The Calcium-Dependent Protein Kinase TaCDPK7 Positively Regulates Wheat Resistance to Puccinia striiformis f. sp. tritici
Abstract
1. Introduction
2. Results
2.1. Mining of CDPK Members from Wheat Genome
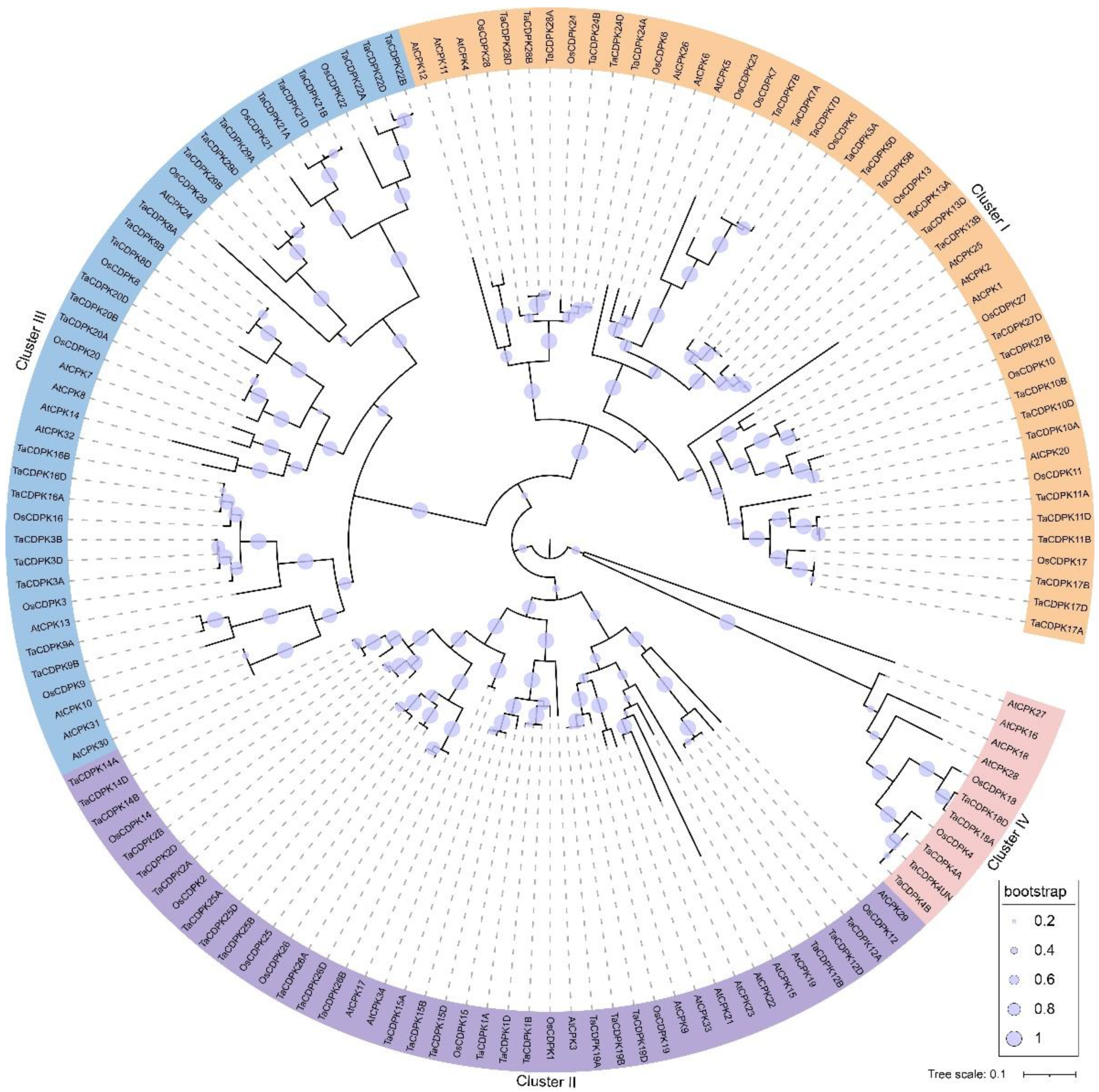
2.2. Phylogeny and Computational Characterization of Wheat CDPKs
2.3. Expression Patterns of TaCDPKs during Wheat–Pst Interaction
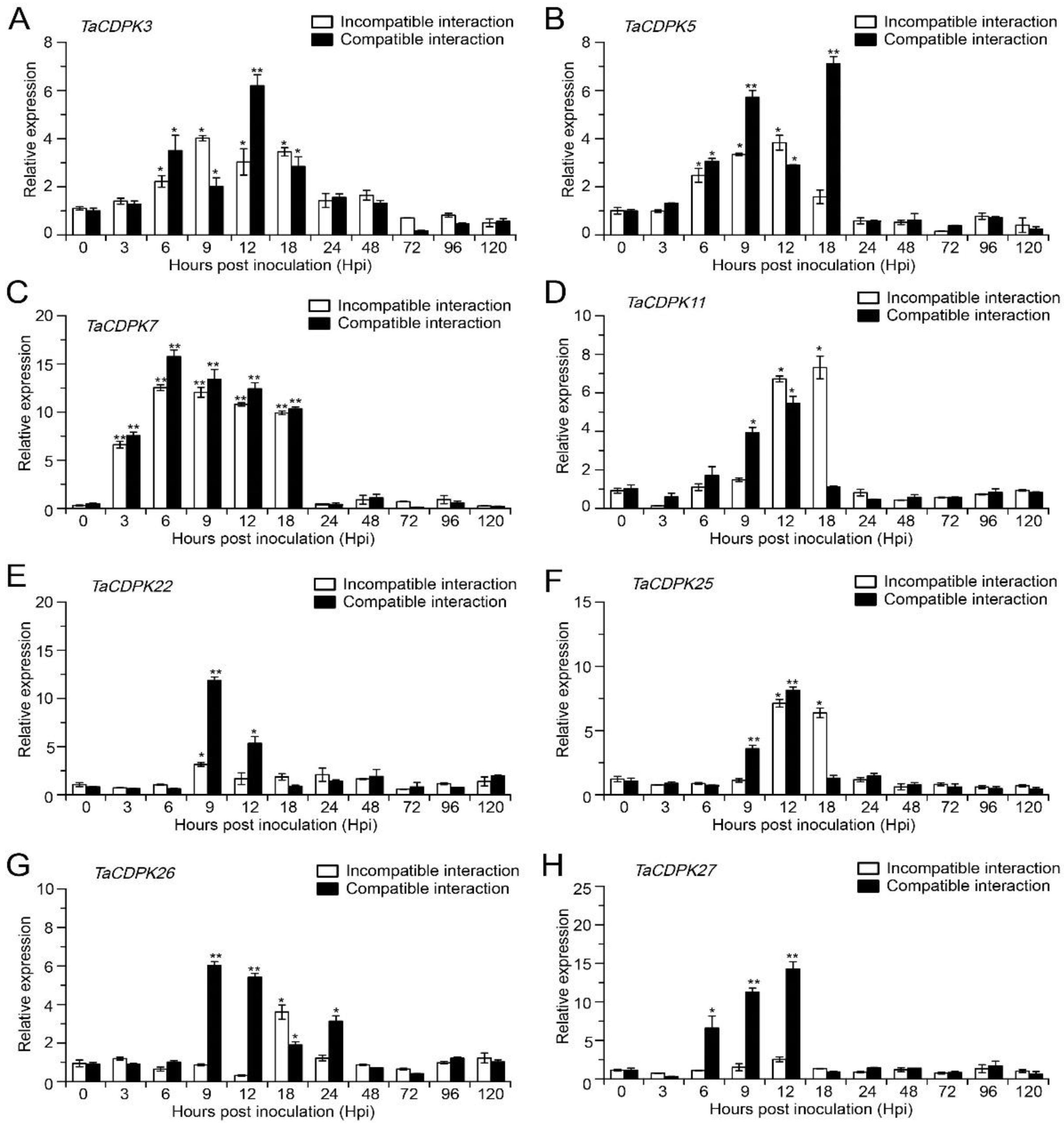
2.4. Transcript Profile of TaCDPKs Challenged with Pst (qRT-PCR Analysis)
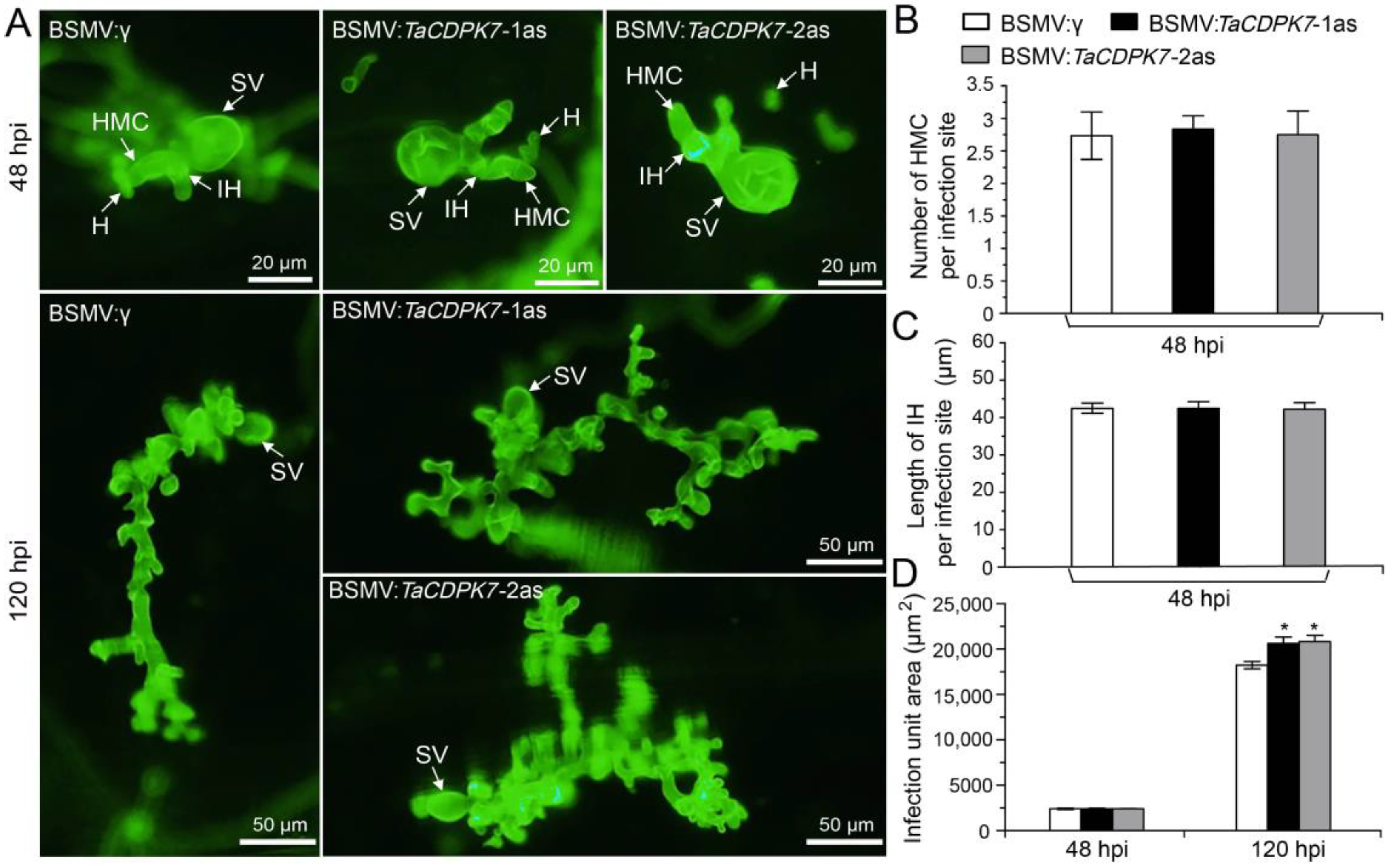
2.5. Silencing of TaCDPK7 Enhances Wheat Susceptibility to Pst
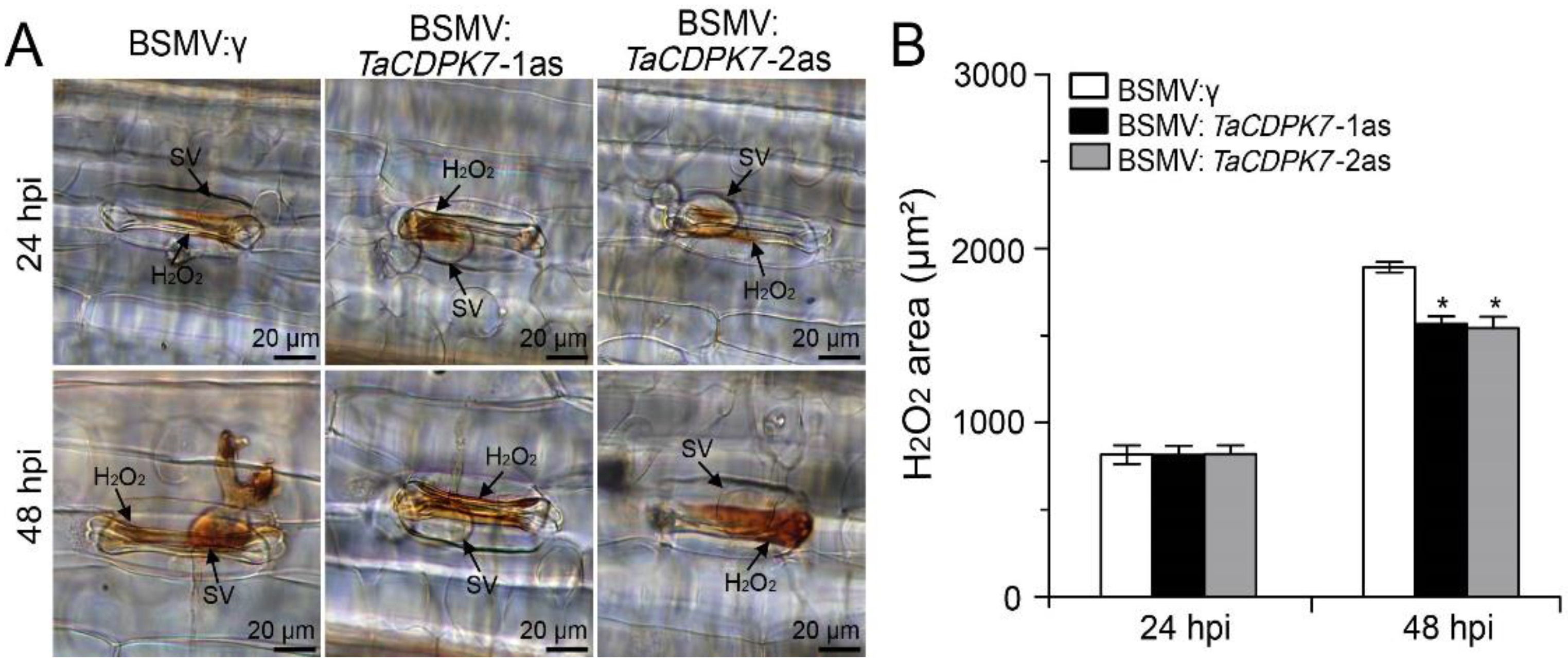
2.6. Knockdown of TaCDPK7 Declines ROS Accumulation
2.7. Knockdown of TaCDPK7 Improves Pst Growth
2.8. Transcript Levels of PR and ROS-Related Genes Are Affected in TaCDPK7-Silenced Plants
3. Discussion
4. Materials and Methods
4.1. Plant Material and Fungal Isolates
4.2. RNA, DNA Extraction, and cDNA Synthesis
4.3. Mining of CDPKs from Wheat Genome
4.4. Phylogeny and Gene Structure Analysis
4.5. The Conserved Motif, Cis-Acting Regulatory Elements, and Synteny Analysis
4.6. Expression Profiling of TaCDPK Family Challenged with Pst
4.7. Real-Time PCR (qRT-PCR) Analysis
4.8. BSMV-Mediated Gene Silencing of TaCDPK7
4.9. Histology of Fungal Infection and Host Response
4.10. Statistical Analysis and Graphical Presentation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, X.Q.; Cox, K.L., Jr.; He, P. Functions of calcium-dependent protein kinases in plant innate immunity. Plants 2014, 3, 160–176. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Kudla, J. Calcium decoding mechanisms in plants. Biochimie 2011, 93, 2054–2059. [Google Scholar] [CrossRef]
- Cheng, S.H.; Willmann, M.R.; Chen, H.C.; Sheen, J. Calcium signaling through protein kinases. The Arabidopsis calcium-dependent protein kinase gene family. Plant Physiol. 2002, 129, 469–485. [Google Scholar] [CrossRef]
- Hamel, L.P.; Sheen, J.; Séguin, A. Ancient signals: Comparative genomics of green plant CDPKs. Trends Plant Sci. 2014, 19, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Simeunovic, A.; Mair, A.; Wurzinger, B.; Teige, M. Know where your clients are: Subcellular localization and targets of calcium-dependent protein kinases. J. Exp. Bot. 2016, 67, 3855–3872. [Google Scholar] [CrossRef] [PubMed]
