The Effect of Oil-Based Cannabis Extracts on Metabolic Parameters and Microbiota Composition of Mice Fed a Standard and a High-Fat Diet
Abstract
:1. Introduction
2. Results
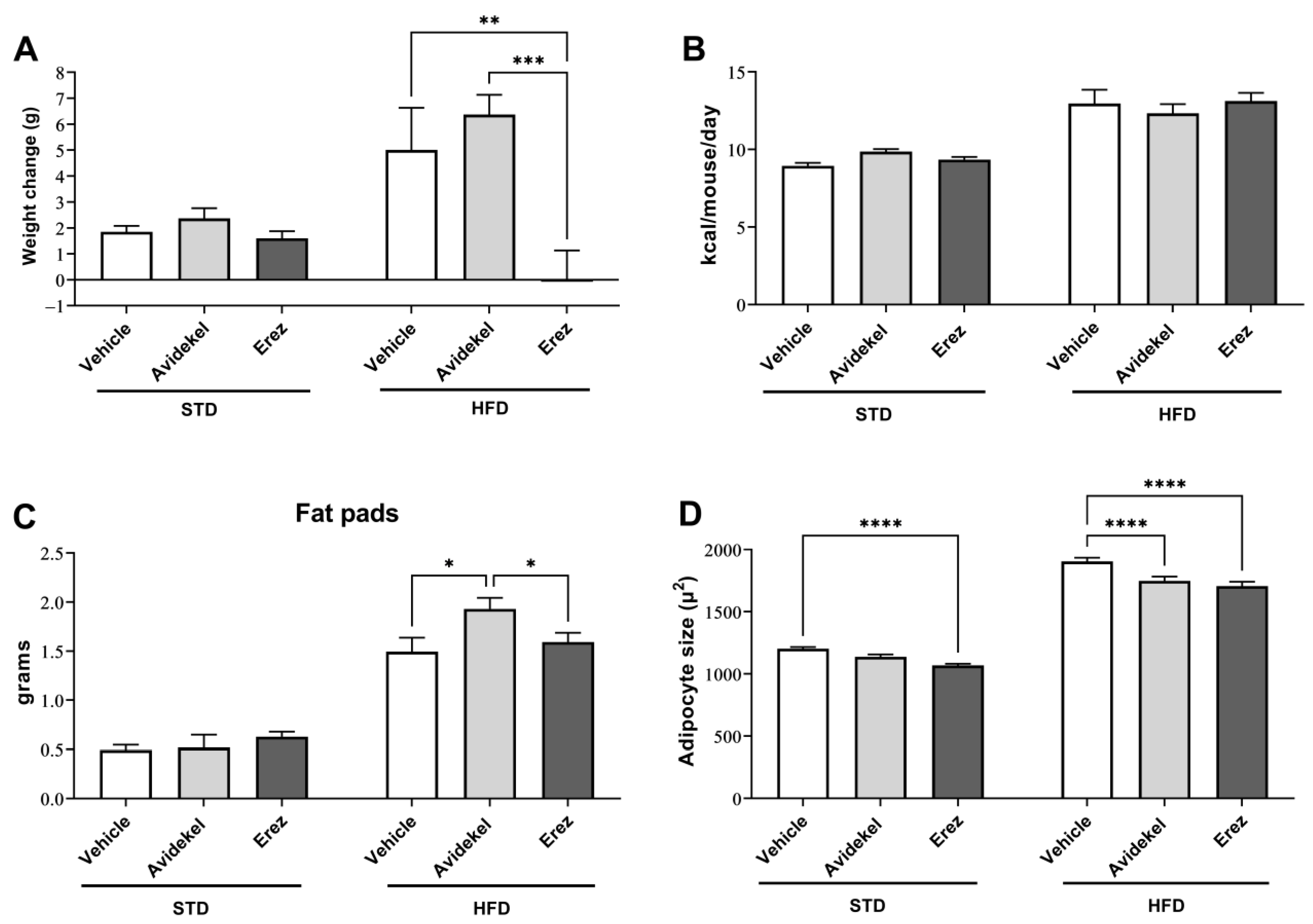
2.1. Erez (THC-Enriched) but Not Avidekel (CBD-Enriched) Reduces Weight Gain
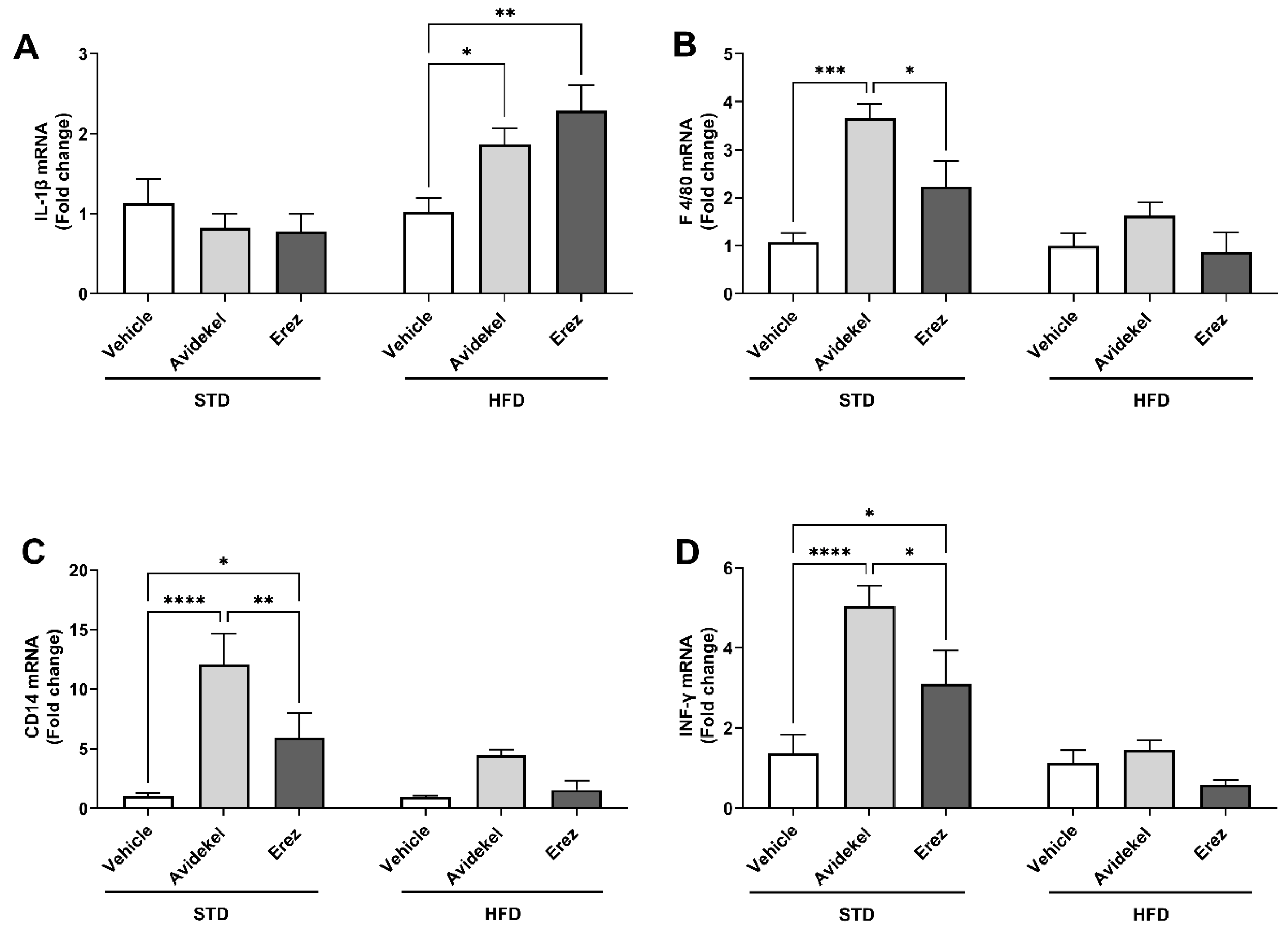
2.2. Avidekel Increases Adipose Tissue Inflammation in STD Mice
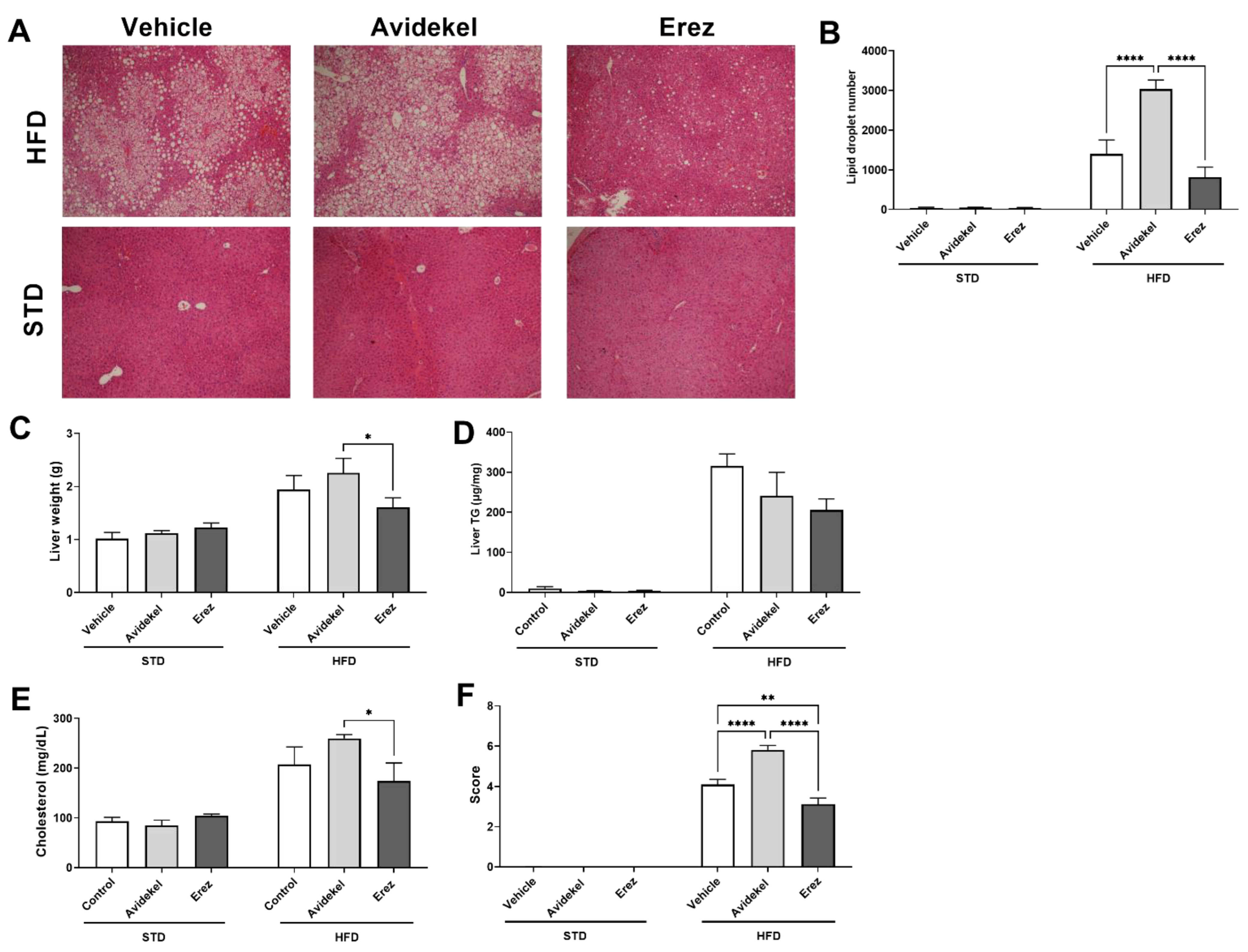
2.3. Erez Extract Improves Diet-Induced Liver Steatosis
2.4. Erez Extract Reduces Liver Levels of DHEA and PEA in High-Fat Diet-Fed Mice
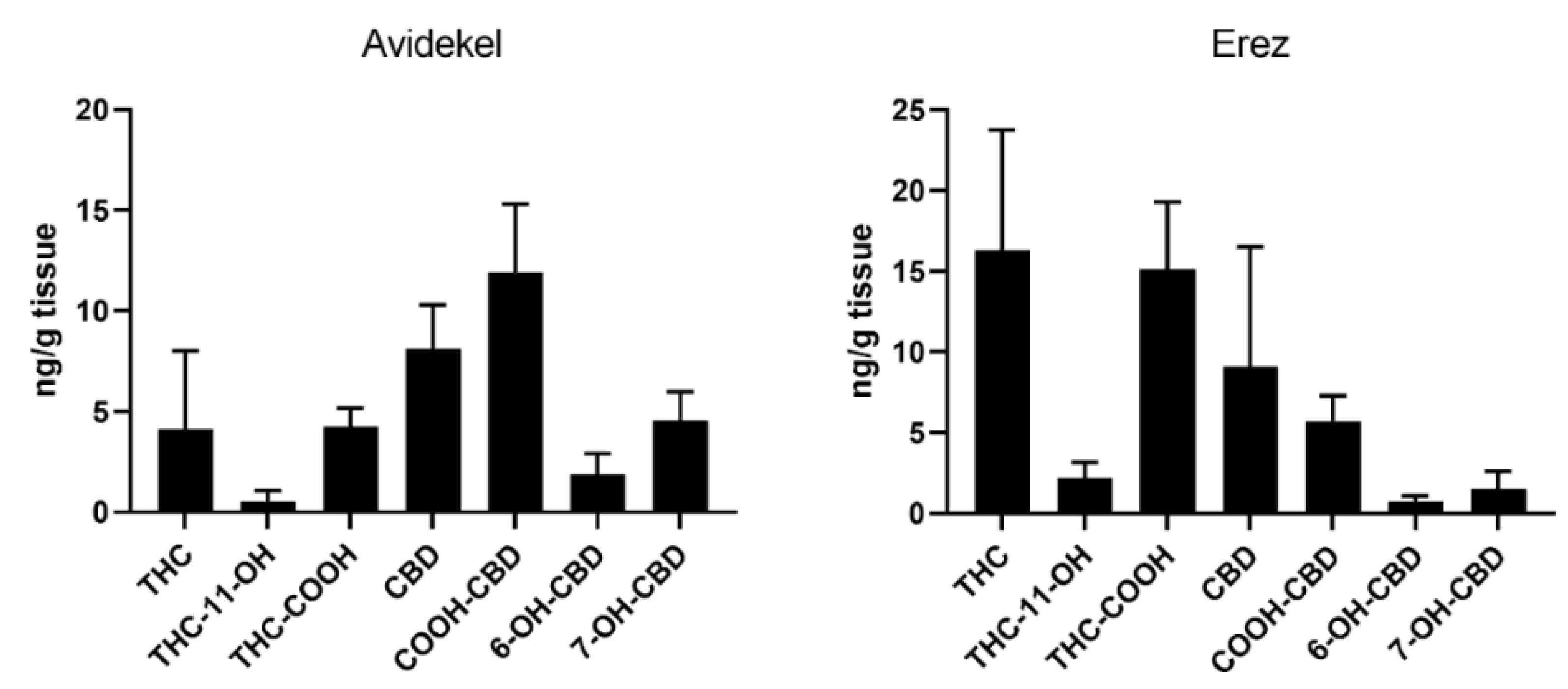
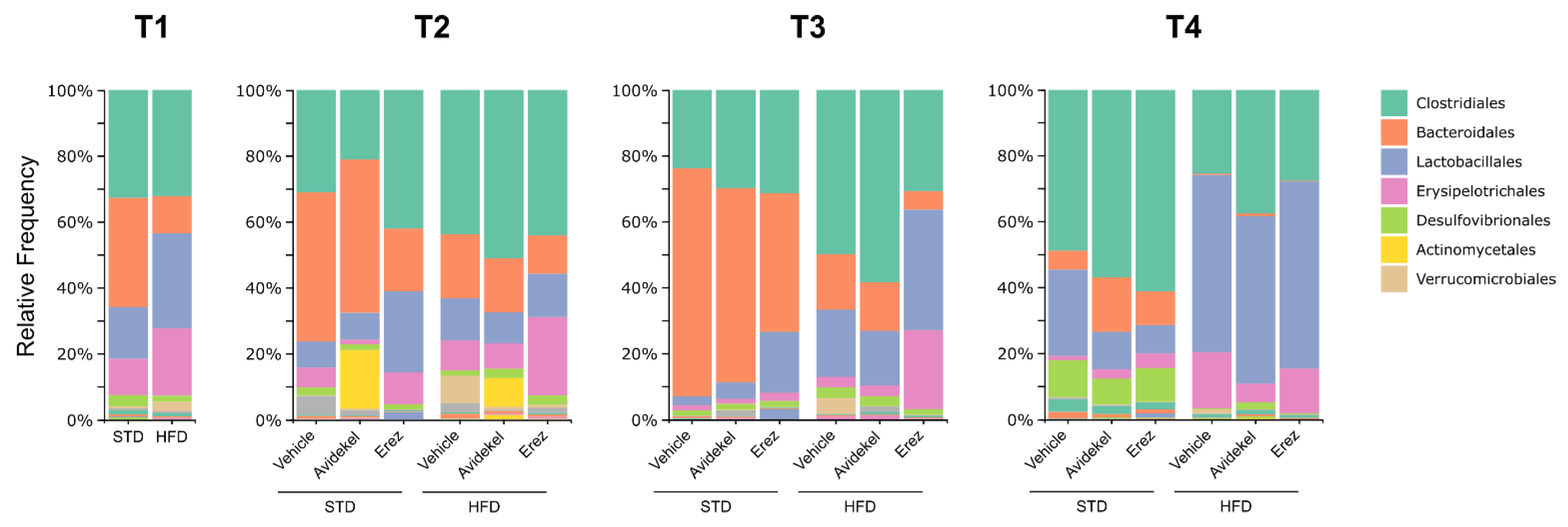
2.5. Effect of Avidekel and Erez Extracts on the Microbiota
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Study Compounds
4.3. Treatments
4.4. DNA Extraction and Sequencing from Mouse Feces
4.5. Liver Triglyceride Quantification
4.6. Histology and Microscopy
4.7. Liquid Chromatography–High-Resolution Mass Spectrometry (LC–HRMS) Chemical Analysis
4.8. RNA Extraction and Real-Time Quantitative PCR
4.9. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2015 Obesity Collaborators; Afshin, A.; Forouzanfar, M.H.; Reitsma, M.B.; Sur, P.; Estep, K.; Lee, A.; Marczak, L.; Mokdad, A.H.; Moradi-Lakeh, M.; et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [CrossRef]
- Mazier, W.; Saucisse, N.; Gatta-Cherifi, B.; Cota, D. The Endocannabinoid System: Pivotal Orchestrator of Obesity and Metabolic Disease. Trends Endocrinol. Metab. 2015, 26, 524–537. [Google Scholar] [CrossRef]
- Maruvada, P.; Leone, V.; Kaplan, L.M.; Chang, E.B. The Human Microbiome and Obesity: Moving beyond Associations. Cell Host Microbe 2017, 22, 589–599. [Google Scholar] [CrossRef]
- Silvestri, C.; Di Marzo, V. The Endocannabinoid System in Energy Homeostasis and the Etiopathology of Metabolic Disorders. Cell Metab. 2013, 17, 475–490. [Google Scholar] [CrossRef]
