Improved Solubility and Stability of a Thermostable Carbonic Anhydrase via Fusion with Marine-Derived Intrinsically Disordered Solubility Enhancers
Abstract
:1. Introduction
2. Results and Discussion
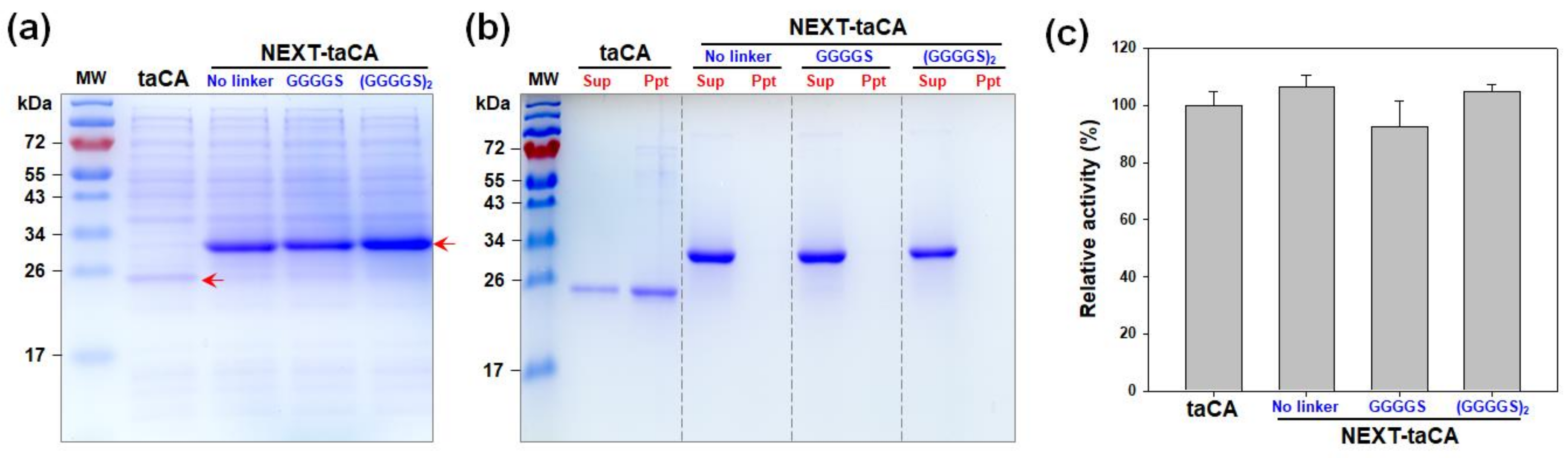
2.1. Effects of Length of Flexible Linker
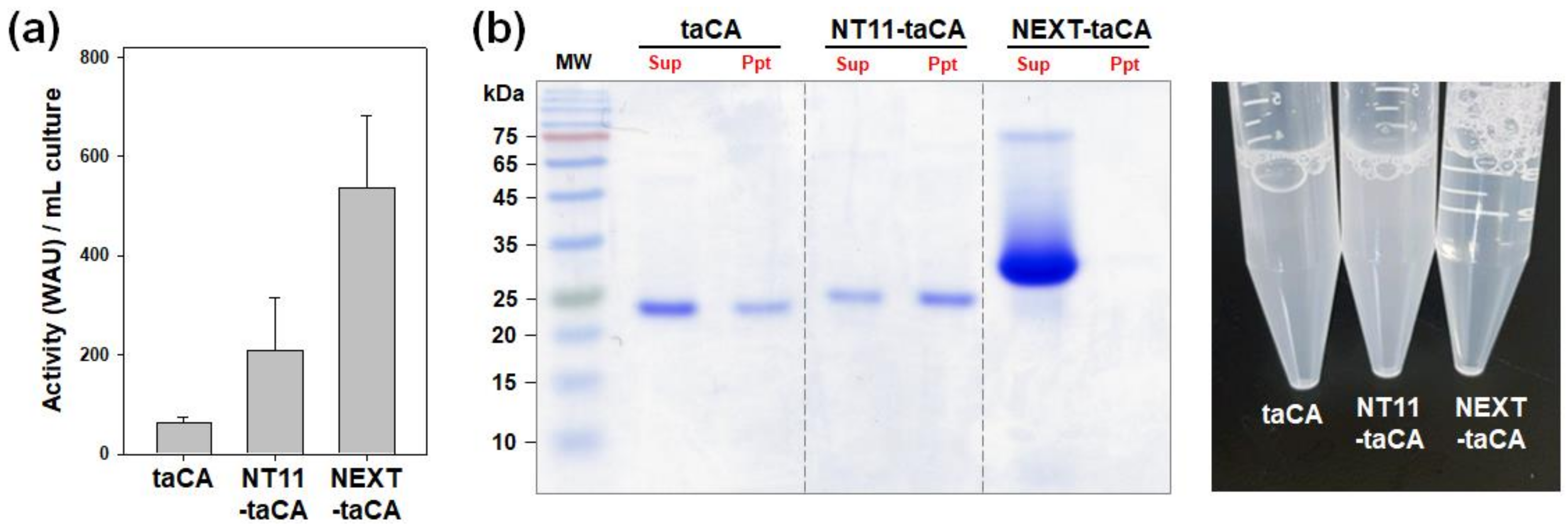
2.2. Comparison of NEXT Tag with NT11 Tag
2.3. Improved Long-Term Thermal Stability of taCA by the Fusion of NEXT Tag
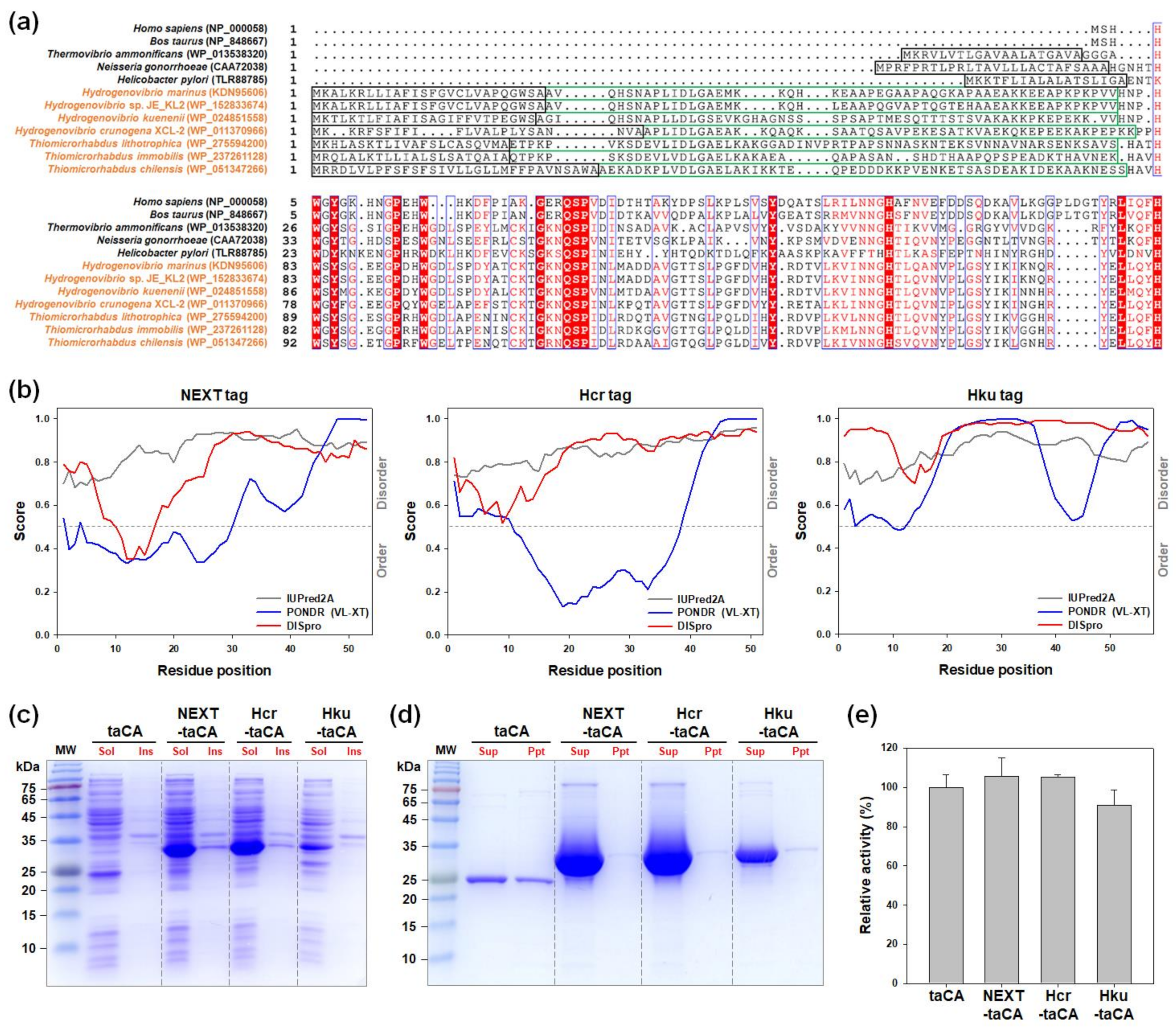
2.4. Bioprospecting of Novel IDP-Based Solubility Enhancers
3. Materials and Methods
3.1. Strains and Construction of Expression Vectors
3.2. Purification of Recombinant Proteins
3.3. In Vitro Solubility Test
3.4. Protein Analyses
3.5. CO2 Hydration Assay
3.6. Thermal Inactivation Test
3.7. In Silico Analyses
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smith, K.S.; Ferry, J.G. Prokaryotic carbonic anhydrases. FEMS Microbiol. Rev. 2000, 24, 335–366. [Google Scholar] [CrossRef] [PubMed]
- Nocentini, A.; Supuran, C.T.; Capasso, C. An overview on the recently discovered iota-carbonic anhydrases. J. Enzyme Inhib. Med. Chem. 2021, 36, 1988–1995. [Google Scholar] [CrossRef] [PubMed]
- Talekar, S.; Jo, B.H.; Dordick, J.S.; Kim, J. Carbonic anhydrase for CO2 capture, conversion and utilization. Curr. Opin. Biotechnol. 2022, 74, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Qi, G.J.; Liu, K.; House, A.; Salmon, S.; Ambedkar, B.; Frimpong, R.A.; Remias, J.E.; Liu, K.L. Laboratory to bench-scale evaluation of an integrated CO2 capture system using a thermostable carbonic anhydrase promoted K2CO3 solvent with low temperature vacuum stripping. Appl. Energy 2018, 209, 180–189. [Google Scholar] [CrossRef]
- Power, I.M.; Harrison, A.L.; Dipple, G.M. Accelerating mineral carbonation using carbonic anhydrase. Environ. Sci. Technol. 2016, 50, 2610–2618. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.Y.; Su, Z.G.; Wang, P.; Ma, G.H.; Zhang, S.P. Tethering of nicotinamide adenine dinucleotide inside hollow nanofibers for high-yield synthesis of methanol from carbon dioxide catalyzed by coencapsulated multienzymes. ACS Nano 2015, 9, 4600–4610. [Google Scholar] [CrossRef]
