Delivery of Adeno-Associated Virus Vectors to the Central Nervous System for Correction of Single Gene Disorders
Abstract
1. Introduction
| Disease | Specific Route | Transgene | Capsid | Dose & Volume | Ages |
|---|---|---|---|---|---|
| AADC deficiency [19,20] | Bilateral putamen [20] | hAADC | AAV2 | 160 µL per hemisphere: 1.81 × 1011 vg 200 µL per hemisphere: 2 × 1011 vg | 1.7–8.4 years 4–19 years |
| Bilateral Substantia nigra compacta and Ventral tegmental area (VTA) [19] | 80 µL per hemisphere: 1.3 × 1011 vg–4.2 × 1011 vg | 4–9 years | |||
| Alhzeimer’s Disease [21] | Basal forebrain (which contains the Nucleus basalis of Meynert) | NGF | AAV2 | 2.0 × 1011 (n = 23) | 55–80 years |
| Batten Disease CLN2 [22] NCT00151216 NCT01161576 NCT01414985 | 12 intraparenchymal locations (six on each side) | hCLN2 | AAV2 | 2.5 × 1012 vg × 12 = 3 × 1012 (n = 10) | 3–18 years |
| AAVrh.10 | 3 × 1012 particle units or 2.4–7.5 × 1010 vg in 150 μL × 12 = 9 × 1011 (n = 8) | 2–18 years 3–18 years | |||
| Canavan Disease [23] | 12 intraparenchymal locations (six on each side) frontal, periventricular, occipital | ASPA | AAV2 | 1.1 × 1012 | 4–83 months |
| Huntington’s disease [24] | Bilateral caudate | miHTT | AAV9 | 6 × 1012–6 × 1013 | 25–65 years |
| Metachromatic leukodystrophy (MLD) NCT01801709 | 12 locatinos in white matter | cuARSA | AAVrh10 | 1 × 1012; 4 × 1012 vg | 6 months–5 years |
| MPS I NCT03580083 | Intraparenchymal | IDUA | AAV9 | 1 × 1010–5 × 1010 gc/g brainmass (n = 5) | >4 months |
| MPS II NCT03566043 | Intraparenchymal | IDS | AAV9 | 1.3 × 1010–2.9 × 1011 moi/mL per site | 4 months–5 years |
| MPS IIIA NCT03612869 | 12 locations in white matter anterior, medial, and posterior to the basal ganglia [25] | hSGSH-IRES-SUMF1 | AAVrh10 | 720 µL of 7.2 × 1011 vg over 12 sites (n = 4) | 18 months to 6 years |
| MPS IIIB NCT03300453 | 16 sites in white matter | NAGLU | AAV5 | 4 × 1012 over 16 sites (n = 7) | 18–60 months |
| Multiple Systems atrophy NCT04680065 | Putamen | GDNF | AAV2 | Dose unknown (n = 9) | 35–75 years |
| Parkinson’s Disease | Bilateral postcommissural putamen CED [26,27] | hAADC | AVV2 | 200 µL over the four injection sites; 9 × 1010–3 × 1011 vg (n = 10) | 57–71 years |
| Unilateral subthalamic nucleus [28] | GAD | AAV2 | 1 × 1012 | 53–65 years | |
| Bilateral putamen [29,30] | hGDNF | AAV2 | 9 × 1010–3 × 1012 vg | >18 yo | |
| Bilateral putamen CED [31] Substantia nigra [32] | Neuturin | AAV2 | 1.3 × 1011–2.4 × 1012 | ||
| Tay-Sachs and Sandhoff Disease (GM2) NCT04669535 | Bilateral thalamic convection-enhanced delivery (CED) [33] | HEXA/HEXB | AAVrh8 | 4.08 × 1013 bilaterally | 6 months–12 years |
| Cisterna Magna [33] and Lumbar intrathecal (IT) | AAVrh8 | 1 × 1014–4.2 × 1013 vg | |||
| Krabbe Disease NCT04771416 | Cisterna Magna | GALC | AAVrh10 | 1.4 × 1011–5.0 × 1011 gc/g brain mass (n = 24) | 1–9 months |
| MPS II NCT04571970 | Cisterna Magna Intracereroventricular | IDS | AAV9 | Dose unkown | 5–17 years |
| Frontotemporal Dementia (FTD) NCT04408625 | Cisterna Magna | GRN | AAV9 | Dose unknown | 30–85 years |
| Canavan Disease NCT04833907 | Intracerebroventricular | ASPA | AAV-oligo001 | 3.7 × 1013 | 3–60 months |
| Parkinson’s Disease NCT04127578 | Cisterna Magna | GBA1 | AAV9 | Ascending dose | 35–80 years |
| Gaucher Disease NCT04411654 | Cisterna Magna | GBA1 | AAV9 | Ascending dose | 0–24 months |
| GM1 NCT04273269 | Cisterna Magna | GLB1 | AAVrh10 | 8 × 1012 vg/kg | <3 years |
| ALS [34] | Lumbar intrathecal (IT) | miR-SOD1 | AAVrh10 | 4.2 × 1014 vg | 22–56 yo |
| CLN3 NCT03770572 | CLN3 | AAV9 | 6 × 1013–1.2 × 1014 vg | 3–10 years | |
| CLN6 NCT02725580 | CLN6 | AAV9 | Dose unknown | >1 year | |
| CLN7 NCT04737460 | CLN7 | AAV9 | 5 × 1014–1 × 1015 vg | 1–18 years | |
| SMA NCT03381729 [35] | SMN | scAAV9 | 6 × 1013–1.2 × 1014–2.4 × 1014 vg | 6–60 months | |
| Giant Axonal Neuropathy NCT02362438 [36] | Gigaxonin | scAAV9 | 3.5 × 1013 vg | 3–99 years | |
