizing and thermal oxidation methods. Biointerface Res. Appl. Chem 2022, 12, 1421–1437. [Google Scholar]
- Burdușel, A.-C.; Gherasim, O.; Grumezescu, A.M.; Mogoantă, L.; Ficai, A.; Andronescu, E. Biomedical Applications of Silver Nanoparticles: An Up-to-Date Overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef]
- Yousef, M.I.; Abuzreda, A.A.; Kamel, M.A.N. Cardiotoxicity and lung toxicity in male rats induced by long-term exposure to iron oxide and silver nanoparticles. Exp Ther Med. 2019, 18, 4329–4339. [Google Scholar] [CrossRef]
- Waiezi, S.; Malek, N.A.N.N.; Asraf, M.H.; Sani, N.S. Preparation, Characterization, and Antibacterial Activity of Green-Biosynthesised Silver Nanoparticles using Clinacanthus nutans Extract. Biointerface Res. Appl. Chem. 2023, 13, 171. [Google Scholar]
- Matar, G.; Akyuz, G.; Kaymazlar, E.; Andaç, M. An investigation of green synthesis of silver nanoparticles using Turkish honey against pathogenic bacterial strains. Biointerface Res. Appl. Chem. 2023, 13, 1–11. [Google Scholar]
- Agudelo, W.; Montoya, Y.; Garcia-Garcia, A.; Bustamante, J. Functionalization of Electrospun Scaffolds with Gold or Silver Nanoparticles for Applications in Cardiovascular Tissue Engineering. In Proceedings of the 2021 Global Medical Engineering Physics Exchanges/Pan American Health Care Exchanges (GMEPE/PAHCE), Sevilla, Spain, 15–20 March 2021; pp. 1–6. [Google Scholar]
- Lu, C.; Liu, Y.; Liu, Y.; Kou, G.; Chen, Y.; Wu, X.; Lv, Y.; Cai, J.; Chen, R.; Luo, J. Silver Nanoparticles Cause Neural and Vascular Disruption by Affecting Key Neuroactive Ligand-Receptor Interaction and VEGF Signaling Pathways. Int. J. Nanomed. 2023, 18, 2693–2706. [Google Scholar] [CrossRef]
- Cho, Y.-M.; Mizuta, Y.; Akagi, J.-i.; Toyoda, T.; Sone, M.; Ogawa, K. Size-dependent acute toxicity of silver nanoparticles in mice. J. Toxicol. Pathol. 2018, 31, 73–80. [Google Scholar] [CrossRef]
- Yasemin, B.-K.; Rabia, C.-K.; Tolga, Z.; Burak, O.; Zeynep, K.; Abdurrahim Can, E.; Serda, K.-G. Assessment of Nano-toxicity and Safety Profiles of Silver Nanoparticles. In Silver Nanoparticles; Khan, M., Ed.; IntechOpen: Rijeka, Yugoslavia, 2018; p. Ch. 10. [Google Scholar]
- Arozal, W.; Monayo, E.R.; Barinda, A.J.; Perkasa, D.P.; Soetikno, V.; Nafrialdi, N.; Louisa, M. Protective effects of silver nanoparticles in isoproterenol-induced myocardial infarction in rats. Front. Med. 2022, 9, 867497. [Google Scholar] [CrossRef]
- Badi’Ah, H.I.; Seedeh, F.; Supriyanto, G.; Zaidan, A.H. Synthesis of silver nanoparticles and the development in analysis method. IOP Publ. 2019, 217, 012005. [Google Scholar] [CrossRef]