- Gao, S.; Mohammad, M.; Yang, H.C.; Xu, J.; Liang, K.; Hou, J.W.; Chen, V. Janus reactors with highly efficient enzymatic CO2 nanocascade at air-liquid interface. ACS Appl. Mater. Interfaces 2017, 9, 42806–42815. [Google Scholar] [CrossRef]
- Yu, S.S.; Lv, P.F.; Xue, P.; Wang, K.; Yang, Q.; Zhou, J.H.; Wang, M.; Wang, L.; Chen, B.Q.; Tan, T.W. Light-driven enzymatic nanosystem for highly selective production of formic acid from CO2. Chem. Eng. J. 2021, 420, 127649. [Google Scholar] [CrossRef]
- Liu, G.H.; Wang, L.R.; Yan, L.H.; Zhao, H.; Li, Y.X.; Zhou, L.Y.; He, Y.; Ma, L.; Liu, Y.T.; Gao, J.; et al. A dual-enzyme microreactor based on encapsulation and covalent bond for enzymatic electrocatalytic CO2 reduction. Chem. Eng. J. 2023, 475, 146186. [Google Scholar] [CrossRef]
- Xu, X.Y.; Kentish, S.E.; Martin, G.J.O. Direct air capture of CO2 by microalgae with buoyant beads encapsulating carbonic anhydrase. ACS Sustain. Chem. Eng. 2021, 9, 9698–9706. [Google Scholar] [CrossRef]
- Jun, S.H.; Yang, J.; Jeon, H.; Kim, H.S.; Pack, S.P.; Jin, E.; Kim, J. Stabilized and immobilized carbonic anhydrase on electrospun nanofibers for enzymatic CO2 conversion and utilization in expedited microalgal growth. Environ. Sci. Technol. 2020, 54, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- You, S.K.; Ko, Y.J.; Shin, S.K.; Hwang, D.H.; Kang, D.H.; Park, H.M.; Han, S.O. Enhanced CO2 fixation and lipid production of Chlorella vulgaris through the carbonic anhydrase complex. Bioresour. Technol. 2020, 318, 124072. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.; Iliuta, I.; Bougie, F.; Pasquier, L.C.; Iliuta, M.C. Techno-economic assessment of enzymatic CO2 capture in hollow fiber membrane contactors with immobilized carbonic anhydrase. Sep. Purif. Technol. 2023, 307, 122702. [Google Scholar] [CrossRef]
- Jo, B.H.; Seo, J.H.; Cha, H.J. Bacterial extremo-α-carbonic anhydrases from deep-sea hydrothermal vents as potential biocatalysts for CO2 sequestration. J. Mol. Catal. B-Enzym. 2014, 109, 31–39. [Google Scholar] [CrossRef]
- Parra-Cruz, R.; Jager, C.M.; Lau, P.L.; Gomes, R.L.; Pordea, A. Rational design of thermostable carbonic anhydrase mutants using molecular dynamics simulations. J. Phys. Chem. B 2018, 122, 8526–8536. [Google Scholar] [CrossRef]