| Adrenomyeloneuropathy (AMN) NCT05394064 | ABCD1 | AAV9 | 1.0 × 1015 vg 3.0 × 1014 vg | 18–65 years | |
| Spinal muscular atrophy NCT03505099 | SMN | scAAV9 | 6.0 × 1013 vg 1.2 × 1014 vg 2.4 × 1014 vg | 6–60 months | |
| Alzheimer’s disease NCT04133454 | IV and IT | hTERT | AAV2 | Dose unknown | >45 years |
| Krabbe NCT04693598 | Intravenous (IV) | hGALC | AAVrh10 | Dose unknown | <12 months |
| Gaucher NCT05324943 | GBA1 | AAVS3 | Dose unknown | >18 years | |
| Spinal muscular atrophy (SMA) [13,37] | SMN | scAAV9 | 1.1 × 1014 vg/kg | <42 days to <6 months (depending on trial) | |
| MPS I NCT02702115 | IDUA | AAV6-ZFN | Does unknown | >5 years | |
| MPS IIIA NCT02716246 NCT04088734 | hSGSH | scAAV9 | 0.5 × 1013–3.00 × 1013 vg/kg | 2–18 years | |
| MPS IIIB NCT03300453 | hNAGLU | AAV9 | 2.0 × 1013–1.00 × 1014 vg/kg | 18 months–60 years | |
| GM1 NCT03952637 | GLB1 | AAV9 | 1.5 × 1012–4.5 × 1013 vg/kg | 6 months–12 years | |
| Canavan NCT04998396 | ASPA | AAV9 | 4.50 × 1013 vg/kg | Up to 30 months | |
| DMD [38] NCT05096221 | micro-dystrophin | rAAVrh74 | 1.0 × 1014–3.0 × 1014 vg/kg | 4–5 years |
2. Routes of Delivery to the Central Nervous System
2.1. Intraparenchymal (IP) Delivery
2.1.1. Convection-Enhanced Delivery
2.1.2. Axonal Transport
2.1.3. Cross Correction
2.1.4. Toxicity Associated with Direct Intraparenchymal Injection
2.1.5. Immune Response Associated with Direct Intraparenchymal Injection
2.2. Intravenous (IV) Delivery
2.2.1. Biodistribution of AAV Delivery
2.2.2. Immune Response Associated with IV Delivery of AAV
2.2.3. Clinical Toxicity Associated with IV Delivery of AAV
2.3. Delivery to the Cerebrospinal Fluid (CSF)
2.3.1. Lumbar Intrathecal (IT) Delivery
2.3.2. Intracerebroventricular (ICV) Delivery
2.3.3. Cisterna Magna (CM) Delivery
2.3.4. Toxicity
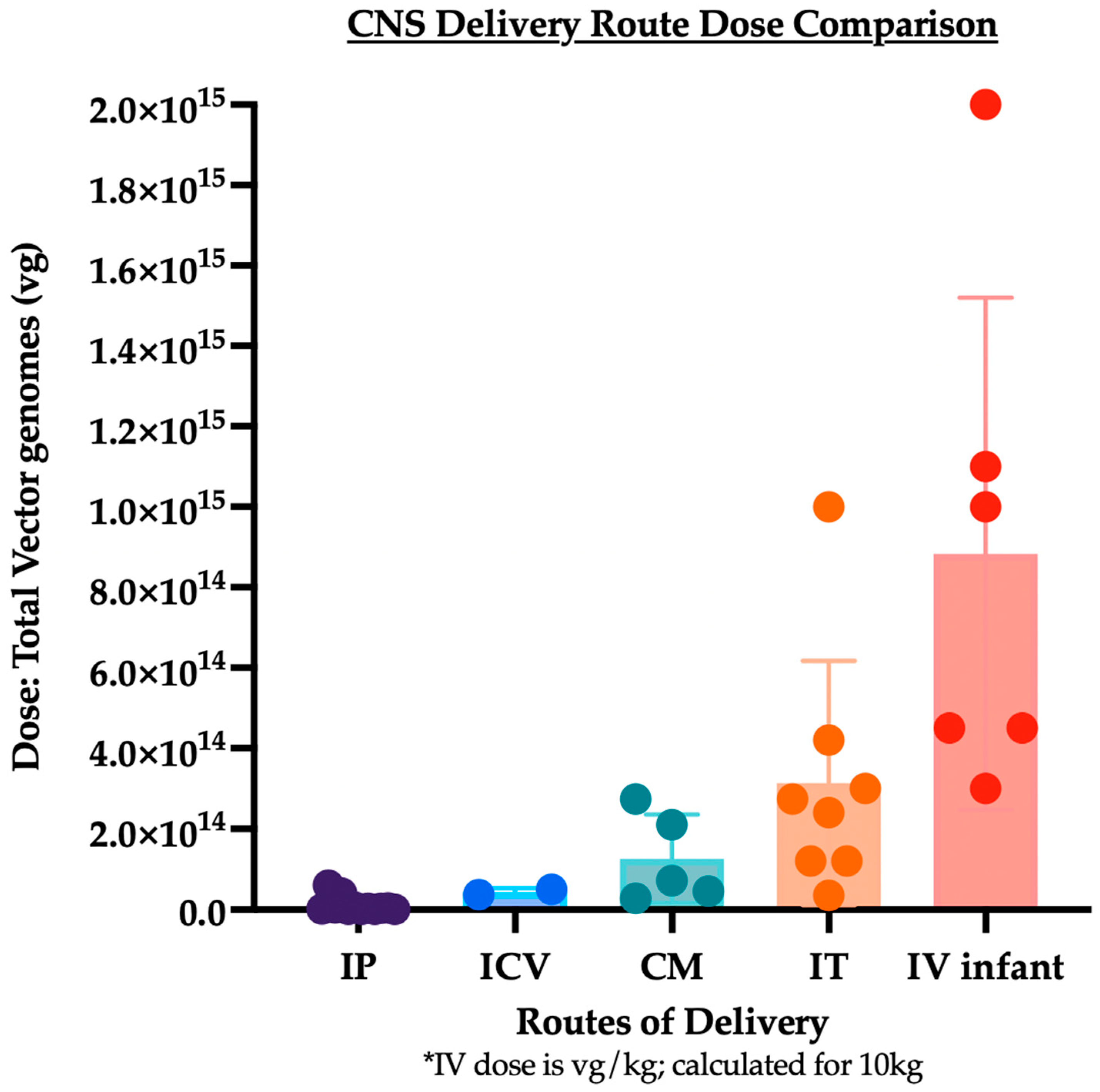
3. Summary and Analysis
4. Literature Review Methods
Author Contributions
Funding
Conflicts of Interest
References
- Kotin, R.M.; Siniscalco, M.; Samulski, R.J.; Zhu, X.D.; Hunter, L.; Laughlin, C.A.; McLaughlin, S.; Muzyczka, N.; Rocchi, M.; Berns, K.I. Site-Specific Integration by Adeno-Associated Virus. Proc. Natl. Acad. Sci. USA 1990, 87, 2211–2215. [Google Scholar] [CrossRef]
- Tatschin, J.-D.; West, M.H.P.; Sandbank, T.; Carter, B.J. A Human Parvovirus, Adeno-Associated Virus, as a Eucaryotic Vector: Transient Expression and Encapsidation of the Procaryotic Gene for Chloramphenicol Acetyltransferase. Mol. Cell. Biol. 1984, 4, 2072–2081. [Google Scholar] [CrossRef]
- Hermonat, P.L.; Muzyczka, N. Use of Adeno-Associated Virus as a Mammalian DNA Cloning Vector: Transduction of Neomycin Resistance into Mammalian Tissue Culture Cells. Proc. Natl. Acad. Sci. USA 1984, 81, 6466–6470. [Google Scholar] [CrossRef]
- Flotte, T.R.; Afione, S.A.; Conrad, C.; McGrath, S.A.; Solow, R.; Oka, H.; Zeitlin, P.L.; Guggino, W.B.; Carter, B.J. Stable in Vivo Expression of the Cystic Fibrosis Transmembrane Conductance Regulator with an Adeno-Associated Virus Vector. Proc. Natl. Acad. Sci. USA 1993, 90, 10613–10617. [Google Scholar] [CrossRef]
- Afione, S.A.; Conrad, C.K.; Kearns, W.G.; Chunduru, S.; Adams, R.; Reynolds, T.C.; Guggino, W.B.; Cutting, G.R.; Carter, B.J.; Flotte, T.R. In Vivo Model of Adeno-Associated Virus Vector Persistence and Rescue. J. Virol. 1996, 70, 3235–3241. [Google Scholar] [CrossRef]
- Kaplitt, M.G.; Leone, P.; Samulski, R.J.; Xiao, X.; Pfaff, D.W.; O’Malley, K.L.; During, M.J. Long-Term Gene Expression and Phenotypic Correction Using Adeno-Associated Virus Vectors in the Mammalian Brain. Nat. Genet. 1994, 8, 148–154. [Google Scholar] [CrossRef]
- Kessler, P.D.; Podsakoff, G.M.; Chen, X.; McQuiston, S.A.; Colosi, P.C.; Matelis, L.A.; Kurtzman, G.J.; Byrne, B.J. Gene Delivery to Skeletal Muscle Results in Sustained Expression and Systemic Delivery of a Therapeutic Protein. Proc. Natl. Acad. Sci. USA 1996, 93, 14082–14087. [Google Scholar] [CrossRef]
