Microbiota-Derived Extracellular Vesicles as a Postbiotic Strategy to Alleviate Diarrhea and Enhance Immunity in Rotavirus-Infected Neonatal Rats
Abstract
:1. Introduction
2. Results
2.1. Evaluation of Body Weight and Morphometric Variables
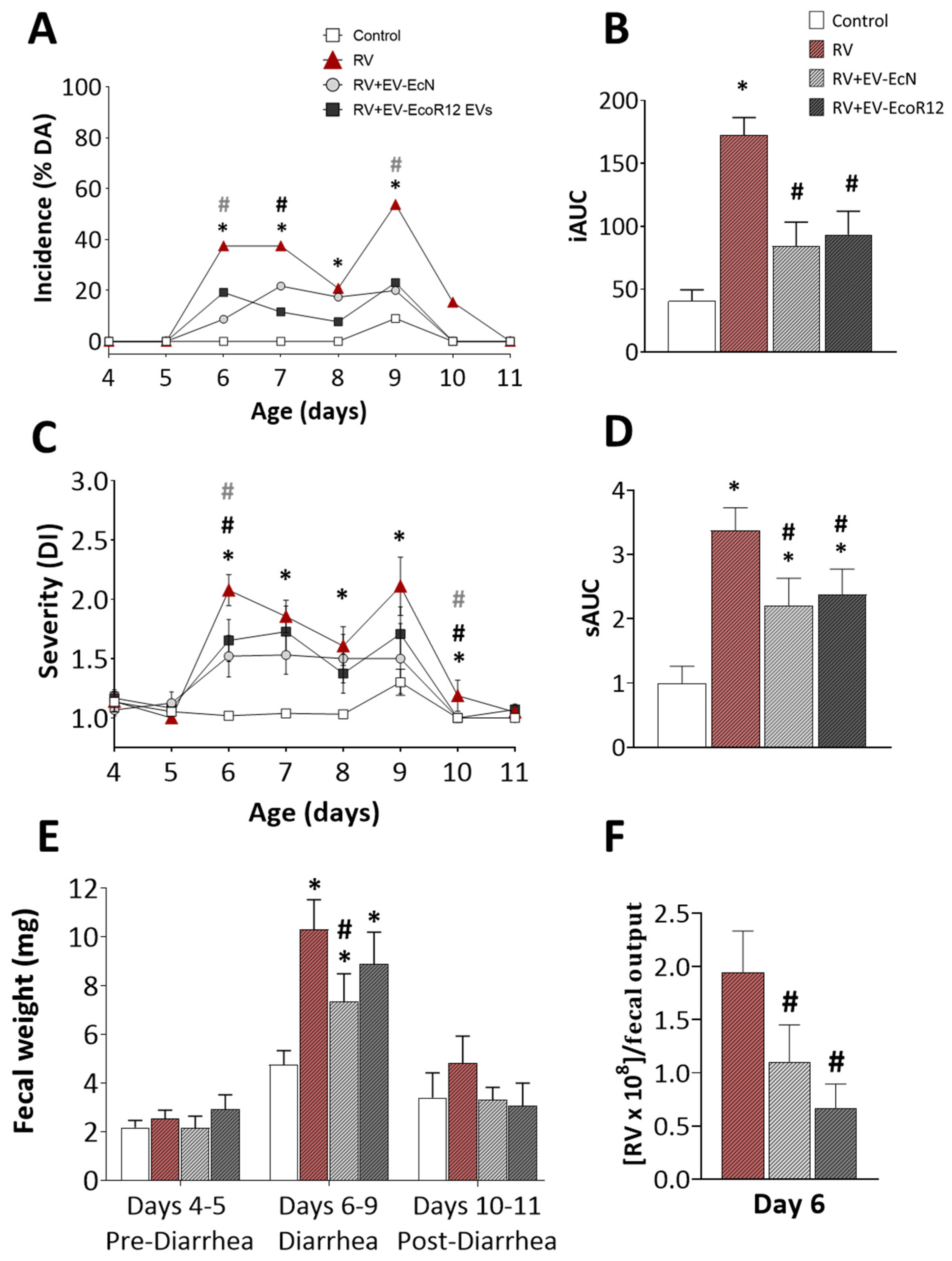
2.2. Clinical Evaluation of Diarrhea
2.3. Viral Shedding and In Vitro Blocking Assay
2.4. Systemic Humoral and Anti-RV Antibody Response
2.5. Cellular Immune Response
2.6. Gene Expression Analysis in Small Intestine
2.7. Intestinal Serotonin (5-HT)
2.8. Histological Analysis of Mucosal Morphology and Goblet Cell Numbers
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Isolation of Extracellular Vesicles
4.2. Virus
4.3. Animals
4.4. Experimental Design
4.5. Clinical Evaluation and Fecal Specimen Collection
4.6. Sample Collection
4.7. Fecal Viral Shedding
4.8. In Vitro Blocking Assay
4.9. Quantification of Total Immunoglobulins and Specific Anti-Rotavirus Antibodies in Plasma
4.10. Lymphocyte Isolation from Spleen and Immunophenotyping by Flow Cytometry
4.11. Gene Expression Analysis by Reverse Transcription–quantitative PCR (RT-qPCR)
4.12. Serotonin Quantification by ELISA
4.13. Histomorphometry Analysis/Mucin Staining
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lundgren, O.; Svensson, L. Pathogenesis of Rotavirus Diarrhea. Microbes Infect. 2001, 3, 13. [Google Scholar] [CrossRef] [PubMed]
- Crawford, S.E.; Ramani, S.; Tate, J.E.; Parashar, U.D.; Svensson, L.; Hagbom, M.; Franco, M.A.; Greenberg, H.B.; O’Ryan, M.; Kang, G.; et al. Rotavirus Infection. Nat. Rev. Dis. Primers 2017, 3, 17083. [Google Scholar] [CrossRef] [PubMed]
- Parashar, U.D.; Gibson, C.J.; Bresee, J.S.; Glass, R.I. Rotavirus and Severe Childhood Diarrhea. Emerg. Infect. Dis. 2006, 12, 304–306. [Google Scholar] [CrossRef]
- Tate, J.E.; Burton, A.H.; Boschi-Pinto, C.; Steele, A.D.; Duque, J.; Parashar, U.D. 2008 Estimate of Worldwide Rotavirus-Associated Mortality in Children Younger Than 5 Years before the Introduction of Universal Rotavirus Vaccination Programmes: A Systematic Review and Meta-Analysis. Lancet Infect. Dis. 2012, 12, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Lundgren, O.; Peregrin, A.T.; Persson, K.; Kordasti, S.; Uhnoo, I.; Svensson, L. Role of the Enteric Nervous System in the Fluid and Electrolyte Secretion of Rotavirus Diarrhea. Science 2000, 287, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Bialowas, S.; Hagbom, M.; Nordgren, J.; Karlsson, T.; Sharma, S.; Magnusson, K.E.; Svensson, L. Rotavirus and Serotonin Cross-Talk in Diarrhoea. PLoS ONE 2016, 11, e0159660. [Google Scholar] [CrossRef] [PubMed]
- Koopman, N.; Katsavelis, D.; Ten Hove, A.S.; Brul, S.; de Jonge, W.J.; Seppen, J. The Multifaceted Role of Serotonin in Intestinal Homeostasis. Int. J. Mol. Sci. 2021, 22, 9487. [Google Scholar] [CrossRef]
- Hagbom, M.; Istrate, C.; Engblom, D.; Karlsson, T.; Rodriguez-Diaz, J.; Buesa, J.; Taylor, J.A.; Loitto, V.M.; Magnusson, K.E.; Ahlman, H.; et al. Rotavirus Stimulates Release of Serotonin (5-HT) from Human Enterochromaffin Cells and Activates Brain Structures Involved in Nausea and Vomiting. PLoS Pathog. 2011, 7, e1002115. [Google Scholar] [CrossRef]
