The Modification of H3K4me3 Enhanced the Expression of CgTLR3 in Hemocytes to Increase CgIL17-1 Production in the Immune Priming of Crassostrea gigas
Abstract
:1. Introduction
2. Results
2.1. The mRNA Expression Levels of CgTLR3, CgMyD88-2, CgRel1, and CgIL17-1 at 6 h after the Secondary Stimulation with Live V. splendidus
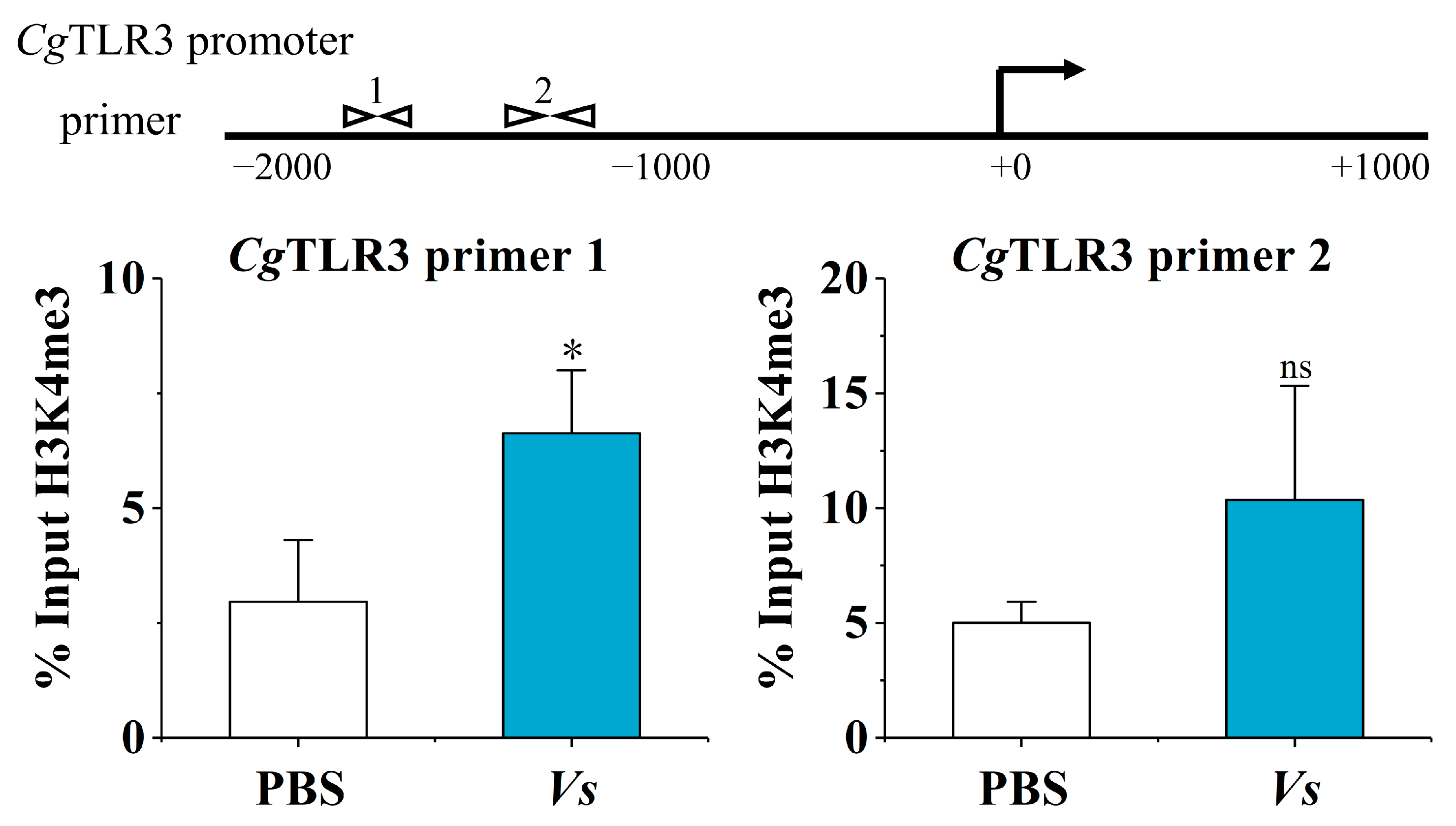
2.2. The H3K4me3 Modification of the CgTLR3 Gene Promoter at 7 d after the Stimulation with Inactivated V. splendidus
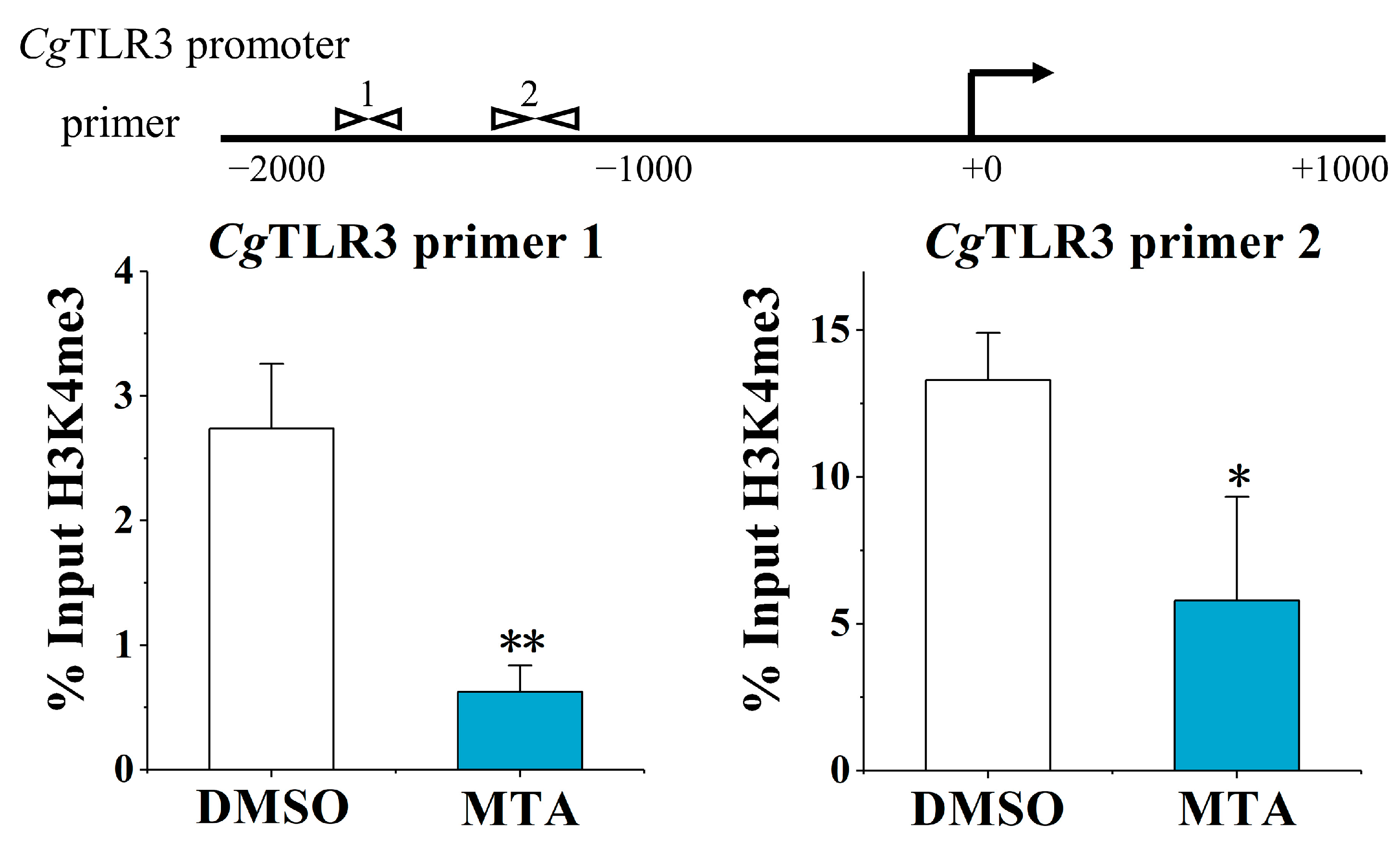
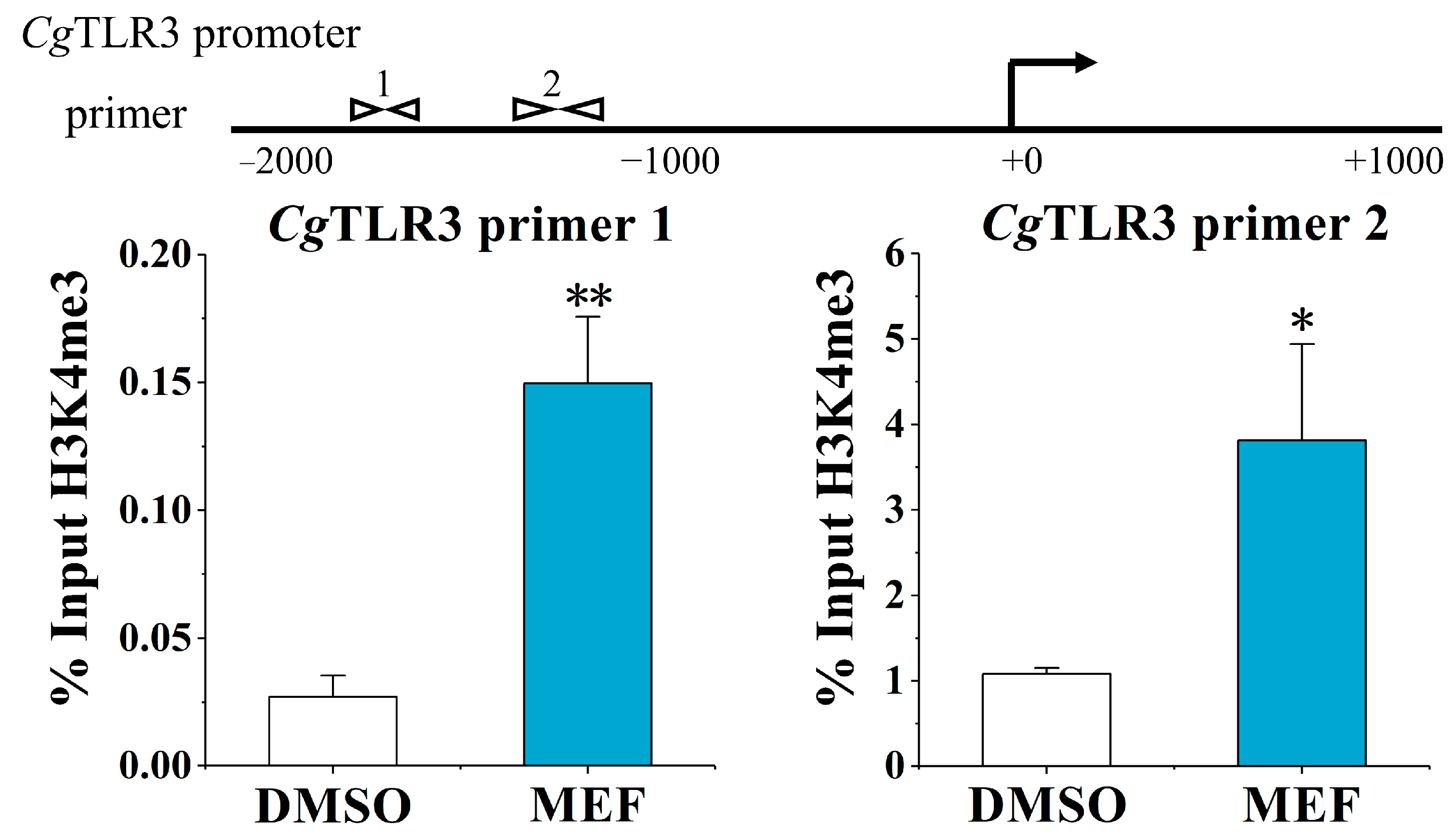
2.3. The Alternation of H3K4me3 Modification Levels at the CgTLR3 Gene Promoter at 7 d after the Treatments with MTA and MEF
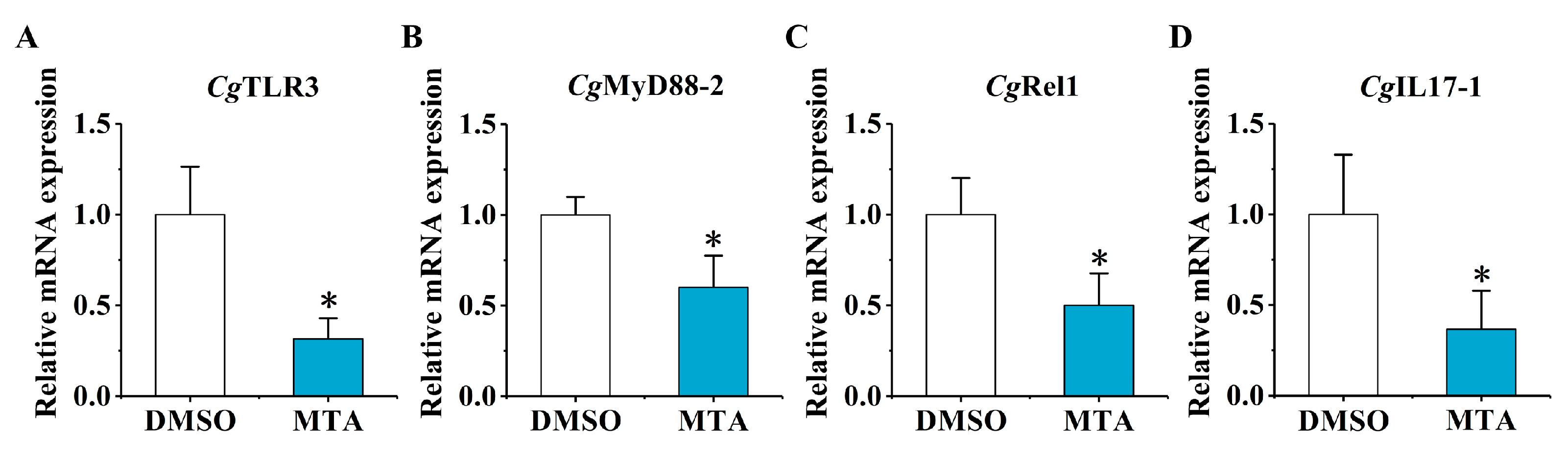
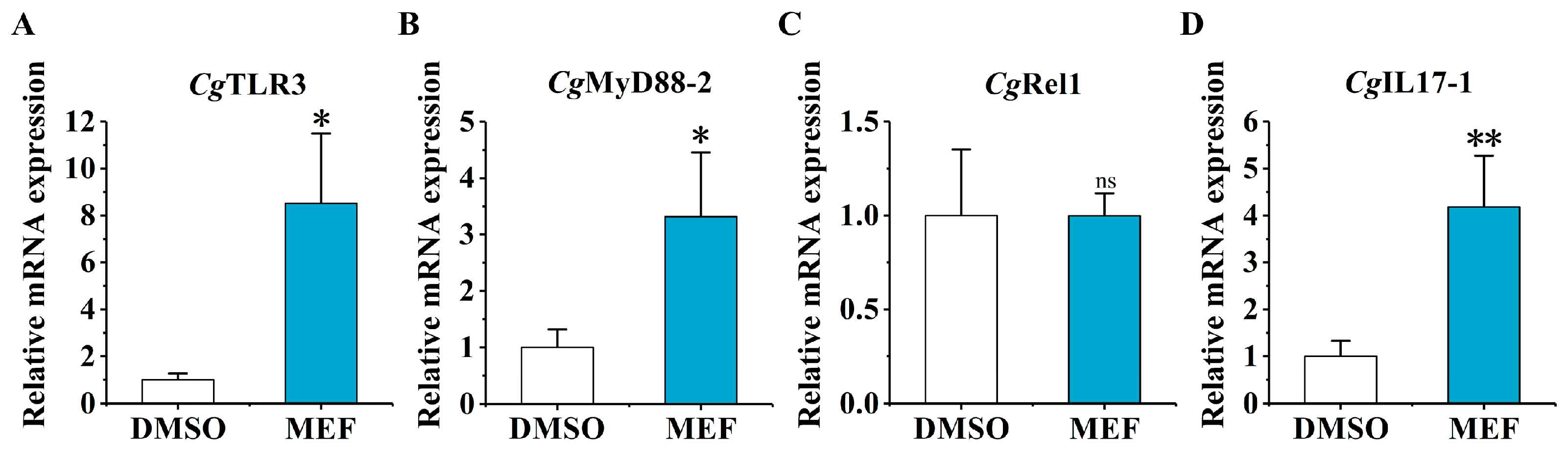
2.4. The mRNA Expression of CgTLR3, CgMyD88-2, CgRel1, and CgIL17-1 at 6 h after the Secondary Stimulation upon the MTA and MEF Treatment
3. Discussion
4. Materials and Methods
4.1. Experimental Animals and Bacteria
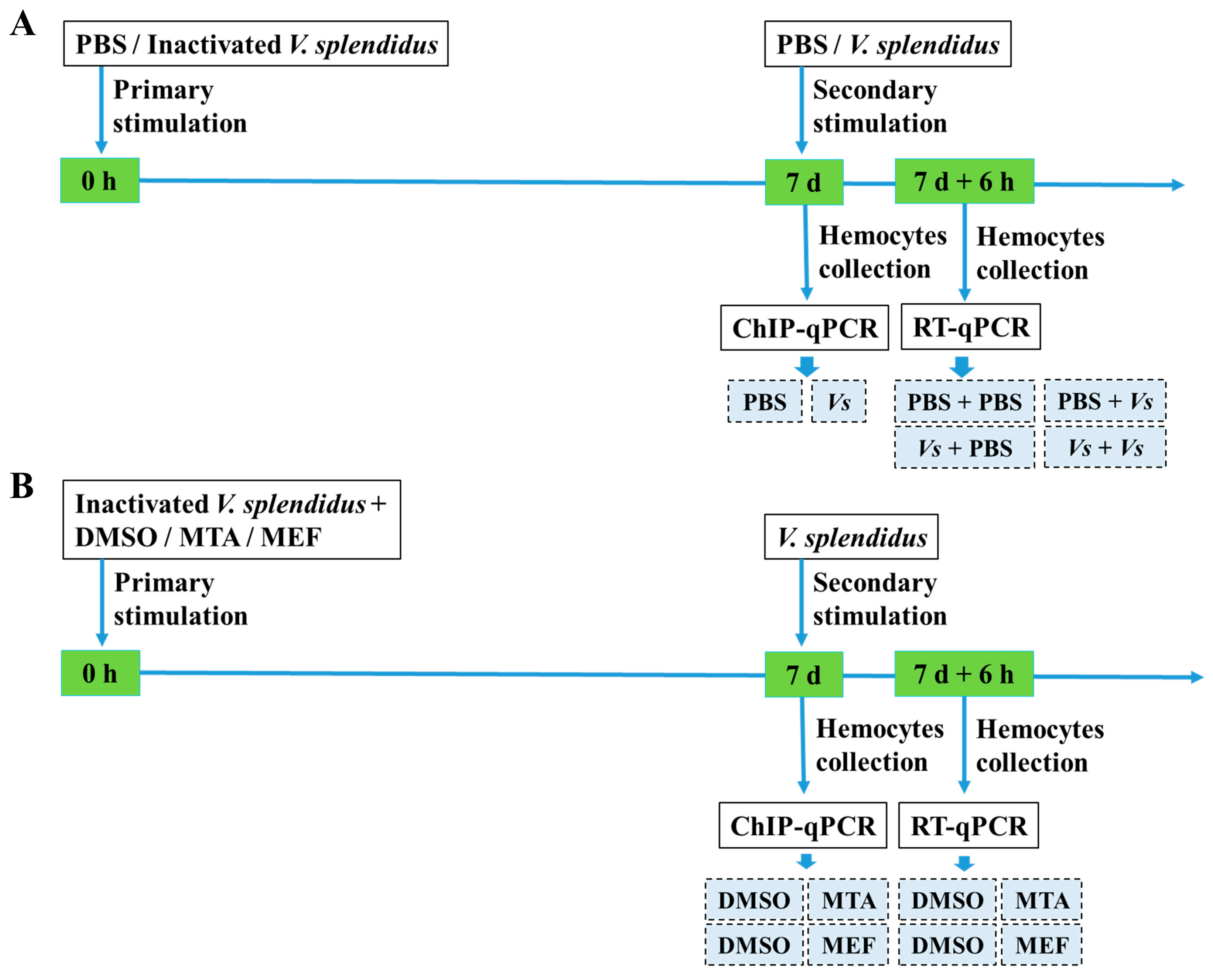
4.2. Immune Priming Induction and Hemocyte Collection
4.3. The Treatments of MTA and MEF
4.4. RNA Isolation and cDNA Synthesis
4.5. Reverse Transcription-Quantitative PCR (RT-qPCR) Analysis
4.6. ChIP-qPCR Assays
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Little, T.J.; Kraaijeveld, A.R. Ecological and evolutionary implications of immunological priming in invertebrates. Trends Ecol. Evol. 2004, 19, 58–60. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Tran, N.T.; Zhu, C.-H.; Yao, D.-F.; Aweya, J.J.; Gong, Y.; Ma, H.-Y.; Zhang, Y.-L.; Li, G.-L.; Li, S.-K. Immune priming in shellfish: A review and an updating mechanistic insight focused on cellular and humoral responses. Aquaculture 2021, 530, 735831. [Google Scholar] [CrossRef]
- Divangahi, M.; Aaby, P.; Khader, S.A.; Barreiro, L.B.; Bekkering, S.; Chavakis, T.; van Crevel, R.; Curtis, N.; DiNardo, A.R.; Dominguez-Andres, J.; et al. Trained immunity, tolerance, priming and differentiation: Distinct immunological processes. Nat. Immunol. 2021, 22, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Ottaviani, E. Invertebrate immunological memory: Could the epigenetic changes play the part of lymphocytes? Invertebr. Surviv. J. 2015, 12, 1–4. [Google Scholar]