- Rabinowitz, J.E.; Rolling, F.; Li, C.; Conrath, H.; Xiao, W.; Xiao, X.; Samulski, R.J. Cross-Packaging of a Single Adeno-Associated Virus (AAV) Type 2 Vector Genome into Multiple AAV Serotypes Enables Transduction with Broad Specificity. J. Virol. 2001, 76, 791–801. [Google Scholar] [CrossRef]
- Gao, G.; Alvira, M.R.; Somanathan, S.; Lu, Y.; Vandenberghe, L.H.; Rux, J.J.; Calcedo, R.; Sanmiguel, J.; Abbas, Z.; Wilson, J.M. Adeno-Associated Viruses Undergo Substantial Evolution in Primates during Natural Infections. Proc. Natl. Acad. Sci. USA 2003, 100, 6081–6086. [Google Scholar] [CrossRef]
- Gao, G.-P.; Alvira, M.R.; Wang, L.; Calcedo, R.; Johnston, J.; Wilson, J.M. Novel Adeno-Associated Viruses from Rhesus Monkeys as Vectors for Human Gene Therapy. Proc. Natl. Acad. Sci. USA 2002, 99, 11854–11859. [Google Scholar] [CrossRef] [PubMed]
- Foust, K.D.; Poirier, A.; Pacak, C.A.; Mandel, R.J.; Flotte, T.R. Neonatal Intraperitoneal or Intravenous Injections of Recombinant Adeno-Associated Virus Type 8 Transduce Dorsal Root Ganglia and Lower Motor Neurons. Hum. Gene Ther. 2008, 19, 61–70. [Google Scholar] [CrossRef]
- Foust, K.D.; Nurre, E.; Montgomery, C.L.; Hernandez, A.; Chan, C.M.; Kaspar, B.K. Intravascular AAV9 Preferentially Targets Neonatal Neurons and Adult Astrocytes. Nat. Biotechnol. 2009, 27, 59–65. [Google Scholar] [CrossRef]
- Mendell, J.R.; Al-Zaidy, S.; Shell, R.; Arnold, W.D.; Rodino-Klapac, L.R.; Prior, T.W.; Lowes, L.; Alfano, L.; Berry, K.; Church, K.; et al. Single-Dose Gene-Replacement Therapy for Spinal Muscular Atrophy. N. Engl. J. Med. 2017, 377, 1713–1722. [Google Scholar] [CrossRef]
- Choudhury, S.R.; Hudry, E.; Maguire, C.A.; Sena-Esteves, M.; Breakefield, X.O.; Grandi, P. Viral Vectors for Therapy of Neurologic Diseases. Neuropharmacology 2017, 120, 63–80. [Google Scholar] [CrossRef]
- Hudry, E.; Vandenberghe, L.H. Therapeutic AAV Gene Transfer to the Nervous System: A Clinical Reality. Neuron 2019, 101, 839–862. [Google Scholar] [CrossRef]
- Ramirez, S.H.; Hale, J.F.; McCarthy, S.; Cardenas, C.L.L.; Dona, K.N.U.G.; Hanlon, K.S.; Hudry, E.; Cruz, D.D.L.; Ng, C.; Das, S.; et al. An Engineered Adeno-Associated Virus Capsid Mediates Efficient Transduction of Pericytes and Smooth Muscle Cells of the Brain Vasculature. Hum. Gene Ther. 2023, 34, 682–696. [Google Scholar] [CrossRef]
- Kimura, S.; Harashima, H. Current Status and Challenges Associated with CNS-Targeted Gene Delivery across the BBB. Pharmaceutics 2020, 12, 1216. [Google Scholar] [CrossRef]
- Keep, R.F.; Jones, H.C.; Hamilton, M.G.; Drewes, L.R. A Year in Review: Brain Barriers and Brain Fluids Research in 2022. Fluids Barriers CNS 2023, 20, 30. [Google Scholar] [CrossRef]
- Pearson, T.S.; Gupta, N.; Sebastian, W.S.; Imamura-Ching, J.; Viehoever, A.; Grijalvo-Perez, A.; Fay, A.J.; Seth, N.; Lundy, S.M.; Seo, Y.; et al. Gene Therapy for Aromatic L-Amino Acid Decarboxylase Deficiency by MR-Guided Direct Delivery of AAV2-AADC to Midbrain Dopaminergic Neurons. Nat. Commun. 2021, 12, 4251. [Google Scholar] [CrossRef]
- Kojima, K.; Nakajima, T.; Taga, N.; Miyauchi, A.; Kato, M.; Matsumoto, A.; Ikeda, T.; Nakamura, K.; Kubota, T.; Mizukami, H.; et al. Gene Therapy Improves Motor and Mental Function of Aromatic L-Amino Acid Decarboxylase Deficiency. Brain 2019, 142, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Rafii, M.S.; Tuszynski, M.H.; Thomas, R.G.; Barba, D.; Brewer, J.B.; Rissman, R.A.; Siffert, J.; Aisen, P.S.; Team, A.-N.S. Adeno-Associated Viral Vector (Serotype 2)–Nerve Growth Factor for Patients with Alzheimer Disease: A Randomized Clinical Trial. JAMA Neurol. 2018, 75, 834. [Google Scholar] [CrossRef]
- Worgall, S.; Sondhi, D.; Hackett, N.R.; Kosofsky, B.; Kekatpure, M.V.; Neyzi, N.; Dyke, J.P.; Ballon, D.; Heier, L.; Greenwald, B.M.; et al. Treatment of Late Infantile Neuronal Ceroid Lipofuscinosis by CNS Administration of a Serotype 2 Adeno-Associated Virus Expressing CLN2 CDNA. Hum. Gene Ther. 2008, 19, 463–474. [Google Scholar] [CrossRef]