- Hagbom, M.; Hellysaz, A.; Istrate, C.; Nordgren, J.; Sharma, S.; de-Faria, F.M.; Magnusson, K.-E.; Svensson, L. The 5-HT 3 Receptor Affects Rotavirus-Induced Motility. J. Virol. 2021, 95, e00751-21. [Google Scholar] [CrossRef]
- Franco, M.A.; Angel, J.; Greenberg, H.B. Immunity and Correlates of Protection for Rotavirus Vaccines. Vaccine 2006, 24, 2718–2731. [Google Scholar] [CrossRef]
- Blutt, S.E.; Miller, A.D.; Salmon, S.L.; Metzger, D.W.; Conner, M.E. IgA Is Important for Clearance and Critical for Protection from Rotavirus Infection. Mucosal Immunol. 2012, 5, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Pollock, L.; Bennett, A.; Jere, K.C.; Mandolo, J.; Dube, Q.; Bar-Zeev, N.; Heyderman, R.S.; Cunliffe, N.A.; Iturriza-Gomara, M. Plasma Rotavirus-Specific IgA and Risk of Rotavirus Vaccine Failure in Infants in Malawi. Clin. Infect. Dis. 2022, 75, 41–46. [Google Scholar] [CrossRef]
- Perez-Schael, I.; Salinas, B.; Tomat, M.; Linhares, A.C.; Guerrero, M.L.; Ruiz-Palacios, G.M.; Bouckenooghe, A.; Yarzábal, J.P. Efficacy of the Human Rotavirus Vaccine RIX4414 in Malnourished Children. J. Infect. Dis. 2007, 196, 537–540. [Google Scholar] [CrossRef] [PubMed]
- Lopman, B.A.; Pitzer, V.E.; Sarkar, R.; Gladstone, B.; Patel, M.; Glasser, J.; Gambhir, M.; Atchison, C.; Grenfell, B.T.; Edmunds, W.J.; et al. Understanding Reduced Rotavirus Vaccine Efficacy in Low Socio-Economic Settings. PLoS ONE 2012, 7, e41720. [Google Scholar] [CrossRef]
- Qadri, F.; Bhuiyan, T.R.; Sack, D.A.; Svennerholm, A.M. Immune Responses and Protection in Children in Developing Countries Induced by Oral Vaccines. Vaccine 2013, 31, 452–460. [Google Scholar] [CrossRef]
- Valdez, Y.; Brown, E.M.; Finlay, B.B. Influence of the Microbiota on Vaccine Effectiveness. Trends Immunol. 2014, 35, 526–537. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, M.; Vimont, A.; Darveau, A.; Fliss, I.; Jean, J. Study of the Ability of Bifidobacteria of Human Origin to Prevent and Treat Rotavirus Infection Using Colonic Cell and Mouse Models. PLoS ONE 2016, 11, e0164512. [Google Scholar] [CrossRef]
- Kandasamy, S.; Vlasova, A.N.; Fischer, D.; Kumar, A.; Chattha, K.S.; Rauf, A.; Shao, L.; Langel, S.N.; Rajashekara, G.; Saif, L.J. Differential Effects of Escherichia coli Nissle and Lactobacillus Rhamnosus Strain GG on Human Rotavirus Binding, Infection, and B Cell Immunity. J. Immunol. 2016, 196, 1780–1789. [Google Scholar] [CrossRef]
- Rigo-Adrover, M.; Saldaña-Ruíz, S.; van Limpt, K.; Knipping, K.; Garssen, J.; Knol, J.; Franch, A.; Castell, M.; Pérez-Cano, F.J. A Combination of ScGOS/LcFOS with Bifidobacterium Breve M-16V Protects Suckling Rats from Rotavirus Gastroenteritis. Eur. J. Nutr. 2017, 56, 1657–1670. [Google Scholar] [CrossRef]
- Azagra-Boronat, I.; Massot-Cladera, M.; Knipping, K.; Garssen, J.; Ben Amor, K.; Knol, J.; Franch, À.; Castell, M.; Rodríguez-Lagunas, M.J.; Pérez-Cano, F.J. Strain-Specific Probiotic Properties of Bifidobacteria and Lactobacilli for the Prevention of Diarrhea Caused by Rotavirus in a Preclinical Model. Nutrients 2020, 12, 498. [Google Scholar] [CrossRef]
- Paim, F.C.; Langel, S.N.; Fischer, D.D.; Kandasamy, S.; Shao, L.; Alhamo, M.A.; Huang, H.C.; Kumar, A.; Rajashekara, G.; Saif, L.J.; et al. Effects of Escherichia coli Nissle 1917 and Ciprofloxacin on Small Intestinal Epithelial Cell MRNA Expression in the Neonatal Piglet Model of Human Rotavirus Infection. Gut Pathog. 2016, 8, 66. [Google Scholar] [CrossRef]
