Biological Properties of Extracts Obtained from In Vitro Culture of Plectranthus scutellarioides in a Cell Model
Abstract
1. Introduction
2. Results
2.1. Aerial Parts and Roots of Plectranthus scutellarioides in In Vitro Culture
2.2. Preliminary Phytochemical Screening of the Aerial Parts and Roots of Plectranthus scutellarioides Extracts
2.3. The Cytotoxic Effect of the Aerial Parts and Roots of Plectranthus scutellarioides Extracts on Breast Cancer Cells (MCF-7), Human Lung Adenocarcinoma Cells (A549) and Normal Human Gingival Fibroblasts (HGF-1)
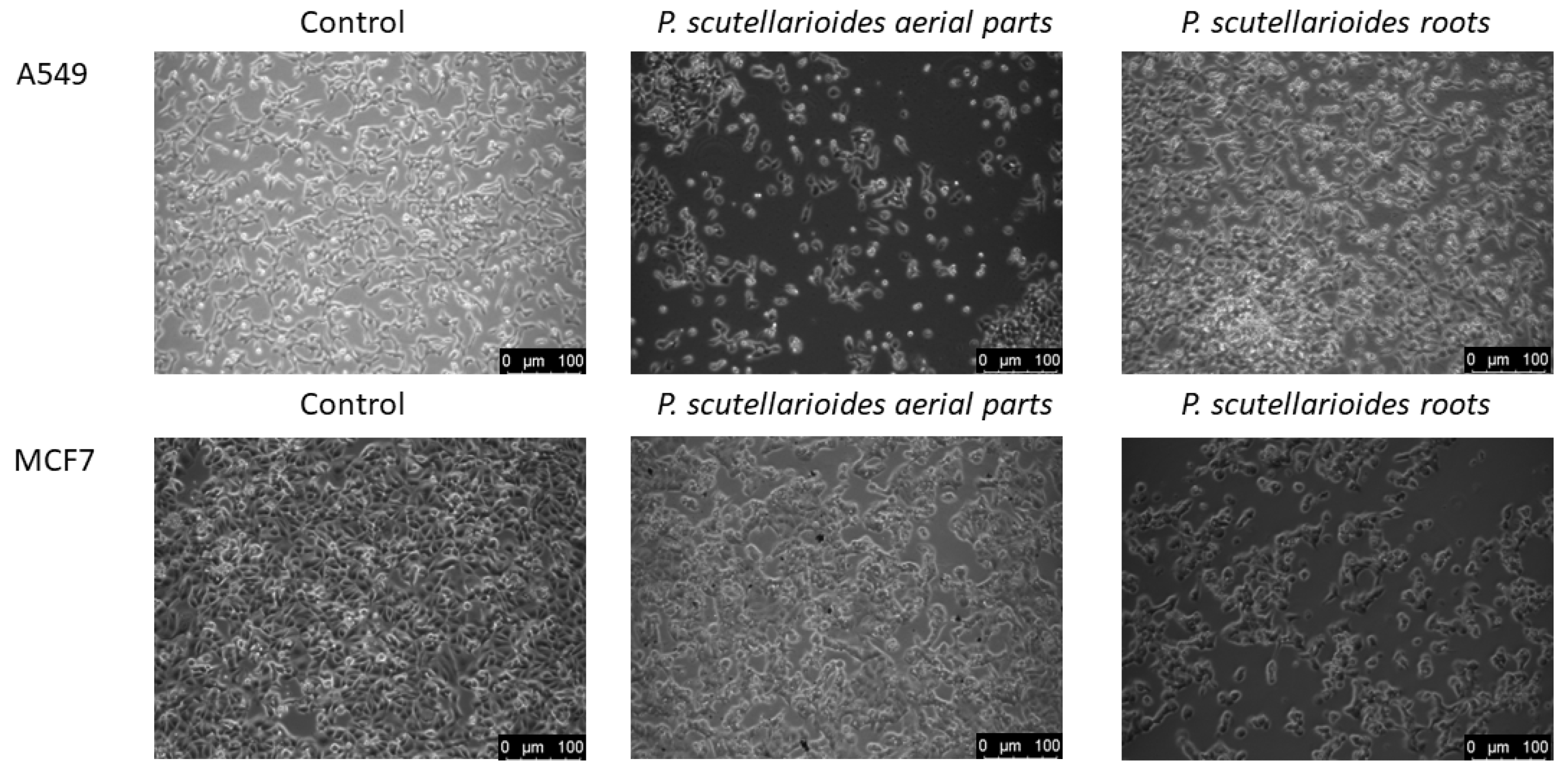
2.4. Morphological Observations under Microscopy after Treatment with the Aerial Part and Root of Plectranthus scutellarioides
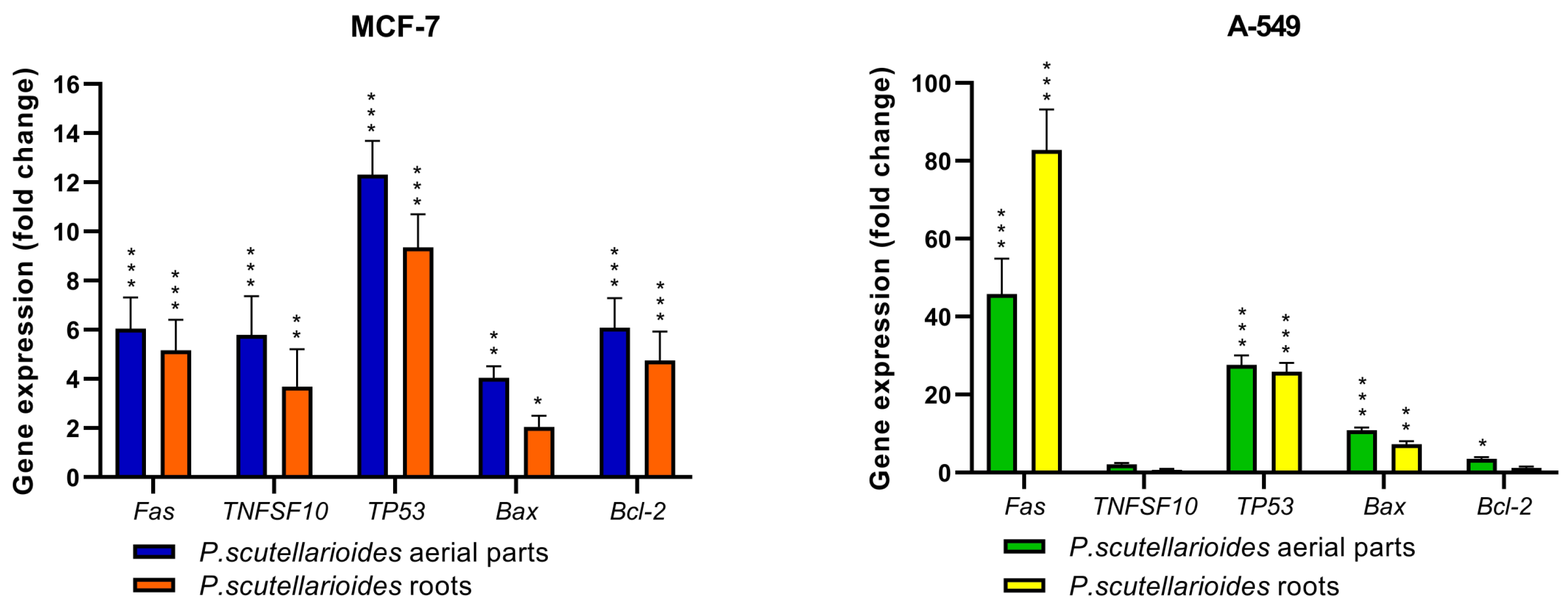
2.5. Apoptotic Genes Expressed following Treatment with Plectranthus scutellarioides Aerial and Root Extracts
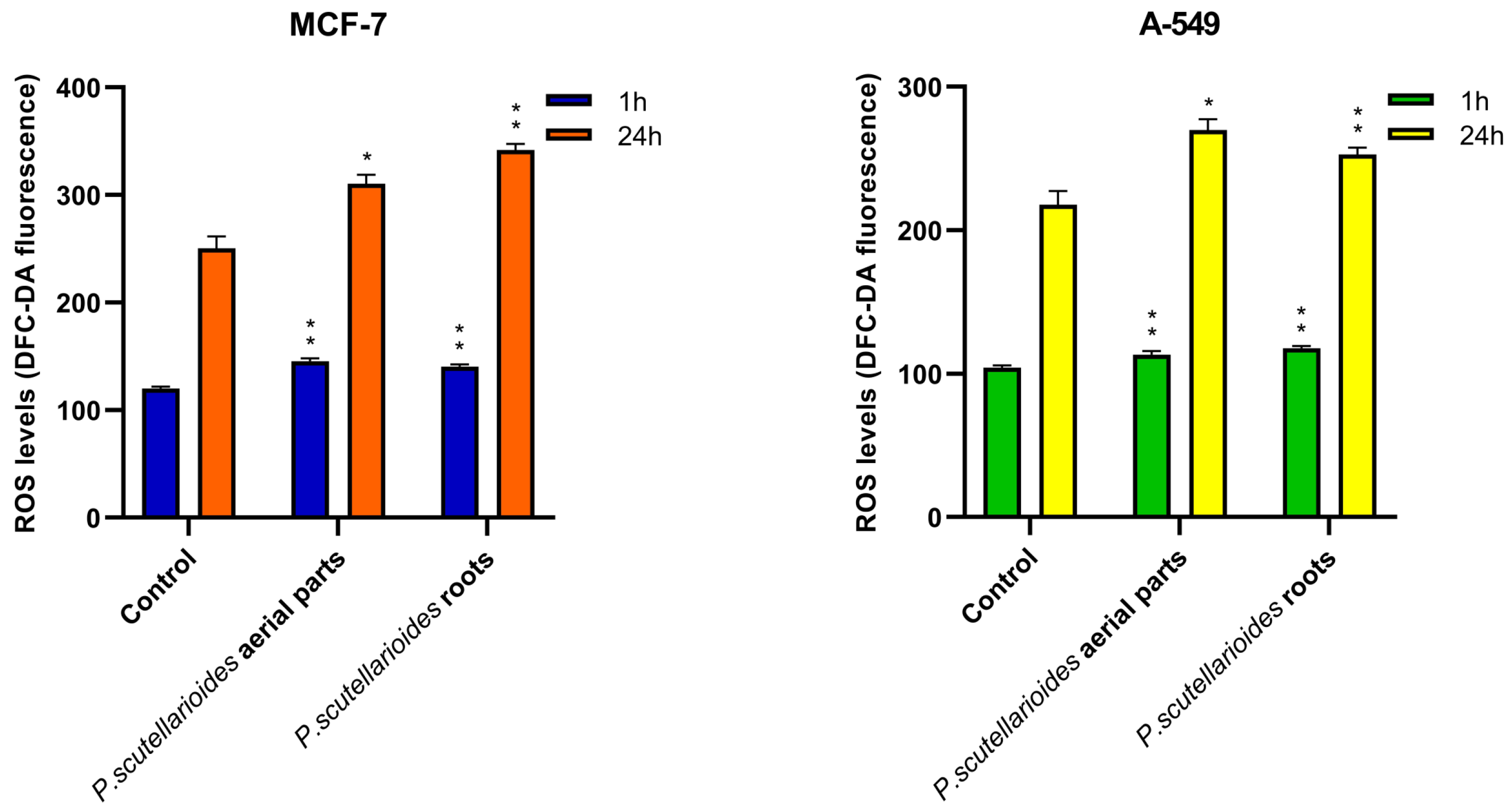
2.6. Measurement of ROS Generation
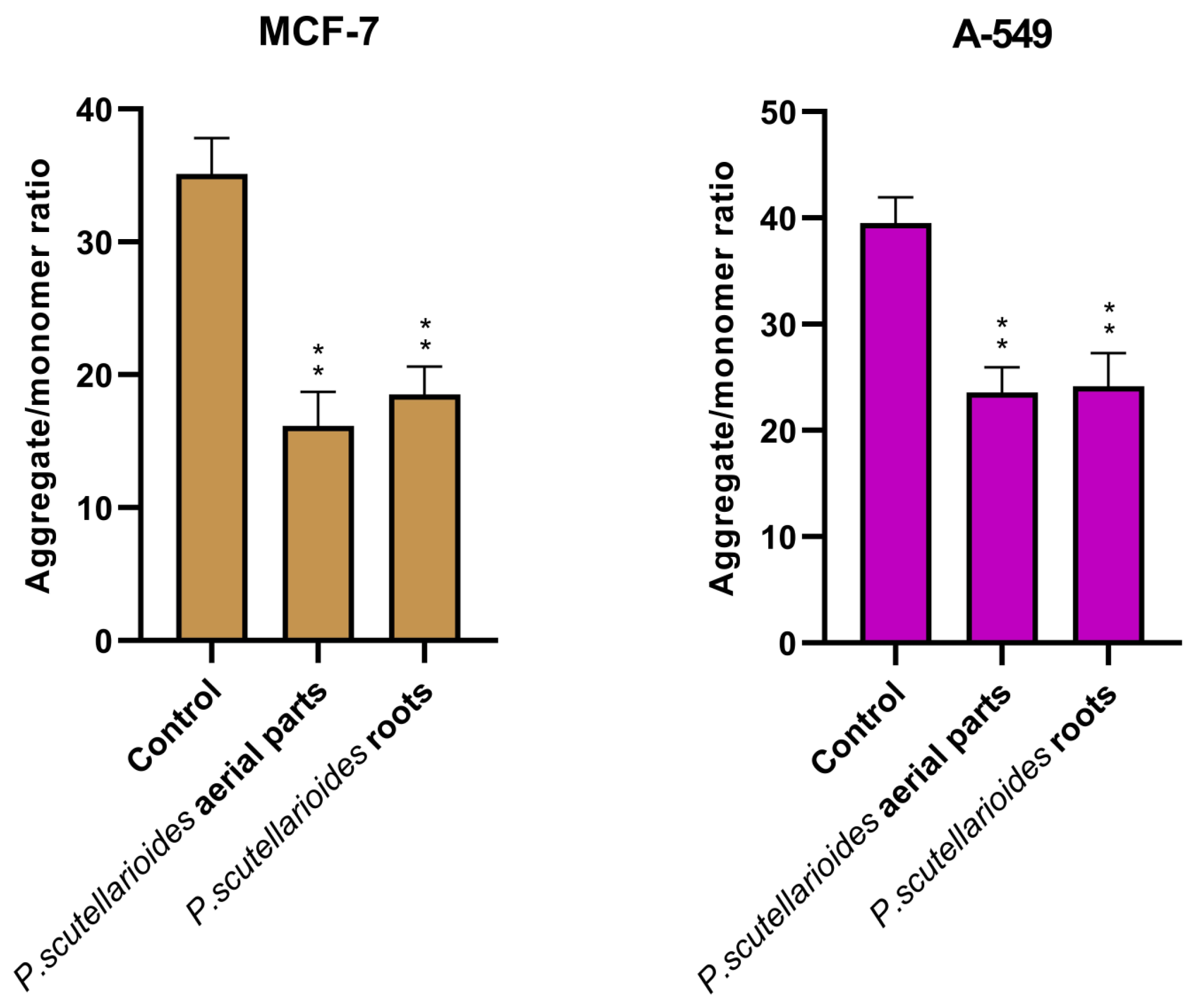
2.7. Measurement of Mitochondrial Membrane Potential (MMP)
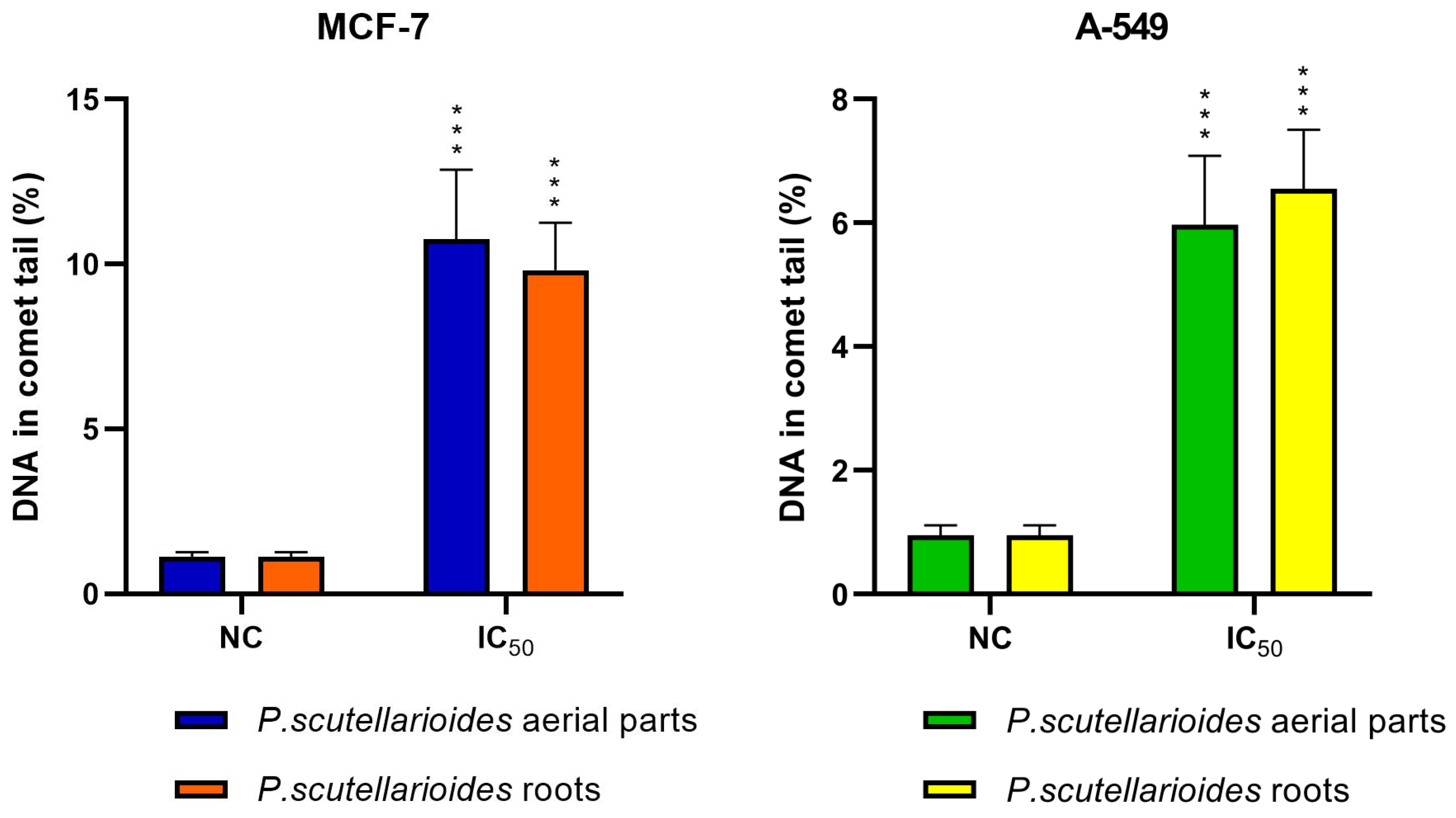
2.8. Measurement of DNA Double Strand Breaks by Comet Assay
2.9. Biocompatibility Studies
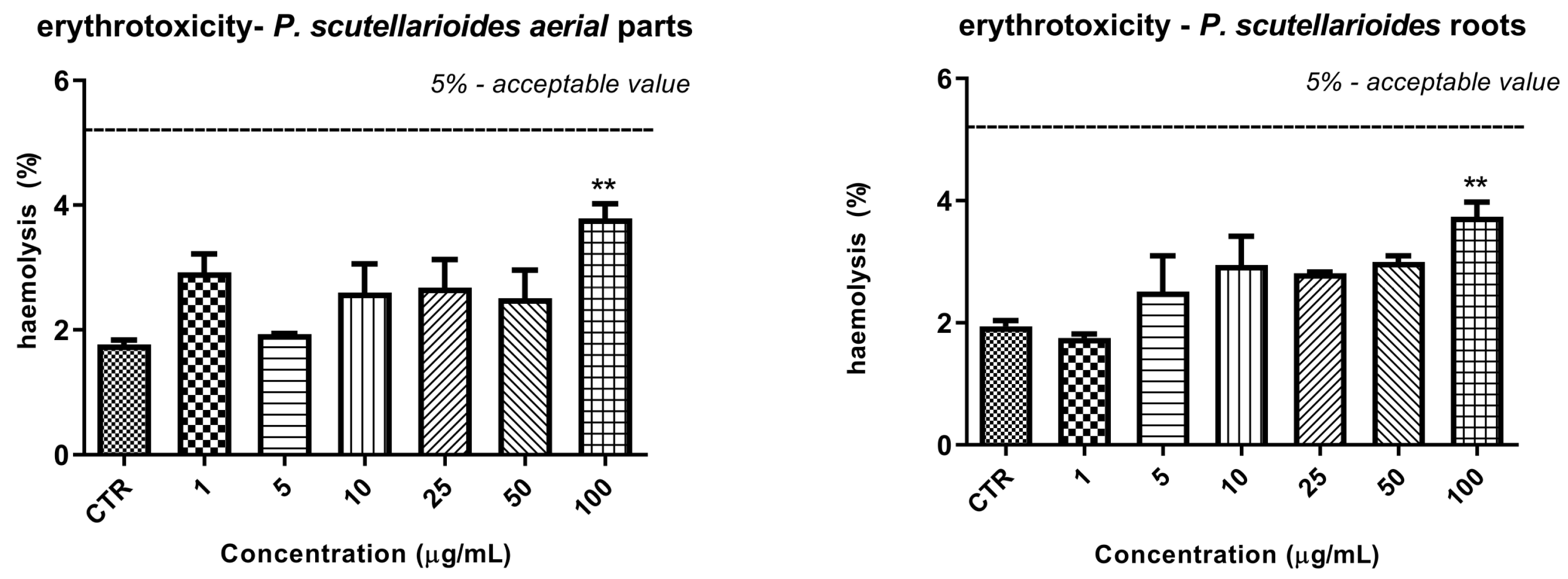
2.10. The Effect of Plectranthus scutellarioides Aerial Part and Root Extracts on RBC Hemolysis
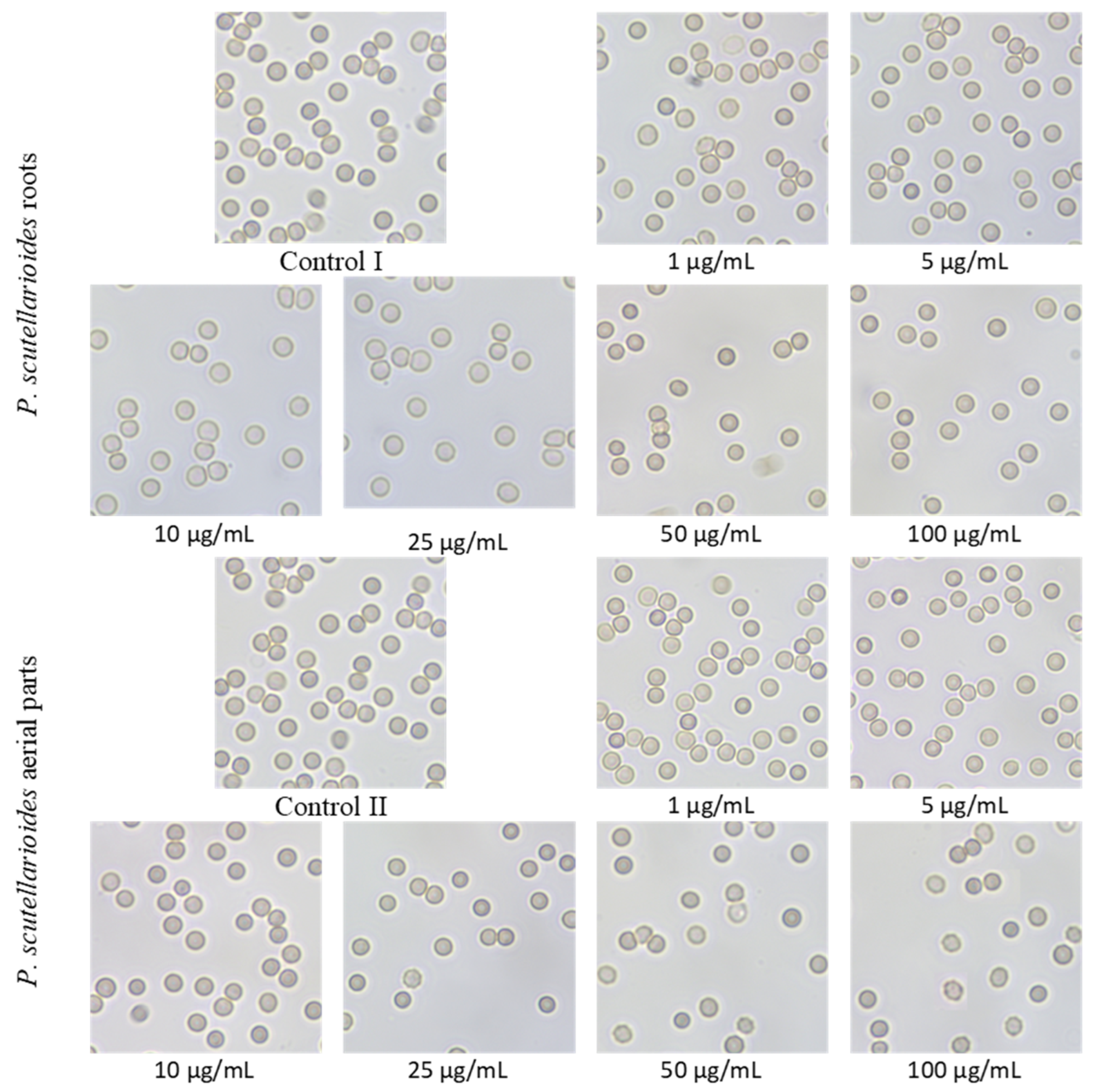
2.11. Microscopic Observations after the Effect of Plectranthus scutellarioides Aerial Part and P. scutellarioides Root Extracts on RBCs
2.12. Effects of Plectranthus scutellarioides Root and Plectranthus scutellarioides Aerial Part Extracts on Parameters of White Thrombus Formation Process
3. Discussion
4. Materials and Methods
4.1. Initiation of Plectranthus scutellarioides in In Vitro Cultures
4.2. Preparation of Aerial Part and Root Extracts of Plectranthus scutellarioides
4.3. Compositional Analyses by HPLC-ESI-QTOF-MS/MS
4.4. Cell Cultures
4.5. Cytotoxicity Effect
4.6. Microscopic Observation
4.7. Isolation of RNA from Cell Lines and Transcription into cDNA
4.8. Gene Expression
4.9. Genotoxicity Assay (Comet Assay)
4.10. Intracellular ROS Measurement
4.11. Assay for the Mitochondrial Membrane Potential (JC-1 Assay)
4.12. Biocompatibility Studies
4.12.1. Biological Materials
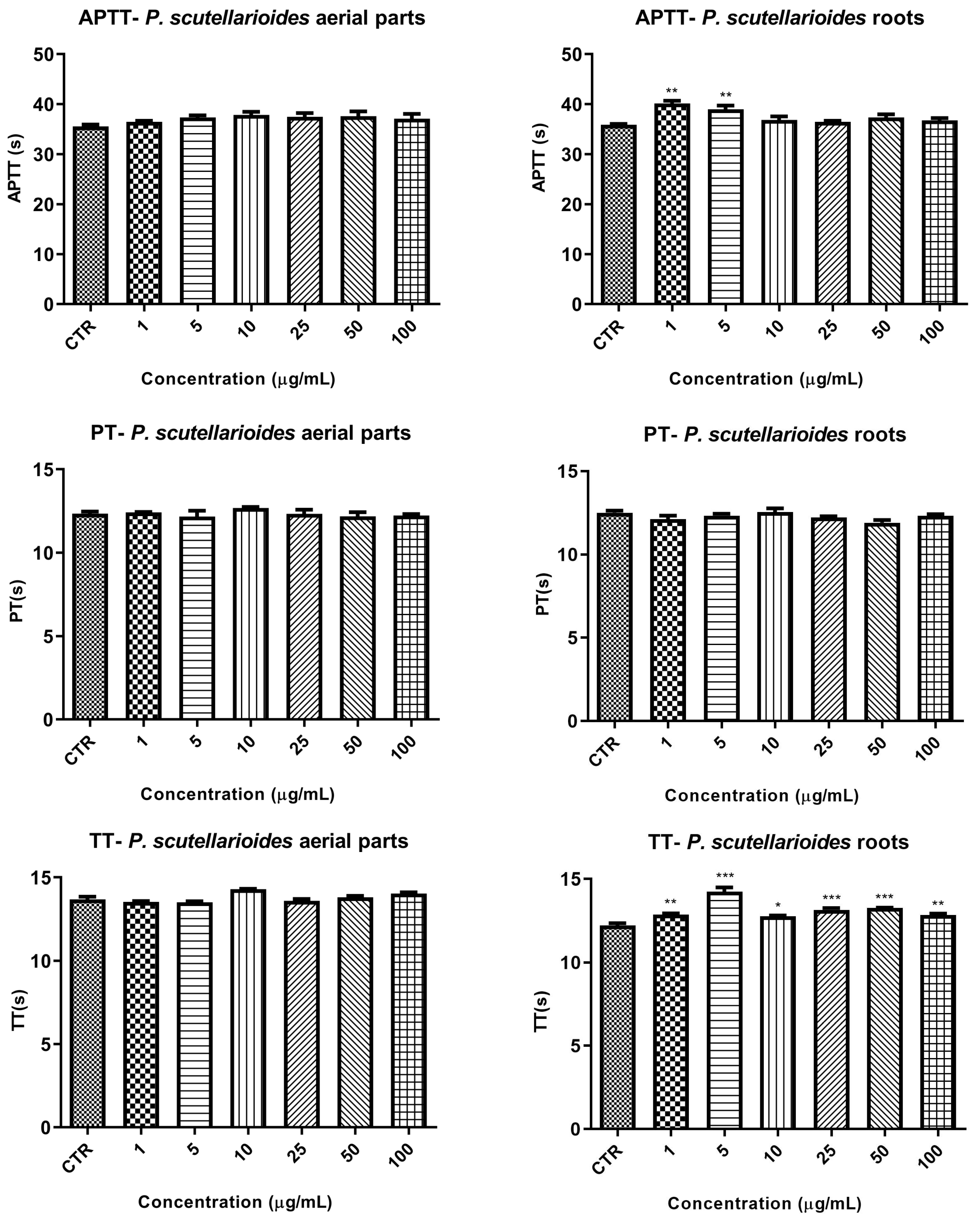
4.12.2. Basic Coagulation Tests: PT, APTT, and TT
4.12.3. Erythrotoxicity
4.12.4. Red Blood Cell Morphology
