Poly(vinyl chloride)/Nanocarbon Composites for Advanced Potentiometric Membrane Sensor Design
Abstract
1. Introduction
2. Results and Discussion
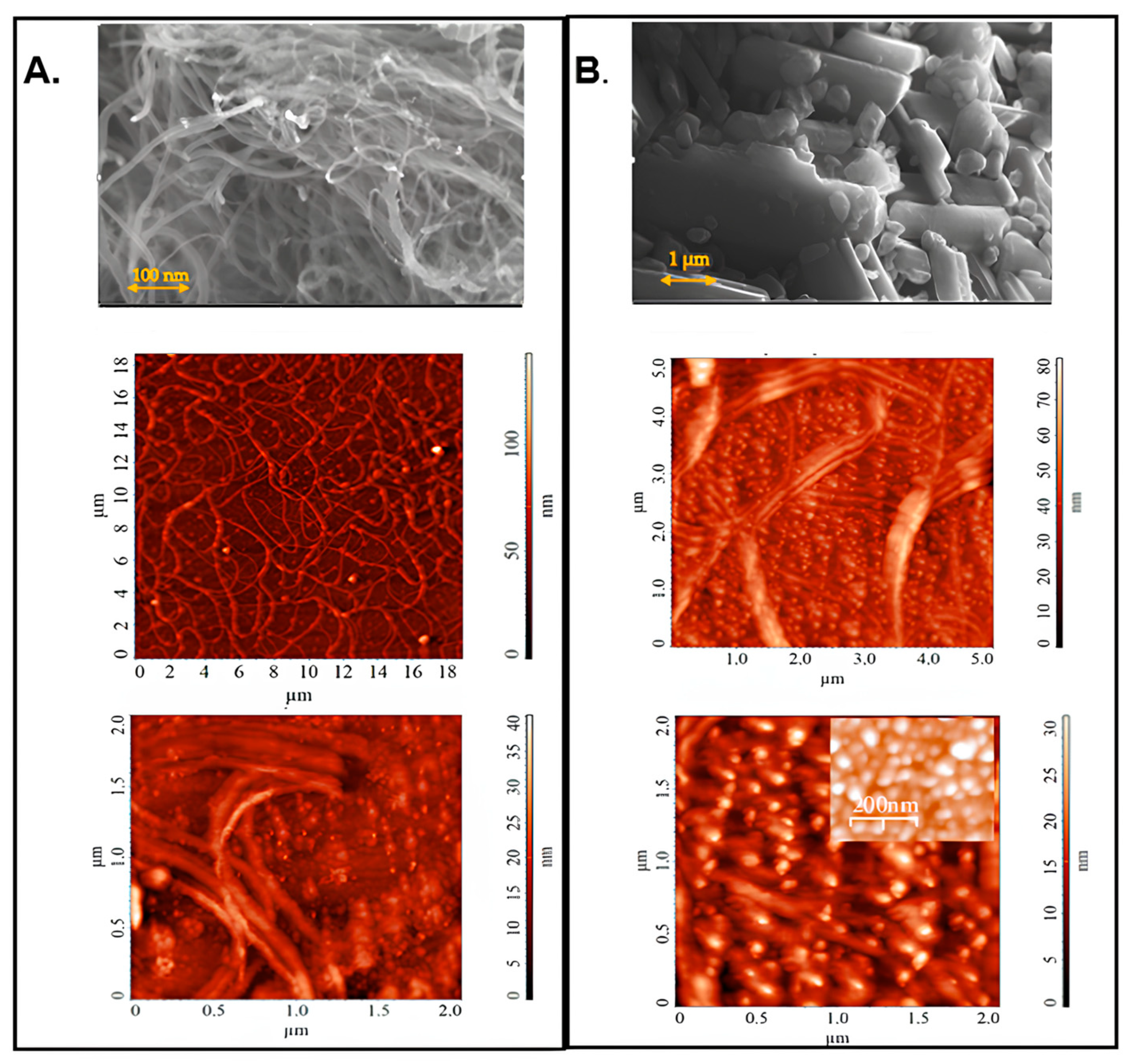
2.1. Performance Evaluation of PVC Nanocomposites with SWCNTs and Fullerene-C60
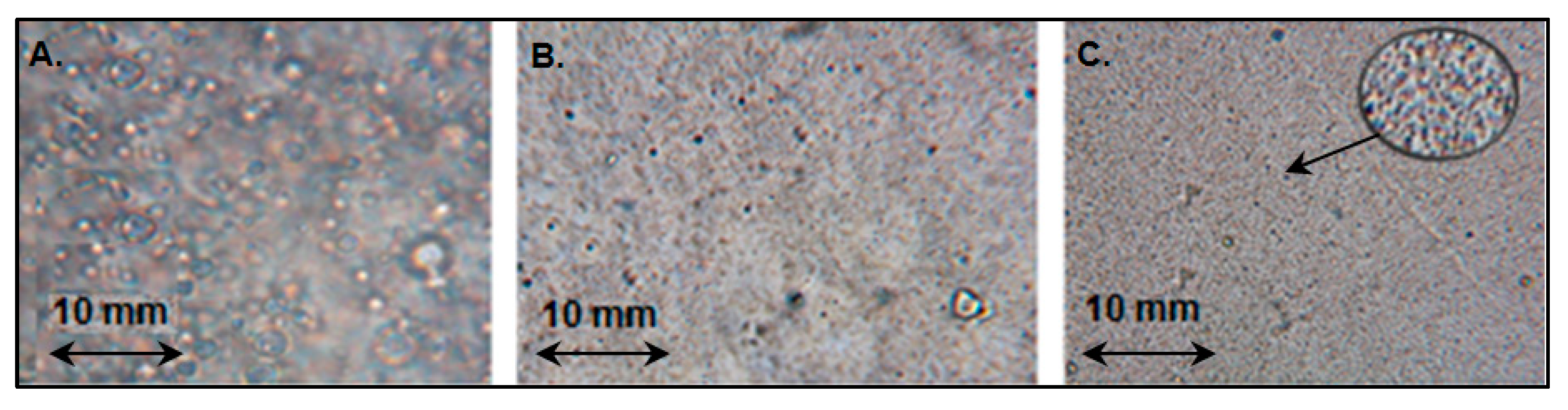
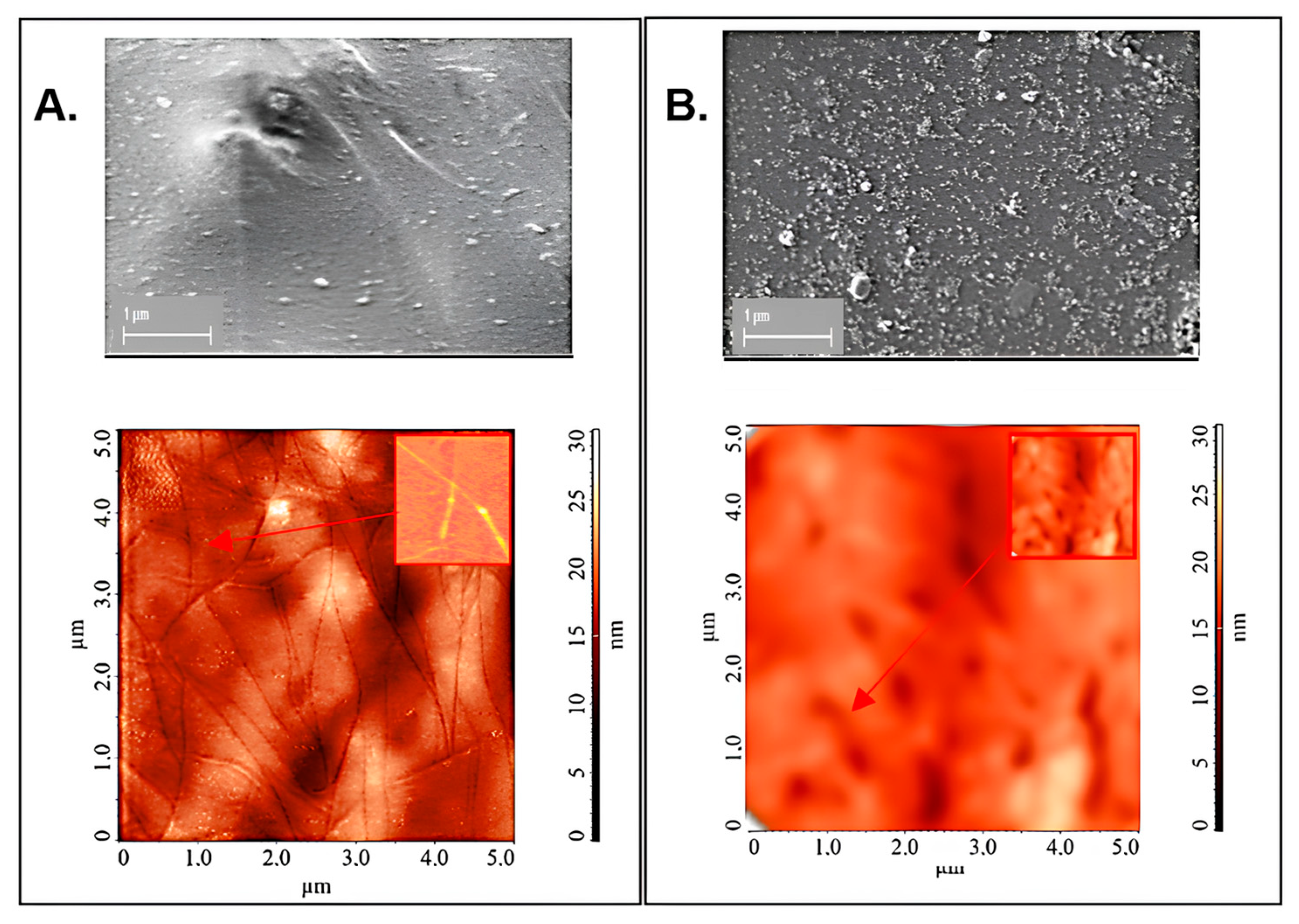
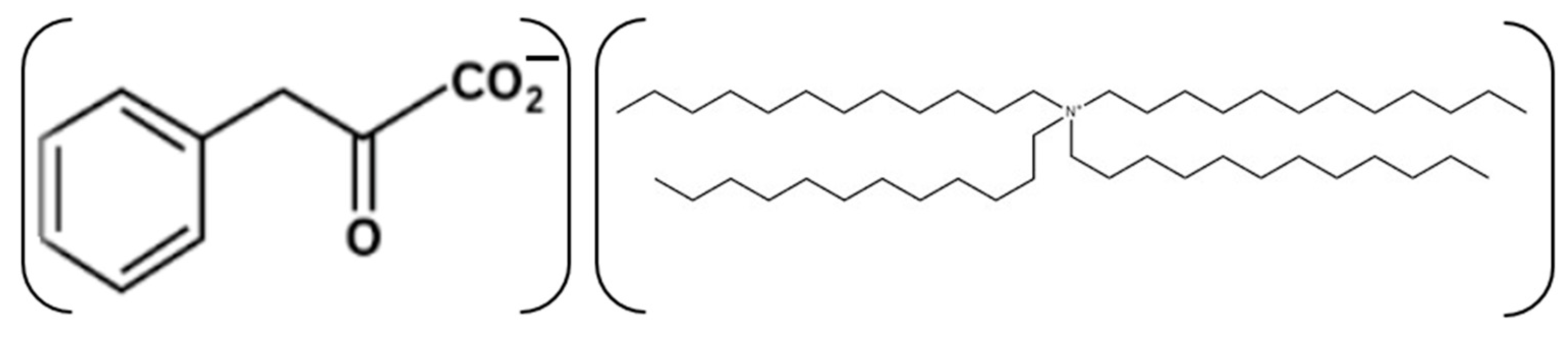
2.2. Application of SWCNTs-C60-Filled pPVC Composite Film for the Development of Potentiometric Membrane Sensor
3. Methods and Materials
3.1. Materials
3.2. Equipment
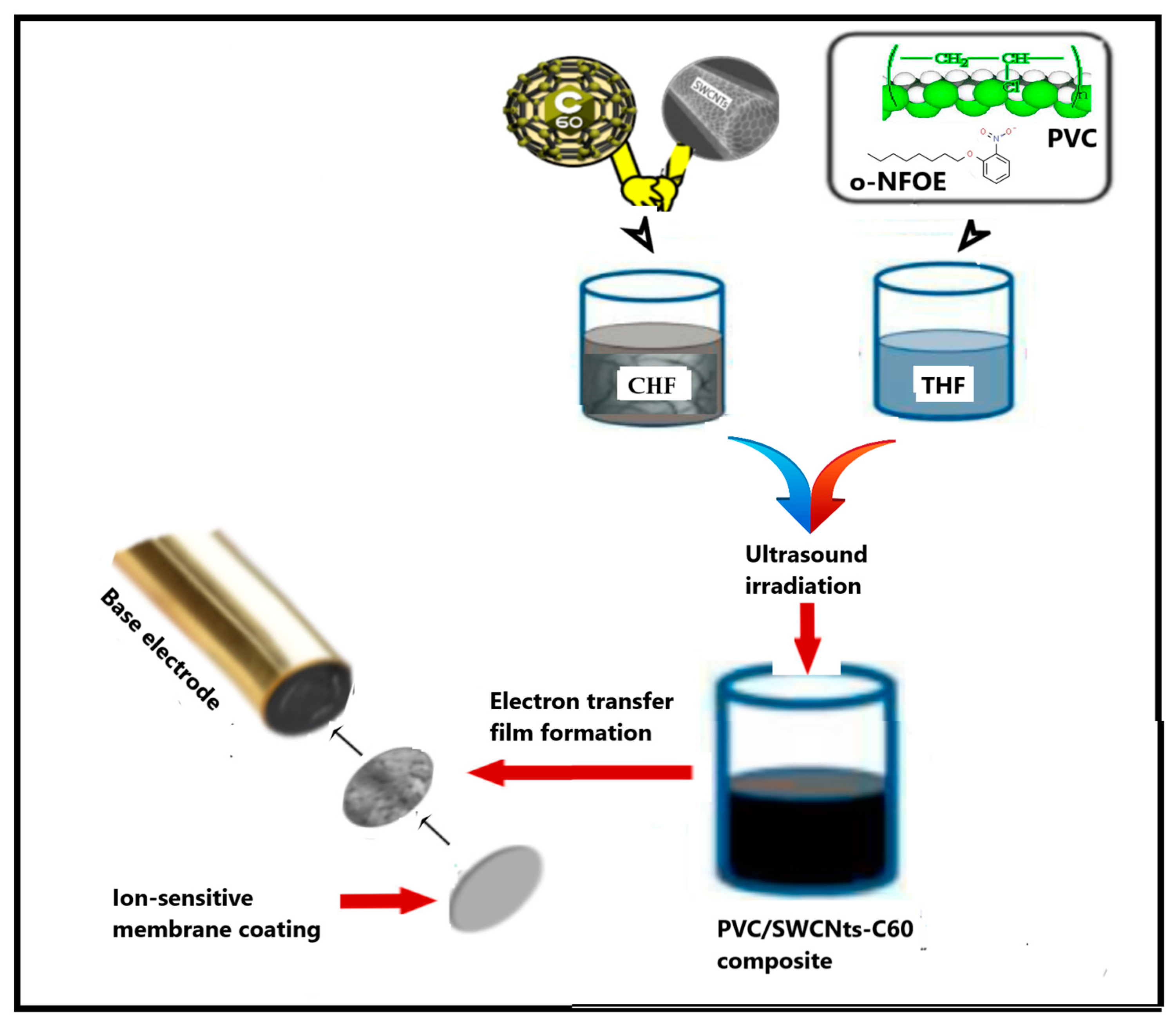
3.3. Methods
3.3.1. Preparation of PVC Nanocomposites
3.3.2. Fabrication of SC-PMS
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Georgakilas, V.; Perman, J.A.; Tucek, J.; Zboril, R. Broad Family of Carbon Nanoallotropes: Classification, Chemistry, and Applications of Fullerenes, Carbon Dots, Nanotubes, Graphene, Nanodiamonds, and Combined Superstructures. Chem. Rev. 2015, 115, 4744–4822. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.H.M.; AL-Oqla, F.M.; Risby, M.S.; Sapuan, S.M. On the Enhancement of the Fatigue Fracture Performance of Polymer Matrix Composites by Reinforcement with Carbon Nanotubes: A Systematic Review. Carbon Lett. 2022, 32, 727–740. [Google Scholar] [CrossRef]
- Hu, Z.; Hong, H. Review on Material Performance of Carbon Nanotube-Modified Polymeric Nanocomposites. Recent Prog. Mater. 2023, 5, 1–20. [Google Scholar] [CrossRef]
- Bikiaris, D.N. Nanocomposites with Different Types of Nanofillers and Advanced Properties for Several Applications. Appl. Nano 2022, 3, 160–162. [Google Scholar] [CrossRef]
- Baptista, F.R.; Belhout, S.A.; Giordani, S.; Quinn, S.J. Recent Developments in Carbon Nanomaterial Sensors. Chem. Soc. Rev. 2015, 44, 4433–4453. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, S.; Jadon, N.; Jain, R. Next-Generation Polymer Nanocomposite-Based Electrochemical Sensors and Biosensors: A Review. TrAC Trends Anal. Chem. 2016, 82, 55–67. [Google Scholar] [CrossRef]