- Melillo, D.; Marino, R.; Italiani, P.; Boraschi, D. Innate immune memory in invertebrate metazoans: A critical appraisal. Front. Immunol. 2018, 9, 1915. [Google Scholar] [CrossRef]
- Gourbal, B.; Pinaud, S.; Beckers, G.J.M.; Van Der Meer, J.W.M.; Conrath, U.; Netea, M.G. Innate immune memory: An evolutionary perspective. Immunol. Rev. 2018, 283, 21–40. [Google Scholar] [CrossRef]
- Zhang, Q.; Cao, X. Epigenetic regulation of the innate immune response to infection. Nat. Rev. Immunol. 2019, 19, 417–432. [Google Scholar] [CrossRef]
- Dominguez-Andres, J.; Fanucchi, S.; Joosten, L.A.B.; Mhlanga, M.M.; Netea, M.G. Advances in understanding molecular regulation of innate immune memory. Curr. Opin. Cell Biol. 2020, 63, 68–75. [Google Scholar] [CrossRef]
- Barski, A.; Cuddapah, S.; Cui, K.; Roh, T.Y.; Schones, D.E.; Wang, Z.; Wei, G.; Chepelev, I.; Zhao, K. High-resolution profiling of histone methylations in the human genome. Cell 2007, 129, 823–837. [Google Scholar] [CrossRef]
- Bernstein, B.E.; Kamal, M.; Lindblad-Toh, K.; Bekiranov, S.; Bailey, D.K.; Huebert, D.J.; McMahon, S.; Karlsson, E.K.; Kulbokas, E.J., 3rd; Gingeras, T.R.; et al. Genomic maps and comparative analysis of histone modifications in human and mouse. Cell 2005, 120, 169–181. [Google Scholar] [CrossRef]
- Foster, S.L.; Hargreaves, D.C.; Medzhitov, R. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature 2007, 447, 972–978. [Google Scholar] [CrossRef]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Joosten, L.A.; Ifrim, D.C.; Saeed, S.; Jacobs, C.; van Loenhout, J.; de Jong, D.; Stunnenberg, H.G.; et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc. Natl. Acad. Sci. USA 2012, 109, 17537–17542. [Google Scholar] [CrossRef]
- Arts, R.J.; Novakovic, B.; Ter Horst, R.; Carvalho, A.; Bekkering, S.; Lachmandas, E.; Rodrigues, F.; Silvestre, R.; Cheng, S.C.; Wang, S.Y.; et al. Glutaminolysis and fumarate accumulation integrate immunometabolic and epigenetic programs in trained immunity. Cell Metab. 2016, 24, 807–819. [Google Scholar] [CrossRef]