4.12.5. Total Thrombus-Formation Analysis System (T-TAS)
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Teoh, E.S. Secondary Metabolites of Plants. In Medicinal Orchids of Asia; Springer: Cham, Switzerland, 2015; Volume 5, pp. 59–73. [Google Scholar] [CrossRef]
- Seca, A.M.L.; Pinto, D.C.G.A. Biological Potential and Medical Use of Secondary Metabolites. Medicines 2019, 6, 66. [Google Scholar] [CrossRef] [PubMed]
- Hussein, R.A.; El-Anssary, A.A. Plants Secondary Metabolites: The Key Drivers of the Pharmacological Actions of Medicinal Plants. In Herbal Medicine; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Barbosa, M.O.; Wilairatana, P.; Leite, G.M.L.; Delmondes, G.A.; Silva, L.Y.S.D.; Júnior, S.C.A.; Dantas, L.B.R.; Bezerra, D.S.; Beltrão, I.C.S.L.; Dias, D.Q.; et al. Plectranthus Species with Anti-Inflammatory and Analgesic Potential: A Systematic Review on Ethnobotanical and Pharmacological Findings. Molecules 2023, 28, 5653. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, G.; Swamy, M.K.; Sinniah, U.R. Plectranthus amboinicus (Lour.) Spreng: Botanical, Phytochemical, Pharmacological and Nutritional Significance. Molecules 2016, 21, 369. [Google Scholar] [CrossRef]
- Paton, A.J.; Mwanyambo, M.; Govaerts, R.H.A.; Smitha, K.; Suddee, S.; Phillipson, P.B.; Wilson, T.C.; Forster, P.I.; Culham, A. Nomenclatural changes in Coleus and Plectranthus (Lamiaceae): A tale of more than two genera. PhytoKeys 2019, 129, 1–158. [Google Scholar] [CrossRef] [PubMed]
- Astuti, A.D.; Yasir, B.; Subehan; Alam, G. Comparison of two varieties of Plectranthus scutellarioides based on extraction method, phytochemical compound, and cytotoxicity. J. Phys. Conf. Ser. 2019, 1341, 072012. [Google Scholar] [CrossRef]
- Lukhoba, C.W.; Simmonds, M.S.; Paton, A.J. Plectranthus: A review of ethnobotanical uses. J. Ethnopharmacol. 2006, 103, 1–24. [Google Scholar] [CrossRef]
- Schultes, R.E. Psychoactive plants in need of chemical and pharmacological study. Proc. Plant Sci. 1984, 93, 281–304. [Google Scholar] [CrossRef]
- Namsa, N.D.; Tag, H.; Mandal, M.; Kalita, P.; Das, A.K. An ethnobotanical study of traditional anti-inflammatory plants used by the Lohit community of Arunachal Pradesh, India. J. Ethnopharmacol. 2009, 125, 234–245. [Google Scholar] [CrossRef]
- Bismelah, N.A.; Ahmad, R.; Mohamed Kassim, Z.H.; Ismail, N.H.; Rasol, N.E. The antibacterial effect of Plectranthus scutellarioides (L.) R.Br. leaves extract against bacteria associated with peri-implantitis. J. Tradit. Complement. Med. 2022, 12, 556–566. [Google Scholar] [CrossRef]