- Manjunatha, J.G.; Hussain, C.M. (Eds.) Carbon Nanomaterials-Based Sensors: Emerging Research Trends in Devices and Applications, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Das, R.; Pattanayak, A.J.; Swain, S.K. Polymer Nanocomposites for Sensor Devices. In Polymer-Based Nanocomposites for Energy and Environmental Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 205–218. [Google Scholar]
- Bölükbaşı, Ö.S.; Yola, B.B.; Boyacıoğlu, H.; Yola, M.L. A Novel Paraoxon Imprinted Electrochemical Sensor Based on MoS2NPs@MWCNTs and Its Application to Tap Water Samples. Food Chem. Toxicol. 2022, 163, 112994. [Google Scholar] [CrossRef]
- Wardak, C.; Pietrzak, K.; Morawska, K.; Grabarczyk, M. Ion-Selective Electrodes with Solid Contact Based on Composite Materials: A Review. Sensors 2023, 23, 5839. [Google Scholar] [CrossRef]
- Lyu, Y.; Gan, S.; Bao, Y.; Zhong, L.; Xu, J.; Wang, W.; Liu, Z.; Ma, Y.; Yang, G.; Niu, L. Solid-Contact Ion-Selective Electrodes: Response Mechanisms, Transducer Materials and Wearable Sensors. Membranes 2020, 10, 128. [Google Scholar] [CrossRef]
- Cheong, Y.H.; Ge, L.; Lisak, G. Highly Reproducible Solid Contact Ion Selective Electrodes: Emerging Opportunities for Potentiometry—A Review. Anal. Chim. Acta 2021, 1162, 338304. [Google Scholar] [CrossRef]
- Matos, M.A.; Tagarielli, V.L.; Pinho, S.T. On the electrical conductivity of composites with a polymeric matrix and a non-uniform concentration of carbon nanotubes. Compos. Sci. Technol. 2020, 188, 108003. [Google Scholar] [CrossRef]
- Mousavi, Z.; Bobacka, J.; Lewenstam, A.; Ivaska, A. Poly(3,4-Ethylenedioxythiophene) (PEDOT) Doped with Carbon Nanotubes as Ion-to-Electron Transducer in Polymer Membrane-Based Potassium Ion-Selective Electrodes. J. Electroanal. Chem. 2009, 633, 246–252. [Google Scholar] [CrossRef]
- Kałuża, D.; Jaworska, E.; Mazur, M.; Maksymiuk, K.; Michalska, A. Multi-walled Carbon Nanotubes–Poly(3-octylthiophene-2,5-diyl) Nanocomposite Transducer for Ion-Selective Electrodes: Raman Spectroscopy Insight into the Transducer/Membrane Interface. Anal. Chem. 2019, 91, 9010–9017. [Google Scholar] [CrossRef] [PubMed]
