New Advances in Rapid Pretreatment for Small Dense LDL Cholesterol Measurement Using Shear Horizontal Surface Acoustic Wave (SH-SAW) Technology
Abstract
1. Introduction
2. Results
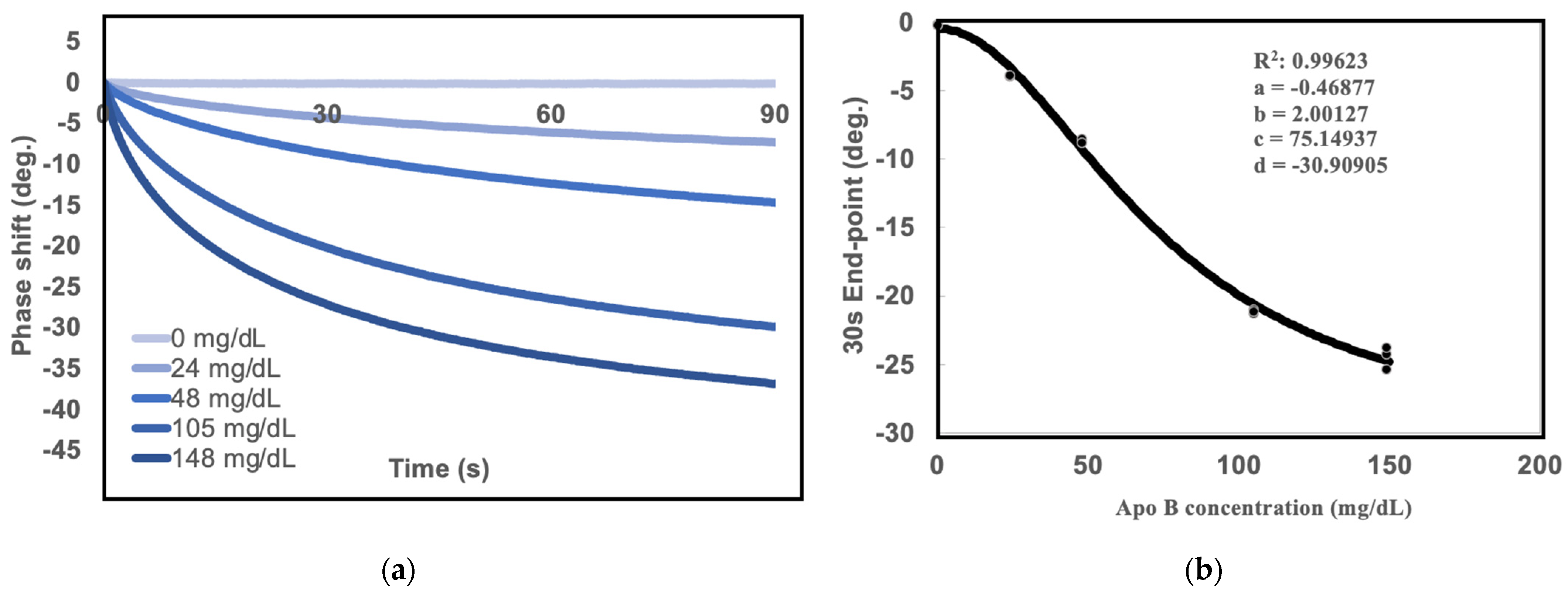
2.1. Measurements of Standard Curves for SH-SAW Biosensor
2.2. Comparison of sd-LDL-apoB by SH-SAW Biosensor under Different Pretreatment Methods
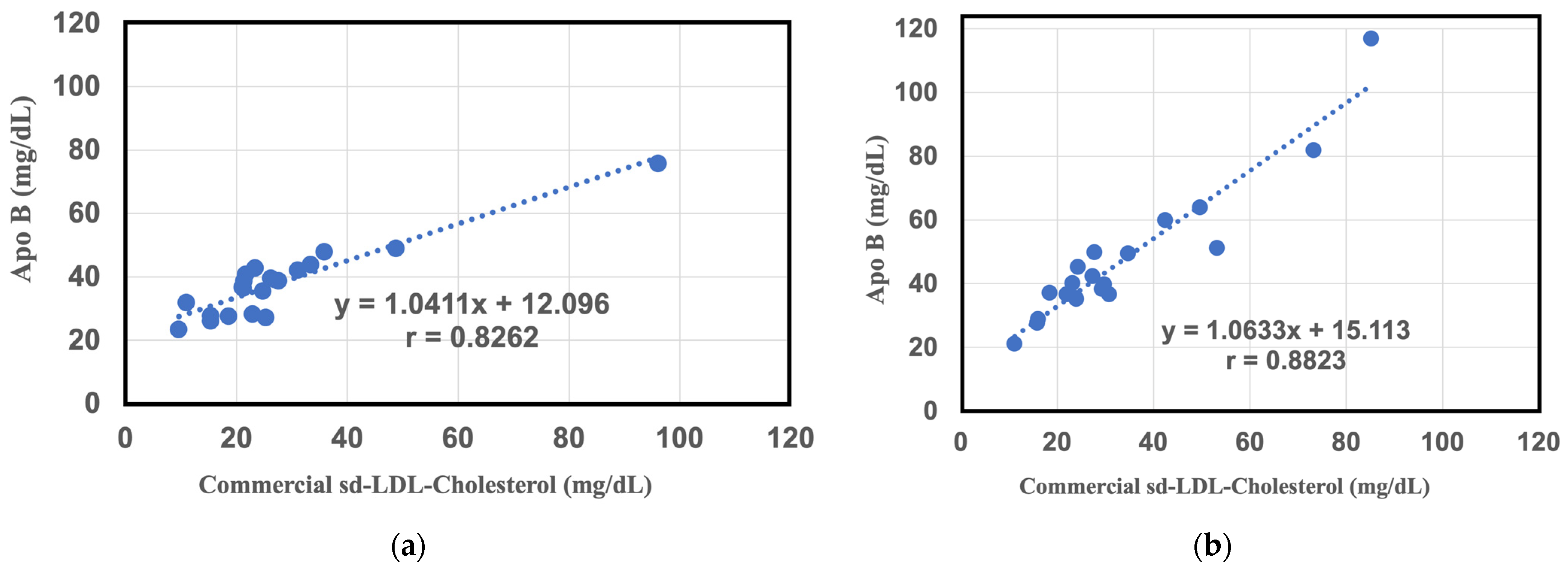
2.3. Comparative Study between Commercial sd-LDL-Cholesterol Assay Results and sd-LDL-ApoB Results from the SH-SAW Biosensor in a Large Study Group
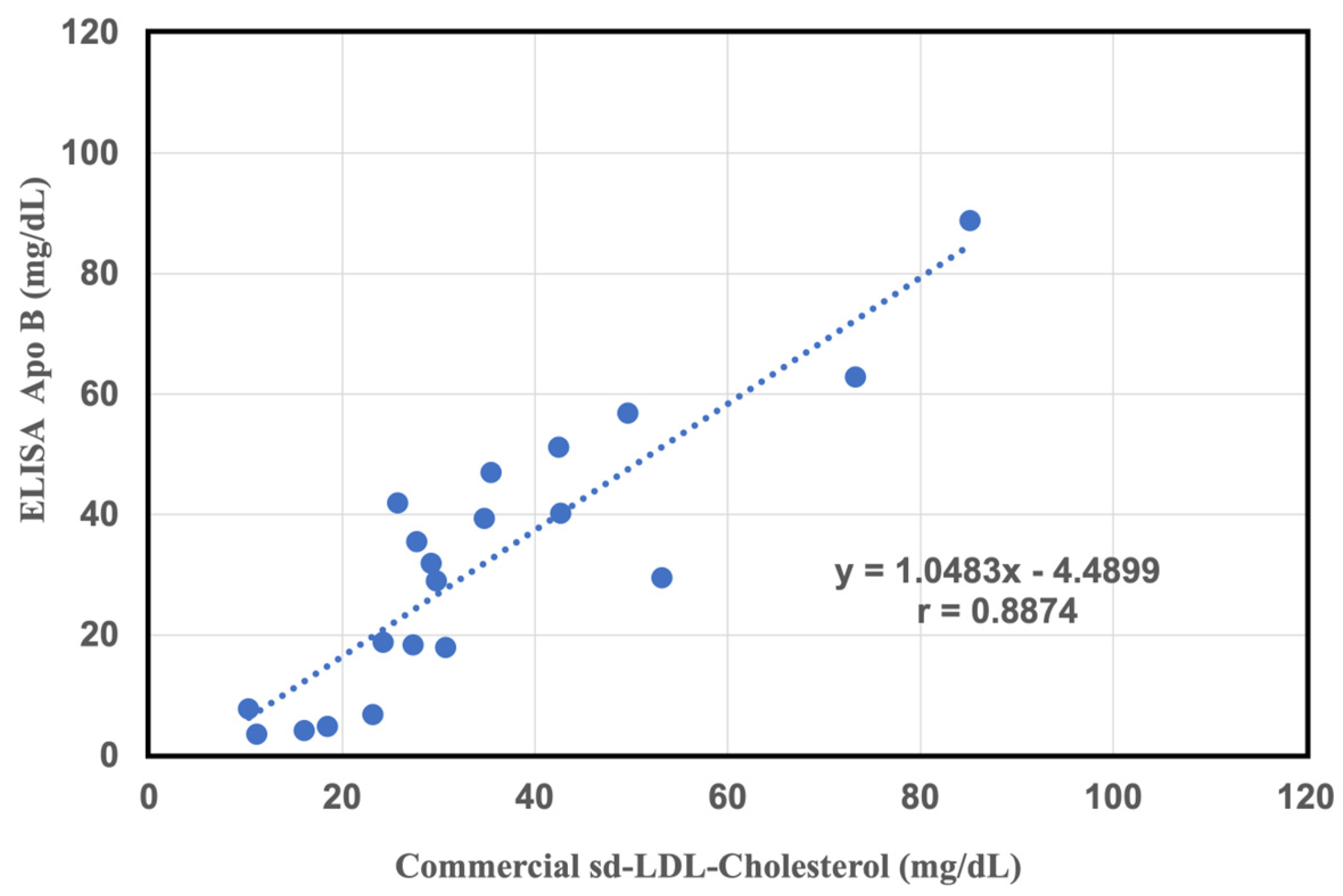
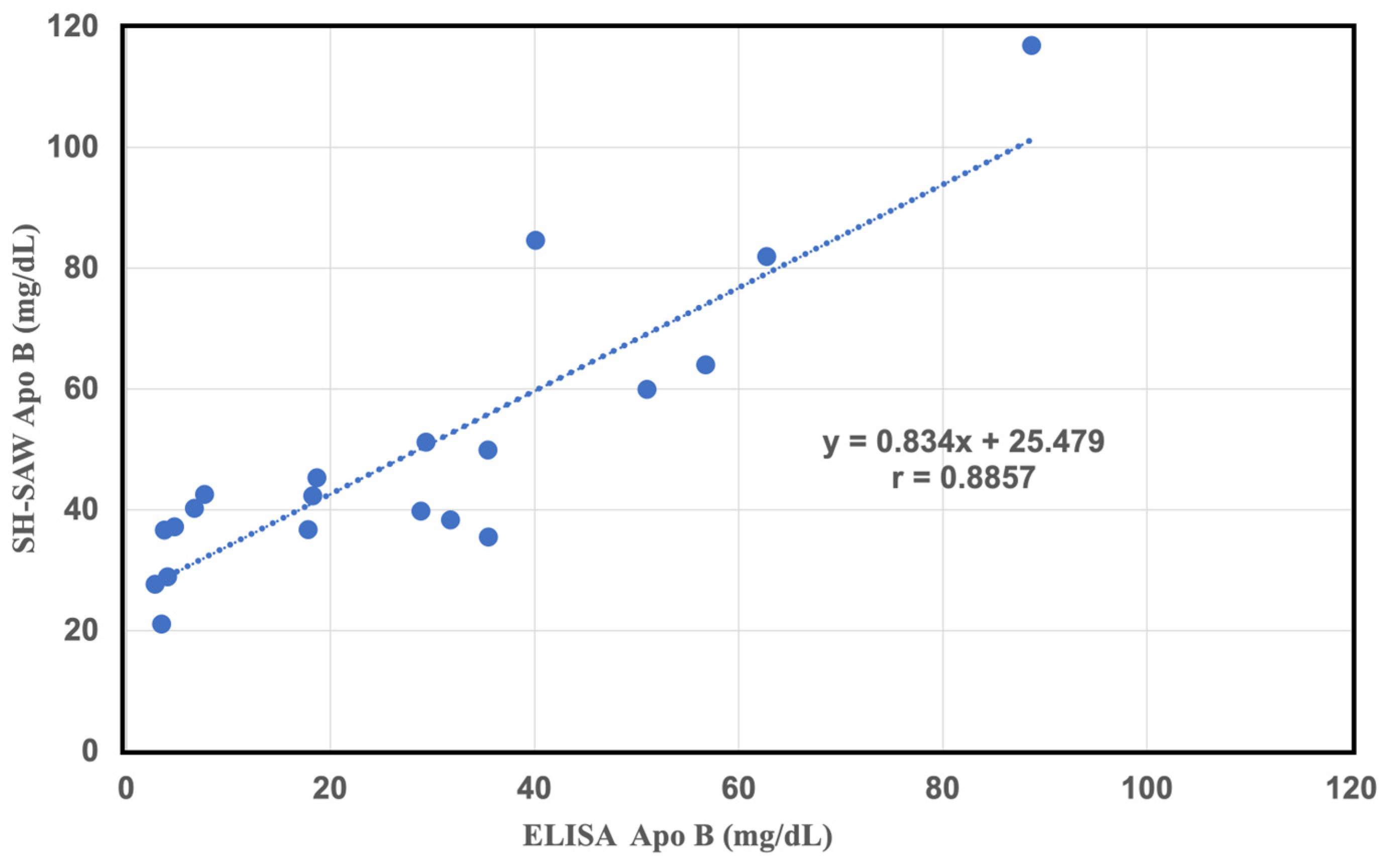
2.4. Comparative Study of a Commercial of sd-LDL-ApoB Kit and an Apolipoprotein B-100 ELISA Kit
2.5. Comparison Study between LDL Subfractions LDL-6 Apo-B, and sd-LDL-ApoB Results by SH-SAW Biosensor in a Large Study Group
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. NMR Analysis
4.3. SH-SAW Biosensor Chips Assay
4.4. Establishment of the SH-S.A.W. 4PL Standard Regression Curve
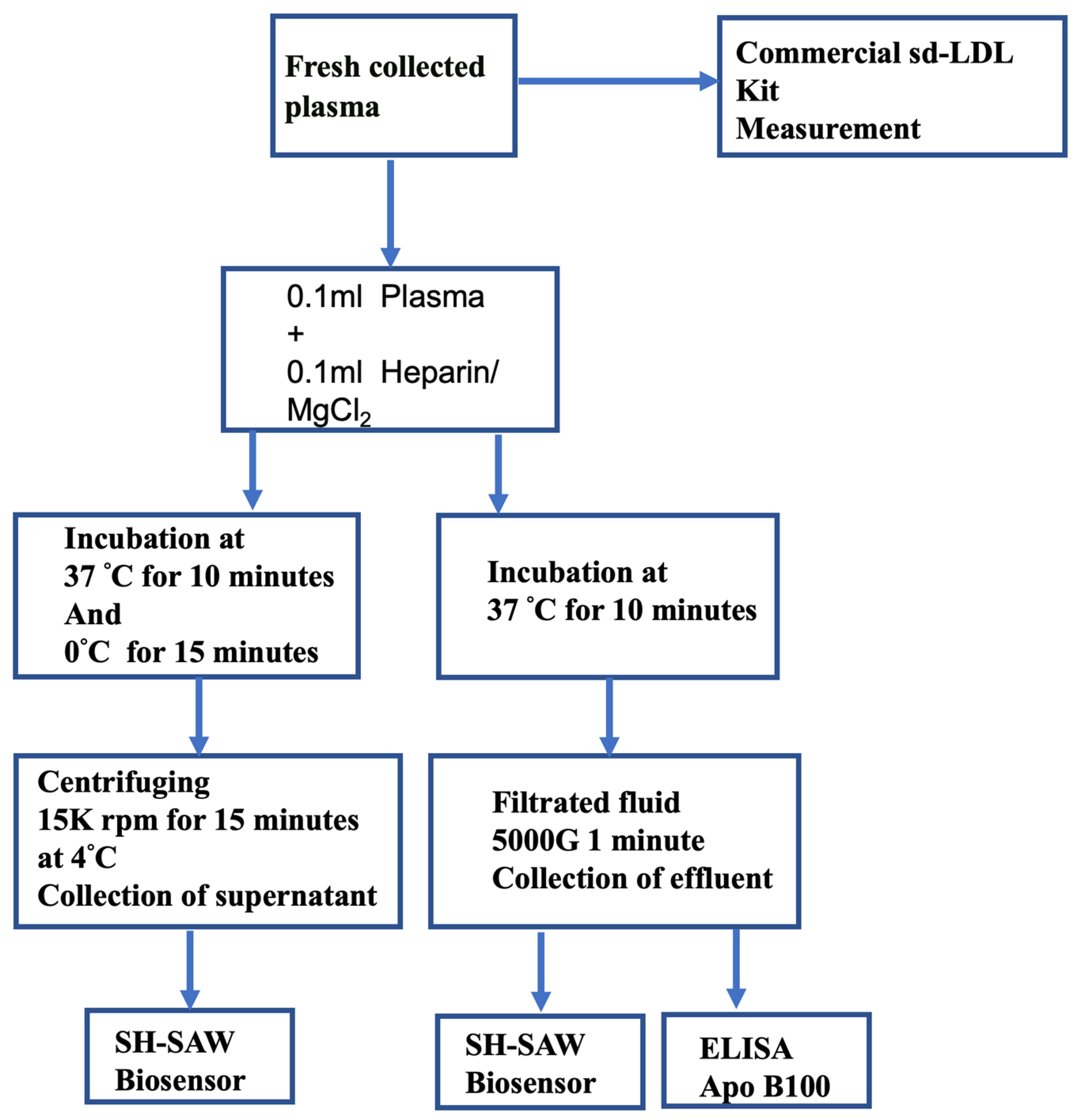
4.5. Centrifugation Assay Procedure
4.6. Filtration Assay Procedure
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Di Cesare, M.; Khang, Y.-H.; Asaria, P.; Blakely, T.; Cowan, M.J.; Farzadfar, F.; Guerrero, R.; Ikeda, N.; Kyobutungi, C.; Msyamboza, K.P. Inequalities in non-communicable diseases and effective responses. Lancet 2013, 381, 585–597. [Google Scholar] [CrossRef]