- Austenaa, L.; Barozzi, I.; Chronowska, A.; Termanini, A.; Ostuni, R.; Prosperini, E.; Stewart, A.F.; Testa, G.; Natoli, G. The histone methyltransferase Wbp7 controls macrophage function through GPI glycolipid anchor synthesis. Immunity 2012, 36, 572–585. [Google Scholar] [CrossRef]
- Netea, M.G.; Quintin, J.; van der Meer, J.W. Trained immunity: A memory for innate host defense. Cell Host Microbe 2011, 9, 355–361. [Google Scholar] [CrossRef]
- Vandenbon, A.; Kumagai, Y.; Lin, M.; Suzuki, Y.; Nakai, K. Waves of chromatin modifications in mouse dendritic cells in response to LPS stimulation. Genome Biol. 2018, 19, 138. [Google Scholar] [CrossRef]
- Bekkering, S.; Quintin, J.; Joosten, L.A.; van der Meer, J.W.; Netea, M.G.; Riksen, N.P. Oxidized low-density lipoprotein induces long-term proinflammatory cytokine production and foam cell formation via epigenetic reprogramming of monocytes. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1731–1738. [Google Scholar] [CrossRef]
- van der Heijden, C.; Groh, L.; Keating, S.T.; Kaffa, C.; Noz, M.P.; Kersten, S.; van Herwaarden, A.E.; Hoischen, A.; Joosten, L.A.B.; Timmers, H.; et al. Catecholamines induce trained immunity in monocytes in vitro and in vivo. Circ Res. 2020, 127, 269–283. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, Q.; Liu, Y.; Li, X.; Zhao, K.; Ding, Y.; Li, Z.; Shen, Q.; Wang, C.; Li, N.; et al. H3K4me3 demethylase Kdm5a is required for NK cell activation by associating with p50 to suppress SOCS1. Cell Rep. 2016, 15, 288–299. [Google Scholar] [CrossRef]
- Quintin, J.; Saeed, S.; Martens, J.H.A.; Giamarellos-Bourboulis, E.J.; Ifrim, D.C.; Logie, C.; Jacobs, L.; Jansen, T.; Kullberg, B.J.; Wijmenga, C.; et al. Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe 2012, 12, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Takeda, K.; Akira, S. TIR domain-containing adaptors define the specificity of TLR signaling. Mol. Immunol. 2004, 40, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Verstrepen, L.; Bekaert, T.; Chau, T.L.; Tavernier, J.; Chariot, A.; Beyaert, R. TLR-4, IL-1R and TNF-R signaling to NF-kappaB: Variations on a common theme. Cell Mol. Life Sci. 2008, 65, 2964–2978. [Google Scholar] [CrossRef]