- Adibkia, K.; Ehsani, A.; Jodaei, A.; Fathi, E.; Farahzadi, R.; Barzegar-Jalali, M. Silver nanoparticles induce the cardiomyogenic differentiation of bone marrow derived mesenchymal stem cells via telomere length extension. Beilstein J. Nanotechnol. 2021, 12, 786–797. [Google Scholar] [CrossRef]
- Agudelo, W.; Montoya, Y.; Bustamante, J. Using a non-reducing sugar in the green synthesis of gold and silver nanoparticles by the chemical reduction method. Dyna 2018, 85, 69–78. [Google Scholar] [CrossRef]
- Minarchick, V.C.; Stapleton, P.A.; Sabolsky, E.M.; Nurkiewicz, T.R. Cerium Dioxide Nanoparticle Exposure Improves Microvascular Dysfunction and Reduces Oxidative Stress in Spontaneously Hypertensive Rats. Front. Physiol. 2015, 6, 339. [Google Scholar] [CrossRef] [PubMed]
- Barker, E.; Shepherd, J.; Asencio, I.O. The Use of Cerium Compounds as Antimicrobials for Biomedical Applications. Molecules 2022, 27, 2678. [Google Scholar] [CrossRef]
- Dekkers, S.; Miller, M.R.; Schins, R.P.F.; Römer, I.; Russ, M.; Vandebriel, R.J.; Lynch, I.; Belinga-Desaunay, M.-F.; Valsami-Jones, E.; Connell, S.P.; et al. The effect of zirconium doping of cerium dioxide nanoparticles on pulmonary and cardiovascular toxicity and biodistribution in mice after inhalation. Nanotoxicology 2017, 11, 794–808. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, P.; Tal, A.; Skallberg, A.; Brommesson, C.; Hu, Z.; Boyd, R.; Olovsson, W.; Fairley, N.; Abrikosov, I.; Zhang, X.; et al. Cerium oxide nanoparticles with antioxidant capabilities and gadolinium integration for MRI contrast enhancement. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- McDonagh, P.R.; Gobalakrishnan, S.; Rabender, C.; Vijayaragavan, V.; Zweit, J. Molecular Imaging Investigations of Polymer-Coated Cerium Oxide Nanoparticles as a Radioprotective Therapeutic Candidate. Pharmaceutics 2023, 15, 2144. [Google Scholar] [CrossRef] [PubMed]
- Dhall, A.; Self, W. Cerium Oxide Nanoparticles: A Brief Review of Their Synthesis Methods and Biomedical Applications. Antioxidants 2018, 7, 97. [Google Scholar] [CrossRef]
- Banavar, S.; Deshpande, A.; Sur, S.; Andreescu, S. Ceria nanoparticle theranostics: Harnessing antioxidant properties in biomedicine and beyond. J. Phys. Mater. 2021, 4, 042003. [Google Scholar] [CrossRef]
- Alahmmar, M.; Prabhakaran, P.; Jaganathan, S.; Nik, N.A.N. Fabrication and characterization of polycaprolactone with retinoic acid and cerium oxide for anticancer applications. Biointerface Res. Appl. Chem 2023, 13, 1–15. [Google Scholar]