- Klimecka, M.; Muszyńska, G. Structure and functions of plant calcium-dependent protein kinases. Acta Biochim. 2007, 54, 219–233. [Google Scholar] [CrossRef]
- Asano, T.; Hayashi, N.; Kobayashi, M.; Aoki, N.; Miyao, A.; Mitsuhara, I.; Ichikawa, H.; Komatsu, S.; Hirochika, H.; Kikuchi, S. A rice calcium-dependent protein kinase OsCPK12 oppositely modulates salt-stress tolerance and blast disease resistance. Plant J. 2012, 69, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Goher, F.; Paulsmeyer, M.; Hu, C.G.; Zhang, J.Z. Calcium (Ca2+) sensors and MYC2 are crucial players during jasmonates-mediated abiotic stress tolerance in plants. Plant Biol. 2023, 25, 1025–1034. [Google Scholar] [CrossRef]
- Zou, J.J.; Wei, F.J.; Wang, C.; Wu, J.J.; Ratnasekera, D.; Liu, W.X.; Wu, W.H. Arabidopsis calcium-dependent protein kinase CPK10 functions in abscisic acid-and Ca2+-mediated stomatal regulation in response to drought stress. Plant Physiol. 2010, 154, 1232–1243. [Google Scholar] [CrossRef]
- Coca, M.; Segundo, B.S. AtCPK1 calcium-dependent protein kinase mediates pathogen resistance in Arabidopsis. Plant J. 2010, 63, 526–540. [Google Scholar] [CrossRef]
- Schulz, P.; Herde, M.; Romeis, T. Calcium-dependent protein kinases: Hubs in plant stress signaling and development. Plant Physiol. 2013, 163, 523–530. [Google Scholar] [CrossRef]
- Boudsocq, M.; Sheen, J. CDPKs in immune and stress signaling. Trends Plant Sci. 2013, 18, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Geng, S.F.; Li, A.L.; Tang, L.C.; Yin, L.J.; Wu, L.; Lei, C.L.; Guo, X.P.; Zhang, X.; Jiang, G.H.; Zhai, W.X.; et al. TaCPK2-A, a calcium-dependent protein kinase gene that is required for wheat powdery mildew resistance enhances bacterial blight resistance in transgenic rice. J. Exp. Bot. 2013, 64, 3125–3136. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.Y.; Jiao, J.L.; Wang, W.W.; Jie, X.R.; Wang, H.Z. Silencing of the calcium-dependent protein kinase TaCDPK27 improves wheat resistance to powdery mildew. BMC Plant Biol. 2023, 23, 134. [Google Scholar] [CrossRef] [PubMed]