- Pasare, C.; Medzhitov, R. Toll-like receptors and acquired immunity. Semin. Immunol. 2004, 16, 23–26. [Google Scholar] [CrossRef]
- Davis, F.M.; Kimball, A.; denDekker, A.; Joshi, A.D.; Boniakowski, A.E.; Nysz, D.; Allen, R.M.; Obi, A.; Singer, K.; Henke, P.K.; et al. Histone methylation directs myeloid TLR4 expression and regulates wound healing following cutaneous tissue injury. J. Immunol. 2019, 202, 1777–1785. [Google Scholar] [CrossRef] [PubMed]
- Piatek, P.; Tarkowski, M.; Namiecinska, M.; Riva, A.; Wieczorek, M.; Michlewska, S.; Dulska, J.; Domowicz, M.; Kulinska-Michalska, M.; Lewkowicz, N.; et al. H3K4me3 histone ChIP-Seq analysis reveals molecular mechanisms responsible for neutrophil dysfunction in HIV-infected individuals. Front. Immunol. 2021, 12, 682094. [Google Scholar] [CrossRef]
- Escoubet-Lozach, L.; Benner, C.; Kaikkonen, M.U.; Lozach, J.; Heinz, S.; Spann, N.J.; Crotti, A.; Stender, J.; Ghisletti, S.; Reichart, D.; et al. Mechanisms establishing TLR4-responsive activation states of inflammatory response genes. PLoS Genet. 2011, 7, e1002401. [Google Scholar] [CrossRef] [PubMed]
- Jeljeli, M.M.; Adamopoulos, I.E. Innate immune memory in inflammatory arthritis. Nat. Rev. Rheumatol. 2023, 19, 627–639. [Google Scholar] [CrossRef]
- Moorlag, S.; Roring, R.J.; Joosten, L.A.B.; Netea, M.G. The role of the interleukin-1 family in trained immunity. Immunol. Rev. 2018, 281, 28–39. [Google Scholar] [CrossRef]
- Huang, X.D.; Zhang, H.; He, M.X. Comparative and evolutionary analysis of the interleukin 17 gene family in invertebrates. PLoS ONE 2015, 10, e0132802. [Google Scholar] [CrossRef]
