Caffeic Acid Phosphanium Derivatives: Potential Selective Antitumor, Antimicrobial and Antiprotozoal Agents
Abstract
1. Introduction
2. Results and Discussion
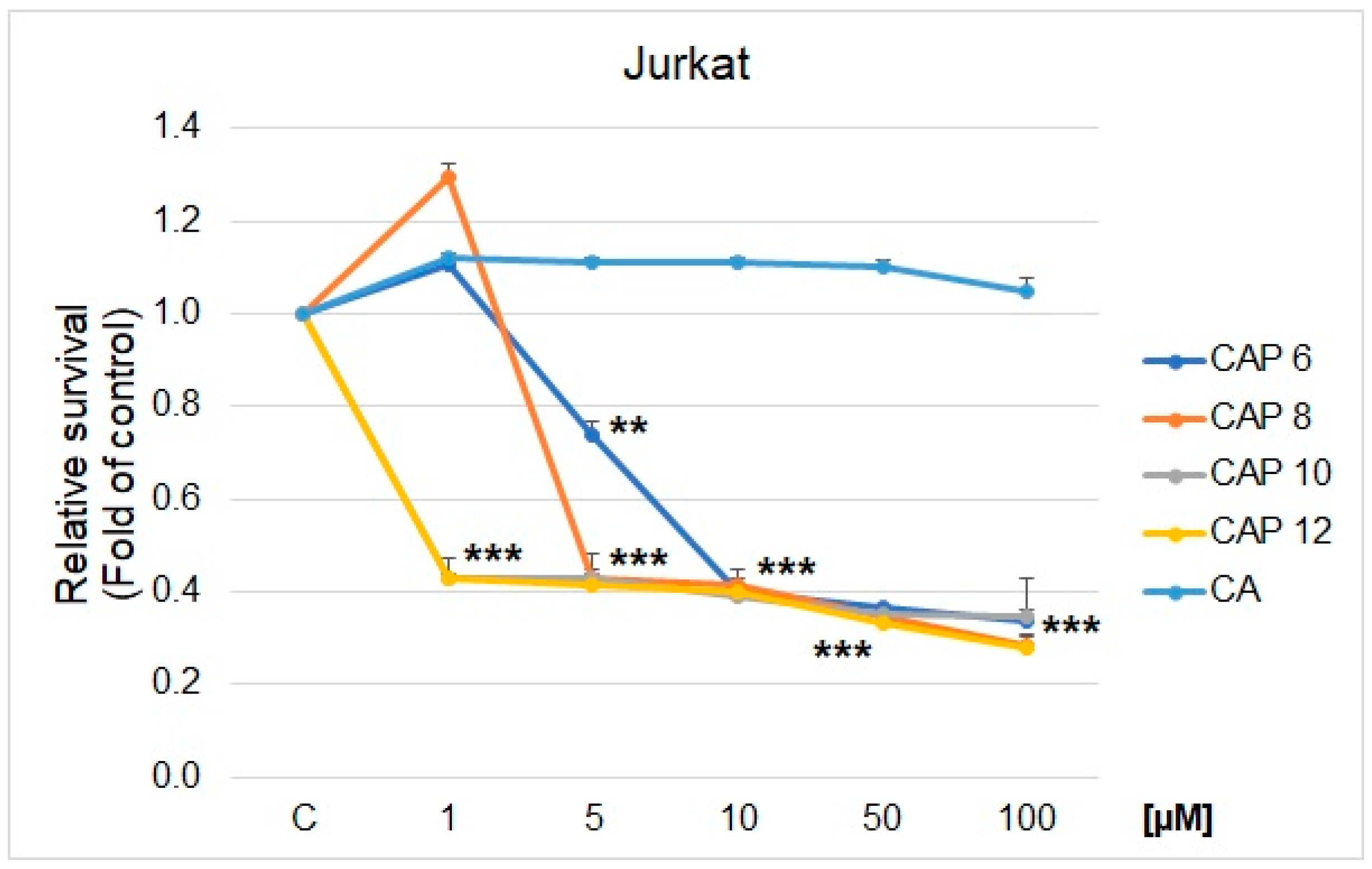
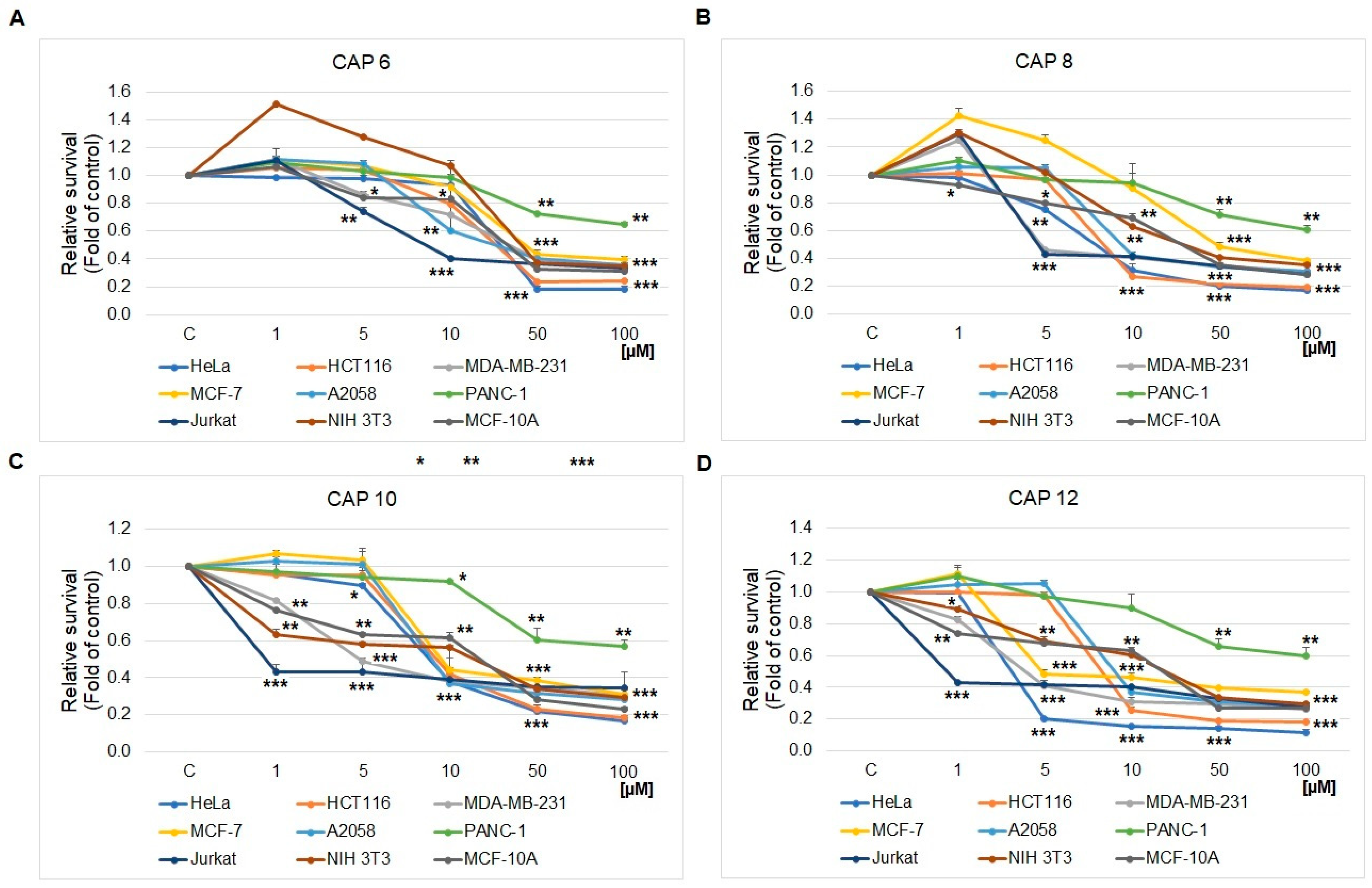
2.1. MTS Cell Proliferation/Viability Assay
2.2. Antibacterial and Antifungal Activity
2.3. Anti-Acanthamoeba Activity
3. Materials and Methods
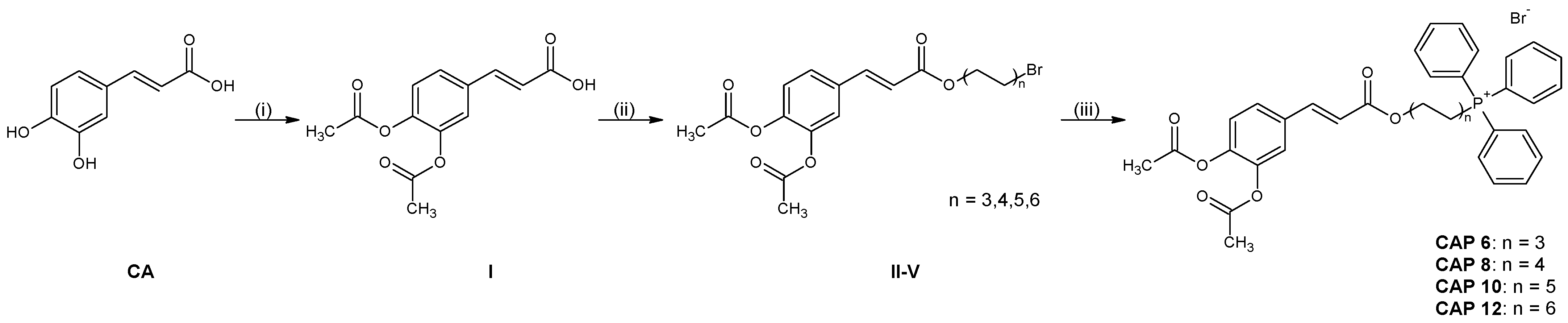
3.1. Synthesis
3.1.1. Acetylation of Caffeic Acid
- (2E)-3-(3,4-diacetyloxyphenyl)prop-2-enoic acid. Yield: 88.9%; 1H NMR (300 MHz, CDCl3) δ: 2.27 (s, 3H), 2.32 (s, 3H), 6.39 (d, J = 16.2 Hz, 1H) 7.23–7.45 (m, 3H), 7.72 (d, J = 15.9 Hz, 1H); 13C NMR (75 MHz, CDCl3) δ: 20.6, 20.7, 118.4, 123.0, 