- Vlasova, A.N.; Shao, L.; Kandasamy, S.; Fischer, D.D.; Rauf, A.; Langel, S.N.; Chattha, K.S.; Kumar, A.; Huang, H.C.; Rajashekara, G.; et al. Escherichia coli Nissle 1917 Protects Gnotobiotic Pigs against Human Rotavirus by Modulating PDC and NK-Cell Responses. Eur. J. Immunol. 2016, 46, 2426–2437. [Google Scholar] [CrossRef] [PubMed]
- Villena, J.; Vizoso-Pinto, M.G.; Kitazawa, H. Intestinal Innate Antiviral Immunity and Immunobiotics: Beneficial Effects against Rotavirus Infection. Front. Immunol. 2016, 7, 563. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, S.; Vlasova, A.N.; Fischer, D.D.; Chattha, K.S.; Shao, L.; Kumar, A.; Langel, S.N.; Rauf, A.; Huang, H.C.; Rajashekara, G.; et al. Unraveling the Differences between Gram-Positive and Gram-Negative Probiotics in Modulating Protective Immunity to Enteric Infections. Front. Immunol. 2017, 8, 334. [Google Scholar] [CrossRef]
- Sonnenborn, U. Escherichia coli Strain Nissle 1917-from Bench to Bedside and Back: History of a Special Escherichia coli Strain with Probiotic Properties. FEMS Microbiol. Lett. 2016, 363, 19. [Google Scholar] [CrossRef] [PubMed]
- Behrouzi, A.; Mazaheri, H.; Falsafi, S.; Tavassol, Z.H.; Moshiri, A.; Siadat, S.D. Intestinal Effect of the Probiotic Escherichia coli Strain Nissle 1917 and Its OMV. J. Diabetes Metab. Disord. 2020, 19, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Scaldaferri, F.; Gerardi, V.; Mangiola, F.; Lopetuso, L.R.; Pizzoferrato, M.; Petito, V.; Papa, A.; Stojanovic, J.; Poscia, A.; Cammarota, G.; et al. Role and Mechanisms of Action of Escherichia coli Nissle 1917 in the Maintenance of Remission in Ulcerative Colitis Patients: An Update. World J. Gastroenterol. 2016, 22, 5505–5511. [Google Scholar] [CrossRef]
- Henker, J.; Laass, M.; Blokhin, B.M.; Bolbot, Y.K.; Maydannik, V.G.; Elze, M.; Wolff, C.; Schulze, J. The Probiotic Escherichia coli Strain Nissle 1917 (EcN) Stops Acute Diarrhoea in Infants and Toddlers. Eur. J. Pediatr. 2007, 166, 311–318. [Google Scholar] [CrossRef]
- Michael, H.; Paim, F.C.; Miyazaki, A.; Langel, S.N.; Fischer, D.D.; Chepngeno, J.; Goodman, S.D.; Rajashekara, G.; Saif, L.J.; Vlasova, A.N. Escherichia coli Nissle 1917 Administered as a Dextranomar Microsphere Biofilm Enhances Immune Responses against Human Rotavirus in a Neonatal Malnourished Pig Model Colonized with Human Infant Fecal Microbiota. PLoS ONE 2021, 16, e0246193. [Google Scholar] [CrossRef]
- Michael, H.; Srivastava, V.; Deblais, L.; Amimo, J.O.; Chepngeno, J.; Saif, L.J.; Rajashekara, G.; Vlasova, A.N. The Combined Escherichia coli Nissle 1917 and Tryptophan Treatment Modulates Immune and Metabolome Responses to Human Rotavirus Infection in a Human Infant Fecal Microbiota-Transplanted Malnourished Gnotobiotic Pig Model. mSphere 2022, 7, e0027022. [Google Scholar] [CrossRef]
- Michael, H.; Miyazaki, A.; Langel, S.N.; Amimo, J.O.; Kick, M.K.; Chepngeno, J.; Paim, F.C.; Fischer, D.D.; Rajashekara, G.; Saif, L.J.; et al. Escherichia coli Nissle 1917 Enhances Efficacy of Oral Attenuated Human Rotavirus Vaccine in a Gnotobiotic Piglet Model. Vaccines 2022, 10, 83. [Google Scholar] [CrossRef] [PubMed]