- Leone, P.; Shera, D.; McPhee, S.W.; Francis, J.S.; Kolodny, E.H.; Bilaniuk, L.T.; Wang, D.J.; Assadi, M.; Goldfarb, O.; Goldman, H.W.; et al. Long-term follow-up after gene therapy for canavan disease. Sci. Transl. Med. 2012, 4, 165ra163. [Google Scholar] [CrossRef]
- Thomson, S.B.; Stam, A.; Brouwers, C.; Fodale, V.; Bresciani, A.; Vermeulen, M.; Mostafavi, S.; Petkau, T.L.; Hill, A.; Yung, A.; et al. AAV5-MiHTT-Mediated Huntingtin Lowering Improves Brain Health in a Huntington’s Disease Mouse Model. Brain 2022, 146, 2298–2315. [Google Scholar] [CrossRef]
- Tardieu, M.; Zérah, M.; Husson, B.; de Bournonville, S.; Deiva, K.; Adamsbaum, C.; Vincent, F.; Hocquemiller, M.; Broissand, C.; Furlan, V.; et al. Intracerebral Administration of Adeno-Associated Viral Vector Serotype Rh.10 Carrying Human SGSH and SUMF1 CDNAs in Children with Mucopolysaccharidosis Type IIIA Disease: Results of a Phase I/II Trial. Hum. Gene Ther. 2014, 25, 506–516. [Google Scholar] [CrossRef]
- Christine, C.W.; Starr, P.A.; Larson, P.S.; Eberling, J.L.; Jagust, W.J.; Hawkins, R.A.; VanBrocklin, H.F.; Wright, J.F.; Bankiewicz, K.S.; Aminoff, M.J. Safety and Tolerability of Putaminal AADC Gene Therapy for Parkinson Disease. Neurology 2009, 73, 1662–1669. [Google Scholar] [CrossRef] [PubMed]
- Eberling, J.L.; Jagust, W.J.; Christine, C.W.; Starr, P.; Larson, P.; Bankiewicz, K.S.; Aminoff, M.J. Results from a Phase I Safety Trial of HAADC Gene Therapy for Parkinson Disease. Neurology 2008, 70, 1980–1983. [Google Scholar] [CrossRef]
- Kaplitt, M.G.; Feigin, A.; Tang, C.; Fitzsimons, H.L.; Mattis, P.; Lawlor, P.A.; Bland, R.J.; Young, D.; Strybing, K.; Eidelberg, D.; et al. Safety and Tolerability of Gene Therapy with an Adeno-Associated Virus (AAV) Borne GAD Gene for Parkinson’s Disease: An Open Label, Phase I Trial. Lancet 2007, 369, 2097–2105. [Google Scholar] [CrossRef]
- Mahato, A.K.; Sidorova, Y.A. Glial Cell Line-Derived Neurotrophic Factors (GFLs) and Small Molecules Targeting RET Receptor for the Treatment of Pain and Parkinson’s Disease. Cell Tissue Res. 2020, 382, 147–160. [Google Scholar] [CrossRef]
- Lang, A.E.; Gill, S.; Patel, N.K.; Lozano, A.; Nutt, J.G.; Penn, R.; Brooks, D.J.; Hotton, G.; Moro, E.; Heywood, P.; et al. Randomized Controlled Trial of Intraputamenal Glial Cell Line–Derived Neurotrophic Factor Infusion in Parkinson Disease. Ann. Neurol. 2006, 59, 459–466. [Google Scholar] [CrossRef]
- Marks, W.J.; Bartus, R.T.; Siffert, J.; Davis, C.S.; Lozano, A.; Boulis, N.; Vitek, J.; Stacy, M.; Turner, D.; Verhagen, L.; et al. Gene Delivery of AAV2-Neurturin for Parkinson’s Disease: A Double-Blind, Randomised, Controlled Trial. Lancet Neurol. 2010, 9, 1164–1172. [Google Scholar] [CrossRef]
- Bartus, R.T.; Baumann, T.L.; Siffert, J.; Herzog, C.D.; Alterman, R.; Boulis, N.; Turner, D.A.; Stacy, M.; Lang, A.E.; Lozano, A.M.; et al. Safety/Feasibility of Targeting the Substantia Nigra with AAV2-Neurturin in Parkinson Patients. Neurology 2013, 80, 1698–1701. [Google Scholar] [CrossRef]
- Flotte, T.R.; Cataltepe, O.; Puri, A.; Batista, A.R.; Moser, R.; McKenna-Yasek, D.; Douthwright, C.; Gernoux, G.; Blackwood, M.; Mueller, C.; et al. AAV Gene Therapy for Tay-Sachs Disease. Nat. Med. 2022, 28, 251–259. [Google Scholar] [CrossRef]
- Mueller, C.; Berry, J.D.; McKenna-Yasek, D.M.; Gernoux, G.; Owegi, M.A.; Pothier, L.M.; Douthwright, C.L.; Gelevski, D.; Luppino, S.D.; Blackwood, M.; et al. SOD1 Suppression with Adeno-Associated Virus and MicroRNA in Familial ALS. N. Engl. J. Med. 2020, 383, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Finkel, R.S.; Darras, B.T.; Mendell, J.R.; Day, J.W.; Kuntz, N.L.; Connolly, A.M.; Zaidman, C.M.; Crawford, T.O.; Butterfield, R.J.; Shieh, P.B.; et al. Intrathecal Onasemnogene Abeparvovec for Sitting, Nonambulatory Patients with Spinal Muscular Atrophy: Phase I Ascending-Dose Study (STRONG). J. Neuromuscul. Dis. 2023, 10, 389–404. [Google Scholar] [CrossRef] [PubMed]
- Bailey, R.M.; Armao, D.; Kalburgi, S.N.; Gray, S.J. Development of Intrathecal AAV9 Gene Therapy for Giant Axonal Neuropathy. Mol. Ther.—Methods Clin. Dev. 2018, 9, 160–171. [Google Scholar] [CrossRef]