- Roosita, K.; Kusharto, C.M.; Sekiyama, M.; Fachrurozi, Y.; Ohtsuka, R. Medicinal plants used by the villagers of a Sundanese community in West Java, Indonesia. J. Ethnopharmacol. 2008, 115, 72–81. [Google Scholar] [CrossRef]
- Pakadang, S.R.; Wahjuni, C.U.; Notobroto, H.B.; Winarni, D.; Dwiyanti, R.; Sabir, M.; Mochammad Hatta, M. Immunomodulator potential of miana leaves (Coleus scutellarioides (L) Benth) in prevention of tuberculosis infection. Am. J. Microbiol. Res. 2015, 3, 129–134. [Google Scholar]
- Hanum, F.S.; Witaningrum, A.M.; Puspitasari, Y. Effect of Plectranthus scutellarioides (L.) Leaf Extract as Natural Antibacterial Against Staphylococcus aureus and Escherichia coli Isolated From Dairy Cattle with Subclinical Mastitis. J. Basic Med. Vet. 2022, 2, 90–97. [Google Scholar] [CrossRef]
- Fakhriati, S.G.; Ida, M.; Moektiwardoyo, M.; Ayu, A.C. Inhibition of Nitric Oxide Production in Lipopolysaccharide-Induced Macrophages Cell by Plectranthus scutellarioides (L.) R.Br Leaves. Res. J. Chem. Environ. 2018, 22, 38–42. [Google Scholar]
- Hasnain, A.; Naqvi, S.A.H.; Ayesha, S.I.; Khalid, F.; Ellahi, M.; Iqbal, S.; Hassan, M.Z.; Abbas, A.; Adamski, R.; Markowska, D.; et al. Plants in vitro propagation with its applications in food, pharmaceuticals and cosmetic industries; current scenario and future approaches. Front. Plant Sci. 2022, 13, 1009395, Correction in Front. Plant Sci. 2023, 14, 1197747. [Google Scholar] [CrossRef] [PubMed]
- Yancheva, S.; Kondakova, V. Plant Tissue Culture Technology: Present and Future Development. In Bioprocessing of Plant In Vitro Systems. Reference Series in Phytochemistry; Pavlov, A., Bley, T., Eds.; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Hussain, A.; Ahmed, I.; Nazir, H.; Ullah, I. Plant Tissue Culture: Current Status and Opportunities. In Recent Advances in Plant In Vitro Culture; InTech: London, UK, 2012. [Google Scholar] [CrossRef]
- Sahu, R.; Dewanjee, S. Differential physiological and biochemical responses under variable culture conditions in micro-propagated Solenostemon scutellarioides: An important ornamental plant. Nat. Prod. Bioprospect. 2012, 2, 160–165. [Google Scholar] [CrossRef]