- Wegh, C.A.M.; Geerlings, S.Y.; Knol, J.; Roeselers, G.; Belzer, C. Postbiotics and Their Potential Applications in Early Life Nutrition and Beyond. Int. J. Mol. Sci. 2019, 20, 4673. [Google Scholar] [CrossRef]
- Żółkiewicz, J.; Marzec, A.; Ruszczyński, M.; Feleszko, W. Postbiotics—A Step beyond Pre- and Probiotics. Nutrients 2020, 12, 2189. [Google Scholar] [CrossRef] [PubMed]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Malagón-Rojas, J.N.; Mantziari, A.; Salminen, S.; Szajewska, H. Postbiotics for Preventing and Treating Common Infectious Diseases in Children: A Systematic Review. Nutrients 2020, 12, 389. [Google Scholar] [CrossRef] [PubMed]
- Morales-Ferré, C.; Azagra-Boronat, I.; Massot-Cladera, M.; Tims, S.; Knipping, K.; Garssen, J.; Knol, J.; Franch, À.; Castell, M.; Pérez-Cano, F.J.; et al. Preventive Effect of a Postbiotic and Prebiotic Mixture in a Rat Model of Early Life Rotavirus Induced-Diarrhea. Nutrients 2022, 14, 1163. [Google Scholar] [CrossRef]
- Sartorio, M.G.; Pardue, E.J.; Feldman, M.F.; Haurat, M.F. Bacterial Outer Membrane Vesicles: From Discovery to Applications. Annu. Rev. Microbiol. 2021, 75, 609–630. [Google Scholar] [CrossRef]
- Toyofuku, M.; Schild, S.; Kaparakis-Liaskos, M.; Eberl, L. Composition and Functions of Bacterial Membrane Vesicles. Nat. Rev. Microbiol. 2023, 21, 415–430. [Google Scholar] [CrossRef]
- Ñahui Palomino, R.A.; Vanpouille, C.; Costantini, P.E.; Margolis, L. Microbiota–Host Communications: Bacterial Extracellular Vesicles as a Common Language. PLoS Pathog. 2021, 17, e1009508. [Google Scholar] [CrossRef]
- Kaparakis-Liaskos, M.; Ferrero, R.L. Immune Modulation by Bacterial Outer Membrane Vesicles. Nat. Rev. Immunol. 2015, 15, 375–387. [Google Scholar] [CrossRef]
- Díaz-Garrido, N.; Badia, J.; Baldomà, L. Microbiota-Derived Extracellular Vesicles in Interkingdom Communication in the Gut. J. Extracell. Vesicles 2021, 10, e12161. [Google Scholar] [CrossRef]
- Bitto, N.J.; Kaparakis-Liaskos, M. The Therapeutic Benefit of Bacterial Membrane Vesicles. Int. J. Mol. Sci. 2017, 18, 1287. [Google Scholar] [CrossRef]
- González-Lozano, E.; García-García, J.; Gálvez, J.; Hidalgo-García, L.; Rodríguez-Nogales, A.; Rodríguez-Cabezas, M.E.; Sánchez, M. Novel Horizons in Postbiotics: Lactobacillaceae Extracellular Vesicles and Their Applications in Health and Disease. Nutrients 2022, 14, 5296. [Google Scholar] [CrossRef] [PubMed]
- Olivo-Martínez, Y.; Bosch, M.; Badia, J.; Baldomà, L. Modulation of the Intestinal Barrier Integrity and Repair by Microbiota Extracellular Vesicles through the Differential Regulation of Trefoil Factor 3 in LS174T Goblet Cells. Nutrients 2023, 15, 2437. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Garrido, N.; Fábrega, M.J.; Vera, R.; Giménez, R.; Badia, J.; Baldomà, L. Membrane Vesicles from the Probiotic Nissle 1917 and Gut Resident Escherichia coli Strains Distinctly Modulate Human Dendritic Cells and Subsequent T Cell Responses. J. Funct. Foods 2019, 61, 103495. [Google Scholar] [CrossRef]
- Diaz-Garrido, N.; Badia, J.; Baldomà, L. Modulation of Dendritic Cells by Microbiota Extracellular Vesicles Influences the Cytokine Profile and Exosome Cargo. Nutrients 2022, 14, 344. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ruiz, S.; Sáez-Fuertes, L.; Casanova-Crespo, S.; Rodríguez-Lagunas, M.J.; Pérez-Cano, F.J.; Badia, J.; Baldoma, L. Microbiota-Derived Extracellular Vesicles Promote Immunity and Intestinal Maturation in Suckling Rats. Nutrients 2023, 15, 4701. [Google Scholar] [CrossRef]