- Al-Zaidy, S.A.; Kolb, S.J.; Lowes, L.; Alfano, L.N.; Shell, R.; Church, K.R.; Nagendran, S.; Sproule, D.M.; Feltner, D.E.; Wells, C.; et al. AVXS-101 (Onasemnogene Abeparvovec) for SMA1: Comparative Study with a Prospective Natural History Cohort. J. Neuromuscul. Dis. 2019, 6, 307–317. [Google Scholar] [CrossRef]
- Hoy, S.M. Delandistrogene Moxeparvovec: First Approval. Drugs 2023, 83, 1323–1329. [Google Scholar] [CrossRef] [PubMed]
- Souweidane, M.M.; Fraser, J.F.; Arkin, L.M.; Sondhi, D.; Hackett, N.R.; Kaminsky, S.M.; Heier, L.; Kosofsky, B.E.; Worgall, S.; Crystal, R.G.; et al. Gene Therapy for Late Infantile Neuronal Ceroid Lipofuscinosis: Neurosurgical Considerations: Clinical Article. J. Neurosurg. Pediatr. 2010, 6, 115–122. [Google Scholar] [CrossRef]
- Salegio, E.A.; Samaranch, L.; Kells, A.P.; Mittermeyer, G.; Sebastian, W.S.; Zhou, S.; Beyer, J.; Forsayeth, J.; Bankiewicz, K.S. Axonal Transport of Adeno-Associated Viral Vectors Is Serotype-Dependent. Gene Ther. 2013, 20, 348–352. [Google Scholar] [CrossRef]
- Bradbury, A.M.; Peterson, T.A.; Gross, A.L.; Wells, S.Z.; McCurdy, V.J.; Wolfe, K.G.; Dennis, J.C.; Brunson, B.L.; Gray-Edwards, H.; Randle, A.N.; et al. AAV-Mediated Gene Delivery Attenuates Neuroinflammation in Feline Sandhoff Disease. Neuroscience 2017, 340, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Gray-Edwards, H.L.; Randle, A.N.; Maitland, S.A.; Benatti, H.R.; Hubbard, S.M.; Canning, P.F.; Vogel, M.B.; Brunson, B.L.; Hwang, M.; Ellis, L.E.; et al. Adeno-Associated Virus Gene Therapy in a Sheep Model of Tay–Sachs Disease. Hum. Gene Ther. 2018, 29, 312–326. [Google Scholar] [CrossRef]
- Cearley, C.N.; Wolfe, J.H. A Single Injection of an Adeno-Associated Virus Vector into Nuclei with Divergent Connections Results in Widespread Vector Distribution in the Brain and Global Correction of a Neurogenetic Disease. J. Neurosci. 2007, 27, 9928–9940. [Google Scholar] [CrossRef] [PubMed]
- Kells, A.P.; Hadaczek, P.; Yin, D.; Bringas, J.; Varenika, V.; Forsayeth, J.; Bankiewicz, K.S. Efficient Gene Therapy-Based Method for the Delivery of Therapeutics to Primate Cortex. Proc. Natl. Acad. Sci. USA 2009, 106, 2407–2411. [Google Scholar] [CrossRef]
- Gao, G.; Vandenberghe, L.H.; Alvira, M.R.; Lu, Y.; Calcedo, R.; Zhou, X.; Wilson, J.M. Clades of Adeno-Associated Viruses Are Widely Disseminated in Human Tissues. J. Virol. 2004, 78, 6381–6388. [Google Scholar] [CrossRef]
- Lonser, R.R.; Akhter, A.S.; Zabek, M.; Elder, J.B.; Bankiewicz, K.S. Direct Convective Delivery of Adeno-Associated Virus Gene Therapy for Treatment of Neurological Disorders. J. Neurosurg. 2021, 134, 1751–1763. [Google Scholar] [CrossRef]
- Sudhakar, V.; Naidoo, J.; Samaranch, L.; Bringas, J.R.; Lonser, R.R.; Fiandaca, M.S.; Bankiewicz, K.S. Infuse-as-You-Go Convective Delivery to Enhance Coverage of Elongated Brain Targets: Technical Note. J. Neurosurg. 2020, 133, 530–537. [Google Scholar] [CrossRef]
- Keam, S.J. Eladocagene Exuparvovec: First Approval. Drugs 2022, 82, 1427–1432. [Google Scholar] [CrossRef]
- Hwu, W.-L.; Muramatsu, S.; Tseng, S.-H.; Tzen, K.-Y.; Lee, N.-C.; Chien, Y.-H.; Snyder, R.O.; Byrne, B.J.; Tai, C.-H.; Wu, R.-M. Gene Therapy for Aromatic L-Amino Acid Decarboxylase Deficiency. Sci. Transl. Med. 2012, 4, 134ra61. [Google Scholar] [CrossRef] [PubMed]
- Acsády, L. Organization of Thalamic Inputs. In The Thalamus; Halassa, M.M., Ed.; Cambridge University Press: Cambridge, UK, 2022; pp. 27–44. [Google Scholar] [CrossRef]
- Baek, R.C.; Broekman, M.L.D.; Leroy, S.G.; Tierney, L.A.; Sandberg, M.A.; d’Azzo, A.; Seyfried, T.N.; Sena-Esteves, M. AAV-Mediated Gene Delivery in Adult GM1-Gangliosidosis Mice Corrects Lysosomal Storage in CNS and Improves Survival. PLoS ONE 2010, 5, e13468. [Google Scholar] [CrossRef]