- Bundó, M.; Coca, M. Enhancing blast disease resistance by overexpression of the calcium-dependent protein kinase OsCPK4 in rice. Plant Biotechnol. J. 2016, 14, 1357–1367. [Google Scholar] [CrossRef]
- Romeis, T.; Ludwig, A.A.; Martin, R.; Jones, J.D. Calcium-dependent protein kinases play an essential role in a plant defence response. EMBO J. 2001, 20, 5556–5567. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Yoshioka, M.; Asai, S.; Nomura, H.; Kuchimura, K.; Mori, H.; Doke, N.; Yoshioka, H. StCDPK5 confers resistance to late blight pathogen but increases susceptibility to early blight pathogen in potato via reactive oxygen species burst. New Phytol. 2012, 196, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.J.; Li, P.; Shimono, M.; Corrion, A.; Higaki, T.; He, S.Y.; Day, B. Arabidopsis calcium-dependent protein kinase 3 regulates actin cytoskeleton organization and immunity. Nat. Commun. 2020, 11, 6234. [Google Scholar] [CrossRef] [PubMed]
- Boudsocq, M.; Willmann, M.R.; McCormack, M.; Lee, H.; Shan, L.; He, P.; Bush, J.; Cheng, S.H.; Sheen, J. Differential innate immune signalling via Ca2+ sensor protein kinases. Nature 2010, 464, 418–422. [Google Scholar] [CrossRef]
- Freymark, G.; Diehl, T.; Miklis, M.; Romeis, T.; Panstruga, R. Antagonistic control of powdery mildew host cell entry by barley calcium-dependent protein kinases (CDPKs). Mol. Plant Microbe Interact. 2007, 20, 1213–1221. [Google Scholar] [CrossRef]
- Clavijo, B.J.; Venturini, L.; Schudoma, C.; Accinelli, G.G.; Kaithakottil, G.; Wright, J.; Borrill, P.; Kettleborough, G.; Heavens, D.; Chapman, H. An improved assembly and annotation of the allohexaploid wheat genome identifies complete families of agronomic genes and provides genomic evidence for chromosomal translocations. Genome Res. 2017, 27, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Li, A.L.; Zhu, Y.F.; Tan, X.M.; Wang, X.; Wei, B.; Guo, H.Z.; Zhang, Z.L.; Chen, X.B.; Zhao, G.Y.; Kong, X.Y.; et al. Evolutionary and functional study of the CDPK gene family in wheat (Triticum aestivum L.). Plant Mol. Biol. 2008, 66, 429–443. [Google Scholar] [CrossRef]
- Appels, R.; Eversole, K.; Stein, N.; Feuillet, C.; Keller, B.; Rogers, J.; Pozniak, C.J.; Choulet, F.; Distelfeld, A. Shifting the limits in wheat research and breeding using a fully annotated reference genome. Science 2018, 361, eaar7191. [Google Scholar]