- Jin, J.L.; Zhang, H.W.; Cao, Y.X.; Liu, H.H.; Hua, Q.; Li, Y.F.; Zhang, Y.; Wu, N.Q.; Zhu, C.G.; Xu, R.X.; et al. Association of small dense low-density lipoprotein with cardiovascular outcome in patients with coronary artery disease and diabetes: A prospective, observational cohort study. Cardiovasc. Diabetol. 2020, 19, 45. [Google Scholar] [CrossRef]
- Holvoet, P.; Vanhaecke, J.; Janssens, S.; Van de Werf, F.; Collen, D. Oxidized LDL and malondialdehyde-modified LDL in patients with acute coronary syndromes and stable coronary artery disease. Circulation 1998, 98, 1487–1494. [Google Scholar] [CrossRef]
- Barquera, S.; Pedroza-Tobias, A.; Medina, C.; Hernandez-Barrera, L.; Bibbins-Domingo, K.; Lozano, R.; Moran, A.E. Global Overview of the Epidemiology of Atherosclerotic Cardiovascular Disease. Arch. Med. Res. 2015, 46, 328–338. [Google Scholar] [CrossRef]
- Tanaga, K.; Bujo, H.; Inoue, M.; Mikami, K.; Kotani, K.; Takahashi, K.; Kanno, T.; Saito, Y. Increased circulating malondialdehyde-modified LDL levels in patients with coronary artery diseases and their association with peak sizes of LDL particles. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 662–666. [Google Scholar] [CrossRef]
- Wilson, P.W. High-density lipoprotein, low-density lipoprotein and coronary artery disease. Am. J. Cardiol. 1990, 66, A7–A10. [Google Scholar] [CrossRef]