- Rosani, U.; Varotto, L.; Gerdol, M.; Pallavicini, A.; Venier, P. IL-17 signaling components in bivalves: Comparative sequence analysis and involvement in the immune responses. Dev. Comp. Immunol. 2015, 52, 255–268. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Zhang, Y.; Xiang, Z.; Tong, Y.; Qu, F.; Yu, Z. Genomic characterization and expression analysis of five novel IL-17 genes in the Pacific oyster, Crassostrea gigas. Fish Shellfish Immunol. 2014, 40, 455–465. [Google Scholar] [CrossRef]
- Roberts, S.; Gueguen, Y.; de Lorgeril, J.; Goetz, F. Rapid accumulation of an interleukin 17 homolog transcript in Crassostrea gigas hemocytes following bacterial exposure. Dev. Comp. Immunol. 2008, 32, 1099–1104. [Google Scholar] [CrossRef]
- Xin, L.; Zhang, H.; Du, X.; Li, Y.; Li, M.; Wang, L.; Wang, H.; Qiu, L.; Song, L. The systematic regulation of oyster CgIL17-1 and CgIL17-5 in response to air exposure. Dev. Comp. Immunol. 2016, 63, 144–155. [Google Scholar] [CrossRef]
- Cao, W.; Wang, W.; Fan, S.; Li, J.; Li, Q.; Wu, S.; Wang, L.; Song, L. The receptor CgIL-17R1 expressed in granulocytes mediates the CgIL-17 induced haemocytes proliferation in Crassostrea gigas. Dev. Comp. Immunol. 2022, 131, 104376. [Google Scholar] [CrossRef]
- Wang, W.; Wang, L.; Liu, Z.; Song, X.; Yi, Q.; Yang, C.; Song, L. The involvement of TLR signaling and anti-bacterial effectors in enhanced immune protection of oysters after Vibrio splendidus pre-exposure. Dev. Comp. Immunol. 2020, 103, 103498. [Google Scholar] [CrossRef]
- Bachère, E.; Rosa, R.D.; Schmitt, P.; Poirier, A.C.; Merou, N.; Charrière, G.M.; Destoumieux-Garzón, D. The new insights into the oyster antimicrobial defense: Cellular, molecular and genetic view. Fish Shellfish Immunol. 2015, 46, 50–64. [Google Scholar] [CrossRef]
- Allam, B.; Raftos, D. Immune responses to infectious diseases in bivalves. J. Invertebr. Pathol. 2015, 131, 121–136. [Google Scholar] [CrossRef]
- Green, T.J.; Helbig, K.; Speck, P.; Raftos, D.A. Primed for success: Oyster parents treated with poly(I:C) produce offspring with enhanced protection against Ostreid herpesvirus type I infection. Mol. Immunol. 2016, 78, 113–120. [Google Scholar] [CrossRef]
- Green, T.J.; Speck, P. Antiviral defense and innate immune memory in the oyster. Viruses 2018, 10, 133. [Google Scholar] [CrossRef]