124.0, 126.7, 132.9, 142.5, 143.9, 145.0, 168.0, 168.1, 171.6. The NMR spectra are in good agreement with the literature [47].
3.1.2. General Procedure for Esterification of (2E)-3-(3,4-Diacetyloxyphenyl)prop-2-enoic Acid
- 6-bromohexyl-(2E)-3-[3,4-di(acetoxy)phenyl]prop-2-enoate. Yield: 60.8%; 1H NMR (300 MHz, CDCl3, TMS) δ: 1.39–1.52 (m, 4H), 1.65–1.78 (m, 2H), 1.85–1.92 (m, 2H), 2.30 (s, 3H), 2.31 (s, 3H), 3.42 (t, J = 6.9 Hz, 2H), 4.20 (t, J = 6.9 Hz, 2H), 6.38 (d, J = 15.9 Hz, 1H), 7.20–7.28 (m, 1H), 7.35-7.42 (m, 2H), 7.61 (d, J = 15.9 Hz, 1H); 13C NMR: (75 MHz, CDCl3, TMS) δ: 20.6(1), 20.6(5), 25.2, 27.8, 28.5, 32.6, 33.7, 64.6, 119.4, 122.7, 123.9, 126.4, 133.3, 142.4, 142.7, 143.5, 166.6, 168.0, 168.1.