- Di Marzo, V. New Approaches and Challenges to Targeting the Endocannabinoid System. Nat. Rev. Drug Discov. 2018, 17, 623–639. [Google Scholar] [CrossRef]
- Mehrpouya-Bahrami, P.; Chitrala, K.N.; Ganewatta, M.S.; Tang, C.; Murphy, E.A.; Enos, R.T.; Velazquez, K.T.; McCellan, J.; Nagarkatti, M.; Nagarkatti, P. Blockade of CB1 Cannabinoid Receptor Alters Gut Microbiota and Attenuates Inflammation and Diet-Induced Obesity. Sci. Rep. 2017, 7, 15645. [Google Scholar] [CrossRef]
- Muccioli, G.G.; Naslain, D.; Bäckhed, F.; Reigstad, C.S.; Lambert, D.M.; Delzenne, N.M.; Cani, P.D. The Endocannabinoid System Links Gut Microbiota to Adipogenesis. Mol. Syst. Biol. 2010, 6, 392. [Google Scholar] [CrossRef]
- Stanislawski, M.A.; Dabelea, D.; Lange, L.A.; Wagner, B.D.; Lozupone, C.A. Gut Microbiota Phenotypes of Obesity. NPJ Biofilms Microbiomes 2019, 5, 18. [Google Scholar] [CrossRef]
- Di Marzo, V.; Piscitelli, F. The Endocannabinoid System and Its Modulation by Phytocannabinoids. Neurotherapeutics 2015, 12, 692–698. [Google Scholar] [CrossRef]
- Amin, M.R.; Ali, D.W. Pharmacology of Medical Cannabis. In Recent Advances in Cannabinoid Physiology and Pathology; Bukiya, A.N., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 151–165. ISBN 978-3-030-21737-2. [Google Scholar]
- Abrams, D.I.; Guzman, M. Cannabis in Cancer Care. Clin. Pharmacol. Ther. 2015, 97, 575–586. [Google Scholar] [CrossRef]
- MacCallum, C.A.; Russo, E.B. Practical Considerations in Medical Cannabis Administration and Dosing. Eur. J. Intern. Med. 2018, 49, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Poyatos, L.; Pérez-Acevedo, A.P.; Papaseit, E.; Pérez-Mañá, C.; Martin, S.; Hladun, O.; Siles, A.; Torrens, M.; Busardo, F.P.; Farré, M. Oral Administration of Cannabis and Δ-9-Tetrahydrocannabinol (THC) Preparations: A Systematic Review. Medicina 2020, 56, 309. [Google Scholar] [CrossRef]
