Oxidative Stress: The Role of Antioxidant Phytochemicals in the Prevention and Treatment of Diseases
Abstract
:1. Introduction
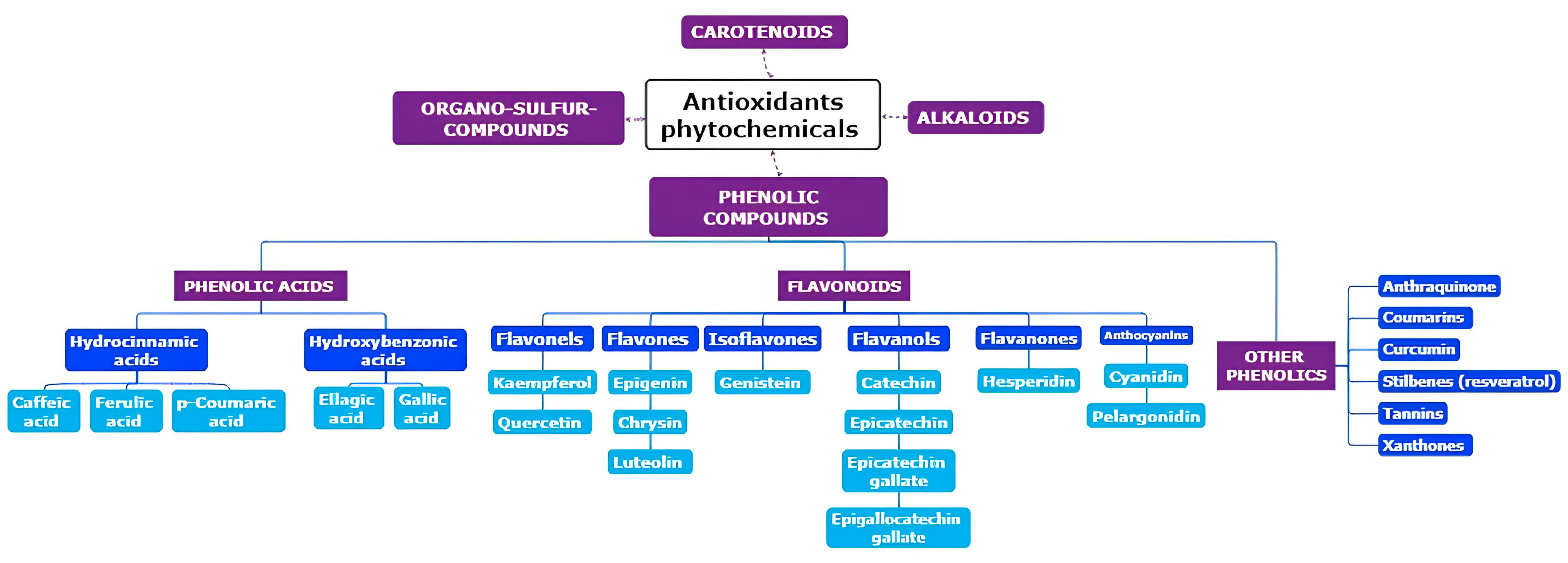
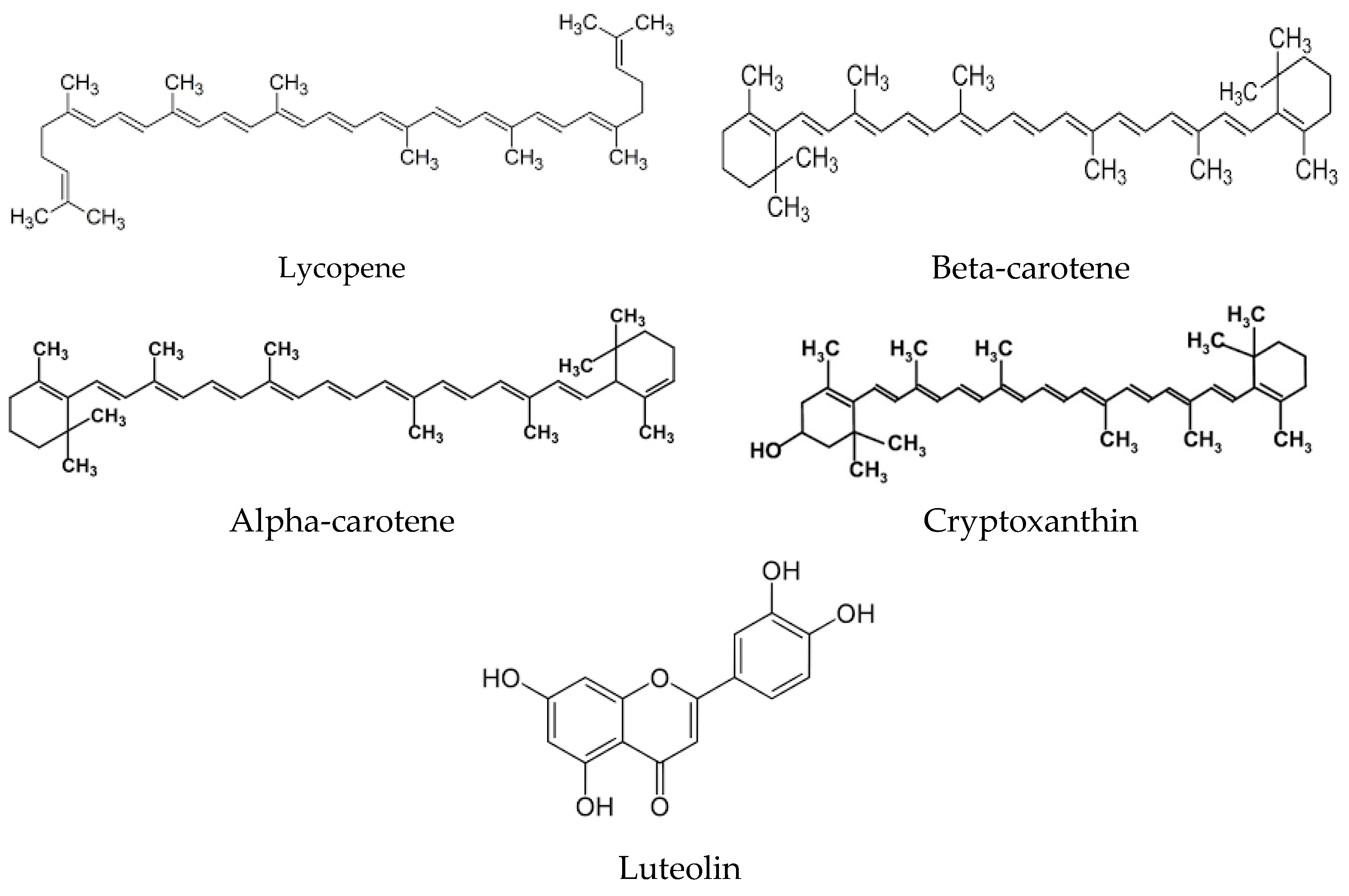
2. Origins of Phytochemicals with Antioxidant Properties: Exploring Their Source and Nature
3. Bioavailability of Phytochemicals
4. Bioaccessibility of Phytochemicals
5. Phytochemicals in the Prevention and Treatment of Human Disease
6. Discussion
7. Methods
Author Contributions
Funding
Conflicts of Interest
References
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Turkan, I. ROS and RNS: Key Signalling Molecules in Plants. J. Exp. Bot. 2018, 69, 3313–3315. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive Oxygen Species (ROS) as Pleiotropic Physiological Signalling Agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Hanke, G. ROS Production and Signalling in Chloroplasts: Cornerstones and Evolving Concepts. Plant J. 2022, 111, 642–661. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxid. Med. Cell Longev. 2016, 2016, e1245049. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell Longev. 2017, 2017, e8416763. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef]
- Shadel, G.S.; Horvath, T.L. Mitochondrial ROS Signalling in Organismal Homeostasis. Cell 2015, 163, 560–569. [Google Scholar] [CrossRef]
- Yang, S.; Lian, G. ROS and Diseases: Role in Metabolism and Energy Supply. Mol. Cell. Biochem. 2020, 467, 1–12. [Google Scholar] [CrossRef]