- Ohno, K.; Samaranch, L.; Hadaczek, P.; Bringas, J.R.; Allen, P.C.; Sudhakar, V.; Stockinger, D.E.; Snieckus, C.; Campagna, M.V.; Sebastian, W.S.; et al. Kinetics and MR-Based Monitoring of AAV9 Vector Delivery into Cerebrospinal Fluid of Nonhuman Primates. Mol. Ther.—Methods Clin. Dev. 2019, 13, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Rocco, M.T.; Akhter, A.S.; Ehrlich, D.J.; Scott, G.C.; Lungu, C.; Munjal, V.; Aquino, A.; Lonser, R.R.; Fiandaca, M.S.; Hallett, M.; et al. Long-Term Safety of MRI-Guided Administration of AAV2-GDNF and Gadoteridol in the Putamen of Individuals with Parkinson’s Disease. Mol. Ther. J. Am. Soc. Gene Ther. 2023, 30, 3632–3638. [Google Scholar] [CrossRef]
- Bankiewicz, K.S.; Sudhakar, V.; Samaranch, L.; Sebastian, W.S.; Bringas, J.; Forsayeth, J. AAV Viral Vector Delivery to the Brain by Shape-Conforming MR-Guided Infusions. J. Control. Release 2016, 240, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Teng, Q.; Federici, T.; Boulis, N.M. Gene Therapy of the Central Nervous System. In Gene Therapy of the Central Nervous System: From Bench to Bedside; Section IV: Gene Therapy for Pain and Spinal Cord Diseases; Academic Press: Cambridge, MA, USA, 2006; pp. 253–271. [Google Scholar] [CrossRef]
- Castle, M.J.; Gershenson, Z.T.; Giles, A.R.; Holzbaur, E.L.F.; Wolfe, J.H. Adeno-Associated Virus Serotypes 1, 8, and 9 Share Conserved Mechanisms for Anterograde and Retrograde Axonal Transport. Hum. Gene Ther. 2014, 25, 705–720. [Google Scholar] [CrossRef] [PubMed]
- Salegio, E.A.; Samaranch, L.; Kells, A.P.; Forsayeth, J.; Bankiewicz, K. Guided Delivery of Adeno-Associated Viral Vectors into the Primate Brain. Adv. Drug Deliv. Rev. 2012, 64, 598–604. [Google Scholar] [CrossRef]
- Castle, M.J.; Perlson, E.; Holzbaur, E.L.; Wolfe, J.H. Long-Distance Axonal Transport of AAV9 Is Driven by Dynein and Kinesin-2 and Is Trafficked in a Highly Motile Rab7-Positive Compartment. Mol. Ther. 2014, 22, 554–566. [Google Scholar] [CrossRef]
- Castle, M.J.; Turunen, H.T.; Vandenberghe, L.H.; Wolfe, J.H. Controlling AAV Tropism in the Nervous System with Natural and Engineered Capsids. Methods Mol. Biol. 2015, 1382, 133–149. [Google Scholar] [CrossRef]
- Chen, F.; Vitry, S.; Hocquemiller, M.; Desmaris, N.; Ausseil, J.; Heard, J.-M. α-l-Iduronidase Transport in Neurites. Mol. Genet. Metab. 2006, 87, 349–358. [Google Scholar] [CrossRef]
- Leinekugel, P.; Michel, S.; Conzelmann, E.; Sandhoff, K. Quantitative Correlation between the Residual Activity of β-Hexosaminidase A and Arylsulfatase A and the Severity of the Resulting Lysosomal Storage Disease. Hum. Genet. 1992, 88, 513–523. [Google Scholar] [CrossRef]
- Bugiani, M.; Abbink, T.E.M.; Edridge, A.W.D.; Hoek, L.; Hillen, A.E.J.; Til, N.P.; Hu-A-Ng, G.V.; Breur, M.; Aiach, K.; Drevot, P.; et al. Focal Lesions Following Intracerebral Gene Therapy for Mucopolysaccharidosis IIIA. Ann. Clin. Transl. Neurol. 2023, 10, 904–917. [Google Scholar] [CrossRef]
- Sevin, C.; Roujeau, T.; Cartier, N.; Baugnon, T.; Adamsbaum, C.; Piraud, M.; Martino, S.; Mouiller, P.; Couzinié, C.; Bellesme, C.; et al. Intracerebral Gene Therapy in Children with Metachromatic Leukodystrophy: Results of a Phase I/II Trial. Mol. Genet. Metab. 2018, 123, S129. [Google Scholar] [CrossRef]
- Gougeon, M.-L.; Poirier-Beaudouin, B.; Ausseil, J.; Zérah, M.; Artaud, C.; Heard, J.-M.; Deiva, K.; Tardieu, M. Cell-Mediated Immunity to NAGLU Transgene Following Intracerebral Gene Therapy in Children with Mucopolysaccharidosis Type IIIB Syndrome. Front. Immunol. 2021, 12, 655478. [Google Scholar] [CrossRef] [PubMed]
- Mingozzi, F.; High, K.A. Immune Responses to AAV Vectors: Overcoming Barriers to Successful Gene Therapy. Blood 2013, 122, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Mendell, J.R.; Al-Zaidy, S.A.; Rodino-Klapac, L.R.; Goodspeed, K.; Gray, S.J.; Kay, C.N.; Boye, S.L.; Boye, S.E.; George, L.A.; Salabarria, S.; et al. Current Clinical Applications of In Vivo Gene Therapy with AAVs. Mol. Ther. 2021, 29, 464–488. [Google Scholar] [CrossRef]
- Nathwani, A.C.; Tuddenham, E.G.; Rangarajan, S.; Rosales, C.; McIntosh, J.; Linch, D.C.; Chowdary, P.; Riddell, A.; Pie, A.J.; Harrington, C.; et al. Adenovirus-Associated Virus Vector–Mediated Gene Transfer in Hemophilia B. N. Engl. J. Med. 2011, 365, 2357–2365. [Google Scholar] [CrossRef]