- Bautista, J.L.; Yu, S.; Tian, L. Flavonoids in Cannabis sativa: Biosynthesis, Bioactivities, and Biotechnology. ACS Omega 2021, 6, 5119–5123. [Google Scholar] [CrossRef]
- Alshaarawy, O.; Anthony, J.C. Are Cannabis Users Less Likely to Gain Weight? Results from a National 3-Year Prospective Study. Int. J. Epidemiol. 2019, 48, 1695–1700. [Google Scholar] [CrossRef] [PubMed]
- Rajavashisth, T.B.; Shaheen, M.; Norris, K.C.; Pan, D.; Sinha, S.K.; Ortega, J.; Friedman, T.C. Decreased Prevalence of Diabetes in Marijuana Users: Cross-Sectional Data from the National Health and Nutrition Examination Survey (NHANES) III. BMJ Open 2012, 2, e000494. [Google Scholar] [CrossRef]
- Alshaarawy, O.; Anthony, J.C. Brief Report: Cannabis Smoking and Diabetes Mellitus: Results from Meta-Analysis with Eight Independent Replication Samples. Epidemiology 2015, 26, 597–600. [Google Scholar] [CrossRef] [PubMed]
- Adejumo, A.C.; Alliu, S.; Ajayi, T.O.; Adejumo, K.L.; Adegbala, O.M.; Onyeakusi, N.E.; Akinjero, A.M.; Durojaiye, M.; Bukong, T.N. Cannabis Use Is Associated with Reduced Prevalence of Non-Alcoholic Fatty Liver Disease: A Cross-Sectional Study. PLoS ONE 2017, 12, e0176416. [Google Scholar] [CrossRef] [PubMed]
- Cluny, N.L.; Keenan, C.M.; Reimer, R.A.; Le Foll, B.; Sharkey, K.A. Prevention of Diet-Induced Obesity Effects on Body Weight and Gut Microbiota in Mice Treated Chronically with Δ9-Tetrahydrocannabinol. PLoS ONE 2015, 10, e0144270. [Google Scholar] [CrossRef]
- Levendal, R.A.; Schumann, D.; Donath, M.; Frost, C.L. Cannabis Exposure Associated with Weight Reduction and β-Cell Protection in an Obese Rat Model. Phytomedicine 2012, 19, 575–582. [Google Scholar] [CrossRef]