- Ardalani, M.; Shamsipur, M.; Besharati-Seidani, A. A New Generation of Highly Sensitive Potentiometric Sensors Based on Ion Imprinted Polymeric Nanoparticles/Multiwall Carbon Nanotubes/Polyaniline/Graphite Electrode for Sub-Nanomolar Detection of Lead(II) Ions. J. Electroanal. Chem. 2020, 879, 114788. [Google Scholar] [CrossRef]
- Paczosa-Bator, B. Ion-Selective Electrodes with Superhydrophobic Polymer/Carbon Nanocomposites as Solid Contact. Carbon 2015, 95, 879–887. [Google Scholar] [CrossRef]
- Boeva, Z.A.; Lindfors, T. Few-Layer Graphene and Polyaniline Composite as Ion-to-Electron Transducer in Silicone Rubber Solid-Contact Ion-Selective Electrodes. Sens. Actuators B Chem. 2016, 224, 624–631. [Google Scholar] [CrossRef]
- Abd-Rabboh, H.S.M.; Kamel, A.H.; Amr, A.E.-G.E. All-Solid-State Calcium Sensors Modified with Polypyrrol (PPY) and Graphene Oxide (GO) as Solid-Contact Ion-to-Electron Transducers. Chemosensors 2020, 8, 93. [Google Scholar] [CrossRef]
- Pietrzak, K.; Morawska, K.; Malinowski, S.; Wardak, C. Chloride Ion-Selective Electrode with Solid-Contact Based on Polyaniline Nanofibers and Multiwalled Carbon Nanotubes Nanocomposite. Membranes 2022, 12, 1150. [Google Scholar] [CrossRef]
- Zhizhin, K.Y.; Turyshev, E.S.; Kopytin, A.V.; Shpigun, L.K.; Kuznetsov, N.T.; Simonenko, N.P. Polymer Nanocarbon Materials as Ion-to-electron Transducers in Solid-contact Ion-selective Electrodes. Nanosyst. Phys. Chem. Math. 2022, 13, 688–697. [Google Scholar] [CrossRef]
- Turyshev, E.S.; Shpigun, L.K.; Kopytin, A.V.; Zhizhin, K.Y.; Kuznetsov, N.T.; Simonenko, N.P. Development of Solid-Contact Potentiometric Sensor Utilized Nanocarbon-filled Poly(vinyl chloride) Membrane as Ion-to-electron Transducing Layer. Austin J. Anal. Pharm. Chem. 2023, 10, 1154. [Google Scholar]
- Edraki, M.; Sheydaei, M.; Alinia-Ahandani, E.; Nezhadghaffar-Borhani, E. Polyvinyl chloride: Chemical modification and investigation of structural and thermal properties. J. Sulfur Chem. 2021, 42, 397–409. [Google Scholar] [CrossRef]
- Lewandowski, K.; Skórczewska, K. A Brief Review of Poly(Vinyl Chloride) (PVC) Recycling. Polymers 2022, 14, 3035. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Mu, Y.H.; Tang, Q.; Li, C.Q. Preparation and Performance of PVC/CNT Nanocomposite. Adv. Polym. Technol. 2016, 37, 358–364. [Google Scholar] [CrossRef]