- Shcherbakov, A.B.; Zholobak, N.M.; Ivanov, V.K. 8-Biological, biomedical and pharmaceutical applications of cerium oxide. In Cerium Oxide (CeO2): Synthesis, Properties and Applications; Scirè, S., Palmisano, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 279–358. [Google Scholar]
- Estevez, A.Y.; Erlichman, J.S. Cerium oxide nanoparticles for the treatment of neurological oxidative stress diseases. In Oxidative Stress: Diagnostics, Prevention, and Therapy; ACS Publications: Washington, DC, USA, 2011; pp. 255–288. [Google Scholar]
- Li, X.; Han, Z.; Wang, T.; Ma, C.; Li, H.; Lei, H.; Yang, Y.; Wang, Y.; Pei, Z.; Liu, Z. Cerium oxide nanoparticles with antioxidative neurorestoration for ischemic stroke. Biomaterials 2022, 291, 121904. [Google Scholar] [CrossRef]
- S Jairam, L.; Chandrashekar, A.; Prabhu, T.N.; Kotha, S.B.; Girish, M.S.; Devraj, I.M.; Dhanya Shri, M.; Prashantha, K. A review on biomedical and dental applications of cerium oxide nanoparticles―Unearthing the potential of this rare earth metal. J. Rare Earths 2023, 41, 1645–1661. [Google Scholar] [CrossRef]
- Chen, S.; Hou, Y.; Cheng, G.; Zhang, C.; Wang, S.; Zhang, J. Cerium oxide nanoparticles protect endothelial cells from apoptosis induced by oxidative stress. Biol. Trace Elem. Res. 2013, 154, 156–166. [Google Scholar] [CrossRef]
- Singh, K.R.B.; Nayak, V.; Sarkar, T.; Singh, R.P. Cerium oxide nanoparticles: Properties, biosynthesis and biomedical application. RSC Adv. 2020, 10, 27194–27214. [Google Scholar] [CrossRef]
- Pagliari, F.; Mandoli, C.; Forte, G.; Magnani, E.; Pagliari, S.; Nardone, G.; Licoccia, S.; Minieri, M.; Di Nardo, P.; Traversa, E. Cerium oxide nanoparticles protect cardiac progenitor cells from oxidative stress. ACS Nano 2012, 6, 3767–3775. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Azfer, A.; Rogers, L.M.; Wang, X.; Kolattukudy, P.E. Cardioprotective effects of cerium oxide nanoparticles in a transgenic murine model of cardiomyopathy. Cardiovasc. Res. 2007, 73, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Nashat, N.; Haider, Z. Therapeutic applications of nanozymes and their role in cardiovascular disease. Int. J. Nanomater. Nanotechnol. Nanomed. 2021, 7, 009–018. [Google Scholar]
- Jain, A.; Behera, M.; Mahapatra, C.; Sundaresan, N.R.; Chatterjee, K. Nanostructured polymer scaffold decorated with cerium oxide nanoparticles toward engineering an antioxidant and anti-hypertrophic cardiac patch. Mater. Sci. Eng. C 2021, 118, 111416. [Google Scholar] [CrossRef] [PubMed]
- Pagliari, F.; Nardone, G.; Traversa, E.; Nardo, P. Cerium Dioxide Nanoparticles Protect Cardiac Progenitor Cells against the Oxidative Stress; River Publishers: Hague, The Netherlands, 2022; pp. 25–35. [Google Scholar]