- Ignatowska-Jankowska, B.; Jankowski, M.M.; Swiergiel, A.H. Cannabidiol Decreases Body Weight Gain in Rats: Involvement of CB2 Receptors. Neurosci. Lett. 2011, 490, 82–84. [Google Scholar] [CrossRef]
- Liao, J.-T.; Huang, Y.-W.; Hou, C.-Y.; Wang, J.-J.; Wu, C.-C.; Hsieh, S.-L. D-Limonene Promotes Anti-Obesity in 3T3-L1 Adipocytes and High-Calorie Diet-Induced Obese Rats by Activating the AMPK Signaling Pathway. Nutrients 2023, 15, 267. [Google Scholar] [CrossRef]
- Franco-Arroyo, N.N.; Viveros-Paredes, J.M.; Zepeda-Morales, A.S.M.; Roldán, E.; Márquez-Aguirre, A.L.; Zepeda-Nuño, J.S.; Velázquez-Juárez, G.; Fafutis-Morris, M.; López-Roa, R.I. β-Caryophyllene, a Dietary Cannabinoid, Protects Against Metabolic and Immune Dysregulation in a Diet-Induced Obesity Mouse Model. J. Med. Food 2022, 25, 993–1002. [Google Scholar]
- Eitan, A.; Gover, O.; Sulimani, L.; Meiri, D.; Schwartz, B. The Effect of Orally Administered Δ9-Tetrahydrocannabinol (THC) and Cannabidiol (CBD) on Obesity Parameters in Mice. Int. J. Mol. Sci. 2023, 24, 13797. [Google Scholar] [CrossRef]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose Tissue Inflammation and Metabolic Dysfunction in Obesity. Am. J. Physiol.-Cell Physiol. 2020, 320, C375–C391. [Google Scholar] [CrossRef]
- Bradbury, M.W. Lipid Metabolism and Liver Inflammation. I. Hepatic Fatty Acid Uptake: Possible Role in Steatosis. Am. J. Physiol.-Gastrointest. Liver Physiol. 2006, 290, G194–G198. [Google Scholar] [CrossRef]
- Liang, W.; Menke, A.L.; Driessen, A.; Koek, G.H.; Lindeman, J.H.; Stoop, R.; Havekes, L.M.; Kleemann, R.; van den Hoek, A.M. Establishment of a General NAFLD Scoring System for Rodent Models and Comparison to Human Liver Pathology. PLoS ONE 2014, 9, e115922. [Google Scholar] [CrossRef] [PubMed]