- Alvarez, C.S.; Giménez, R.; Cañas, M.A.; Vera, R.; Díaz-Garrido, N.; Badia, J.; Baldomà, L. Extracellular Vesicles and Soluble Factors Secreted by Escherichia coli Nissle 1917 and ECOR63 Protect against Enteropathogenic E. coli-Induced Intestinal Epithelial Barrier Dysfunction. BMC Microbiol. 2019, 19, 166. [Google Scholar] [CrossRef]
- Pérez-Cano, F.J.; Castell, M.; Castellote, C.; Franch, À. Characterization of Clinical and Immune Response in a Rotavirus Diarrhea Model in Suckling Lewis Rats. Pediatr. Res. 2007, 62, 658–663. [Google Scholar] [CrossRef]
- Boshuizen, J.A.; Reimerink, J.H.J.; Korteland-Van Male, A.M.; Van Ham, V.J.J.; Bouma, J.; Gerwig, G.J.; Koopmans, M.P.G.; Büller, H.A.; Dekker, J.; Einerhand, A.W.C. Homeostasis and Function of Goblet Cells during Rotavirus Infection in Mice. Virology 2005, 337, 210–221. [Google Scholar] [CrossRef]
- Kim, A.H.J.; Hogarty, M.P.; Harris, V.C.; Baldridge, M.T. The Complex Interactions between Rotavirus and the Gut Microbiota. Front. Cell. Infect. Microbiol. 2021, 10, 586751. [Google Scholar] [CrossRef] [PubMed]
- Gaon, D.; Garcia, H.; Winter, L.; Rodriguez, N.; Quintas, R.; Gonzalez, S.N.; Oliver, G. Effect of Lactobacillus Strains and Saccharomyces boulardii on Persistent Diarrhea in Children. Medicina 2003, 63, 293–298. [Google Scholar] [PubMed]
- Grandy, G.; Medina, M.; Soria, R.; Terán, C.G.; Araya, M. Probiotics in the Treatment of Acute Rotavirus Diarrhoea. A Randomized, Double-Blind, Controlled Trial Using Two Different Probiotic Preparations in Bolivian Children. BMC Infect. Dis. 2010, 10, 253. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.K.; Park, J.E.; Kim, M.J.; Seo, J.G.; Lee, J.H.; Ha, N.J. Probiotic Bacteria, B. longum and L. acidophilus Inhibit Infection by Rotavirus In Vitro and Decrease the Duration of Diarrhea in Pediatric Patients. Clin. Res. Hepatol. Gastroenterol. 2015, 39, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, E.; Alizadeh-Navaei, R.; Rezai, M.S. Efficacy of Probiotic Use in Acute Rotavirus Diarrhea in Children: A Systematic Review and Meta-Analysis. Casp. J. Intern. Med. 2015, 6, 187–195. [Google Scholar]
- Huang, R.; Xing, H.Y.; Liu, H.J.; Chen, Z.F.; Tang, B.B. Efficacy of Probiotics in the Treatment of Acute Diarrhea in Children: A Systematic Review and Meta-Analysis of Clinical Trials. Transl. Pediatr. 2021, 10, 3248–3260. [Google Scholar] [CrossRef]
- Carvalho, M.F.; Gill, D. Rotavirus Vaccine Efficacy: Current Status and Areas for Improvement. Hum. Vaccin. Immunother. 2019, 15, 1237–1250. [Google Scholar] [CrossRef]
- Raya Tonetti, F.; Arce, L.; Salva, S.; Alvarez, S.; Takahashi, H.; Kitazawa, H.; Vizoso-Pinto, M.G.; Villena, J. Immunomodulatory Properties of Bacterium-like Particles Obtained from Immunobiotic Lactobacilli: Prospects for Their Use as Mucosal Adjuvants. Front. Immunol. 2020, 11, 15. [Google Scholar] [CrossRef]
- Azagra-Boronat, I.; Massot-Cladera, M.; Knipping, K.; van’t Land, B.; Stahl, B.; Garssen, J.; Rodríguez-Lagunas, M.J.; Franch, À.; Castell, M.; Pérez-Cano, F.J. Supplementation with 2′-FL and ScGOS/LcFOS Ameliorates Rotavirus-Induced Diarrhea in Suckling Rats. Front. Cell. Infect. Microbiol. 2018, 8, 372. [Google Scholar] [CrossRef]