- Guerra-Ojeda, S.; Marchio, P.; Rueda, C.; Suarez, A.; Garcia, H.; Victor, V.M.; Juez, M.; Martin-Gonzalez, I.; Vila, J.M.; Mauricio, M.D. Cerium dioxide nanoparticles modulate antioxidant defences and change vascular response in the human saphenous vein. Free Radic. Biol. Med. 2022, 193, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Nemmar, A.; Al-Salam, S.; Beegam, S.; Yuvaraju, P.; Ali, B.H. Aortic Oxidative Stress, Inflammation and DNA Damage Following Pulmonary Exposure to Cerium Oxide Nanoparticles in a Rat Model of Vascular Injury. Biomolecules 2019, 9, 376. [Google Scholar] [CrossRef] [PubMed]
- Naha, P.C.; Hsu, J.C.; Kim, J.; Shah, S.; Bouché, M.; Si-Mohamed, S.; Rosario-Berrios, D.N.; Douek, P.; Hajfathalian, M.; Yasini, P.; et al. Dextran-Coated Cerium Oxide Nanoparticles: A Computed Tomography Contrast Agent for Imaging the Gastrointestinal Tract and Inflammatory Bowel Disease. ACS Nano 2020, 14, 10187–10197. [Google Scholar] [CrossRef]
- Popov, A.L.; Abakumov, M.A.; Savintseva, I.V.; Ermakov, A.M.; Popova, N.R.; Ivanova, O.S.; Kolmanovich, D.D.; Baranchikov, A.E.; Ivanov, V.K. Biocompatible dextran-coated gadolinium-doped cerium oxide nanoparticles as MRI contrast agents with high T1 relaxivity and selective cytotoxicity to cancer cells. J. Mater. Chem. B 2021, 9, 6586–6599. [Google Scholar] [CrossRef]
- Briggs, A.; Corde, S.; Oktaria, S.; Brown, R.; Rosenfeld, A.; Lerch, M.; Konstantinov, K.; Tehei, M. Cerium oxide nanoparticles: Influence of the high-Z component revealed on radioresistant 9L cell survival under X-ray irradiation. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 1098–1105. [Google Scholar] [CrossRef]
- McDonagh, P.R.; Sundaresan, G.; Yang, L.; Sun, M.; Mikkelsen, R.; Zweit, J. Biodistribution and PET imaging of 89-zirconium labeled cerium oxide nanoparticles synthesized with several surface coatings. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1429–1440. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Sohail; Raja, N.I.; Asad, M.J.; Mashwani, Z.-U.-R. Antioxidant and hypoglycemic potential of phytogenic cerium oxide nanoparticles. Scientific Reports 2023, 13, 4514. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Chen, Z.; Yang, S.; Liu, T.; Yin, L.; Pu, Y.; Liang, G. Nanomaterials-induced toxicity on cardiac myocytes and tissues, and emerging toxicity assessment techniques. Sci. Total Environ. 2021, 800, 149584. [Google Scholar] [CrossRef] [PubMed]