- Mallat, A.; Teixeira-Clerc, F.; Deveaux, V.; Manin, S.; Lotersztajn, S. The Endocannabinoid System as a Key Mediator during Liver Diseases: New Insights and Therapeutic Openings. Br. J. Pharmacol. 2011, 163, 1432–1440. [Google Scholar] [CrossRef]
- Reese, A.T.; Dunn, R.R. Drivers of Microbiome Biodiversity: A Review of General Rules, Feces, and Ignorance. mBio 2018, 9, e01294-18. [Google Scholar] [CrossRef]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel Disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef]
- Silva Sofrás, F.M.; Desimone, M.F. Entourage Effect and Analytical Chemistry: Chromatography as a Tool in the Analysis of the Secondary Metabolism of Cannabis sativa L. Curr. Pharm. Des. 2023, 29, 394–406. [Google Scholar] [CrossRef]
- Colizzi, M.; Bhattacharyya, S. Cannabis Use and the Development of Tolerance: A Systematic Review of Human Evidence. Neurosci. Biobehav. Rev. 2018, 93, 1–25. [Google Scholar] [CrossRef]
- Lin, L.; Jung, K.-M.; Lee, H.-L.; Le, J.; Colleluori, G.; Wood, C.; Palese, F.; Squire, E.; Ramirez, J.; Su, S. Adolescent Exposure to Low-Dose THC Disrupts Energy Balance and Adipose Organ Homeostasis in Adulthood. Cell Metab. 2023, 35, 1227–1241.e7. [Google Scholar] [CrossRef]
- Islam, A.U.S.; Hellman, B.; Nyberg, F.; Amir, N.; Jayaraj, R.L.; Petroianu, G.; Adem, A. Myrcene Attenuates Renal Inflammation and Oxidative Stress in the Adrenalectomized Rat Model. Molecules 2020, 25, 4492. [Google Scholar] [CrossRef]
- Deveaux, V.; Cadoudal, T.; Ichigotani, Y.; Teixeira-Clerc, F.; Louvet, A.; Manin, S.; Van Nhieu, J.T.; Belot, M.P.; Zimmer, A.; Even, P.; et al. Cannabinoid CB2 Receptor Potentiates Obesity-Associated Inflammation, Insulin Resistance and Hepatic Steatosis. PLoS ONE 2009, 4, e5844. [Google Scholar] [CrossRef]
- Gallily, R.; Yekhtin, Z.; Hanuš, L.O. Overcoming the Bell-shaped Dose-response of Cannabidiol by Using Cannabis Extract Enriched in Cannabidiol. Pharmacol. Pharm. 2015, 6, 75–85. [Google Scholar] [CrossRef]