- 8-bromooctyl-(2E)-3-[3,4-di(acetoxy)phenyl]prop-2-enoate. Yield: 48.8%; 1H NMR (300 MHz, CDCl3) δ: 1.35–1.45 (m, 8H), 1.67–1.70 (m, 2H), 1.84–1.89 (m, 2H), 2.31 (s, 3H), 2.32 (s, 3H), 3.41 (t, J = 6.9 Hz, 2H), 4.20 (t, J = 6.6 Hz, 2H), 6.38 (d, J = 15.9 Hz, 1H), 7.21–7.42 (m, 3H), 7.61 (d, J = 15.9 Hz, 1H); 13C NMR (75 MHz, CDCl3) δ: 20.6(1), 20.6(4), 25.9, 28.1, 28.6, 29.1, 32.8, 34.0, 64.8, 119.5, 122.7, 123.9, 126.4, 133.4, 142.4, 142.6, 143.4, 166.7, 168.0, 168.1.
- 10-bromodecyl-(2E)-3-[3,4-di(acetoxy)phenyl]prop-2-enoate. Yield: 54.8%; 1H NMR (300 MHz, CDCl3) δ: 1.22–1.45 (m, 12H), 1.62–1.75 (m, 2H), 1.81–1.92 (m, 2H), 2.30 (s, 3H), 2.31 (s, 3H), 3.41, (t, J = 6.9 Hz, 2H), 4.19 (t, J = 6.9 Hz, 2H), 6.38 (d, J = 15.9 Hz, 1H), 7.21–7.30 (m, 1H), 7.35–7.42 (m, 2H), 7.61 (d, J = 15.9 Hz, 1H); 13C NMR: (CDCl3, TMS) δ: 20.6, 20.7, 25.9, 28.1, 28.7, 28.7, 29.2, 29.3, 29.4, 32.8, 34.0, 64.8, 119.5, 122.7, 123.9, 126.4, 133.4, 142.4, 142.6, 143.4, 166.7, 168.0, 168.1.
- 12-bromododecyl-(2E)-3-[3,4-di(acetoxy)phenyl]prop-2-enoate. Yield: 41.8%; 1H NMR (300 MHz, CDCl3) δ: 1.26–1.56 (m, 16H), 1.67–1.69 (m, 2H), 1.83–1.88 (m, 2H), 2.30 (s, 3H), 2.31 (s, 3H), 3.41 (t, J = 6.9 Hz, 2H), 4.19 (t, J = 6.9 Hz, 2H), 6.38 (d, J = 15.9 Hz, 1H), 7.21–7.41 (m, 3H), 7.60 (d, J = 15.9 Hz, 1H); 13C NMR (75 MHz, CDCl3) δ: 20.6, 25.9, 28.2, 28.7, 28.8, 29.3, 29.4(8), 29.5, 32.8, 34.1, 64.9, 119.5, 122.7, 123.9, 126.4, 133.4, 142.5, 143.4, 166.7, 168.0, 168.1.
3.1.3. General Procedure for Quaternization of ω-Bromoalkyl (2E)-3-[3,4-Di(acetoxy)phenyl]prop-2-enoates with Cfuane
- P,P,P-triphenyl-6-{(2E)-3-[3,4-(diacetyloxy)penyl]prop-2-enoyloxy}hexane-1-phosphanium bromide CAP 6. Yield: 73.6%; 1H NMR (300 MHz, CDCl3) δ: 1.35–1.45 (m, 2H), 1.58–1.75 (m, 6H), 2.30 (s, 3H), 2.31 (s, 3H), 3.78–3.89 (m, 2H), 4.13 (t, J = 6.6 Hz, 2H), 6.35 (d, J = 15.9 Hz, 1H), 7.28–7.36 (m, 1H), 7.36–7.43 (m, 2H), 7.62 (d, J = 15.9 Hz, 1H), 7.69–7.90 (m, 15H); 13C NMR: (CDCl3, TMS) δ: 20.7, 22.4, 22.6, 22.9 (d, J = 30.4 Hz), 25.7, 28.3, 30.0 (d, J = 16.1 Hz), 64.5, 118.4 (d, J = 85.1 Hz), 119.4 122.7, 123.9, 126.4, 130.5 (d, J = 12.5 Hz), 133.3, 133.7 (d, J = 9.9 Hz), 135.0 (d, J = 3.0 Hz), 142.4, 142.7, 143.5, 166.6, 168.0, 168.1; 31P NMR: (CDCl3, TMS) δ: 24.4.
- P,P,P-triphenyl-8-{(2E)-3-[3,4-(diacetyloxy)penyl]prop-2-enoyloxy}okctane-1-phosphanium bromide CAP 8. Yield: 67.0%; 1H NMR (300 MHz, CDCl3) δ: 1.26–1.29 (m, 6H), 1.63–1.81 (m, 6H), 2.17 (s, 3H), 2.30 (s, 3H), 3.78 (t, J = 5.4 Hz, 2H), 4.14 (t, J = 6.6 Hz, 2H), 6.37 (d, J = 15.9 Hz, 1H), 7.20–7.42 (m, 3H), 7.59 (d, J = 15.9 Hz, 1H) 7.67–7.88 (m, 15H); 13C NMR (75 MHz, CDCl3) δ: 20.7, 22.4, 22.6, 22.9 (d, J = 30.1 Hz), 25.8, 28.6, 28.8, 29.1, 30.2 (d, J = 15.8 Hz), 64.7, 118.4 (d, J = 85.2 Hz), 119.5, 122.7, 123.9, 126.4, 130.5 (d, J = 12.5 Hz), 133.3, 133.7 (d, J = 9.9 Hz), 135.0 (d, J = 2.9 Hz), 142.4, 142.6, 143.4, 166.7, 168.0, 168.1; 31P NMR (121,47 MHz, CDCl3) δ: 24.4.