- Parra-Cruz, R.; Lau, P.L.; Loh, H.S.; Pordea, A. Engineering of Thermovibrio ammonificans carbonic anhydrase mutants with increased thermostability. J. CO2 Util. 2020, 37, 1–8. [Google Scholar] [CrossRef]
- Voyer, N.; Daigle, R.; Madore, É.; Fradette, S. Variants of Thermovibrio ammonificans Carbonic Anhydrase and CO2 Capture Methods Using Thermovibrio ammonificans Carbonic Anhydrase Variants. U.S. Patent US10415028B2, 17 September 2019. [Google Scholar]
- Jo, B.H. An intrinsically disordered peptide tag that confers an unusual solubility to aggregation-prone proteins. Appl. Environ. Microbiol. 2022, 88, e00097-22. [Google Scholar] [CrossRef]
- Hwang, I.S.; Kim, J.H.; Jo, B.H. Enhanced production of a thermostable carbonic anhydrase in Escherichia coli by using a modified NEXT tag. Molecules 2021, 26, 5830. [Google Scholar] [CrossRef]
- Jo, B.H.; Im, S.K.; Cha, H.J. Halotolerant carbonic anhydrase with unusual N-terminal extension from marine Hydrogenovibrio marinus as novel biocatalyst for carbon sequestration under high-salt environments. J. CO2 Util. 2018, 26, 415–424. [Google Scholar] [CrossRef]
- Santner, A.A.; Croy, C.H.; Vasanwala, F.H.; Uversky, V.N.; Van, Y.Y.; Dunker, A.K. Sweeping away protein aggregation with entropic bristles: Intrinsically disordered protein fusions enhance soluble expression. Biochemistry 2012, 51, 7250–7262. [Google Scholar] [CrossRef]
- Nguyen, T.K.M.; Ki, M.R.; Son, R.G.; Pack, S.P. The NT11, a novel fusion tag for enhancing protein expression in Escherichia coli. Appl. Microbiol. Biotechnol. 2019, 103, 2205–2216. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zaro, J.L.; Shen, W.C. Fusion protein linkers: Property, design and functionality. Adv. Drug Deliv. Rev. 2013, 65, 1357–1369. [Google Scholar] [CrossRef]
- Trevino, S.R.; Scholtz, J.M.; Pace, C.N. Measuring and increasing protein solubility. J. Pharm. Sci. 2008, 97, 4155–4166. [Google Scholar] [CrossRef] [PubMed]
- Golovanov, A.P.; Hautbergue, G.M.; Wilson, S.A.; Lian, L.Y. A simple method for improving protein solubility and long-term stability. J. Am. Chem. Soc. 2004, 126, 8933–8939. [Google Scholar] [CrossRef] [PubMed]