- Gilgun-Sherki, Y.; Melamed, E.; Offen, D. Oxidative Stress Induced-Neurodegenerative Diseases: The Need for Antioxidants That Penetrate the Blood Brain Barrier. Neuropharmacology 2001, 40, 959–975. [Google Scholar] [CrossRef]
- Reshi, Z.A.; Ahmad, W.; Lukatkin, A.S.; Javed, S.B. From Nature to Lab: A Review of Secondary Metabolite Biosynthetic Pathways, Environmental Influences, and In Vitro Approaches. Metabolites 2023, 13, 895. [Google Scholar] [CrossRef]
- Bourgaud, F.; Gravot, A.; Milesi, S.; Gontier, E. Production of Plant Secondary Metabolites: A Historical Perspective. Plant Sci. 2001, 161, 839–851. [Google Scholar] [CrossRef]
- Jamwal, K.; Bhattacharya, S.; Puri, S. Plant Growth Regulator Mediated Consequences of Secondary Metabolites in Medicinal Plants. J. Appl. Res. Med. Aromat. Plants 2018, 9, 26–38. [Google Scholar] [CrossRef]
- Jan, R.; Asaf, S.; Numan, M.; Lubna; Kim, K.-M. Plant Secondary Metabolite Biosynthesis and Transcriptional Regulation in Response to Biotic and Abiotic Stress Conditions. Agronomy 2021, 11, 968. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, H.; Xie, J.; Lv, J.; Zhang, G.; Hu, L.; Luo, S.; Li, L.; Yu, J. The Roles of Cruciferae Glucosinolates in Disease and Pest Resistance. Plants 2021, 10, 1097. [Google Scholar] [CrossRef] [PubMed]
- Davinelli, S.; Scapagnini, G. The Pharma-Nutritional Role of Antioxidant Phytochemicals in Health and Disease. Antioxidants 2022, 11, 1081. [Google Scholar] [CrossRef] [PubMed]