- Chand, D.; Mohr, F.; McMillan, H.; Tukov, F.F.; Montgomery, K.; Kleyn, A.; Sun, R.; Tauscher-Wisniewski, S.; Kaufmann, P.; Kullak-Ublick, G. Hepatotoxicity Following Administration of Onasemnogene Abeparvovec (AVXS-101) for the Treatment of Spinal Muscular Atrophy. J. Hepatol. 2021, 74, 560–566. [Google Scholar] [CrossRef]
- Keeler, A.M.; Flotte, T.R. Recombinant Adeno-Associated Virus Gene Therapy in Light of Luxturna (and Zolgensma and Glybera): Where Are We, and How Did We Get Here? Annu. Rev. Virol. 2019, 6, 601–621. [Google Scholar] [CrossRef]
- Ertl, H.C.J. Immunogenicity and Toxicity of AAV Gene Therapy. Front. Immunol. 2022, 13, 975803. [Google Scholar] [CrossRef]
- Arjomandnejad, M.; Dasgupta, I.; Flotte, T.R.; Keeler, A.M. Immunogenicity of Recombinant Adeno-Associated Virus (AAV) Vectors for Gene Transfer. BioDrugs 2023, 37, 311–329. [Google Scholar] [CrossRef]
- Ogbonmide, T.; Rathore, R.; Rangrej, S.B.; Hutchinson, S.; Lewis, M.; Ojilere, S.; Carvalho, V.; Kelly, I.; Ogbonmide, T.S. Gene Therapy for Spinal Muscular Atrophy (SMA): A Review of Current Challenges and Safety Considerations for Onasemnogene Abeparvovec (Zolgensma). Cureus 2023, 15, e36197. [Google Scholar] [CrossRef]
- Chand, D.H.; Zaidman, C.; Arya, K.; Millner, R.; Farrar, M.A.; Mackie, F.E.; Goedeker, N.L.; Dharnidharka, V.R.; Dandamudi, R.; Reyna, S.P. Thrombotic Microangiopathy Following Onasemnogene Abeparvovec for Spinal Muscular Atrophy: A Case Series. J. Pediatr. 2021, 231, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Guillou, J.; de Pellegars, A.; Porcheret, F.; Frémeaux-Bacchi, V.; Allain-Launay, E.; Debord, C.; Denis, M.; Péréon, Y.; Barnérias, C.; Desguerre, I.; et al. Fatal Thrombotic Microangiopathy Case Following Adeno-Associated Viral SMN Gene Therapy. Blood Adv. 2022, 6, 4266–4270. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.M.; Flotte, T.R. Moving Forward after Two Deaths in a Gene Therapy Trial of Myotubular Myopathy. Hum. Gene Ther. 2020, 31, 695–696. [Google Scholar] [CrossRef] [PubMed]
- Gessler, D.J.; Li, D.; Xu, H.; Su, Q.; Sanmiguel, J.; Tuncer, S.; Moore, C.; King, J.; Matalon, R.; Gao, G. Redirecting N-acetylaspartate metabolism in the central nervous system normalizes myelination and rescues Canavan disease. JCI Insight 2017, 2, e90807. [Google Scholar] [CrossRef]
- Corti, M.; Byrne, B.J.; Gessler, D.J.; Thompson, G.; Norman, S.; Lammers, J.; Coleman, K.E.; Liberati, C.; Elder, M.E.; Escolar, M.L.; et al. Case Report of Adeno-Associated Virus-Mediated Gene Therapy in a Patient with Canavan Disease Using Simultaneous Dual Route of Administration and Immune Modulation. Mol. Ther.—Methods Clin. Dev. 2023, 30, 303–314. [Google Scholar] [CrossRef]
- Chen, X.; Lim, D.A.; Lawlor, M.W.; Dimmock, D.; Vite, C.H.; Lester, T.; Tavakkoli, F.; Sadhu, C.; Prasad, S.; Gray, S.J. Biodistribution of Adeno-Associated Virus Gene Therapy Following Cerebrospinal Fluid-Directed Administration. Hum. Gene Ther. 2023, 34, 94–111. [Google Scholar] [CrossRef]
- Wright, J.F. Quantification of CpG Motifs in RAAV Genomes: Avoiding the Toll. Mol. Ther. 2020, 28, 1756–1758. [Google Scholar] [CrossRef]
- Sands, M.S. Adeno-Associated Virus, Methods and Protocols. Methods Mol. Biol. 2011, 807, 141–157. [Google Scholar] [CrossRef]
- Mingozzi, F.; Maus, M.V.; Hui, D.J.; Sabatino, D.E.; Murphy, S.L.; Rasko, J.E.J.; Ragni, M.V.; Manno, C.S.; Sommer, J.; Jiang, H.; et al. CD8+ T-Cell Responses to Adeno-Associated Virus Capsid in Humans. Nat. Med. 2007, 13, 419–422. [Google Scholar] [CrossRef]
- Shieh, P.B.; Kuntz, N.L.; Dowling, J.J.; Müller-Felber, W.; Bönnemann, C.G.; Seferian, A.M.; Servais, L.; Smith, B.K.; Muntoni, F.; Blaschek, A.; et al. Safety and Efficacy of Gene Replacement Therapy for X-Linked Myotubular Myopathy (ASPIRO): A Multinational, Open-Label, Dose-Escalation Trial. Lancet Neurol. 2023, 22, 1125–1139. [Google Scholar] [CrossRef]