- Kramer, R.M.; Shende, V.R.; Motl, N.; Pace, C.N.; Scholtz, J.M. Toward a molecular understanding of protein solubility: Increased negative surface charge correlates with increased solubility. Biophys. J. 2012, 102, 1907–1915. [Google Scholar] [CrossRef]
- Chan, P.; Curtis, R.A.; Warwicker, J. Soluble expression of proteins correlates with a lack of positively-charged surface. Sci. Rep. 2013, 3, 3333. [Google Scholar] [CrossRef]
- Alvizo, O.; Nguyen, L.J.; Savile, C.K.; Bresson, J.A.; Lakhapatri, S.L.; Solis, E.O.P.; Fox, R.J.; Broering, J.M.; Benoit, M.R.; Zimmerman, S.A.; et al. Directed evolution of an ultrastable carbonic anhydrase for highly efficient carbon capture from flue gas. Proc. Natl. Acad. Sci. USA 2014, 111, 16436–16441. [Google Scholar] [CrossRef] [PubMed]
- Aymard, C.; Belarbi, A. Kinetics of thermal deactivation of enzymes: A simple three parameters phenomenological model can describe the decay of enzyme activity, irrespectively of the mechanism. Enzyme Microb. Technol. 2000, 27, 612–618. [Google Scholar] [CrossRef]
- Scott, K.M.; Williams, J.; Porter, C.M.B.; Russel, S.; Harmer, T.L.; Paul, J.H.; Antonen, K.M.; Bridges, M.K.; Camper, G.J.; Campla, C.K.; et al. Genomes of ubiquitous marine and hypersaline Hydrogenovibrio, Thiomicrorhabdus and Thiomicrospira spp. encode a diversity of mechanisms to sustain chemolithoautotrophy in heterogeneous environments. Environ. Microbiol. 2018, 20, 2686–2708. [Google Scholar] [CrossRef]
- Gill, S.C.; von Hippel, P.H. Calculation of protein extinction coefficients from amino acid sequence data. Anal. Biochem. 1989, 182, 319–326. [Google Scholar] [CrossRef]
- Wilbur, K.M.; Anderson, N.G. Electrometric and colorimetric determination of carbonic anhydrase. J. Biol. Chem. 1948, 176, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Jo, B.H. A colorimetric CO2 hydration assay for facile, accurate, and precise determination of carbonic anhydrase activity. Catalysts 2022, 12, 1391. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Wilkins, M.R.; Gasteiger, E.; Bairoch, A.; Sanchez, J.C.; Williams, K.L.; Appel, R.D.; Hochstrasser, D.F. Protein identification and analysis tools in the ExPASy server. Methods Mol. Biol. 1999, 112, 531–552. [Google Scholar] [PubMed]