- Tao, J.; Qin, Y.; Zhang, P.; Guo, Z. Preparation and Properties of Polyvinyl Chloride/Carbon Nanotubes Composite. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2019, 34, 516–520. [Google Scholar] [CrossRef]
- Yazdani, H.; Smith, B.E.; Hatami, K. Multi-Walled Carbon Nanotube-Filled Polyvinyl Chloride Composites: Influence of Processing Method on Dispersion Quality, Electrical Conductivity and Mechanical Properties. Compos. Part A Appl. Sci. Manuf. 2016, 82, 65–77. [Google Scholar] [CrossRef]
- Bera, R.; Karan, S.K.; Paria, S.; De, A.; Khatua, B.B. PVC Bead Assisted Selective Dispersion of MWCNT for Designing Efficient Electromagnetic Interference Shielding PVC/MWCNT Nanocomposite with Very Low Percolation Threshold. Compos. B Eng. 2019, 167, 377–386. [Google Scholar] [CrossRef]
- Al Naim, A.F.; AlFannakh, H.; Arafat, S.; Ibrahim, S.S. Characterization of PVC/MWCNTs Nanocomposite: Solvent Blend. Sci. Eng. Compos. Mater. 2020, 27, 55–64. [Google Scholar] [CrossRef]
- Abdolmaleki, A.; Mallakpour, S.; Azimi, F. Microwave and Ultrasound-Assisted Synthesis of Poly(Vinyl Chloride)/Riboflavin Modified MWCNTs: Examination of Thermal, Mechanical and Morphology Properties. Ultrason. Sonochemistry 2018, 41, 27–36. [Google Scholar] [CrossRef]
- Skórczewska, K.; Lewandowski, K.; Wilczewski, S. Novel Composites of Poly(Vinyl Chloride) with Carbon Fiber/Carbon Nanotube Hybrid Filler. Materials 2022, 15, 5625. [Google Scholar] [CrossRef]
- Haruna, H.; Pekdemir, M.E.; Tukur, A.; Coşkun, M. Characterization, Thermal and Electrical Properties of Aminated PVC/Oxidized MWCNT Composites Doped with Nanographite. J. Therm. Anal. Calorim. 2020, 139, 3887–3895. [Google Scholar] [CrossRef]
- Wilczewski, S.; Skórczewska, K.; Tomaszewska, J.; Lewandowski, K. Structure and Properties of Poly(vinyl chloride)/Graphene Nanocomposites. Polym. Test. 2020, 81, 106282. [Google Scholar] [CrossRef]
- Wilczewski, S.; Skórczewska, K.; Tomaszewska, J.; Lewandowski, K.; Szulc, J.; Runka, T. Manufacturing Homogenous PVC/Graphene Nanocomposites Using a Novel Dispersion Agent. Polym. Test. 2020, 91, 106868. [Google Scholar] [CrossRef]