- Bhardwaj, S.C.; Singh, G.P.; Gangwar, O.P.; Prasad, P.; Kumar, S. Status of wheat rust research and progress in rust management-Indian context. Agronomy 2019, 9, 892. [Google Scholar] [CrossRef]
- Zhang, G.S.; Zhao, Y.Y.; Kang, Z.S.; Zhao, J. First report of a Puccinia striiformis f. sp. tritici race virulent to wheat stripe rust resistance gene Yr5 in China. Plant Dis. 2020, 104, 284. [Google Scholar] [CrossRef]
- Tekin, M.; Cat, A.; Akan, K.; Catal, M.; Akar, T. A new virulent race of wheat stripe rust pathogen (Puccinia striiformis f. sp. tritici) on the resistance gene Yr5 in Turkey. Plant Dis. 2021, 105, 3292. [Google Scholar] [CrossRef]
- Asano, T.; Tanaka, N.; Yang, G.; Hayashi, N.; Komatsu, S. Genome-wide identification of the rice calcium-dependent protein kinase and its closely related kinase gene families: Comprehensive analysis of the CDPKs gene family in rice. Plant Cell Physiol. 2005, 46, 356–366. [Google Scholar] [CrossRef]
- Holub, E.B. The arms race is ancient history in Arabidopsis, the wildflower. Nat. Rev. Genet. 2001, 2, 516–527. [Google Scholar] [CrossRef]
- Liu, L.; White, M.J.; MacRae, T.H. Transcription factors and their genes in higher plants: Functional domains, evolution and regulation. Eur. J. Biochem. 1999, 262, 247–257. [Google Scholar] [CrossRef]
- Hawku, M.D.; Goher, F.; Islam, M.A.; Guo, J.; He, F.X.; Bai, X.X.; Yuan, P.; Kang, Z.S.; Guo, J. TaAP2-15, an AP2/ERF transcription factor, is positively involved in wheat resistance to Puccinia striiformis f. sp. tritici. Int. J. Mol. Sci. 2021, 22, 2080. [Google Scholar] [CrossRef]
- Bai, X.X.; Peng, H.; Goher, F.; Islam, M.A.; Xu, S.D.; Guo, J.; Kang, Z.S.; Guo, J. A candidate effector protein PstCFEM1 contributes to virulence of stripe rust fungus and impairs wheat immunity. Stress Biol. 2022, 2, 21. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.Y.; Wang, P.C.; Chen, J.; Song, C.P. Comprehensive functional analysis of the catalase gene family in Arabidopsis thaliana. J. Integr. Plant Biol. 2008, 50, 1318–1326. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Q.; Wang, Q.L.; Chen, Q.; Yin, X.; Qian, M.; Sun, X.D.; Yang, Y.P. Genome-wide survey indicates diverse physiological roles of the barley (Hordeum vulgare L.) calcium-dependent protein kinase genes. Sci Rep. 2017, 7, 5306. [Google Scholar] [CrossRef]