- Campos, H.; Genest Jr, J.J.; Blijlevens, E.; McNamara, J.R.; Jenner, J.L.; Ordovas, J.M.; Wilson, P.; Schaefer, E.J. Low density lipoprotein particle size and coronary artery disease. Arterioscler. Thromb. A J. Vasc. Biol. 1992, 12, 187–195. [Google Scholar] [CrossRef]
- Mudd, J.O.; Borlaug, B.A.; Johnston, P.V.; Kral, B.G.; Rouf, R.; Blumenthal, R.S.; Kwiterovich, P.O. Beyond low-density lipoprotein cholesterol: Defining the role of low-density lipoprotein heterogeneity in coronary artery disease. J. Am. Coll. Cardiol. 2007, 50, 1735–1741. [Google Scholar] [CrossRef]
- Hoogeveen, R.C.; Gaubatz, J.W.; Sun, W.; Dodge, R.C.; Crosby, J.R.; Jiang, J.; Couper, D.; Virani, S.S.; Kathiresan, S.; Boerwinkle, E.; et al. Small dense low-density lipoprotein-cholesterol concentrations predict risk for coronary heart disease: The Atherosclerosis Risk In Communities (ARIC) study. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1069–1077. [Google Scholar] [CrossRef]
- Sorokin, A.V.; Patel, N.; Li, H.; Hong, C.G.; Sampson, M.; O’Hagan, R.; Florida, E.M.; Teague, H.L.; Playford, M.P.; Chen, M.Y.; et al. Estimated sdLDL-C for predicting high-risk coronary plaque features in psoriasis: A prospective observational study. Lipids Health Dis. 2023, 22, 55. [Google Scholar] [CrossRef]