- Cho, K.H.; Laux, V.Y.; Wallace-Springer, N.; Clark, D.G.; Folta, K.M.; Colquhoun, T.A. Effects of Light Quality on Vegetative Cutting and In Vitro Propagation of Coleus (Plectranthus scutellarioides). HortScience 2019, 54, 926–935. [Google Scholar] [CrossRef]
- Belniaki, A.C.; Rabel, L.A.d.N.; Gomes, E.N.; Zuffellato-Ribas, K.C. Does the presence of leaves on coleus stem cuttings influence their rooting? Ornam. Hortic. 2018, 24, 206. [Google Scholar] [CrossRef]
- Hussain, M.S.; Fareed, S.; Ansari, S.; Rahman, M.A.; Ahmad, I.Z.; Saeed, M. Current approaches toward production of secondary plant metabolites. J. Pharm. Bioallied Sci. 2012, 4, 10–20. [Google Scholar] [CrossRef]
- Dias, D.A.; Urban, S.; Roessner, U. A Historical Overview of Natural Products in Drug Discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef]
- Les, F.; Cásedas, G.; López, V. Bioactivity of Medicinal Plants and Extracts. Biology 2021, 10, 634. [Google Scholar] [CrossRef]
- Leonti, M.; Casu, L. Traditional medicines and globalization: Current and future perspectives in ethnopharmacology. Front. Pharmacol. 2013, 4, 92. [Google Scholar] [CrossRef] [PubMed]
- Nasim, N.; Sandeep, I.S.; Mohanty, S. Plant-derived natural products for drug discovery: Current approaches and prospects. Nucleus 2022, 65, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Ozyigit, I.I.; Dogan, I.; Hocaoglu-Ozyigit, A.; Yalcin, B.; Erdogan, A.; Yalcin, I.E.; Cabi, E.; Kaya, Y. Production of secondary metabolites using tissue culture-based biotechnological applications. Front. Plant Sci. 2023, 14, 1132555. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Leal, C.A.; Puente-Garza, C.A.; García-Lara, S. In vitro plant tissue culture: Means for production of biological active compounds. Planta 2018, 248, 1–18. [Google Scholar] [CrossRef]
- Rani, G.; Talwar, D.; Nagpal, A.; Virk, G.S. Micropropagation of Coleus blumei from nodal segments and shoot tips. Biol. Plant. 2006, 50, 496–500. [Google Scholar] [CrossRef]
- Bauer, N.; Vuković, R.; Likić, S.; Jelaska, S. Potential of Different Coleus blumei Tissues for Rosmarinic Acid Production. Food Technol. Biotechnol. 2015, 53, 3–10. [Google Scholar] [CrossRef]
- Marcotrigiano, M.; Boyle, T.H.; Morgan, P.A.; Ambach, K.L. Leaf Color Variants from Coleus Shoot Cultures. J. Amer. Soc. Hort. Sci. 1990, 115, 681–686. [Google Scholar] [CrossRef]
- Levita, J.; Sumiwi, S.A.; Pratiwi, T.I.; Ilham, E.; Sidiq, S.P.; Moektiwardoyo, M. Pharmacological Activities of Plectranthus scutellarioides (L.) R.Br. Leaves Extract on Cyclooxygenase and Xanthine Oxidase Enzymes. J. Med. Plants Res. 2016, 10, 261–269. [Google Scholar]