- Feldman, M.; Levy, A.A. Genome evolution due to allopolyploidization in wheat. Genetics 2012, 192, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Chauve, C.; Doyon, J.P.; El-Mabrouk, N. Gene family evolution by duplication, speciation, and loss. J. Comput. Biol. 2008, 15, 1043–1062. [Google Scholar] [CrossRef] [PubMed]
- Ishihama, N.; Yoshioka, H. Post-translational regulation of WRKY transcription factors in plant immunity. Curr. Opin. Plant Biol. 2012, 15, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.Q.; Chen, X.; Lin, W.W.; Chen, S.X.; Lu, D.P.; Niu, Y.J.; Li, L.; Cheng, C.; McCormack, M.; Sheen, J.; et al. Bifurcation of Arabidopsis NLR immune signaling via Ca2+-dependent protein kinases. PLoS Pathog. 2013, 9, e1003127. [Google Scholar] [CrossRef]
- Dubiella, U.; Seybold, H.; Durian, G.; Komander, E.; Lassig, R.; Witte, C.P.; Schulze, W.X.; Romeis, T. Calcium-dependent protein kinase/NADPH oxidase activation circuit is required for rapid defense signal propagation. Proc. Natl. Acad. Sci. USA 2013, 110, 8744–8749. [Google Scholar] [CrossRef]
- Kadota, Y.; Sklenar, J.; Derbyshire, P.; Stransfeld, L.; Asai, S.; Ntoukakis, V.; Jones, J.D.; Shirasu, K.; Menke, F.; Jones, A. Direct regulation of the NADPH oxidase RBOHD by the PRR-associated kinase BIK1 during plant immunity. Mol. Cell 2014, 54, 43–55. [Google Scholar] [CrossRef]
- Luo, X.M.; Xu, N.; Huang, J.K.; Gao, F.; Zou, H.S.; Boudsocq, M.; Coaker, G.; Liu, J. A lectin receptor-like kinase mediates pattern-triggered salicylic acid signaling. Plant Physiol. 2017, 174, 2501–2514. [Google Scholar] [CrossRef]
- Bredow, M.; Monaghan, J. Regulation of plant immune signaling by calcium-dependent protein kinases. Mol. Plant Microbe Interact. 2019, 32, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Monaghan, J.; Matschi, S.; Shorinola, O.; Rovenich, H.; Matei, A.; Segonzac, C.; Malinovsky, F.G.; Rathjen, J.P.; MacLean, D.; Romeis, T. The calcium-dependent protein kinase CPK28 buffers plant immunity and regulates BIK1 turnover. Cell Host Microbe 2014, 16, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Gravino, M.; Savatin, D.V.; Macone, A.; De Lorenzo, G. Ethylene production in Botrytis cinerea-and oligogalacturonide-induced immunity requires calcium-dependent protein kinases. Plant J. 2015, 84, 1073–1086. [Google Scholar] [CrossRef]