- P,P,P-triphenyl-10-{(2E)-3-[3,4-(diacetyloxy)penyl]prop-2-enoyloxy}decane-1-phosphanium bromide CAP 10. Yield: 72.5%; 1H NMR (300 MHz, CDCl3) δ: 1.21–1.42 (m, 12H), 1.55–1.82 (m, 4H), 2.30 (s, 3H), 2.31 (s, 3H), 3.75–3.83 (m, 2H), 4.17 (t, J = 6.9 Hz, 2H), 6.38 (d, J = 16.2 Hz, 1H), 7.21–7.28 (m, 1H), 7.35–7.42 (m, 2H), 7.61 (d, J = 16.2 Hz, 1H), 7.65–7.90 (m, 15H); 13C NMR: (CDCl3, TMS) δ: 206, 22.5, 22.6, 22.9 (d, J = 29.6 Hz), 25.9, 28.6, 29.0(7), 29.1, 29.2, 29.3, 30.4 (d, J = 15.6 Hz), 64.8, 118.4 (d, J = 85.3 Hz), 119.3 122.7, 123.9, 126.4, 130.5 (d, J = 12.5 Hz), 133.3, 133.7 (d, J = 9.8 Hz), 134.9 (d, J = 3.0 Hz), 142.4, 142.6, 143.4, 166.7, 168.0, 168.1; 31P NMR: (CDCl3, TMS) δ: 24.5.
- P,P,P-triphenyl-12-{(2E)-3-[3,4-(diacetyloxy)penyl]prop-2-enoyloxy}dodecane-1-phosphanium bromide CAP 12. Yield: 65.5%; 1H NMR (400 MHz, CDCl3) δ: 1.20–1.37 (m, 16H), 1.62–1.70 (m, 4H), 2.30 (s, 3H), 2.31 (s, 3H), 3.80–3.81 (m, 2H), 4.18 (t, J = 6.4 Hz, 2H), 6.38 (d, J = 15.9 Hz, 1H), 7.22 (d, J = 8.4 Hz, 1H), 7.36 (d, J = 2.0 Hz, 1H), 7.40 (d, J = 8.4 Hz, J = 2.0 Hz, 1H), 7.61 (d, J = 15.9 Hz, 1H), 7.68–7.88 (m, 15H); 13C NMR (100 MHz, CDCl3) δ: 20.6, 20.7, 22.5, 22.9 (d, J = 30.1 Hz), 25.9, 28.7, 29.2, 29.2, 29.2, 29.4, 30,4 (d, J = 15.6 Hz), 64.8, 118.5 (d, J = 85.7 Hz), 119.5, 122.7, 123.9, 126.3, 130.4 (d, J = 12.2 Hz), 133.3, 133.7 (d, J = 9.9 Hz), 134.9 (d, J = 3.1 Hz), 142.4, 142.6, 143.4, 166.7, 168.0, 168.1; 31P NMR (162 MHz, CDCl3) δ: 24.5;
3.1.4. Equilibrium Surface Tension Measurements
3.1.5. HPLC-DAD Analysis of CAPs
3.2. Biological Activities
3.2.1. MTS Cell Proliferation/Viability Assay
3.2.2. Antibacterial and Antifungal Activity Testing
3.2.3. Amoebicidal Activity Assay
3.2.4. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nagy, M.; Mučaji, P.; Grančai, D. Pharmacognosy Biogenesis of Natural Substances, 1st ed.; Osveta: Martin, Slovakia, 2011. [Google Scholar]
- Silva, T.; Oliveira, C.; Borges, F. Caffeic Acid Derivatives, Analogs and Applications: A Patent Review (2009–2013). Expert Opin. Ther. Pat. 2014, 24, 1257–1270. [Google Scholar] [CrossRef]
- Espíndola, K.M.M.; Ferreira, R.G.; Narvaez, L.E.M.; Silva Rosario, A.C.R.; da Silva, A.H.M.; Silva, A.G.B.; Vieira, A.P.O.; Monteiro, M.C. Chemical and Pharmacological Aspects of Caffeic Acid and Its Activity in Hepatocarcinoma. Front. Oncol. 2019, 9, 541. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, S.; Gholami, M.H.; Zabolian, A.; Saleki, H.; Farahani, M.V.; Hamzehlou, S.; Far, F.B.; Sharifzadeh, S.O.; Samarghandian, S.; Khan, H.; et al. Caffeic Acid and Its Derivatives as Potential Modulators of Oncogenic Molecular Pathways: New Hope in the Fight against Cancer. Pharmacol. Res. 2021, 171, 105759. [Google Scholar] [CrossRef] [PubMed]
- Nuhn, P. Naturstoffchemie, 4th ed.; S. Hirzel Verlag: Stuttgart, Germany, 2006. [Google Scholar]
- Lopes, R.; Costa, M.; Ferreira, M.; Gameiro, P.; Fernandes, S.; Catarino, C.; Santos-Silva, A.; Paiva-Martins, F. Caffeic Acid Phenolipids in the Protection of Cell Membranes from Oxidative Injuries. Interaction with the Membrane Phospholipid Bilayer. Biochim. Biophys. Acta (BBA) Biomembr. 2021, 1863, 183727. [Google Scholar] [CrossRef] [PubMed]
- Medina, I.; Undeland, I.; Larsson, K.; Storrø, I.; Rustad, T.; Jacobsen, C.; Kristinová, V.; Gallardo, J.M. Activity of Caffeic Acid in Different Fish Lipid Matrices: A Review. Food Chem. 2012, 131, 730–740. [Google Scholar] [CrossRef]
- Kurin, E.; Mučaji, P.; Nagy, M. In Vitro Antioxidant Activities of Three Red Wine Polyphenols and Their Mixtures: An Interaction Study. Molecules 2012, 17, 14336–14348. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Bamunuarachchi, N.I.; Tabassum, N.; Kim, Y.M. Caffeic Acid and Its Derivatives: Antimicrobial Drugs toward Microbial Pathogens. J. Agric. Food Chem. 2021, 69, 2979–3004. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.-Z.; Deng, G.; Guo, R.; Fu, Z.-M.; Chen, D.-F. Effects of Different Ester Chains on the Antioxidant Activity of Caffeic Acid. Bioorg. Chem. 2020, 105, 104341. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Yu, D.; Eom, S.-H.; Kim, S.-H.; Oh, J.; Jung, W.; Kim, Y.-M. Synergistic Antibacterial Effects of Chitosan-Caffeic Acid Conjugate against Antibiotic-Resistant Acne-Related Bacteria. Mar. Drugs 2017, 15, 167. [Google Scholar] [CrossRef] [PubMed]
- Kępa, M.; Miklasińska-Majdanik, M.; Wojtyczka, R.D.; Idzik, D.; Korzeniowski, K.; Smoleń-Dzirba, J.; Wąsik, T.J. Antimicrobial Potential of Caffeic Acid against Staphylococcus aureus Clinical Strains. Biomed. Res. Int. 2018, 2018, 7413504. [Google Scholar] [CrossRef] [PubMed]
- Ruqaiyyah, S.; Yousuf, A.; Ahmed, K.N. The Development of Drugs against Acanthamoeba Infections. Antimicrob. Agents Chemother. 2016, 60, 6441–6450. [Google Scholar] [CrossRef]