- Lafont, M.; Vergnes, A.; Vidal-Dupiol, J.; de Lorgeril, J.; Gueguen, Y.; Haffner, P.; Petton, B.; Chaparro, C.; Barrachina, C.; Destoumieux-Garzon, D.; et al. A sustained immune response supports long-term antiviral immune priming in the Pacific oyster, Crassostrea gigas. mBio 2020, 11, e02777-19. [Google Scholar] [CrossRef]
- Zhang, T.; Qiu, L.; Sun, Z.; Wang, L.; Zhou, Z.; Liu, R.; Yue, F.; Sun, R.; Song, L. The specifically enhanced cellular immune responses in Pacific oyster (Crassostrea gigas) against secondary challenge with Vibrio splendidus. Dev. Comp. Immunol. 2014, 45, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, X.; Yu, F.; Xiang, Z.; Li, J.; Thorpe, K.L.; Yu, Z. Characteristic and functional analysis of toll-like receptors (TLRs) in the lophotrocozoan, Crassostrea gigas, reveals ancient origin of TLR-mediated innate immunity. PLoS ONE 2013, 8, e76464. [Google Scholar]
- Gerdol, M.; Venier, P.; Edomi, P.; Pallavicini, A. Diversity and evolution of TIR-domain-containing proteins in bivalves and Metazoa: New insights from comparative genomics. Dev. Comp. Immunol. 2017, 70, 145–164. [Google Scholar] [CrossRef] [PubMed]
- Sang, X.; Dong, J.; Chen, F.; Wei, L.; Liu, Y.; Zhang, M.; Huang, B.; Wang, X. Molecular cloning and immune function study of an oyster IκB gene in the NF-κB signaling pathway. Aquaculture 2020, 525, 735322. [Google Scholar] [CrossRef]
- Chen, H.; Cai, X.; Li, R.; Wu, Y.; Qiu, H.; Zheng, J.; Zhou, D.; Fang, J.; Wu, X. A novel toll-like receptor from Crassostrea gigas is involved in innate immune response to Vibrio alginolyticus. Infect. Genet. Evol. 2022, 97, 105159. [Google Scholar] [CrossRef]
- Li, Y.; Sun, J.; Zhang, Y.; Wang, M.; Wang, L.; Song, L. CgRel involved in antibacterial immunity by regulating the production of CgIL17s and CgBigDef1 in the Pacific oyster Crassostrea gigas. Fish Shellfish Immunol. 2020, 97, 474–482. [Google Scholar] [CrossRef]
- Buchmann; Kurt, Evolution of innate immunity: Clues from invertebrates via fish to mammals. Front. Immunol. 2014, 5, 459.