- Nomura, S.O.; Karger, A.B.; Garg, P.; Cao, J.; Bhatia, H.; Duran, E.K.; Duprez, D.; Guan, W.; Tsai, M.Y. Small dense low-density lipoprotein cholesterol compared to other lipoprotein biomarkers for predicting coronary heart disease among individuals with normal fasting glucose: The Multi-Ethnic Study of Atherosclerosis. Am. J. Prev. Cardiol. 2023, 13, 100436. [Google Scholar] [CrossRef]
- Juhi, A.; Jha, K.; Mondal, H. Small Dense Low-Density Lipoprotein Level in Newly Diagnosed Type 2 Diabetes Mellitus Patients With Normal Low-Density Lipoprotein. Cureus 2023, 15, e33924. [Google Scholar] [CrossRef]
- Santos, H.O.; Earnest, C.P.; Tinsley, G.M.; Izidoro, L.F.; Macedo, R.C. Small dense low-density lipoprotein-cholesterol (sdLDL-C): Analysis, effects on cardiovascular endpoints and dietary strategies. Progress Cardiovasc. Dis. 2020, 63, 503–509. [Google Scholar] [CrossRef]
- Senmaru, T.; Fukui, M.; Okada, H.; Mineoka, Y.; Yamazaki, M.; Tsujikawa, M.; Hasegawa, G.; Kitawaki, J.; Obayashi, H.; Nakamura, N. Testosterone deficiency induces markedly decreased serum triglycerides, increased small dense LDL, and hepatic steatosis mediated by dysregulation of lipid assembly and secretion in mice fed a high-fat diet. Metabolism 2013, 62, 851–860. [Google Scholar] [CrossRef]
- Superko, H.R.; Nejedly, M.; Garrett, B. Small LDL and its clinical importance as a new CAD risk factor: A female case study. Progress Cardiovasc. Nurs. 2002, 17, 167–173. [Google Scholar] [CrossRef]