- Azagra-Boronat, I.; Massot-Cladera, M.; Knipping, K.; Van‘T Land, B.; Tims, S.; Stahl, B.; Knol, J.; Garssen, J.; Franch, À.; Castell, M.; et al. Oligosaccharides Modulate Rotavirus-Associated Dysbiosis and TLR Gene Expression in Neonatal Rats. Cells 2019, 8, 876. [Google Scholar] [CrossRef]
- Qasem, A.; Naser, A.E.; Naser, S.A. Enteropathogenic Infections Modulate Intestinal Serotonin Transporter (SERT) Function by Activating Toll-like Receptor 2 (TLR-2) in Crohn’s Disease. Sci. Rep. 2021, 11, 22624. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.M.; Ge, X.Z.; Wang, W.Q.; Wang, T.; Cao, H.L.; Wang, B.L.; Wang, B.M. Lactobacillus Rhamnosus GG Supernatant Upregulates Serotonin Transporter Expression in Intestinal Epithelial Cells and Mice Intestinal Tissues. Neurogastroenterol. Motil. 2015, 27, 1239–1248. [Google Scholar] [CrossRef] [PubMed]
- Yaghoubfar, R.; Behrouzi, A.; Ashrafian, F.; Shahryari, A.; Moradi, H.R.; Choopani, S.; Hadifar, S.; Vaziri, F.; Nojoumi, S.A.; Fateh, A.; et al. Modulation of Serotonin Signaling/Metabolism by Akkermansia Muciniphila and Its Extracellular Vesicles through the Gut-Brain Axis in Mice. Sci. Rep. 2020, 10, 22119. [Google Scholar] [CrossRef] [PubMed]
- Yaghoubfar, R.; Behrouzi, A.; Zare Banadkoki, E.; Ashrafian, F.; Lari, A.; Vaziri, F.; Nojoumi, S.A.; Fateh, A.; Khatami, S.; Siadat, S.D. Effect of Akkermansia Muciniphila, Faecalibacterium Prausnitzii, and Their Extracellular Vesicles on the Serotonin System in Intestinal Epithelial Cells. Probiotics Antimicrob. Proteins 2021, 13, 1546–1556. [Google Scholar] [CrossRef] [PubMed]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous Bacteria from the Gut Microbiota Regulate Host Serotonin Biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Nzakizwanayo, J.; Dedi, C.; Standen, G.; Macfarlane, W.M.; Patel, B.A.; Jones, B.V. Escherichia coli Nissle 1917 Enhances Bioavailability of Serotonin in Gut Tissues through Modulation of Synthesis and Clearance. Sci. Rep. 2015, 5, 17324. [Google Scholar] [CrossRef]
- Engevik, M.A.; Chang-Graham, A.; Hyser, J.M.; Versalovic, J. Serotonin Promotes Epithelial Restitution through Goblet Cell Mediated Secretion of Muc2 and TFF3. FASEB J. 2019, 33, 869.1. [Google Scholar] [CrossRef]
- Kumar, A.; Helmy, Y.A.; Fritts, Z.; Vlasova, A.; Saif, L.J.; Rajashekara, G. Anti-Rotavirus Properties and Mechanisms of Selected Gram-Positive and Gram-Negative Probiotics on Polarized Human Colonic (HT-29) Cells. Probiotics Antimicrob. Proteins 2023, 15, 107–128. [Google Scholar] [CrossRef]
- Desselberger, U.; Huppertz, H.I. Immune Responses to Rotavirus Infection and Vaccination and Associated Correlates of Protection. J. Infect. Dis. 2011, 203, 188–195. [Google Scholar] [CrossRef]
- Dong, C. Cytokine Regulation and Function in T Cells. Annu. Rev. Immunol. 2021, 39, 51–76. [Google Scholar] [CrossRef]
- Li, M.; Zhou, H.; Yang, C.; Wu, Y.; Zhou, X.; Liu, H.; Wang, Y. Bacterial Outer Membrane Vesicles as a Platform for Biomedical Applications: An Update. J. Control. Release 2020, 323, 253–268. [Google Scholar] [CrossRef] [PubMed]