- Hellinga, A.H.; Tsallis, T.; Eshuis, T.; Triantis, V.; Ulfman, L.H.; Neerven, R. In vitro induction of trained innate immunity by bIgG and whey protein extracts. Int. J. Mol. Sci. 2020, 21, 9077. [Google Scholar] [CrossRef]
- Saeed, S.; Quintin, J.; Kerstens, H.H.; Rao, N.A.; Aghajanirefah, A.; Matarese, F.; Cheng, S.C.; Ratter, J.; Berentsen, K.; van der Ent, M.A.; et al. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science 2014, 345, 1251086. [Google Scholar] [CrossRef]
- Gao, D.; Qiu, L.; Gao, Q.; Hou, Z.; Wang, L.; Song, L. Repertoire and evolution of TNF superfamily in Crassostrea gigas: Implications for expansion and diversification of this superfamily in Mollusca. Dev. Comp. Immunol. 2015, 51, 251–260. [Google Scholar] [CrossRef]
- Wang, P.H.; He, J.G. Nucleic Acid Sensing in Invertebrate Antiviral Immunity. Int. Rev. Cell Mol. Biol. 2019, 345, 287–360. [Google Scholar]
- Huang, S.; Yuan, S.; Guo, L.; Yu, Y.; Li, J.; Wu, T.; Liu, T.; Yang, M.; Wu, K.; Liu, H.; et al. Genomic analysis of the immune gene repertoire of amphioxus reveals extraordinary innate complexity and diversity. Genome Res. 2008, 18, 1112–1126. [Google Scholar] [CrossRef]
- Thomas, C. Hemocytes: Forms and functions. East. Oyster Crassostrea Virginica 1996, 1, 75–93. [Google Scholar]
- Mu, D.; Yang, J.; Jiang, Y.; Wang, Z.; Chen, W.; Huang, J.; Zhang, Y.; Liu, Q.; Yang, D. Single-cell transcriptomic analysis reveals neutrophil as orchestrator during β-glucan-induced trained immunity in a teleost fish. J. Immunol. 2022, 209, 783–795. [Google Scholar] [CrossRef]
- Mandraju, R.; Troutman, T.D.; Pasare, C. Toll-like receptor function and signaling. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Blumenthal, A.; Feng, H.; Zhang, Y.-B.; Gui, J.-F.; Lemon, S.M.; Yamane, D. Interferon regulatory factor 1 (IRF1) and anti-pathogen innate immune responses. PLoS Pathog. 2021, 17, e1009220. [Google Scholar]
- Ren, Y.; Ding, D.; Pan, B.; Bu, W. The TLR13-MyD88-NF-κB signalling pathway of Cyclina sinensis plays vital roles in innate immune responses. Fish Shellfish. Immunol. 2017, 70, 720–730. [Google Scholar] [CrossRef]
- Yu, F.; Chen, J.; Lin, J.; Zhong, Z.; Lu, Y.; Zeng, X.; Lei, X. TLR4 involved in immune response against Vibrio Parahaemolyticus by MyD88-dependent pathway in Crassostrea hongkongensis. Fish Shellfish Immunol. 2023, 134, 108591. [Google Scholar] [CrossRef]
- Fan, S.; Wang, W.; Li, J.; Cao, W.; Li, Q.; Wu, S.; Wang, L.; Song, L. The truncated MyD88s negatively regulates TLR2 signal on expression of IL17-1 in oyster Crassostrea gigas. Dev. Comp. Immunol. 2022, 133, 104446. [Google Scholar] [CrossRef]
- Liu, J.; Wang, W.; Kong, N.; Yu, S.; Dong, M.; Yang, W.; Li, Y.; Zhou, X.; Wang, L.; Song, L. A pattern recognition receptor CgTLR3 involves in regulating the proliferation of haemocytes in oyster Crassostrea gigas. Dev. Comp. Immunol. 2023, 147, 104762. [Google Scholar] [CrossRef]
- Hua, Z.; Hou, B. TLR signaling in B-cell development and activation. Cell Mol. Immunol. 2013, 10, 103–106. [Google Scholar] [CrossRef]
- Reis e Sousa, C. Toll-like receptors and dendritic cells: For whom the bug tolls. Semin. Immunol. 2004, 16, 27–34. [Google Scholar] [CrossRef]
- Mills, K.H. TLR-dependent T cell activation in autoimmunity. Nat. Rev. Immunol. 2011, 11, 807–822. [Google Scholar] [CrossRef]
- Lahiri, A.; Das, P.; Chakravortty, D. Engagement of TLR signaling as adjuvant: Towards smarter vaccine and beyond. Vaccine 2008, 26, 6777–6783. [Google Scholar] [CrossRef] [PubMed]
- Pham, L.N.; Dionne, M.S.; Shirasu-Hiza, M.; Schneider, D.S. A specific primed immune response in Drosophila is dependent on phagocytes. PLoS Pathog. 2007, 3, e26. [Google Scholar] [CrossRef]
- Zhang, X.; Zeng, X.; Sun, Y.; Wang, Y.; Zhang, Z. Enhanced immune protection of mud crab Scylla paramamosain in response to the secondary challenge by Vibrio parahaemolyticus. Front. Immunol. 2020, 11, 565958. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, M.; Sun, Y.; Wang, Y.; Zhang, Z. Transcriptomic analysis and discovery of genes involving in enhanced immune protection of Pacific abalone (Haliotis discus hannai) in response to the re-infection of Vibrio parahaemolyticus. Fish Shellfish Immunol. 2022, 125, 128–140. [Google Scholar] [CrossRef]
- Willis, A.R.; Sukhdeo, R.; Reinke, A.W. Remembering your enemies: Mechanisms of within-generation and multigenerational immune priming in Caenorhabditis elegans. FEBS J. 2020, 288, 1759–1770. [Google Scholar] [CrossRef]
- Low, C.F.; Chong, C.M. Peculiarities of innate immune memory in crustaceans. Fish Shellfish Immunol. 2020, 104, 605–612. [Google Scholar] [CrossRef]
- Gomes, F.M.; Tyner, M.D.W.; Barletta, A.B.F.; Saha, B.; Yenkoidiok-Douti, L.; Canepa, G.E.; Molina-Cruz, A.; Barillas-Mury, C. Double peroxidase and histone acetyltransferase AgTip60 maintain innate immune memory in primed mosquitoes. Proc. Natl. Acad. Sci. USA 2021, 118, e2114242118. [Google Scholar] [CrossRef]
- Amarante, A.M.; da Silva, I.C.A.; Carneiro, V.C.; Vicentino, A.R.R.; Pinto, M.A.; Higa, L.M.; Moharana, K.C.; Talyuli, O.A.C.; Venancio, T.M.; de Oliveira, P.L.; et al. Zika virus infection drives epigenetic modulation of immunity by the histone acetyltransferase CBP of Aedes aegypti. PLoS Negl. Trop. Dis. 2022, 16, e0010559. [Google Scholar] [CrossRef] [PubMed]
- Schafer, A.; Baric, R.S. Epigenetic landscape during coronavirus infection. Pathogens 2017, 6, 8. [Google Scholar] [CrossRef]