- Austin, M.A.; Breslow, J.L.; Hennekens, C.H.; Buring, J.E.; Willett, W.C.; Krauss, R.M. Low-density lipoprotein subclass patterns and risk of myocardial infarction. JAMA 1988, 260, 1917–1921. [Google Scholar] [CrossRef]
- Krauss, R.M.; Burke, D.J. Identification of multiple subclasses of plasma low density lipoproteins in normal humans. J. Lipid Res. 1982, 23, 97–104. [Google Scholar] [CrossRef]
- Kanonidou, C. Small dense low-density lipoprotein: Analytical review. Clin. Chim. Acta 2021, 520, 172–178. [Google Scholar] [CrossRef]
- Ito, Y.; Satoh, N.; Ishii, T.; Kumakura, J.; Hirano, T. Development of a homogeneous assay for measurement of high-density lipoprotein-subclass cholesterol. Clin. Chim. Acta 2014, 427, 86–93. [Google Scholar] [CrossRef]
- Rizzo, M.; Berneis, K. The clinical relevance of low-density-lipoproteins size modulation by statins. Cardiovasc. Drugs Ther. 2006, 20, 205–217. [Google Scholar] [CrossRef]
- Rizzo, M.; Berneis, K. Low-density lipoprotein size and cardiovascular risk assessment. Qjm 2006, 99, 1–14. [Google Scholar] [CrossRef]
- Voiculescu, I.; Nordin, A.N. Acoustic wave based MEMS devices for biosensing applications. Biosens. Bioelectron. 2012, 33, 1–9. [Google Scholar] [CrossRef]