- Elsheikha, H.M.; Siddiqui, R.; Khan, N.A. Drug Discovery against Acanthamoeba Infections: Present Knowledge and Unmet Needs. Pathogens 2020, 9, 405. [Google Scholar] [CrossRef] [PubMed]
- Bittner Fialová, S.; Kello, M.; Čoma, M.; Slobodníková, L.; Drobná, E.; Holková, I.; Garajová, M.; Mrva, M.; Zachar, V.; Lukáč, M. Derivatization of Rosmarinic Acid Enhances Its in Vitro Antitumor, Antimicrobial and Antiprotozoal Properties. Molecules 2019, 24, 1078. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Ahmed, S.; Elasbali, A.M.; Adnan, M.; Alam, S.; Hassan, M.I.; Pasupuleti, V.R. Therapeutic Implications of Caffeic Acid in Cancer and Neurological Diseases. Front. Oncol. 2022, 12, 860508. [Google Scholar] [CrossRef]
- Lagoa, R.; Silva, J.; Rodrigues, J.R.; Bishayee, A. Advances in Phytochemical Delivery Systems for Improved Anticancer Activity. Biotechnol. Adv. 2020, 38, 107382. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, P.; Gardana, C.; Pietta, P. Plasma Levels of Caffeic Acid and Antioxidant Status after Red Wine Intake. J. Agric. Food Chem. 2001, 49, 5964–5968. [Google Scholar] [CrossRef] [PubMed]
- Lukáč, M.; Pisárčik, M.; Garajová, M.; Mrva, M.; Dušeková, A.; Vrták, A.; Horáková, R.; Horváth, B.; Devínsky, F. Synthesis, Surface Activity, and Biological Activities of Phosphonium and Metronidazole Salts. J. Surfactants Deterg. 2020, 23, 1025–1032. [Google Scholar] [CrossRef]
- Akita, H.; Nozawa, M.; Mitsuda, A.; Ohsawa, H. A Convenient Synthesis of (+)-Albicanol Based on Enzymatic Function: Total Syntheses of (+)-Albicanyl Acetate, (−)-Albicanyl 3,4-Dihydroxycinnamate, (−)-Drimenol, (−)-Drimenin and (−)-Ambrox. Tetrahedron Asymmetry 2000, 11, 1375–1388. [Google Scholar] [CrossRef]
- De Lucia, D.; Lucio, O.M.; Musio, B.; Bender, A.; Listing, M.; Dennhardt, S.; Koeberle, A.; Garscha, U.; Rizzo, R.; Manfredini, S.; et al. Design, Synthesis and Evaluation of Semi-Synthetic Triazole-Containing Caffeic Acid Analogues as 5-Lipoxygenase Inhibitors. Eur. J. Med. Chem. 2015, 101, 573–583. [Google Scholar] [CrossRef]
- Kruizinga, W.H.; Kellogg, R.M. Preparation of Macrocyclic Lactones by Ring Closure of Cesium Carboxylates. J. Am. Chem. Soc. 1981, 103, 5183–5189. [Google Scholar] [CrossRef]
- Lukáč, M.; Mrva, M.; Garajová, M.; Mojžišová, G.; Varinská, L.; Mojžiš, J.; Sabol, M.; Kubincová, J.; Haragová, H.; Ondriska, F.; et al. Synthesis, Self-Aggregation and Biological Properties of Alkylphosphocholine and Alkylphosphohomocholine Derivatives of Cetyltrimethylammonium Bromide, Cetylpyridinium Bromide, Benzalkonium Bromide (C16) and Benzethonium Chloride. Eur. J. Med. Chem. 2013, 66, 46–55. [Google Scholar] [CrossRef]
- Kabała-Dzik, A.; Rzepecka-Stojko, A.; Kubina, R.; Jastrzębska-Stojko, Ż.; Stojko, R.; Wojtyczka, R.D.; Stojko, J. Comparison of Two Components of Propolis: Caffeic Acid (CA) and Caffeic Acid Phenethyl Ester (CAPE) Induce Apoptosis and Cell Cycle Arrest of Breast Cancer Cells MDA-MB-231. Molecules 2017, 22, 1554. [Google Scholar] [CrossRef]
- Kabała-Dzik, A.; Rzepecka-Stojko, A.; Kubina, R.; Jastrzębska-Stojko, Ż.; Stojko, R.; Wojtyczka, R.D.; Stojko, J. Migration Rate Inhibition of Breast Cancer Cells Treated by Caffeic Acid and Caffeic Acid Phenethyl Ester: An In Vitro Comparison Study. Nutrients 2017, 9, 1144. [Google Scholar] [CrossRef]
- Rezaei-Seresht, H.; Cheshomi, H.; Falanji, F.; Movahedi-Motlagh, F.; Hashemian, M.; Mireskandari, E. Cytotoxic Activity of Caffeic Acid and Gallic Acid against MCF-7 Human Breast Cancer Cells: An in Silico and in Vitro Study. Avicenna J. Phytomed. 2019, 9, 574–586. [Google Scholar] [CrossRef]
- Chen, C.; Kuo, Y.-H.; Lin, C.-C.; Chao, C.-Y.; Pai, M.-H.; Chiang, E.-P.I.; Tang, F.-Y. Decyl Caffeic Acid Inhibits the Proliferation of Colorectal Cancer Cells in an Autophagy-Dependent Manner in Vitro and in Vivo. PLoS ONE 2020, 15, e0232832. [Google Scholar] [CrossRef] [PubMed]
- Kello, M.; Goga, M.; Kotorova, K.; Sebova, D.; Frenak, R.; Tkacikova, L.; Mojzis, J. Screening Evaluation of Antiproliferative, Antimicrobial and Antioxidant Activity of Lichen Extracts and Secondary Metabolites In Vitro. Plants 2023, 12, 611. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Yao, X.; Yao, C.; Zhao, X.; Zuo, H.; Li, Z. Anti-Colon Cancer Effect of Caffeic Acid p-Nitro-Phenethyl Ester in Vitro and in Vivo and Detection of Its Metabolites. Sci. Rep. 2017, 7, 7599. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Omene, C.; Karkoszka, J.; Bosland, M.; Eckard, J.; Klein, C.B.; Frenkel, K. Caffeic Acid Phenethyl Ester (CAPE), Derived from a Honeybee Product Propolis, Exhibits a Diversity of Anti-Tumor Effects in Pre-Clinical Models of Human Breast Cancer. Cancer Lett. 2011, 308, 43–53. [Google Scholar] [CrossRef]
- Duan, J.; Xiaokaiti, Y.; Fan, S.; Pan, Y.; Li, X.; Li, X. Direct Interaction between Caffeic Acid Phenethyl Ester and Human Neutrophil Elastase Inhibits the Growth and Migration of PANC-1 Cells. Oncol. Rep. 2017, 37, 3019–3025. [Google Scholar] [CrossRef]