- Davis, F.M.; denDekker, A.; Kimball, A.; Joshi, A.D.; El Azzouny, M.; Wolf, S.J.; Obi, A.T.; Lipinski, J.; Gudjonsson, J.E.; Xing, X.; et al. Epigenetic regulation of TLR4 in diabetic macrophages modulates immunometabolism and wound repair. J. Immunol. 2020, 204, 2503–2513. [Google Scholar] [CrossRef]
- Fellous, A.; Favrel, P.; Guo, X.; Riviere, G. The Jumonji gene family in Crassostrea gigas suggests evolutionary conservation of Jmj-C histone demethylases orthologues in the oyster gametogenesis and development. Gene 2014, 538, 164–175. [Google Scholar] [CrossRef]
- Gu, X.; Qiao, X.; Yu, S.; Song, X.; Wang, L.; Song, L. Histone lysine-specific demethylase 1 regulates the proliferation of hemocytes in the oyster Crassostrea gigas. Front. Immunol. 2022, 13, 1088149. [Google Scholar] [CrossRef] [PubMed]
- Fellous, A.; Le Franc, L.; Jouaux, A.; Goux, D.; Favrel, P.; Rivière, G. Histone methylation participates in gene expression control during the early development of the Pacific oyster Crassostrea gigas. Genes 2019, 10, 695. [Google Scholar] [CrossRef] [PubMed]
- Armitage, S.A.; Peuss, R.; Kurtz, J. Dscam and pancrustacean immune memory—A review of the evidence. Dev. Comp. Immunol. 2015, 48, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Song, C.; Ning, J.; Liu, Y.; Cui, Z. Identification, functional characterization and the potential role of variable lymphocyte receptor EsVLRA from Eriocheir sinensis in response to secondary challenge after Vibrio parahaemolyticus vaccine. Fish Shellfish Immunol. 2020, 98, 201–209. [Google Scholar] [CrossRef]
- Pinaud, S.; Portela, J.; Duval, D.; Nowacki, F.C.; Olive, M.A.; Allienne, J.F.; Galinier, R.; Dheilly, N.M.; Kieffer-Jaquinod, S.; Mitta, G.; et al. A shift from cellular to humoral responses contributes to innate immune memory in the vector snail Biomphalaria glabrata. PLoS Pathog. 2016, 12, e1005361. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Yang, C.; Jiang, Q.; Zhang, H.; Yue, F.; Huang, M.; Sun, Z.; Song, L. The response of mRNA expression upon secondary challenge with Vibrio anguillarum suggests the involvement of C-lectins in the immune priming of scallop Chlamys farreri. Dev. Comp. Immunol. 2013, 40, 142–147. [Google Scholar] [CrossRef]
- Schulenburg, H.; Boehnisch, C.; Michiels, N.K. How do invertebrates generate a highly specific innate immune response? Mol. Immunol. 2007, 44, 3338–3344. [Google Scholar] [CrossRef]
- Chang, Y.-H.; Kumar, R.; Ng, T.H.; Wang, H.-C. What vaccination studies tell us about immunological memory within the innate immune system of cultured shrimp and crayfish. Dev. Comp. Immunol. 2018, 80, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Lafont, M.; Petton, B.; Vergnes, A.; Pauletto, M.; Segarra, A.; Gourbal, B.; Montagnani, C. Long-lasting antiviral innate immune priming in the Lophotrochozoan Pacific oyster, Crassostrea gigas. Sci. Rep. 2017, 7, 13143. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Qiu, L.; Yu, Z.; Zi, J.; Yue, F.; Wang, L.; Zhang, H.; Teng, W.; Liu, X.; Song, L. Identification and characterisation of pathogenic Vibrio splendidus from Yesso scallop (Patinopecten yessoensis) cultured in a low temperature environment. J. Invertebr. Pathol. 2013, 114, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhu, Q.; Li, L.; Wang, W.; Zhang, G. Identification of HSF1 Target Genes Involved in Thermal Stress in the Pacific Oyster Crassostrea gigas by ChIP-seq. Mar. Biotechnol. 2020, 22, 167–179. [Google Scholar] [CrossRef]
- Moreno, B.; Hevia, H.; Santamaria, M.; Sepulcre, J.; Munoz, J.; Garcia-Trevijano, E.R.; Berasain, C.; Corrales, F.J.; Avila, M.A.; Villoslada, P. Methylthioadenosine reverses brain autoimmune disease. Ann. Neurol. 2006, 60, 323–334. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]

| Primer | Sequence (5′-3′) |
|---|---|
| RT-qPCR primers | |
| CgTLR3-RT-F | TGCCAAAAGCAAATGGTGTGAAT |
| CgTLR3-RT-R | TTTCCCCCAAAACAAACTTCGTC |
| CgMyD88-2-RT-F | CAGATAAACCGCTACGACGCCTA |
| CgMyD88-2-RT-R | ATTTCCGATTCCTTTTGGTGGTC |
| CgRel1-RT-F | TCCGCACACCACCTTACAA |
| CgRel1-RT-R | CGCCTTTATCTTCAGCCTCT |
| CgIL17-1-RT-F | GCGAACGCCACAGTGTCAAA |
| CgIL17-1-RT-R | GACGCTACGAGGAAATACGGAC |
| CgEF-RT-F | AGTCACCAAGGCTGCACAGAAAG |
| CgEF-RT-R | TCCGACGTATTTCTTTGCGATGT |
| ChIP-qPCR primers | |
| CgTLR3-Pro-F1 | CAACATGAATCTCAGCAGACG |
| CgTLR3-Pro-R1 | TTCTTCCCAAACTGCCACA |
| CgTLR3-Pro-F2 | AAGAAGGGGGAGGAGTGCT |
| CgTLR3-Pro-R2 | ATGTGTCTTTAAAAGCCGGTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lian, X.; Li, Y.; Wang, W.; Zuo, J.; Yu, T.; Wang, L.; Song, L. The Modification of H3K4me3 Enhanced the Expression of CgTLR3 in Hemocytes to Increase CgIL17-1 Production in the Immune Priming of Crassostrea gigas. Int. J. Mol. Sci. 2024, 25, 1036. https://doi.org/10.3390/ijms25021036
Lian X, Li Y, Wang W, Zuo J, Yu T, Wang L, Song L. The Modification of H3K4me3 Enhanced the Expression of CgTLR3 in Hemocytes to Increase CgIL17-1 Production in the Immune Priming of Crassostrea gigas. International Journal of Molecular Sciences. 2024; 25(2):1036. https://doi.org/10.3390/ijms25021036
Chicago/Turabian StyleLian, Xingye, Yinan Li, Weilin Wang, Jiajun Zuo, Tianqi Yu, Lingling Wang, and Linsheng Song. 2024. "The Modification of H3K4me3 Enhanced the Expression of CgTLR3 in Hemocytes to Increase CgIL17-1 Production in the Immune Priming of Crassostrea gigas" International Journal of Molecular Sciences 25, no. 2: 1036. https://doi.org/10.3390/ijms25021036