- Toma, K.; Miki, D.; Kishikawa, C.; Yoshimura, N.; Miyajima, K.; Arakawa, T.; Yatsuda, H.; Mitsubayashi, K. Repetitive immunoassay with a surface acoustic wave device and a highly stable protein monolayer for on-site monitoring of airborne dust mite allergens. Anal. Chem. 2015, 87, 10470–10474. [Google Scholar] [CrossRef]
- Jeng, M.-J.; Sharma, M.; Li, Y.-C.; Lu, Y.-C.; Yu, C.-Y.; Tsai, C.-L.; Huang, S.-F.; Chang, L.-B.; Lai, C.-S. Surface acoustic wave sensor for c-reactive protein detection. Sensors 2020, 20, 6640. [Google Scholar] [CrossRef]
- Lo, X.-C.; Li, J.-Y.; Lee, M.-T.; Yao, D.-J. Frequency shift of a SH-SAW biosensor with glutaraldehyde and 3-aminopropyltriethoxysilane functionalized films for detection of epidermal growth factor. Biosensors 2020, 10, 92. [Google Scholar] [CrossRef]
- Cham, B.E. Nature of the interaction between low-density lipoproteins and polyanions and metal ions, as exemplified by heparin and Ca2+. Clin. Chem. 1976, 22, 1812–1816. [Google Scholar] [CrossRef]
- Wieland, H.; Seidel, D. A simple specific method for precipitation of low density lipoproteins. J. Lipid Res. 1983, 24, 904–909. [Google Scholar] [CrossRef]
- Hirano, T.; Ito, Y.; Saegusa, H.; Yoshino, G. A novel and simple method for quantification of small, dense LDL. J. Lipid Res. 2003, 44, 2193–2201. [Google Scholar] [CrossRef]
- Hirano1, T.; Ito, Y.; Yoshino, G. Measurement of Small Dense Low-density Lipoprotein Particles. J. Atheroscler. Thromb. 2005, 12, 67–72. [Google Scholar] [CrossRef]