- Lester, S.N.; Li, K. Toll-like Receptors in Antiviral Innate Immunity. J. Mol. Biol. 2014, 426, 1246–1264. [Google Scholar] [CrossRef] [PubMed]
- Nur, N.A.; Balasubramaniam, V.R.M.T.; Yap, W.B. Potential of Interleukin (IL)-12 Group as Antivirals: Severe Viral Disease Prevention and Management. Int. J. Mol. Sci. 2023, 24, 7350. [Google Scholar] [CrossRef]
- Kühl, A.A.; Erben, U.; Kredel, L.I.; Siegmund, B. Diversity of Intestinal Macrophages in Inflammatory Bowel Diseases. Front. Immunol. 2015, 6, 613. [Google Scholar] [CrossRef] [PubMed]
- Arévalo Sureda, E.; Weström, B.; Pierzynowski, S.G.; Prykhodko, O. Maturation of the Intestinal Epithelial Barrier in Neonatal Rats Coincides with Decreased FcRn Expression, Replacement of Vacuolated Enterocytes and Changed Blimp-1 Expression. PLoS ONE 2016, 11, e0164775. [Google Scholar] [CrossRef]
- Boshuizen, J.A.; Reimerink, J.H.J.; Korteland-van Male, A.M.; van Ham, V.J.J.; Koopmans, M.P.G.; Büller, H.A.; Dekker, J.; Einerhand, A.W.C. Changes in Small Intestinal Homeostasis, Morphology, and Gene Expression during Rotavirus Infection of Infant Mice. J. Virol. 2003, 77, 13005–13016. [Google Scholar] [CrossRef]
- Cortez, V.; Schultz-Cherry, S. The Role of Goblet Cells in Viral Pathogenesis. FEBS J. 2021, 288, 7060–7072. [Google Scholar] [CrossRef]
- Jung, K.; Saif, L.J. Goblet Cell Depletion in Small Intestinal Villous and Crypt Epithelium of Conventional Nursing and Weaned Pigs Infected with Porcine Epidemic Diarrhea Virus. Res. Vet. Sci. 2017, 110, 12–15. [Google Scholar] [CrossRef]
- Golubkova, A.; Hunter, C.J. Development of the Neonatal Intestinal Barrier, Microbiome, and Susceptibility to NEC. Microorganisms 2023, 11, 1247. [Google Scholar] [CrossRef]
- Ochman, H.; Selander, R.K. Standard Reference Strains of Escherichia coli from Natural Populations. J. Bacteriol. 1984, 157, 690–693. [Google Scholar] [CrossRef]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C. AIN-93 Purified Diets for Laboratory Rodents: Final Report of the American Institute of Nutrition Ad Hoc Writing Committee on the Reformulation of the AIN-76A Rodent Diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef] [PubMed]
- Torres-Castro, P.; Grases-Pintó, B.; Abril-Gil, M.; Castell, M.; Rodríguez-Lagunas, M.J.; Pérez-Cano, F.J.; Franch, À. Modulation of the Systemic Immune Response in Suckling Rats by Breast Milk TGF-Β2, EGF and FGF21 Supplementation. Nutrients 2020, 12, 1888. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]





| Day 8 | CON | RV | RV + EV-EcN | RV + EV-EcoR12 |
|---|---|---|---|---|
| Intestine weight | 3.19 ± 0.06 | 3.43 ± 0.06 | 3.41 ± 0.05 | 3.57 ± 0.11 |
| Intestine length | 231.57 ± 7.39 | 210.64 ± 6.5 | 226.35 ± 6.42 | 231.95 ± 10.04 |
| Cecum weight | 0.19 ± 0.04 | 0.20 ± 0.04 | 0.21 ± 0.04 | 0.21 ± 0.04 |
| Thymus weight | 0.29 ± 0.01 | 0.30 ± 0.01 | 0.33 ± 0.03 | 0.29 ± 0.02 |
| Spleen weight | 0.48 ± 0.02 | 0.59 ± 0.02 | 0.61 ± 0.04 * | 0.60 ± 0.03 * |
| Day 16 | CON | RV | RV + EV-EcN | Rv + EV-EcoR12 |
| Intestine weight | 2.94 ± 0.04 | 3.03 ± 0.05 | 3.10± 0.08 | 2.97 ± 0.05 |
| Intestine length | 126.08 ± 5.51 | 119.74 ± 3.04 | 128.97 ± 7.26 | 134.07 ± 4.31 |
| Cecum weight | 0.21 ± 0.01 | 0.21 ± 0.01 | 0.25 ± 0.02 | 0.24 ± 0.02 |
| Thymus weight | 0.52 ± 0.01 | 0.50 ± 0.01 | 0.51 ± 0.02 | 0.51 ± 0.02 |