- Ahmed, R.M.; Sata, M.M.; Taha, E.O. Optical Spectroscopy, Thermal Analysis, and Dynamic Mechanical Properties of Graphene Nanoplatelets Reinforced Poly(vinyl)chloride. J. Mater. Sci. Mater. Electron. 2021, 32, 22699–22717. [Google Scholar] [CrossRef]
- Joshi, G.M.; Deshmukh, K. Optimized Quality Factor of Graphene Oxide-Reinforced PVC Nanocomposite. J. Electron. Mater. 2014, 43, 1161–1165. [Google Scholar] [CrossRef]
- Baibarac, M.; Stingescu, L.; Stroe, M.; Negrila, C.; Matei, E.; Cotet, L.C.; Anghel, I.; Sofran, I.E.; Baia, L. Poly(Vinyl Chloride) Spheres Coated with Graphene Oxide Sheets: From Synthesis to Optical Properties and Their Applications as Flame-Retardant Agents. Polymers 2021, 13, 565. [Google Scholar] [CrossRef]
- Broza, G.; Piszczek, K.; Schulte, K.; Sterzynski, T. Nanocomposites of Poly(Vinyl Chloride) with Carbon Nanotubes (CNT). Compos. Sci. Technol. 2007, 67, 890–894. [Google Scholar] [CrossRef]
- Aljaafari, A.A.; Ibrahim, S.S.; El-Brolossy, T.A. Thermophysical and Electrical Characterization of PVC–SWNT Nanocomposites. Compos. Part A Appl. Sci. Manuf. 2011, 42, 394–399. [Google Scholar] [CrossRef]
- Zanjanijam, A.R.; Bahrami, M.; Hajian, M. Poly(Vinyl Chloride)/Single Wall Carbon Nanotubes Composites: Investigation of Mechanical and Thermal Characteristics. J. Vinyl Addit. Technol. 2016, 22, 128–133. [Google Scholar] [CrossRef]
- Kharlamova, M.V.; Kramberger, C. Phemenology of Filling, Investigation of Growth Kinetics and Electronic Properties for Applications of Filled Single-walled Carbon Nanotubes. Nanomaterials 2023, 13, 314. [Google Scholar] [CrossRef]
- Kharlamova, M.V.; Bati, A.S.R.; Yu, L.P.; Batmunkh, M.; Shapter, J.G. Recent Advances in Applications of Sorted Single-Walled Carbon Nanotubes. Adv. Funct. Mater. 2019, 29, 30. [Google Scholar]
- Gerstman, E.; Hendler-Neumark, A.; Wulf, V. Monitoring the Formation of Fibrin Clots as Part of the Coagulation Cascade Using Fluorescent Single-Walled Carbon Nanotubes. ACS Appl. Mater. Interfaces 2023, 15, 21866–21876. [Google Scholar] [CrossRef] [PubMed]