- Bennett, J.; Dolin, R.; Blaser, M.J. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 9th ed.; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Andrade, M.; Benfeito, S.; Soares, P.; Magalhães e Silva, D.; Loureiro, J.; Borges, A.; Borges, F.; Simões, M. Fine-Tuning of the Hydrophobicity of Caffeic Acid: Studies on the Antimicrobial Activity against Staphylococcus Aureus and Escherichia coli. RSC Adv. 2015, 5, 53915–53925. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings1PII of Original Article: S0169-409X(96)00423-1. The Article Was Originally Published in Advanced Drug Delivery Reviews 23 (1997) 3. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Falk, N.A. Surfactants as Antimicrobials: A Brief Overview of Microbial Interfacial Chemistry and Surfactant Antimicrobial Activity. J. Surfactants Deterg. 2019, 22, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Liptáková, A. Lekárska Mikrobiológia, 1st ed.; Herba: Bratislava, Slovakia, 2019; ISBN 978-80-89631-91-9. [Google Scholar]
- Busscher, H.J.; Weerkamp, A.H.; van der Mei, H.C.; van Pelt, A.W.; de Jong, H.P.; Arends, J. Measurement of the Surface Free Energy of Bacterial Cell Surfaces and Its Relevance for Adhesion. Appl. Environ. Microbiol. 1984, 48, 980–983. [Google Scholar] [CrossRef] [PubMed]
- Garajová, M.; Mrva, M.; Timko, L.; Lukáč, M.; Ondriska, F. Cytomorphological Changes and Susceptibility of Clinical Isolates of Acanthamoeba Spp. to Heterocyclic Alkylphosphocholines. Exp. Parasitol. 2014, 145, S102–S110. [Google Scholar] [CrossRef] [PubMed]
- Julia, W.; Michael, D.; Karin, S.; Andreas, O.; Thomas, H.; Gerhard, W.; Hansjörg, E.; Horst, A. Cytotoxic Activities of Alkylphosphocholines against Clinical Isolates of Acanthamoeba spp. Antimicrob. Agents Chemother. 2002, 46, 695–701. [Google Scholar] [CrossRef]
- Cope, J.R.; Ali, I.K.; Visvesvara, G.S. Pathogenic and Opportunistic Free-Living Ameba Infections. In Hunter’s Tropical Medicine and Emerging Infectious Diseases, 10th ed.; Ryan, E.T., Hill, D.R., Solomon, T., Aronson, N.E., Endy, T.P., Eds.; Elsevier: London, UK, 2020; Chapter 107; pp. 814–820. ISBN 978-0-323-55512-8. [Google Scholar]
- Walvekar, S.; Anwar, A.; Anwar, A.; Sridewi, N.; Khalid, M.; Yow, Y.Y.; Khan, N.A. Anti-Amoebic Potential of Azole Scaffolds and Nanoparticles against Pathogenic acanthamoeba. Acta Trop. 2020, 211, 105618. [Google Scholar] [CrossRef] [PubMed]
- Kikowska, M.; Chanaj-Kaczmarek, J.; Derda, M.; Budzianowska, A.; Thiem, B.; Ekiert, H.; Szopa, A. The Evaluation of Phenolic Acids and Flavonoids Content and Antiprotozoal Activity of Eryngium Species Biomass Produced by Biotechnological Methods. Molecules 2022, 27, 363. [Google Scholar] [CrossRef]
- Sifaoui, I.; López-Arencibia, A.; Martín-Navarro, C.M.; Chammem, N.; Mejri, M.; Lorenzo-Morales, J.; Abderabba, M.; Piñero, J.E. Activity Assessment of Tunisian Olive Leaf Extracts against the Trophozoite Stage of Acanthamoeba. Parasitol. Res. 2013, 112, 2825–2829. [Google Scholar] [CrossRef]
- Sifaoui, I.; López-Arencibia, A.; Martín-Navarro, C.M.; Reyes-Batlle, M.; Wagner, C.; Chiboub, O.; Mejri, M.; Valladares, B.; Abderrabba, M.; Piñero, J.E.; et al. Programmed Cell Death in Acanthamoeba Castellanii Neff Induced by Several Molecules Present in Olive Leaf Extracts. PLoS ONE 2017, 12, e0183795. [Google Scholar] [CrossRef]
- Timko, L.; Pisárčik, M.; Mrva, M.; Garajová, M.; Juhásová, A.; Mojžiš, J.; Mojžišová, G.; Bukovský, M.; Devínsky, F.; Lukáč, M. Synthesis, Physicochemical Properties and Biological Activities of Novel Alkylphosphocholines with Foscarnet Moiety. Bioorg Chem. 2020, 104, 104224. [Google Scholar] [CrossRef]
- Maher, S.; Geoghegan, C.; Brayden, D.J. Safety of Surfactant Excipients in Oral Drug Formulations. Adv. Drug Deliv. Rev. 2023, 202, 115086. [Google Scholar] [CrossRef] [PubMed]
- Avetyan, D.L.; Shatskiy, A.; Kärkäs, M.D.; Stepanova, E.V. Scalable Total Synthesis of Natural Vanillin-Derived Glucoside ω-Esters. Carbohydr. Res. 2022, 522, 108683. [Google Scholar] [CrossRef] [PubMed]
- Lukáč, M.; Devínsky, F.; Pisárčik, M.; Papapetropoulou, A.; Bukovský, M.; Horváth, B. Novel Phospholium-Type Cationic Surfactants: Synthesis, Aggregation Properties and Antimicrobial Activity. J. Surfactants Deterg. 2017, 20, 159–171. [Google Scholar] [CrossRef]
- Stojanovic, A.; Lämmerhofer, M.; Kogelnig, D.; Schiesel, S.; Sturm, M.; Galanski, M.S.; Krachler, R.; Keppler, B.K.; Lindner, W. Analysis of Quaternary Ammonium and Phosphonium Ionic Liquids by Reversed-Phase High-Performance Liquid Chromatography with Charged Aerosol Detection and Unified Calibration. J. Chromatogr. A 2008, 1209, 179–187. [Google Scholar] [CrossRef]
- EUCAST Antimicrobial Susceptibility Testing. Antimicrobial Susceptibility Testing. 2019. Available online: http://www.eucast.org/ast_of_bacteria/ (accessed on 23 June 2022).