| Spleen weight | 0.44 ± 0.02 | 0.50 ± 0.03 | 0.47 ± 0.03 | 0.52 ± 0.03 |
| Clinical Outcome | Variable 1 | RV | RV + EV-EcN | RV + EV-EcoR12 |
|---|---|---|---|---|
| Incidence | MDA | 53.85 | 21.74 | 23.08 |
| Incidence | MDF | 77.77 | 36.36 | 50.00 |
| Severity | MDI | 2.15 ± 0.12 | 1.71 ± 0.16 # | 1.76 ± 0.15 # |
| Duration | DP | 2.06 ± 0.28 | 1.75 ± 0.41 | 1.25 ± 0.25 # |
| Duration | DwD | 1.88 ± 0.22 | 1.63 ± 0.32 | 1.10 ± 0.08 # |
| Lymphocyte Populations in Spleen (%) 1 | CON | RV | Rv + EV-EcN | Rv + EV-EcoR12 |
|---|---|---|---|---|
| 1. B cells (CD45+) | 29.30 ± 1.21 | 25.17 ± 2.13 | 30.73 ± 1.59 | 27.81 ± 2.08 |
| 2. TCRαβ+ cells | 12.48 ± 1.03 | 12.20 ± 1.57 | 15.00 ± 2.01 | 16.93 ± 1.75 |
| 2.1 Th cells (TCRαβ+ CD4+ NK-) | 60.45 ± 1.42 | 55.46 ± 1.31 * | 55.16 ± 1.78 * | 54.89 ± 2.56 * |
| 2.2 Tc cells (TCRαβ+ CD8+ NK-) | 25.65 ± 0.84 | 30.23 ± 1.1 * | 29.97 ± 0.8 * | 31.72 ± 2.01 * |
| 2.3 Tc/Th ratio | 0.44 ± 0.02 | 0.54 ± 0.05 * | 0.55 ± 0.04 * | 0.56 ± 0.03 * |
| 3. TCRγδ+ cells | 1.56 ± 0.05 | 1.5 ± 0.17 | 1.986 ± 0.11 * # | 2.00 ± 0.21 * # |
| 3.1 TCRγδ+ CD8+ | 76.54 ± 1.01 | 75.99 ± 2.43 | 81.60 ± 1.77 * | 85.43 ± 1.48 * # |
| 3.2 TCRγδ+ CD8- | 8.26 ± 0.26 | 9.68 ± 1.12 | 7.77 ± 0.59 | 6.78 ± 0.74 |
| 4. NKT cells | 1.88 ± 0.1 | 1.95 ± 0.28 | 2.45 ± 0.36 | 2.67 ± 0.19 * # |
| 4.1 NKT CD8+ | 87.82 ± 0.75 | 89.41 ± 0.8 | 89.25 ± 1.24 | 89 ± 1.15 |
| 4.2 NKT CD8- | 5.21 ± 0.3 | 4.16 ± 0.52 | 5.05 ± 0.68 | 4.83 ± 0.67 |
| 5. NK cells | 5.13 ± 0.16 | 4.21 ± 0.24 | 5.03 ± 0.34 | 4.11 ± 0.38 |
| 5.1 NK CD8+ | 22.64 ± 1.18 | 28.21 ± 4.48 | 34.54 ± 3.15 * (# p = 0.09) | 43.78 ± 3.14 * # |
| 5.2 NK CD8- | 36.44 ± 0.82 | 38.53 ± 2.74 | 34.53 ± 1.91 | 31.25 ± 2.67 |
| 6. Treg cells | 45.17 ± 3.48 | 42.33 ± 5.11 | 45.81 ± 3.46 | 45.28 ± 3.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Ruiz, S.; Olivo-Martínez, Y.; Cordero, C.; Rodríguez-Lagunas, M.J.; Pérez-Cano, F.J.; Badia, J.; Baldoma, L. Microbiota-Derived Extracellular Vesicles as a Postbiotic Strategy to Alleviate Diarrhea and Enhance Immunity in Rotavirus-Infected Neonatal Rats. Int. J. Mol. Sci. 2024, 25, 1184. https://doi.org/10.3390/ijms25021184
Martínez-Ruiz S, Olivo-Martínez Y, Cordero C, Rodríguez-Lagunas MJ, Pérez-Cano FJ, Badia J, Baldoma L. Microbiota-Derived Extracellular Vesicles as a Postbiotic Strategy to Alleviate Diarrhea and Enhance Immunity in Rotavirus-Infected Neonatal Rats. International Journal of Molecular Sciences. 2024; 25(2):1184. https://doi.org/10.3390/ijms25021184
Chicago/Turabian StyleMartínez-Ruiz, Sergio, Yenifer Olivo-Martínez, Cecilia Cordero, María J. Rodríguez-Lagunas, Francisco J. Pérez-Cano, Josefa Badia, and Laura Baldoma. 2024. "Microbiota-Derived Extracellular Vesicles as a Postbiotic Strategy to Alleviate Diarrhea and Enhance Immunity in Rotavirus-Infected Neonatal Rats" International Journal of Molecular Sciences 25, no. 2: 1184. https://doi.org/10.3390/ijms25021184
APA StyleMartínez-Ruiz, S., Olivo-Martínez, Y., Cordero, C., Rodríguez-Lagunas, M. J., Pérez-Cano, F. J., Badia, J., & Baldoma, L. (2024). Microbiota-Derived Extracellular Vesicles as a Postbiotic Strategy to Alleviate Diarrhea and Enhance Immunity in Rotavirus-Infected Neonatal Rats. International Journal of Molecular Sciences, 25(2), 1184. https://doi.org/10.3390/ijms25021184