- Huang, Y.; Das, P.K.; Bhethanabotla, V.R. Surface acoustic waves in biosensing applications. Sens. Actuators Rep. 2021, 3, 100041. [Google Scholar] [CrossRef]
- Jiménez, B.; Holmes, E.; Heude, C.; Tolson, R.F.; Harvey, N.; Lodge, S.L.; Chetwynd, A.J.; Cannet, C.; Fang, F.; Pearce, J.T. Quantitative lipoprotein subclass and low molecular weight metabolite analysis in human serum and plasma by 1H NMR spectroscopy in a multilaboratory trial. Anal. Chem. 2018, 90, 11962–11971. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.-H.; Peng, Y.-C.; Lin, S.-M.; Yatsuda, H.; Liu, S.-H.; Liu, S.-J.; Kuo, C.-Y.; Wang, R. Measurements of Anti-SARS-CoV-2 Antibody Levels after Vaccination Using a SH-SAW Biosensor. Biosensors 2022, 12, 599. [Google Scholar] [CrossRef] [PubMed]
- Yatsuda, H.; Kogai, T.; Goto, M.; Kano, K.; Yoshimura, N. Immunosensor Using 250 MHz Shear Horizontal Surface Acoustic Wave Delay Line. In Proceedings of the 2018 Asia-Pacific Microwave Conference (APMC), Kyoto, Japan, 6–9 November 2018; pp. 566–568. [Google Scholar]
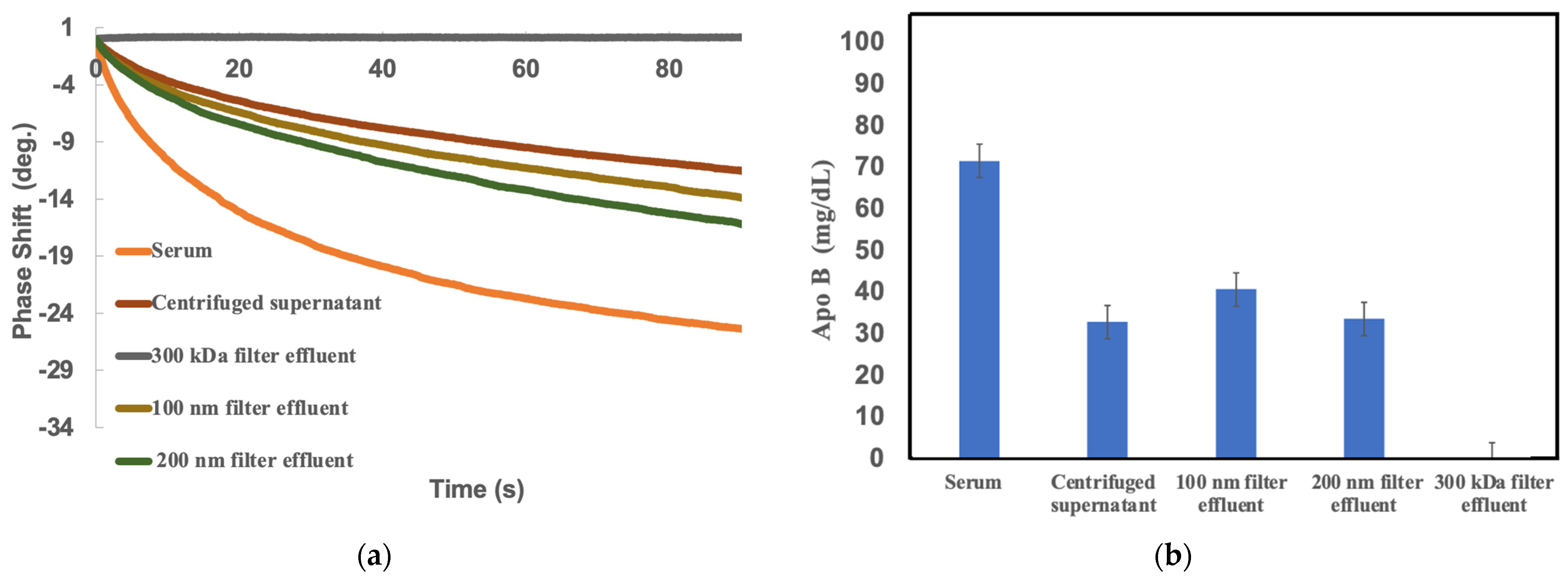

| Samples | LDL Cholesterol (mg/dL) | Apo-B100 (mg/dL) | LDL-ApoB (mg/dL) | sd-LDL-ApoB (* LDL-6) (mg/dL) |
|---|---|---|---|---|
| Serum | 76.9 | 63.2 | 49.7 | 14.8 |
| Centrifuged Supernatant | 9.0 | 33.0 | 18.4 | 2.9 |
| 300 KDa Effluent | 0.0 | 29.6 | 15.9 | 0.0 |
| 100nm Effluent | 7.4 | 29.7 | 13.7 | 1.0 |