- Kyte, J.; Doolittle, R.F. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 1982, 157, 105–132. [Google Scholar] [CrossRef] [PubMed]
- Teufel, F.; Almagro Armenteros, J.J.; Johansen, A.R.; Gislason, M.H.; Pihl, S.I.; Tsirigos, K.D.; Winther, O.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 6.0 predicts all five types of signal peptides using protein language models. Nat. Biotechnol. 2022, 40, 1023–1025. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [PubMed]
- Robert, X.; Gouet, P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 2014, 42, W320–W324. [Google Scholar] [CrossRef]
- Meszaros, B.; Erdos, G.; Dosztanyi, Z. IUPred2A: Context-dependent prediction of protein disorder as a function of redox state and protein binding. Nucleic Acids Res. 2018, 46, W329–W337. [Google Scholar] [CrossRef]
- Romero, P.; Obradovic, Z.; Li, X.; Garner, E.C.; Brown, C.J.; Dunker, A.K. Sequence complexity of disordered protein. Proteins 2001, 42, 38–48. [Google Scholar] [CrossRef]
- Cheng, J.; Sweredoski, M.J.; Baldi, P. Accurate prediction of protein disordered regions by mining protein structure data. Data Min. Knowl. Discov. 2005, 11, 213–222. [Google Scholar] [CrossRef]

| Kinetic Mode | Pre-Exponential Parameters | Inactivation Rate Constants (Half-Life in Day) | |
|---|---|---|---|
| DvCA10 | Biphasic | x1: 0.58 | k1: 0.3064 d−1 (2.3 d) |
| x2: 0.42 | k2: 0.0272 d−1 (25.5 d) | ||
| taCA | Monophasic | 1 | k: 0.0867 d−1 (8.0 d) |
| NEXT-taCA | Monophasic | 1 | k: 0.0667 d−1 (10.4 d) |
| Fusion Tag | Amino Acid Length | Molecular Mass (kDa) | pI | Charged Amino Acids | Net Charge | Mean Hydropathy |
|---|---|---|---|---|---|---|
| NEXT | 53 | 5.5 | 8.1 | 28% | +1 | 0.397 |
| Hcr | 51 | 5.5 | 9.0 | 39% | +2 | 0.367 |
| Hku | 57 | 5.8 | 9.2 | 21% | +2 | 0.406 |
| Strains, Plasmids, or Primers | Genotypes, Relevant Characteristics, or Sequences | Source or References |
|---|---|---|
| Strains | ||
| E. coli TOP10 | F− mcrA Δ(mrr-hsdRMS-mcrBC) Φ80lacZΔM15 ΔlacX74 recA1 araD139 Δ(ara-leu)7697 galU galK rpsL(Strr) endA1 nupG | Thermo Fisher Scientific, Waltham, MA, USA |
| E. coli BL21(DE3) | F− ompT hsdSB(rB− mB−) gal dcm lon λ(DE3), carrying T7 RNA polymerase gene | Novagen, Madison, WI, USA |
| Plasmids | ||
| pGEM-T Easy | pUC ori, Ampr, TA cloning vector, | Promega, Madison, WI, USA |