- Ito, T.; Rakainsa, S.K.; Nisa, K.; Morita, H. Three new abietane-type diterpenoids from the leaves of Indonesian Plectranthus scutellarioides. Fitoterapia 2018, 127, 146–150. [Google Scholar] [CrossRef]
- Rodríguez-Ferreiro, A.O.; Ochoa-Pacheco, A.; Méndez-Rodriguez, D.; Ortiz-Beatón, E.; Font-Salmo, O.; Guisado-Bourzac, F.; Molina-Bertrán, S.; Monzote, L.; Cos, P.; Foubert, K.; et al. LC-MS Characterization and Biological Activities of Cuban Cultivars of Plectranthus neochilus Schltr. Plants 2022, 11, 134. [Google Scholar] [CrossRef]
- Ślusarczyk, S.; Cieślak, A.; Yanza, Y.R.; Szumacher-Strabel, M.; Varadyova, Z.; Stafiniak, M.; Wojnicz, D.; Matkowski, A. Phytochemical Profile and Antioxidant Activities of Coleus amboinicus Lour. Cultivated in Indonesia and Poland. Molecules 2021, 26, 2915. [Google Scholar] [CrossRef] [PubMed]
- El-hawary, S.S.; El-sofany, R.H.; Abdel-Monem, A.R.; Ashour, R.S.; Sleem, A.A. Polyphenolics content and biological activity of Plectranthus amboinicus (Lour.) spreng growing in Egypt (Lamiaceae). Pharmacogn. J. 2012, 4, 45–54. [Google Scholar] [CrossRef]
- Cretton, S.; Saraux, N.; Monteillier, A.; Righi, D.; Marcourt, L.; Genta-Jouve, G.; Wolfender, J.L.; Cuendet, M.; Christen, P. Anti-inflammatory and antiproliferative diterpenoids from Plectranthus scutellarioides. Phytochemistry 2018, 154, 39–46. [Google Scholar] [CrossRef]
- Safarzadeh, E.; Sandoghchian Shotorbani, S.; Baradaran, B. Herbal medicine as inducers of apoptosis in cancer treatment. Adv. Pharm. Bull. 2014, 4 (Suppl. S1), 421–427. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, G.E.S.; Md Akim, A.; Sung, Y.Y.; Sifzizul, T.M.T. Cancer and apoptosis: The apoptotic activity of plant and marine natural products and their potential as targeted cancer therapeutics. Front. Pharmacol. 2022, 13, 842376. [Google Scholar] [CrossRef] [PubMed]
- Burmistrova, O.; Perdomo, J.; Simões, M.F.; Rijo, P.; Quintana, J.; Estévez, F. The abietane diterpenoid parvifloron D from Plectranthus ecklonii is a potent apoptotic inducer in human leukemia cells. Phytomedicine 2015, 11, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Darsan, B.; Menon, V.; Gopalakrishnan, K. Terpenoids Isolated From the Shoot of Plectranthus hadiensis Induces Apoptosis in Human Colon Cancer Cells Via the Mitochondria-Dependent Pathway. Nutr. Cancer 2015, 4, 697–705. [Google Scholar] [CrossRef]