- Voermans, N.C.; Ferreiro, A.; Aartsema-Rus, A.; Jungbluth, H. Gene Therapy for X-Linked Myotubular Myopathy: The Challenges. Lancet Neurol. 2023, 22, 1089–1091. [Google Scholar] [CrossRef]
- Duan, D. Lethal Immunotoxicity in High-Dose Systemic AAV Therapy. Mol. Ther. 2023, 31, 3123–3126. [Google Scholar] [CrossRef] [PubMed]
- Philippidis, A. Novartis Confirms Deaths of Two Patients Treated with Gene Therapy Zolgensma. Hum. Gene Ther. 2022, 33, 842–844. [Google Scholar] [CrossRef] [PubMed]
- Flotte, T.R. Revisiting the “New” Inflammatory Toxicities of Adeno-Associated Virus Vectors. Hum. Gene Ther. 2020, 31, 398–399. [Google Scholar] [CrossRef]
- Rossano, J.; Lin, K.; Epstein, S.; Battiprolu, P.; Ricks, D.; Syed, A.A.; Waldron, A.; Schwartz, J.; Greenberg, B. Safety Profile of The First Pediatric Cardiomyopathy Gene Therapy Trial: RP-A501 (AAV9:LAMP2B) For Danon Disease. J. Card. Fail. 2023, 29, 554. [Google Scholar] [CrossRef]
- Lek, A.; Wong, B.; Keeler, A.; Blackwood, M.; Ma, K.; Huang, S.; Sylvia, K.; Batista, A.R.; Artinian, R.; Kokoski, D.; et al. Death after High-Dose RAAV9 Gene Therapy in a Patient with Duchenne’s Muscular Dystrophy. N. Engl. J. Med. 2023, 389, 1203–1210. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarty, P.; Rosario, A.; Cruz, P.; Siemienski, Z.; Ceballos-Diaz, C.; Crosby, K.; Jansen, K.; Borchelt, D.R.; Kim, J.-Y.; Jankowsky, J.L.; et al. Capsid Serotype and Timing of Injection Determines AAV Transduction in the Neonatal Mice Brain. PLoS ONE 2013, 8, e67680. [Google Scholar] [CrossRef] [PubMed]
- Iliff, J.J.; Wang, M.; Liao, Y.; Plogg, B.A.; Peng, W.; Gundersen, G.A.; Benveniste, H.; Vates, G.E.; Deane, R.; Goldman, S.A.; et al. A Paravascular Pathway Facilitates CSF Flow through the Brain Parenchyma and the Clearance of Interstitial Solutes, Including Amyloid β. Sci. Transl. Med. 2012, 4, 147ra111. [Google Scholar] [CrossRef]
- Mestre, H.; Mori, Y.; Nedergaard, M. The Brain’s Glymphatic System: Current Controversies. Trends Neurosci. 2020, 43, 458–466. [Google Scholar] [CrossRef]
- Hinderer, C.; Bell, P.; Vite, C.H.; Louboutin, J.-P.; Grant, R.; Bote, E.; Yu, H.; Pukenas, B.; Hurst, R.; Wilson, J.M. Widespread Gene Transfer in the Central Nervous System of Cynomolgus Macaques Following Delivery of AAV9 into the Cisterna Magna. Mol. Ther.—Methods Clin. Dev. 2014, 1, 14051. [Google Scholar] [CrossRef]
- Hinderer, C.; Bell, P.; Katz, N.; Vite, C.H.; Louboutin, J.-P.; Bote, E.; Yu, H.; Zhu, Y.; Casal, M.L.; Bagel, J.; et al. Evaluation of Intrathecal Routes of Administration for Adeno-Associated Viral Vectors in Large Animals. Hum. Gene Ther. 2018, 29, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Taghian, T.; Marosfoi, M.G.; Puri, A.S.; Cataltepe, O.I.; King, R.M.; Diffie, E.B.; Maguire, A.S.; Martin, D.R.; Fernau, D.; Batista, A.R.; et al. A Safe and Reliable Technique for CNS Delivery of AAV Vectors in the Cisterna Magna. Mol. Ther. 2020, 28, 411–421. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daci, R.; Flotte, T.R. Delivery of Adeno-Associated Virus Vectors to the Central Nervous System for Correction of Single Gene Disorders. Int. J. Mol. Sci. 2024, 25, 1050. https://doi.org/10.3390/ijms25021050
Daci R, Flotte TR. Delivery of Adeno-Associated Virus Vectors to the Central Nervous System for Correction of Single Gene Disorders. International Journal of Molecular Sciences. 2024; 25(2):1050. https://doi.org/10.3390/ijms25021050
Chicago/Turabian StyleDaci, Rrita, and Terence R. Flotte. 2024. "Delivery of Adeno-Associated Virus Vectors to the Central Nervous System for Correction of Single Gene Disorders" International Journal of Molecular Sciences 25, no. 2: 1050. https://doi.org/10.3390/ijms25021050
APA StyleDaci, R., & Flotte, T. R. (2024). Delivery of Adeno-Associated Virus Vectors to the Central Nervous System for Correction of Single Gene Disorders. International Journal of Molecular Sciences, 25(2), 1050. https://doi.org/10.3390/ijms25021050