- Manickam, V.; Periyasamy, M.; Dhakshinamoorthy, V.; Panneerselvam, L.; Perumal, E. Recurrent exposure to ferric oxide nanoparticles alters myocardial oxidative stress, apoptosis and necrotic markers in male mice. Chem. -Biol. Interact. 2017, 278, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Gong, S.; Li, J.; Wang, Y.; Zhang, X.; Zheng, H.; Zhang, Q.; You, J.; Huang, Z.; Chen, Y. Co-loading antioxidant N-acetylcysteine attenuates cytotoxicity of iron oxide nanoparticles in hypoxia/reoxygenation cardiomyocytes. Int. J. Nanomed. 2019, 14, 6103–6115. [Google Scholar] [CrossRef]
- Wang, Z.; Tang, M. Research progress on toxicity, function, and mechanism of metal oxide nanoparticles on vascular endothelial cells. J. Appl. Toxicol. JAT 2021, 41, 683–700. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xue, Y.; Ni, Y.; Ning, F.; Shang, L.; Ma, A. Size dependent effects of Gold Nanoparticles in ISO-induced Hyperthyroid Rats. Sci. Rep. 2018, 8, 10960. [Google Scholar] [CrossRef] [PubMed]
- De Jong, W.H.; Hagens, W.I.; Krystek, P.; Burger, M.C.; Sips, A.J.A.M.; Geertsma, R.E. Particle size-dependent organ distribution of gold nanoparticles after intravenous administration. Biomaterials 2008, 29, 1912–1919. [Google Scholar] [CrossRef]
- Abdelhalim, M.A.K. Exposure to gold nanoparticles produces cardiac tissue damage that depends on the size and duration of exposure. Lipids Health Dis. 2011, 10, 205. [Google Scholar] [CrossRef]
- Pan, Y.; Leifert, A.; Ruau, D.; Neuss, S.; Bornemann, J.; Schmid, G.; Brandau, W.; Simon, U.; Jahnen-Dechent, W. Gold nanoparticles of diameter 1.4 nm trigger necrosis by oxidative stress and mitochondrial damage. Small 2009, 5, 2067–2076. [Google Scholar] [CrossRef]
- Pan, Y.; Leifert, A.; Graf, M.; Schiefer, F.; Thoröe-Boveleth, S.; Broda, J.; Halloran, M.C.; Hollert, H.; Laaf, D.; Simon, U.; et al. High-sensitivity real-time analysis of nanoparticle toxicity in green fluorescent protein-expressing zebrafish. Small 2013, 9, 863–869. [Google Scholar] [CrossRef]
- Olugbodi, J.O.; Lawal, B.; Bako, G.; Onikanni, A.S.; Abolenin, S.M.; Mohammud, S.S.; Ataya, F.S.; Batiha, G.E.-S. Effect of sub-dermal exposure of silver nanoparticles on hepatic, renal and cardiac functions accompanying oxidative damage in male Wistar rats. Sci. Rep. 2023, 13, 10539. [Google Scholar] [CrossRef]
- Lin, C.X.; Yang, S.Y.; Gu, J.L.; Meng, J.; Xu, H.Y.; Cao, J.M. The acute toxic effects of silver nanoparticles on myocardial transmembrane potential, I(Na) and I(K1) channels and heart rhythm in mice. Nanotoxicology 2017, 11, 827–837. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, F.; Yalamarty, S.S.K.; Filipczak, N.; Jin, Y.; Li, X. Nano Silver-Induced Toxicity and Associated Mechanisms. Int. J. Nanomed. 2022, 17, 1851–1864. [Google Scholar] [CrossRef]