- Sitarek, P.; Synowiec, E.; Kowalczyk, T.; Bangay, G.; Śliwiński, T.; Picot, L.; Princiotto, S.; Rijo, P. Anticancer Properties of Plectranthus ornatus-Derived Phytochemicals Inducing Apoptosis via Mitochondrial Pathway. Int. J. Mol. Sci. 2022, 23, 11653. [Google Scholar] [CrossRef]
- Śliwiński, T.; Sitarek, P.; Skała, E.; Isca, V.M.S.; Synowiec, E.; Kowalczyk, T.; Bijak, M.; Rijo, P. Diterpenoids from Plectranthus spp. as Potential Chemotherapeutic Agents via Apoptosis. Pharmaceuticals 2020, 13, 123. [Google Scholar] [CrossRef]
- Bhatt, P.; Joseph, G.S.; Negi, P.S.; Varadaraj, M.C. Chemical Composition and Nutraceutical Potential of Indian Borage (Plectranthus amboinicus) Stem Extract. J. Chem. 2013, 2013, 320329. [Google Scholar] [CrossRef]
- Sitarek, P.; Skała, E.; Toma, M.; Wielanek, M.; Szemraj, J.; Nieborowska-Skorska, M.; Kolasa, M.; Skorski, T.; Wysokińska, H.; Śliwiński, T. A preliminary study of apoptosis induction in glioma cells via alteration of the Bax/Bcl-2-p53 axis by transformed and non-transformed root extracts of Leonurus sibiricus L. Tumour Biol. 2016, 37, 8753–8764. [Google Scholar] [CrossRef] [PubMed]
- Sitarek, P.; Synowiec, E.; Kowalczyk, T.; Śliwiński, T.; Skała, E. An In Vitro Estimation of the Cytotoxicity and Genotoxicity of Root Extract from Leonurus sibiricus L. Overexpressing AtPAP1 against Different Cancer Cell Lines. Molecules 2018, 23, 2049. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, T.; Sitarek, P.; Toma, M.; Rijo, P.; Domínguez-Martín, E.; Falcó, I.; Sánchez, G.; Śliwiński, T. Enhanced Accumulation of Betulinic Acid in Transgenic Hairy Roots of Senna Obtusifolia Growing in the Sprinkle Bioreactor and Evaluation of Their Biological Properties in Various Biological Models. Chem. Biodivers. 2021, 18, e2100455. [Google Scholar] [CrossRef]
- Moongkarndi, P.; Kosem, N.; Kaslungka, S.; Luanratana, O.; Pongpan, N.; Neungton, N. Antiproliferation, antioxidation and induction of apoptosis by Garcinia mangostana (mangosteen) on SKBR3 human breast cancer cell line. J. Ethnopharmacol. 2004, 90, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Błasiak, J.; Synowiec, E.; Tarnawska, J.; Czarny, P.; Popławski, T.; Reiter, R.J. Dental methacrylates may exert genotoxic effects via the oxidative induction of DNA strand breaks and the inhibition of their repair. Mol. Biol. Rep. 2012, 39, 7487–7496. [Google Scholar] [CrossRef]