- Forel, S.; Sacco, L.; Castan, A.; Florea, I.; Cojocaru, C.S. Simple and Rapid Gas Sensing Using a Single-walled Carbon Nanotube Field-effect Transistor-based Logic Inverter. Nanoscale Adv. 2021, 3, 1582–1587. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.Y.; Hou, P.X.; Zhang, F.; Liu, C.; Cheng, H.M. Gas Sensors Based on Single-wall Carbon Nanotubes. Molecules 2022, 27, 5381. [Google Scholar] [CrossRef] [PubMed]
- Çapar, N.; Polat, İ.; Yola, B.B.; Atar, N.; Yola, M.L. A Novel Molecular Imprinted QCM Sensor Based on MoSNPs-MWCNT Nanocomposite for Zearalenone Determination. Microchim. Acta 2023, 190, 262. [Google Scholar] [CrossRef]
- Wang, H.; Boghossian, A.A. Covalent Conjugation of Proteins onto Fluorescent Single-Walled Carbon Nanotubes for Biological and Medical Applications. Mater. Adv. 2023, 4, 823–834. [Google Scholar] [CrossRef] [PubMed]
- Rusen, E.; Marculescu, B.; Butac, L.; Preda, N.; Mihut, L. The Synthesis and Characterization of Poly Vinyl Chloride Chemically Modified with C60. Fuller. Nanotub. Carbon Nanostructures 2008, 16, 178–185. [Google Scholar] [CrossRef]
- Fan, X.; Soin, N.; Li, H.; Li, H.; Xia, X.; Geng, J. Fullerene (C60) Nanowires: Preparation, Characterization, and Potential Applications. Energy Environ. Mater. 2020, 3, 469–491. [Google Scholar] [CrossRef]
- Smith, B.W.; Monthioux, M.; Luzzi, D.E. Carbon nanotube encapsulated fullerenes: A unique class of hybrid materials. Chem. Phys. Lett 1999, 315, 31–36. [Google Scholar] [CrossRef]
- Saldıvar-Guerra, E.; Vivaldo-Lima, E. (Eds.) Handbook of Polymer Synthesis, Characterization, and Processing; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013. [Google Scholar]
- Silvano, L.T.; Vittorazzo, A.L., Jr.; Araujo, R.G. Effect of Preparation Method on the Electrical and Mechanical Properties of PVC/Carbon Nanotubes Nanocomposites. Mater. Res. 2018, 5, 21. [Google Scholar] [CrossRef]
- Bilalis, P.; Katsigiannopoulos, D.; Avgeropoulos, A.; Sakellariou, G. Non-covalent Functionalization of Carbon Nanotubes with Polymers. RSC Adv. 2014, 4, 2911–2934. [Google Scholar] [CrossRef]