- Li, S.; Han, X.F.; Yang, L.Y.; Deng, X.X.; Wu, H.J.; Zhang, M.M.; Liu, Y.B.; Zhang, S.Q.; Xu, J. Mitogen-activated protein kinases and calcium-dependent protein kinases are involved in wounding-induced ethylene biosynthesis in Arabidopsis thaliana. Plant Cell Environ. 2018, 41, 134–147. [Google Scholar] [CrossRef]
- Lachaud, C.; Prigent, E.; Thuleau, P.; Grat, S.; Silva, D.D.; Briere, C.; Mazars, C.; Cotelle, V. 14-3-3-regulated Ca2+-dependent protein kinase CPK3 is required for sphingolipid-induced cell death in Arabidopsis. Cell Death Differ. 2013, 20, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Perraki, A.; Gronnier, J.; Gouguet, P.; Boudsocq, M.; Deroubaix, A.F.; Simon, V.; German-Retana, S.; Legrand, A.; Habenstein, B.; Zipfel, C.; et al. REM1.3’s phospho-status defines its plasma membrane nanodomain organization and activity in restricting PVX cell-to-cell movement. PLoS Pathog. 2018, 14, e1007378. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, L.; Zhang, J.; Liu, P.; Chen, M.; Chen, Z.H.; Zhong, K.L.; Liu, J.Q.; Chen, J.P.; Yang, J. TaTHI2 interacts with Ca2+-dependent protein kinase TaCPK5 to suppress virus infection by regulating ROS accumulation. Plant Biotechnol. J. 2023. [Google Scholar] [CrossRef]
- Wei, X.N.; Shen, F.D.; Hong, Y.T.; Rong, W.; Du, L.P.; Liu, X.; Xu, H.J.; Ma, L.J.; Zhang, Z.Y. The wheat calcium- dependent protein kinase TaCPK7-D positively regulates host resistance to sharp eyespot disease. Mol. Plant Pathol. 2016, 17, 1252–1264. [Google Scholar] [CrossRef]
- Scofield, S.R.; Huang, L.; Brandt, A.S.; Gill, B.S. Development of a virus-induced gene-silencing system for hexaploid wheat and its use in functional analysis of the Lr21-mediated leaf rust resistance pathway. Plant Physiol. 2005, 138, 2165–2173. [Google Scholar] [CrossRef]
- Garcia-Brugger, A.; Lamotte, O.; Vandelle, E.; Bourque, S.; Lecourieux, D.; Poinssot, B.; Wendehenne, D.; Pugin, A. Early signaling events induced by elicitors of plant defenses. Mol. Plant Microbe Interact. 2006, 19, 711–724. [Google Scholar] [CrossRef]