- Ramirez-Lee, M.A.; Aguirre-Bañuelos, P.; Martinez-Cuevas, P.P.; Espinosa-Tanguma, R.; Chi-Ahumada, E.; Martinez-Castañon, G.A.; Gonzalez, C. Evaluation of cardiovascular responses to silver nanoparticles (AgNPs) in spontaneously hypertensive rats. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 385–395. [Google Scholar] [CrossRef]
- Bostan, H.B.; Rezaee, R.; Valokala, M.G.; Tsarouhas, K.; Golokhvast, K.; Tsatsakis, A.M.; Karimi, G. Cardiotoxicity of nano-particles. Life Sci. 2016, 165, 91–99. [Google Scholar] [CrossRef]
- Espinosa-Cristobal, L.F.; Martinez-Castañon, G.A.; Loyola-Rodriguez, J.P.; Patiño-Marin, N.; Reyes-Macías, J.F.; Vargas-Morales, J.M.; Ruiz, F. Toxicity, distribution, and accumulation of silver nanoparticles in Wistar rats. J. Nanoparticle Res. 2013, 15, 1702. [Google Scholar] [CrossRef]
- Rzigalinski, B.A.; Hockey, K.; Klein, L.M.; Sholar, C.A.; Himler, J.; Billings, M.J.; Cook, J. Cerium Oxide Nanoparticles for the Treatment and Prevention of Stroke and Cardiovascular Disease. U.S. Patent 9,649,337, 2017; Application granted, 23 April 2019. [Google Scholar]
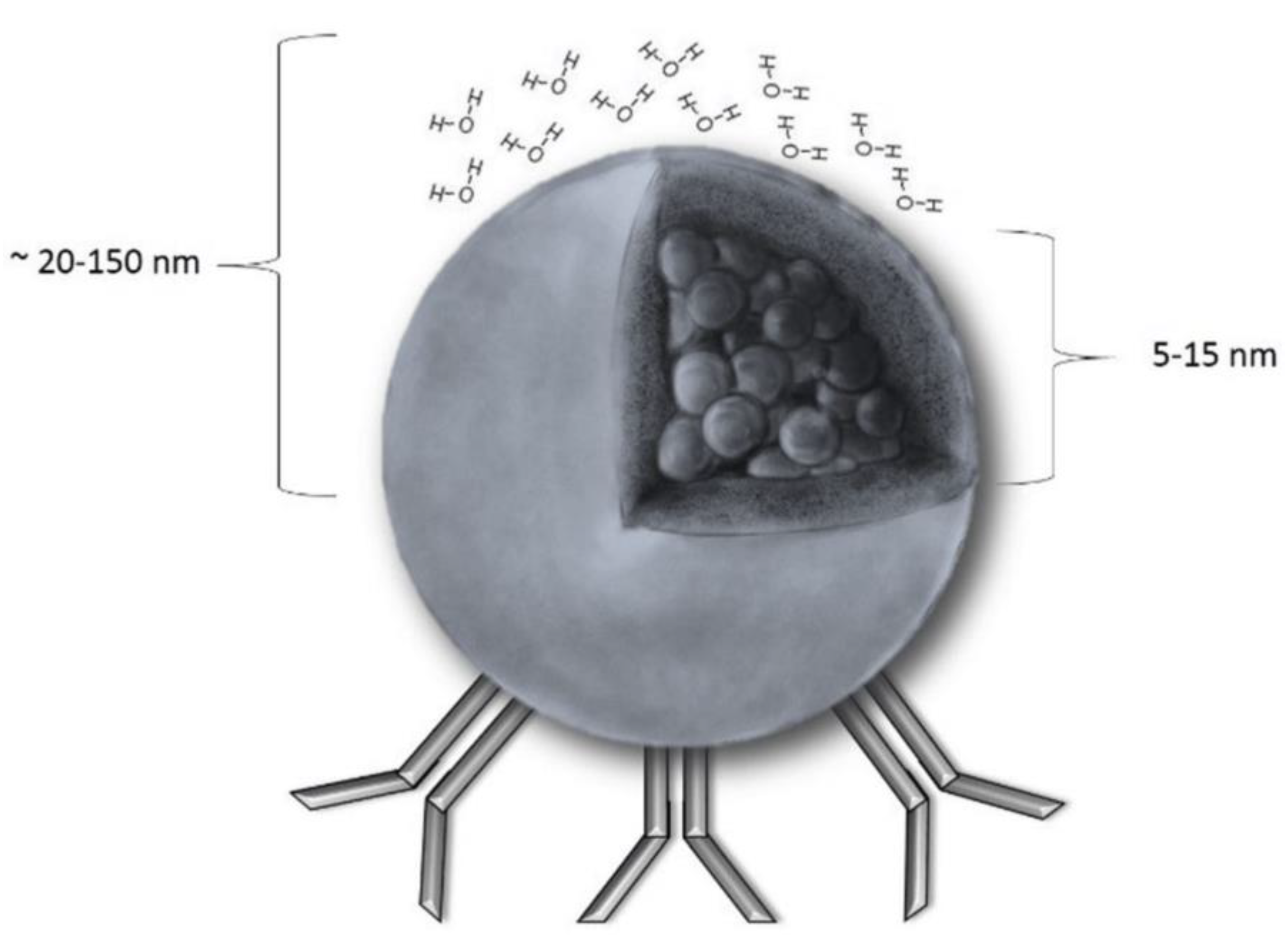
| Iron Oxide Nanoparticles | Applications |
|---|---|
| Fe3O4-PFH-DiR@CS-DS NPs | Magnetic resonance imaging, near-infrared fluorescence (NIRF) |
| 2,3-dimercaptosuccinic acid modified Fe2O3 NPs | Protection of the heart from ischemic damage |
| IONPs surface modified with dextran | Macrophages infiltration |
| Magneto fluorescent nanoparticles (cross-linked iron oxide [CLIO]-Cy5.5) | MRI and fluorescence tomography |
| Fe@CNPs | Penetration of the internal structures of atherosclerotic plaques |
| Ultrasmall superparamagnetic particles of iron oxide | Improvement of the MRI |
| SPION-antiCD34/Nes | Increase CD133+ EPC accumulation at MI sites |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scafa Udriște, A.; Burdușel, A.C.; Niculescu, A.-G.; Rădulescu, M.; Grumezescu, A.M. Metal-Based Nanoparticles for Cardiovascular Diseases. Int. J. Mol. Sci. 2024, 25, 1001. https://doi.org/10.3390/ijms25021001
Scafa Udriște A, Burdușel AC, Niculescu A-G, Rădulescu M, Grumezescu AM. Metal-Based Nanoparticles for Cardiovascular Diseases. International Journal of Molecular Sciences. 2024; 25(2):1001. https://doi.org/10.3390/ijms25021001
Chicago/Turabian StyleScafa Udriște, Alexandru, Alexandra Cristina Burdușel, Adelina-Gabriela Niculescu, Marius Rădulescu, and Alexandru Mihai Grumezescu. 2024. "Metal-Based Nanoparticles for Cardiovascular Diseases" International Journal of Molecular Sciences 25, no. 2: 1001. https://doi.org/10.3390/ijms25021001