| 200nm Effluent | 13.5 | 36.2 | 19.6 | 3.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chou, T.-H.; Cheng, C.-H.; Lo, C.-J.; Young, G.-H.; Liu, S.-H.; Wang, R.Y.-L. New Advances in Rapid Pretreatment for Small Dense LDL Cholesterol Measurement Using Shear Horizontal Surface Acoustic Wave (SH-SAW) Technology. Int. J. Mol. Sci. 2024, 25, 1044. https://doi.org/10.3390/ijms25021044
Chou T-H, Cheng C-H, Lo C-J, Young G-H, Liu S-H, Wang RY-L. New Advances in Rapid Pretreatment for Small Dense LDL Cholesterol Measurement Using Shear Horizontal Surface Acoustic Wave (SH-SAW) Technology. International Journal of Molecular Sciences. 2024; 25(2):1044. https://doi.org/10.3390/ijms25021044
Chicago/Turabian StyleChou, Tai-Hua, Chia-Hsuan Cheng, Chi-Jen Lo, Guang-Huar Young, Szu-Heng Liu, and Robert Y-L Wang. 2024. "New Advances in Rapid Pretreatment for Small Dense LDL Cholesterol Measurement Using Shear Horizontal Surface Acoustic Wave (SH-SAW) Technology" International Journal of Molecular Sciences 25, no. 2: 1044. https://doi.org/10.3390/ijms25021044
APA StyleChou, T.-H., Cheng, C.-H., Lo, C.-J., Young, G.-H., Liu, S.-H., & Wang, R. Y.-L. (2024). New Advances in Rapid Pretreatment for Small Dense LDL Cholesterol Measurement Using Shear Horizontal Surface Acoustic Wave (SH-SAW) Technology. International Journal of Molecular Sciences, 25(2), 1044. https://doi.org/10.3390/ijms25021044