- Smiałowski, A.; Bijak, M. Repeated imipramine treatment increases the responsivity of the rat hippocampus to dopamine. An in vitro study. J. Neural Transm. 1986, 66, 187–196. [Google Scholar] [CrossRef]
- Kroemer, G.; Galluzzi, L.; Brenner, C. Mitochondrial Membrane Permeabilization in Cell Death. Physiol. Rev. 2007, 87, 99–163. [Google Scholar] [CrossRef]
- Markowicz-Piasecka, M.; Sikora, J.; Mateusiak, Ł.; Mikiciuk-Olasik, E.; Huttunen, K.M. New prodrugs of metformin do not influence the overall haemostasis potential and integrity of the erythrocyte membrane. Eur. J. Pharmacol. 2017, 811, 208–221. [Google Scholar] [CrossRef]


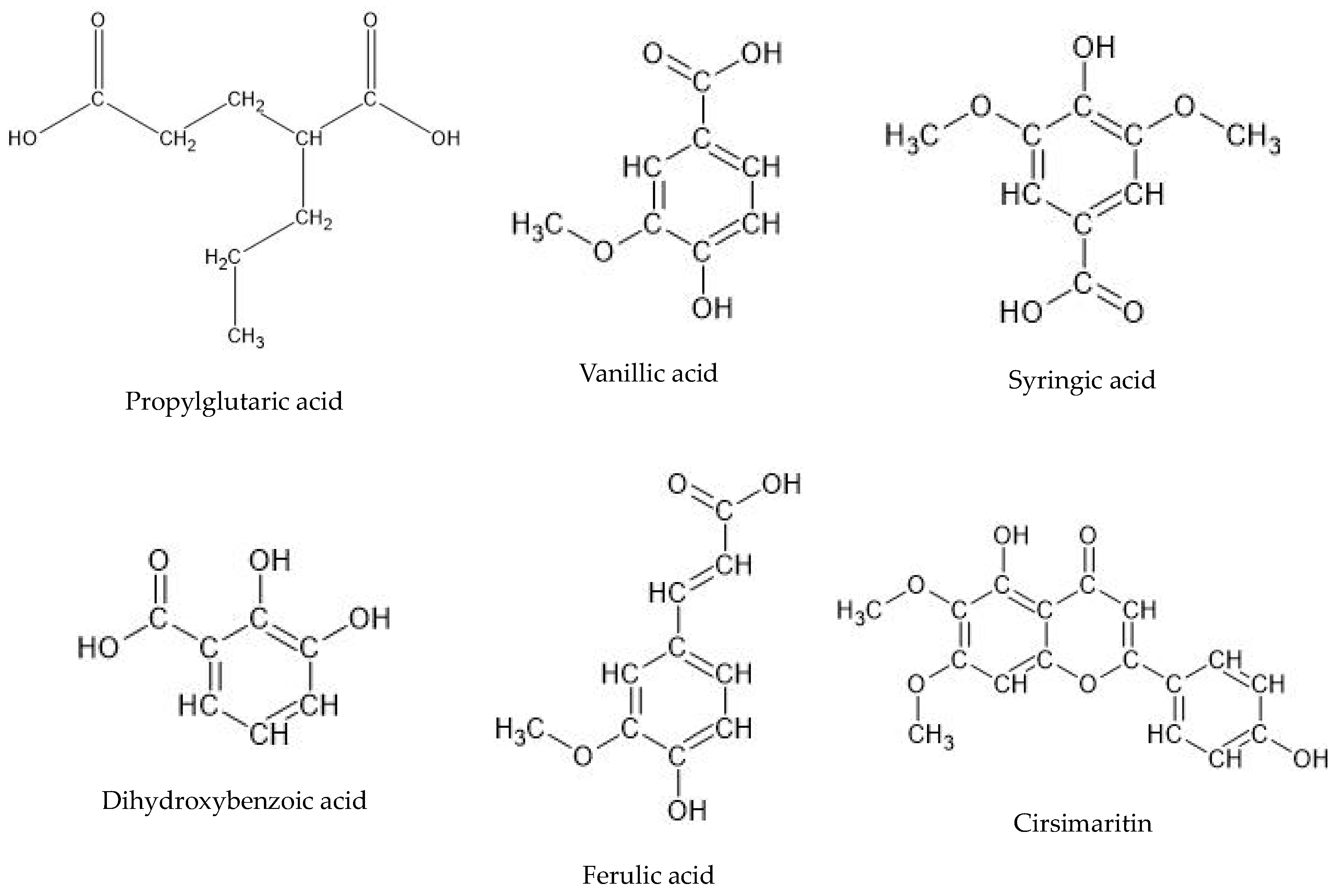

| No. | Ion.+/− | Rt (min) | Molecular Formula | m/z Theoretical | m/z Experimental | Error | DBE | MS/MS Spectrum | Proposed Compound | Root | Aerial Part |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | - | 6.1 | C15H12O6 | 287.0561 | 287.0565 | −1.35 | 10 | ND | Eriodyctiol | ++ | ++ |
| 2 | - | 7.5 | C8H14O4 | 173.0455 | 173.0468 | −7.2 | 3 | 155, 130, 111 | Propylglutaric acid | +++ | +++ |
| 3 | - | 11.5 | C24H32O10 | 479.1923 | 479.1952 | −6.1 | 9 | ND | 6,11,12,14,16-Pentahydroxy-3,17diacetyl-8,11,13-abietatrien-7-one | ++ | ++ |
| 4 | - | 12.2 | C8H8O4 | 167.035 | 167.0363 | −7.84 | 5 | 149, 123, 121, 109 | Vanillic acid | +++ | +++ |
| 5 | - | 12.3 | C24H30O10 | 477.1766 | 477.1732 | 7.15 | 10 | ND | 6,11,12,14,16-Pentahydroxy-3,17-diacetyl5,8,11,13-abietatetraen-7-one | ++ | ++ |
| 6 | - | 12.3 | C9H10O5 | 197.0455 | 197.0470 | −7.34 | 5 | 179, 135, 123 | Syringic acid | +++ | +++ |
| 7 | - | 12.9 | C16H18O9 | 353.0878 | 353.0903 | −7.04 | 8 | ND | Chlorogenic acid | ++ | +++ |
| 8 | - | 13.1 | C7H6O4 | 153.0193 | 153.0200 | −4.34 | 5 | 123, 109 | Dihydroxybenzoic acid | +++ | +++ |
| 9 | - | 13.7 | C22H28O8 | 419.1711 | 419.1673 | 9.14 | 9 | ND | 3,6,12-Trihydroxy-2-acetyl-8,12-abietadien7,11,14-trione | + | + |
| 10 | - | 15.1 | C7H6O3 | 137.0244 | 137.0245 | −0.6 | 5 | 108 | Hydroxybenzoic acid | ++ | +++ |
| 11 | - | 17.2 | C18H16O8 | 359.0772 | 359.0780 | −2.11 | 11 | 179, 135 | Rosmarinic acid isomer | + | +++ |
| 12 | - | 17.8 | C9H8O4 | 179.0350 | 179.0361 | −9.21 | 6 | 135 | Caffeic acid | ++ | +++ |
| 13 | - | 19.3 | C9H8O3 | 163.0401 | 163.0413 | −7.51 | 6 | 119, 108 | Coumaric acid | + | ++ |
| 14 | - | 20.9 | C18H16O8 | 359.0772 | 359.0788 | −433 | 11 | 197, 161, 135 | Rosmarinic acid | ++ | +++ |