- Muthumalage, T.; Rahman, I. Cannabidiol Differentially Regulates Basal and LPS-Induced Inflammatory Responses in Macrophages, Lung Epithelial Cells, and Fibroblasts. Toxicol. Appl. Pharmacol. 2019, 382, 114713. [Google Scholar] [CrossRef]
- Tovar, R.; Gavito, A.L.; Vargas, A.; Soverchia, L.; Hernandez-Folgado, L.; Jagerovic, N.; Baixeras, E.; Ciccocioppo, R.; Rodríguez de Fonseca, F.; Decara, J. Palmitoleoylethanolamide Is an Efficient Anti-Obesity Endogenous Compound: Comparison with Oleylethanolamide in Diet-Induced Obesity. Nutrients 2021, 13, 2589. [Google Scholar] [CrossRef]
- Annunziata, C.; Lama, A.; Pirozzi, C.; Cavaliere, G.; Trinchese, G.; Di Guida, F.; Nitrato Izzo, A.; Cimmino, F.; Paciello, O.; De Biase, D. Palmitoylethanolamide Counteracts Hepatic Metabolic Inflexibility Modulating Mitochondrial Function and Efficiency in Diet-Induced Obese Mice. FASEB J. 2020, 34, 350–364. [Google Scholar] [CrossRef]
- Caraceni, P.; Viola, A.; Piscitelli, F.; Giannone, F.; Berzigotti, A.; Cescon, M.; Domenicali, M.; Petrosino, S.; Giampalma, E.; Riili, A.; et al. Circulating and Hepatic Endocannabinoids and Endocannabinoid-Related Molecules in Patients with Cirrhosis. Liver Int. 2010, 30, 816–825. [Google Scholar] [CrossRef]
- Shmarakov, I.O.; Jiang, H.; Liu, J.; Fernandez, E.J.; Blaner, W.S. Hepatic Stellate Cell Activation: A Source for Bioactive Lipids. Biochim. Et Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2019, 1864, 629–642. [Google Scholar] [CrossRef]
- de Bus, I.; van Krimpen, S.; Hooiveld, G.J.; Boekschoten, M.V.; Poland, M.; Witkamp, R.F.; Albada, B.; Balvers, M.G.J. Immunomodulating Effects of 13- and 16-Hydroxylated Docosahexaenoyl Ethanolamide in LPS Stimulated RAW264.7 Macrophages. Biochim. Et Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2021, 1866, 158908. [Google Scholar] [CrossRef] [PubMed]