- EUCAST Antifungal Susceptibility Testing. Antifungal Susceptibility Testing. 2019. Available online: http://www.eucast.org/ast_of_fungi// (accessed on 23 June 2022).
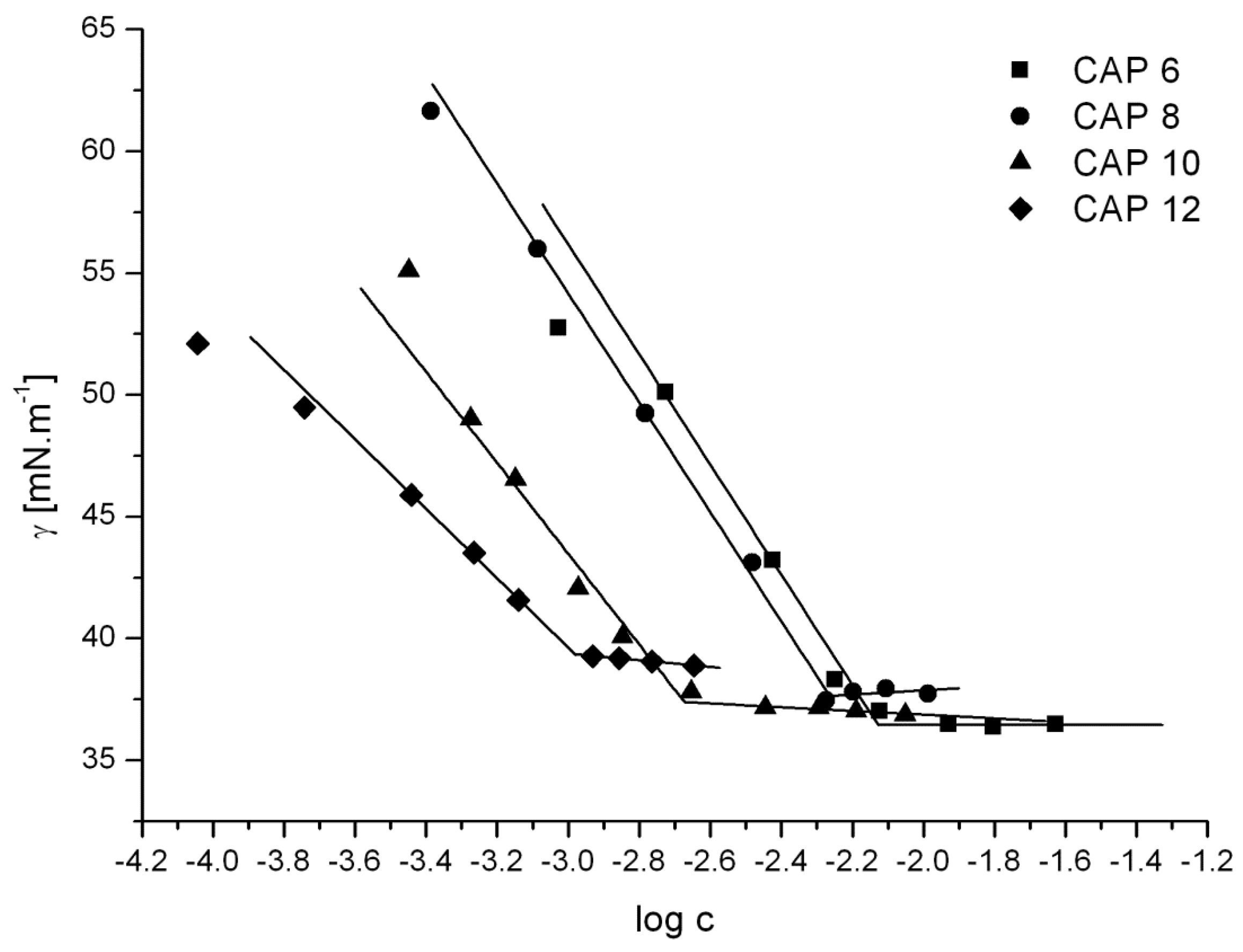
| Compound | cmc [mol·dm−3] | γcmc [mN·m−1] | Acmc [nm2] |
|---|---|---|---|
| CAP 6 | 7.5 × 10−3 | 36.4 | 0.84 |
| CAP 8 | 5.2 × 10−3 | 37.7 | 0.82 |
| CAP 10 | 2.2 × 10−3 | 37.1 | 1.02 |
| CAP 12 | 1.0 × 10−3 | 39.4 | 1.33 |
| Compound | Cancer Cell Lines/IC50 (μM) | Control Cell Lines/IC50 (μM) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HeLa | HCT116 | MDA-MB-231 | MCF-7 | A2058 | PANC-1 | Jurkat | NIH 3T3 | MCF-10A | |
| CAP 6 | 34.0 ± 1.8 | 31.0 ± 0.4 | 37.0 ± 0.3 | 44.5 ± 0.1 | 30.5 ± 1.5 | 98.8 ± 2.4 | 8.5 ± 0.2 | 44.4 ± 2.6 | 53.7 ± 0.2 |
| CAP 8 | 7.1 ± 0.3 | 8.3 ± 0.6 | 4.8 ± 0.5 | 48.7 ± 0.1 | 9.4 ± 0.3 | 103.2 ± 1.3 | 6.9 ± 0.3 | 38.2 ± 1.8 | 42.3 ± 1.1 |
| CAP 10 | 8.0 ± 0.7 | 9.2 ± 0.3 | 4.9 ± 0.5 | 9.5 ± 0.2 | 9.0 ± 0.5 | 91.2 ± 2.6 | 0.9 ± 0.4 | 28.0 ± 1.3 | 31.1 ± 2.3 |
| CAP 12 | 3.3 ± 1.1 | 8.3 ± 0.6 | 3.5 ± 0.6 | 4.9 ± 0.7 | 9.0 ± 0.5 | 110.7 ± 3.2 | 0.9 ± 0.2 | 32.5 ± 0.9 | 33.7 ± 1.5 |
| CA | >300 | >300 | >300 | >300 | >300 | >300 | >300 | >300 | >300 |
| CisPt [28] | 35.4 | 7.4 | 7.1 | 29.7 | 21.5 | 16.5 | 6.3 | 40.6 | 25.9 |
| Compound | Microbial strains/MIC (μM) | |||||||
| Staphylococcus aureus (MR) CCM 4750 | S. aureus (MS) CCM 4223 | Enterococcus faecalis CCM 4224 | Pseudomonas aeruginosa CCM 3955 | Escherichia coli CCM 3954 | Klebsiella pneumoniae CCM 4415 | Proteus mirabilis CCM 7188 | Candida albicans CCM 8267 | |
| CA | 6938 | 6938 | 13877 | 3469 | 6938 | 6938 | 6938 | 13,877 |
| CAP 6 | 14 | 14 | 57 | 453 | 227 | 28 | 453 | 57 |
| CAP 8 | 27 | 14 | 27 | 871 | 435 | 27 | 218 | 27 |
| CAP 10 | 13 | 13 | 26 | 838 | 419 | 105 | 210 | 13 |
| CAP 12 | 25 | 50 | 25 | 404 | 808 | 404 | 404 | 25 |
| Compound | Microbial strains/MBC or MFC (μM) | |||||||
| Staphylococcus aureus (MR) CCM 4750 | S. aureus (MS) CCM 4223 | Enterococcus faecalis CCM 4224 | Pseudomonas aeruginosa CCM 3955 | Escherichia coli CCM 3954 | Klebsiella pneumoniae CCM 4415 | Proteus mirabilis CCM 7188 | Candida albicans CCM 8267 | |