- Yaya, A.; Tekley, A.; Annan, E.; Efavi, J.K.; Tiburu, E.K.; Onwona-Agyeman, B.; Jensen, L.R. Dispersion and Functionalization of Single-Walled Carbon Nanotubes (SWCNTs) for Nanocomposite Applications. Matériaux Tech. 2016, 104, 607. [Google Scholar] [CrossRef]
- Komov, V.P.; Khalchitsky, S.E.; Dubina, M.V. Disturbances in Metabolism of Phenylalanine and Tyrosine as an Important Factor in the Etiology and Pathogenesis of Psychoneurological Disorders Associated with Liver Diseases. Int. J. BioMed. 2015, 5, 65–70. [Google Scholar] [CrossRef]
- Bulycheva, I.A.; Halchitsky, S.E. Pathochemistry of Aromatic Amino Acid Metabolism in Alcoholic Disease. Nat. Tech. Sci. 2008, 5, 44. [Google Scholar]
- Okada, S.; Saito, S.; Oshiyama, A. Energetics and Electronic Structures of Encapsulated C60 in a Carbon Nanotube. Phys. Rev. Lett. 2001, 86, 3835–3838. [Google Scholar] [CrossRef]
- Yang, J.; Lee, J.; Lee, J.; Yi, W. Conductivity and Field Emission Enhancement of C60-encapsulated Single-walled Carbon Nanotubes. Diam. Relat. Mater. 2018, 90, 75. [Google Scholar] [CrossRef]
- John, B. Polymer Nanocomposite-based Electrochemical Sensors and Biosensors. In Nanorods Nanocomposites; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Shao, Y.; Ying, Y.; Ping, J. Recent Advances in Solid-contact Ion-selective Electrodes: Functional Materials, Transduction Mechanisms, and Development Trends. Chem. Soc. Rev. 2020, 49, 4405–4465. [Google Scholar] [CrossRef]
- Bakker, E. The Phase-Boundary Potential Model. Talanta 2004, 63, 3–20. [Google Scholar] [CrossRef]
- Watanabe, K.; Noguchi, O.; Okada, K.; Katsu, T. In situ Determination of Phenylpyruvate in Urine Using a Phenylpyruvate-selective Membrane Electrode Constructed with a Heptyl-4-trifluoroacetylbenzoate Neutral Carrier. Anal. Sci. 1997, 3, 209–212. [Google Scholar] [CrossRef]
- Thirumavalavana, S.; Mani, K.S.; Sagadevan, S.S. Studies on Hall Effect and DC Conductivity Measurements of Semiconductor Thin Films prepared by Chemical Bath Deposition (CBD) method. J. Nano Electron. Phys. 2015, 7, 04024. [Google Scholar]



| PNC Components | Sensing Species | Limit of Detection, M | References | |
|---|---|---|---|---|
| Type of CNPs | Polymer Matrix | |||