| pET-22b(+) | T7lac promoter, pBR322 ori, Ampr, parental expression vector harboring PelB signal sequence | Novagen, Madison, WI, USA |
| pET-taCA | Expression plasmid carrying taCA gene | [18] |
| pET-NEXT-taCA | Expression plasmid carrying NEXT-tagged taCA gene with the (GGGGS)2 linker | [18] |
| pET-NEXT-taCAno link | Expression plasmid carrying NEXT-tagged taCA gene without linker | This study |
| pET-NEXT-taCAshort link | Expression plasmid carrying NEXT-tagged taCA gene with the GGGGS linker | This study |
| pET-NT11-taCA | Expression plasmid carrying NT11-tagged taCA gene | [22] |
| pET-DvCA10 | Expression plasmid carrying DvCA10 gene | This study |
| pET-Hcr-taCA | Expression plasmid carrying Hcr-tagged taCA gene with the (GGGGS)2 linker | This study |
| pET-Hku-taCA | Expression plasmid carrying Hku-tagged taCA gene with the (GGGGS)2 linker | This study |
| Primers a | ||
| NEXT-Forward | CATATGGCTGTTCAACATAGCAATGCCCC | [18] |
| NEXT-no link-Reverse | CCATGGCCACAACGGGTTTTGGTTTAG | This study |
| NEXT-short link-Reverse | CCATGGAGCCTCCACCGCCCACAACGGGTTTTGGTTTAG | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, B.H. Improved Solubility and Stability of a Thermostable Carbonic Anhydrase via Fusion with Marine-Derived Intrinsically Disordered Solubility Enhancers. Int. J. Mol. Sci. 2024, 25, 1139. https://doi.org/10.3390/ijms25021139
Jo BH. Improved Solubility and Stability of a Thermostable Carbonic Anhydrase via Fusion with Marine-Derived Intrinsically Disordered Solubility Enhancers. International Journal of Molecular Sciences. 2024; 25(2):1139. https://doi.org/10.3390/ijms25021139
Chicago/Turabian StyleJo, Byung Hoon. 2024. "Improved Solubility and Stability of a Thermostable Carbonic Anhydrase via Fusion with Marine-Derived Intrinsically Disordered Solubility Enhancers" International Journal of Molecular Sciences 25, no. 2: 1139. https://doi.org/10.3390/ijms25021139
APA StyleJo, B. H. (2024). Improved Solubility and Stability of a Thermostable Carbonic Anhydrase via Fusion with Marine-Derived Intrinsically Disordered Solubility Enhancers. International Journal of Molecular Sciences, 25(2), 1139. https://doi.org/10.3390/ijms25021139