| 15 | - | 21.1 | C10H10O4 | 193.0506 | 193.0492 | 7.38 | 6 | 178, 161, 134 | Ferulic acid | +++ | +++ |
| 16 | - | 22.3 | C17H14O6 | 313.0718 | 313.0708 | 3.06 | 11 | 293, 194, 161 | Cirsimaritin | +++ | +++ |
| OST—Occlusion Start Time (s) | OT—Occlusion Time (s) ** | AUC—Area under the Curve ** | |||||
|---|---|---|---|---|---|---|---|
| REPS | AEPS | REPS | AEPS | REPS | AEPS | ||
| CTR | 402 ± 16.9 | 335 ± 8.5 | 466 ± 5.7 | 399 ± 3.5 | 1373 ± 5.7 | 1455 ± 30.4 | |
| 1 µg/mL | 421 ± 10.7 | 292 ± 15.3 | 464 ± 10.6 | 443 ± 7.9 | 1365 ± 6.4 | 1389 ± 53.4 | |
| 10 µg/mL | 405 ± 24.0 | 302 ± 3.5 | 452 ± 27.6 | 408 ± 16.2 | 1380 ± 26.9 | 1389 ± 53.4 | |
| 100 µg/mL | 408 ± 55.2 | 360 ± 8.8 | 472 ± 43.8 | 467 ± 15.2 | 1371 ± 48.1 | 1369 ± 45.2 | |
| interpretation | low risk of adverse events | * | 357–729 s | 1257–1422 | |||
| high risk of bleeding | * | >729 s | <1257 | ||||
| high risk of thromboembolic events | * | <357 s | >1422 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalczyk, T.; Sikora, J.; Merecz-Sadowska, A.; Kukula-Koch, W.; Synowiec, E.; Majda, A.; Juda, D.; Śliwiński, T.; Sitarek, P. Biological Properties of Extracts Obtained from In Vitro Culture of Plectranthus scutellarioides in a Cell Model. Int. J. Mol. Sci. 2024, 25, 1043. https://doi.org/10.3390/ijms25021043
Kowalczyk T, Sikora J, Merecz-Sadowska A, Kukula-Koch W, Synowiec E, Majda A, Juda D, Śliwiński T, Sitarek P. Biological Properties of Extracts Obtained from In Vitro Culture of Plectranthus scutellarioides in a Cell Model. International Journal of Molecular Sciences. 2024; 25(2):1043. https://doi.org/10.3390/ijms25021043
Chicago/Turabian StyleKowalczyk, Tomasz, Joanna Sikora, Anna Merecz-Sadowska, Wirginia Kukula-Koch, Ewelina Synowiec, Agata Majda, Dawid Juda, Tomasz Śliwiński, and Przemysław Sitarek. 2024. "Biological Properties of Extracts Obtained from In Vitro Culture of Plectranthus scutellarioides in a Cell Model" International Journal of Molecular Sciences 25, no. 2: 1043. https://doi.org/10.3390/ijms25021043
APA StyleKowalczyk, T., Sikora, J., Merecz-Sadowska, A., Kukula-Koch, W., Synowiec, E., Majda, A., Juda, D., Śliwiński, T., & Sitarek, P. (2024). Biological Properties of Extracts Obtained from In Vitro Culture of Plectranthus scutellarioides in a Cell Model. International Journal of Molecular Sciences, 25(2), 1043. https://doi.org/10.3390/ijms25021043