| MWCNTs | PEDOT | K+ | 1.0 × 10−6 | [14] |
| MWCNTs | POT | K+ | 1.6 × 10−7 | [15] |
| MWCNTs-COOH | Nafion | Pb2+ | 6.7 × 10−9 | [16] |
| Gr-Carbon black | Fluorinated acrylic copolymer | K+ | 7.5 × 10−7 | [17] |
| Gr | Polyaniline | Ca2+ | 5.0 × 10−8 | [18] |
| GrO | Polypyrrole | Ca2+ | 2.3 × 10−7 | [19] |
| MWCNTs | Polyaniline/Nanofibers | Cl- | 2.7 × 10−6 | [20] |
| Fullerene-C60, | Nafion, PVC | Procaine | 1.0 × 10−7 | [21] |
| Concentration of PPA, M | Response Time, s | Potential Drift, mV | |||
|---|---|---|---|---|---|
| 24 h | 7 Days | 1 Month | 3 Months | ||
| 1 × 10−3 | 2.2 | ≈±0.3 | ±0.7 | ±3.6 | ±5.3 |
| 1 × 10−4 | 2.4 | ≈±0.3 | ±1.0 | ±4.0 | ±5.5 |
| 1 × 10−5 | 3.6 | ≈±0.3 | ±1.2 | ±4.2 | ±6.2 |
| 1 × 10−6 | 5.1 | ≈±0.4 | ±1.4 | ±4.5 | ±6.7 |
| 1 × 10−7 | 9.0 | ≈±0.5 | ±1.5 | ±5.6 | ±8.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhizhin, K.Y.; Turyshev, E.S.; Shpigun, L.K.; Gorobtsov, P.Y.; Simonenko, N.P.; Simonenko, T.L.; Kuznetsov, N.T. Poly(vinyl chloride)/Nanocarbon Composites for Advanced Potentiometric Membrane Sensor Design. Int. J. Mol. Sci. 2024, 25, 1124. https://doi.org/10.3390/ijms25021124
Zhizhin KY, Turyshev ES, Shpigun LK, Gorobtsov PY, Simonenko NP, Simonenko TL, Kuznetsov NT. Poly(vinyl chloride)/Nanocarbon Composites for Advanced Potentiometric Membrane Sensor Design. International Journal of Molecular Sciences. 2024; 25(2):1124. https://doi.org/10.3390/ijms25021124
Chicago/Turabian StyleZhizhin, Konstantin Yu., Evgeniy S. Turyshev, Liliya K. Shpigun, Philipp Yu. Gorobtsov, Nikolay P. Simonenko, Tatiana L. Simonenko, and Nikolay T. Kuznetsov. 2024. "Poly(vinyl chloride)/Nanocarbon Composites for Advanced Potentiometric Membrane Sensor Design" International Journal of Molecular Sciences 25, no. 2: 1124. https://doi.org/10.3390/ijms25021124
APA StyleZhizhin, K. Y., Turyshev, E. S., Shpigun, L. K., Gorobtsov, P. Y., Simonenko, N. P., Simonenko, T. L., & Kuznetsov, N. T. (2024). Poly(vinyl chloride)/Nanocarbon Composites for Advanced Potentiometric Membrane Sensor Design. International Journal of Molecular Sciences, 25(2), 1124. https://doi.org/10.3390/ijms25021124