- Manor, O.; Dai, C.L.; Kornilov, S.A.; Smith, B.; Price, N.D.; Lovejoy, J.C.; Gibbons, S.M.; Magis, A.T. Health and Disease Markers Correlate with Gut Microbiome Composition across Thousands of People. Nat. Commun. 2020, 11, 5206. [Google Scholar] [CrossRef]
- Scott, J.C.; Rosen, A.F.G.; Moore, T.M.; Roalf, D.R.; Satterthwaite, T.D.; Calkins, M.E.; Ruparel, K.; Gur, R.E.; Gur, R.C. Cannabis Use in Youth Is Associated with Limited Alterations in Brain Structure. Neuropsychopharmacology 2019, 44, 1362–1369. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.R.; Sanders, J.G.; McDonald, D.; Amir, A.; Ladau, J.; Locey, K.J.; Prill, R.J.; Tripathi, A.; Gibbons, S.M.; Ackermann, G.; et al. A Communal Catalogue Reveals Earth’s Multiscale Microbial Diversity. Nature 2017, 551, 457–463. [Google Scholar] [CrossRef]
- McDonald, D.; Price, M.N.; Goodrich, J.; Nawrocki, E.P.; DeSantis, T.Z.; Probst, A.; Andersen, G.L.; Knight, R.; Hugenholtz, P. An Improved Greengenes Taxonomy with Explicit Ranks for Ecological and Evolutionary Analyses of Bacteria and Archaea. ISME J. 2012, 6, 610–618. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Berman, P.; Sulimani, L.; Gelfand, A.; Amsalem, K.; Lewitus, G.M.; Meiri, D. Cannabinoidomics—An Analytical Approach to Understand the Effect of Medical Cannabis Treatment on the Endocannabinoid Metabolome. Talanta 2020, 219, 121336. [Google Scholar] [CrossRef]

| Lipid Family | ECs (ng/g Tissue) | Vehicle—HFD | Avidekel—HFD | Erez—HFD | |
|---|---|---|---|---|---|
| Liver | NAEs | AEA | 23.8 ± 4.8 | 22.9 ± 3.9 | 15.8 ± 2.9 |
| DHEA | 6 ± 1.5 a | 5.1 ± 0.9 ab | 3.3 ± 0.2 b | ||
| LEA | 36.8 ± 7.8 | 23.3 ± 4.7 | 23.5 ± 3.8 | ||
| OEA | 33 ± 2.4 | 35 ± 3.8 | 21 ± 3.1 | ||
| PEA | 157 ± 16 a | 126 ± 17 ab | 91 ± 13 b | ||