| CA | 13,877 | 13,877 | 27,753 | 3469 | 13,877 | 6938 | 6938 | 13,877 |
| CAP 6 | 14 | 14 | 57 | 453 | 227 | 28 | 453 | 57 |
| CAP 8 | 27 | 14 | 27 | 871 | 435 | 27 | 435 | 27 |
| CAP 10 | 26 | 13 | 26 | 676 | 419 | 105 | 419 | 13 |
| CAP 12 | 25 | 50 | 25 | 808 | 808 | 404 | 404 | 25 |
| Compound | A. lugdunensis (AcaVNAK02) | A. quina (AcaVNAK03) |
|---|---|---|
| CA | >105 | 3005.0 ± 1514.3 |
| CAP 6 | 74.2 ± 18.4 | 44.1 ± 9.2 |
| CAP 8 | 99.8 ± 23.7 | 94.1 ± 14.5 |
| CAP 10 | 833.8 ± 303.2 | 291.1 ± 36.8 |
| CAP 12 | 3124.8 ± 941.0 | 3323.9 ± 2055.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lukáč, M.; Slobodníková, L.; Mrva, M.; Dušeková, A.; Garajová, M.; Kello, M.; Šebová, D.; Pisárčik, M.; Kojnok, M.; Vrták, A.; et al. Caffeic Acid Phosphanium Derivatives: Potential Selective Antitumor, Antimicrobial and Antiprotozoal Agents. Int. J. Mol. Sci. 2024, 25, 1200. https://doi.org/10.3390/ijms25021200
Lukáč M, Slobodníková L, Mrva M, Dušeková A, Garajová M, Kello M, Šebová D, Pisárčik M, Kojnok M, Vrták A, et al. Caffeic Acid Phosphanium Derivatives: Potential Selective Antitumor, Antimicrobial and Antiprotozoal Agents. International Journal of Molecular Sciences. 2024; 25(2):1200. https://doi.org/10.3390/ijms25021200
Chicago/Turabian StyleLukáč, Miloš, Lívia Slobodníková, Martin Mrva, Aneta Dušeková, Mária Garajová, Martin Kello, Dominika Šebová, Martin Pisárčik, Marián Kojnok, Andrej Vrták, and et al. 2024. "Caffeic Acid Phosphanium Derivatives: Potential Selective Antitumor, Antimicrobial and Antiprotozoal Agents" International Journal of Molecular Sciences 25, no. 2: 1200. https://doi.org/10.3390/ijms25021200
APA StyleLukáč, M., Slobodníková, L., Mrva, M., Dušeková, A., Garajová, M., Kello, M., Šebová, D., Pisárčik, M., Kojnok, M., Vrták, A., Kurin, E., & Bittner Fialová, S. (2024). Caffeic Acid Phosphanium Derivatives: Potential Selective Antitumor, Antimicrobial and Antiprotozoal Agents. International Journal of Molecular Sciences, 25(2), 1200. https://doi.org/10.3390/ijms25021200