- Siou, H.; Jiang, J.Z.; Chen, B.H.; Kuo, C.H.; Ho, S.L. Overexpression of a constitutively active truncated form of OsCDPK1 confers disease resistance by affecting OsPR10a expression in rice. Sci. Rep. 2018, 8, 403. [Google Scholar] [CrossRef]
- Bai, X.X.; Huang, X.L.; Tian, S.X.; Peng, H.; Zhan, G.M.; Goher, F.; Guo, J.; Kang, Z.; Guo, J. RNAi-mediated stable silencing of TaCSN5 confers broad-spectrum resistance to Puccinia striiformis f. sp. tritici. Mol. Plant Pathol. 2021, 22, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.M.; Zhang, Y.P.; Han, D.J.; Kang, Z.S.; Li, G.P.; Gao, A.Z.; Chen, P.D. SSR and STS markers for wheat stripe rust resistance gene Yr26. Euphytica. 2008, 159, 359–366. [Google Scholar] [CrossRef]
- Bai, X.X.; Zhan, G.M.; Tian, S.X.; Peng, H.; Cui, X.Y.; Islam, M.A.; Goher, F.; Ma, Y.Z.; Kang, Z.; Xu, Z.S. Transcription factor BZR2 activates chitinase Cht20. 2 transcription to confer resistance to wheat stripe rust. Plant Physiol. 2021, 187, 2749–2762. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.T.; Goher, F.; Chen, L.; Song, J.C.; Zhao, J.Q. Genome-wide identification, phylogeny and expression analysis of subtilisin (SBT) gene family under wheat biotic and abiotic stress. Plants 2023, 12, 3065. [Google Scholar] [CrossRef]
- Huang, X.L.; Bai, X.X.; Qian, C.W.; Liu, S.; Goher, F.; He, F.X.; Zhao, G.S.; Pei, G.L.; Zhao, H.; Wang, J.F.; et al. TaUAM3, a UDP-Ara mutases protein, positively regulates wheat resistance to the stripe rust fungus. Food Energy Secur. 2023, 12, e456. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, 293–296. [Google Scholar] [CrossRef]
- Khan, F.S.; Zeng, R.F.; Gan, Z.M.; Zhang, J.Z.; Hu, C.G. Genome-wide identification and expression profiling of the WOX gene family in Citrus sinensis and functional analysis of a CsWUS member. Int. J. Mol. Sci. 2021, 22, 4919. [Google Scholar] [CrossRef]
- Hu, B.; Jin, J.P.; Guo, A.Y.; Zhang, H.; Luo, J.C.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, 202–208. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, 296–303. [Google Scholar] [CrossRef]