| SEA | 134 ± 27 | 117 ± 24 | 85 ± 13 | ||
| 2-MAGs | 2-AG | 1764 ± 572 | 526 ± 64 | 691 ± 37 | |
| 2-LG | 10,362 ± 4853 | 2362 ± 387 | 4023 ± 759 | ||
| NAAs | NL-Gly | 122 ± 41 | 101 ± 17 | 70 ± 13 | |
| NP-Gly | 366 ± 70 | 393 ± 47 | 206 ± 35 | ||
| NA-Gly | 117 ± 44 | 115 ± 21 | 98 ± 23 | ||
| NDH-Gly | 106 ± 33 | 77 ± 16 | 58 ± 10 | ||
| NA-Ser | 46 ± 19 | 37 ± 8.9 | 27 ± 6.9 | ||
| NA-GABA | 6 ± 2.5 | 6 ± 1.6 | 2.6 ± 0.3 | ||
| NA-Ala | 91 ± 32 | 88 ± 15 | 69 ± 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eitan, A.; Gover, O.; Sulimani, L.; Meiri, D.; Shterzer, N.; Mills, E.; Schwartz, B. The Effect of Oil-Based Cannabis Extracts on Metabolic Parameters and Microbiota Composition of Mice Fed a Standard and a High-Fat Diet. Int. J. Mol. Sci. 2024, 25, 1073. https://doi.org/10.3390/ijms25021073
Eitan A, Gover O, Sulimani L, Meiri D, Shterzer N, Mills E, Schwartz B. The Effect of Oil-Based Cannabis Extracts on Metabolic Parameters and Microbiota Composition of Mice Fed a Standard and a High-Fat Diet. International Journal of Molecular Sciences. 2024; 25(2):1073. https://doi.org/10.3390/ijms25021073
Chicago/Turabian StyleEitan, Adi, Ofer Gover, Liron Sulimani, David Meiri, Naama Shterzer, Erez Mills, and Betty Schwartz. 2024. "The Effect of Oil-Based Cannabis Extracts on Metabolic Parameters and Microbiota Composition of Mice Fed a Standard and a High-Fat Diet" International Journal of Molecular Sciences 25, no. 2: 1073. https://doi.org/10.3390/ijms25021073
APA StyleEitan, A., Gover, O., Sulimani, L., Meiri, D., Shterzer, N., Mills, E., & Schwartz, B. (2024). The Effect of Oil-Based Cannabis Extracts on Metabolic Parameters and Microbiota Composition of Mice Fed a Standard and a High-Fat Diet. International Journal of Molecular Sciences, 25(2), 1073. https://doi.org/10.3390/ijms25021073