- Artimo, P.; Jonnalagedda, M.; Arnold, K.; Baratin, D.; Csardi, G.; De Castro, E.; Duvaud, S.; Flegel, V.; Fortier, A.; Gasteiger, E. ExPASy: SIB bioinformatics resource portal. Nucleic Acids Res. 2012, 40, W597–W603. [Google Scholar] [CrossRef] [PubMed]
- Higo, K.; Ugawa, Y.; Iwamoto, M.; Korenaga, T. Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Res. 1999, 27, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.H.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Islam, M.A.; Lin, H.C.; Ji, C.; Duan, Y.H.; Liu, P.; Zeng, Q.; Day, B.; Kang, Z.S.; Guo, J. Genome-wide identification of cyclic nucleotide-gated ion channel gene family in wheat and functional analyses of TaCNGC14 and TaCNGC16. Front. Plant Sci. 2018, 9, 18. [Google Scholar] [CrossRef]
- Zheng, W.M.; Huang, L.L.; Huang, J.Q.; Wang, X.J.; Chen, X.M.; Zhao, J.; Guo, J.; Zhuang, H.; Qiu, C.Z.; Liu, J.; et al. High genome heterozygosity and endemic genetic recombination in the wheat stripe rust fungus. Nat. Commun. 2013, 4, 2673. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goher, F.; Bai, X.; Liu, S.; Pu, L.; Xi, J.; Lei, J.; Kang, Z.; Jin, Q.; Guo, J. The Calcium-Dependent Protein Kinase TaCDPK7 Positively Regulates Wheat Resistance to Puccinia striiformis f. sp. tritici. Int. J. Mol. Sci. 2024, 25, 1048. https://doi.org/10.3390/ijms25021048
Goher F, Bai X, Liu S, Pu L, Xi J, Lei J, Kang Z, Jin Q, Guo J. The Calcium-Dependent Protein Kinase TaCDPK7 Positively Regulates Wheat Resistance to Puccinia striiformis f. sp. tritici. International Journal of Molecular Sciences. 2024; 25(2):1048. https://doi.org/10.3390/ijms25021048
Chicago/Turabian StyleGoher, Farhan, Xingxuan Bai, Shuai Liu, Lefan Pu, Jiaojiao Xi, Jiaqi Lei, Zhensheng Kang, Qiaojun Jin, and Jun Guo. 2024. "The Calcium-Dependent Protein Kinase TaCDPK7 Positively Regulates Wheat Resistance to Puccinia striiformis f. sp. tritici" International Journal of Molecular Sciences 25, no. 2: 1048. https://doi.org/10.3390/ijms25021048
APA StyleGoher, F., Bai, X., Liu, S., Pu, L., Xi, J., Lei, J., Kang, Z., Jin, Q., & Guo, J. (2024). The Calcium-Dependent Protein Kinase TaCDPK7 Positively Regulates Wheat Resistance to Puccinia striiformis f. sp. tritici. International Journal of Molecular Sciences, 25(2), 1048. https://doi.org/10.3390/ijms25021048