- Davinelli, S.; Calabrese, V.; Zella, D.; Scapagnini, G. Epigenetic Nutraceutical Diets in Alzheimer’s Disease. J. Nutr. Health Aging 2014, 18, 800–805. [Google Scholar] [CrossRef] [PubMed]
- Davinelli, S.; Maes, M.; Corbi, G.; Zarrelli, A.; Willcox, D.C.; Scapagnini, G. Dietary Phytochemicals and Neuro-Inflammaging: From Mechanistic Insights to Translational Challenges. Immun. Ageing 2016, 13, 16. [Google Scholar] [CrossRef]
- Davinelli, S.; Stefani, D.D.; Vivo, I.D.; Scapagnini, G. Polyphenols as Caloric Restriction Mimetics Regulating Mitochondrial Biogenesis and Mitophagy. Trends Endocrinol. Metab. 2020, 31, 536–550. [Google Scholar] [CrossRef]
- Davinelli, S.; Ali, S.; Solfrizzi, V.; Scapagnini, G.; Corbi, G. Carotenoids and Cognitive Outcomes: A Meta-Analysis of Randomized Intervention Trials. Antioxidants 2021, 10, 223. [Google Scholar] [CrossRef]
- Deng, G.-F.; Xu, X.-R.; Guo, Y.-J.; Xia, E.-Q.; Li, S.; Wu, S.; Chen, F.; Ling, W.-H.; Li, H.-B. Determination of Antioxidant Property and Their Lipophilic and Hydrophilic Phenolic Contents in Cereal Grains. J. Funct. Foods 2012, 4, 906–914. [Google Scholar] [CrossRef]
- Guo, Y.-J.; Deng, G.-F.; Xu, X.-R.; Wu, S.; Li, S.; Xia, E.-Q.; Li, F.; Chen, F.; Ling, W.-H.; Li, H.-B. Antioxidant Capacities, Phenolic Compounds and Polysaccharide Contents of 49 Edible Macro-Fungi. Food Funct. 2012, 3, 1195–1205. [Google Scholar] [CrossRef]
- Fu, L.; Xu, B.-T.; Xu, X.-R.; Gan, R.-Y.; Zhang, Y.; Xia, E.-Q.; Li, H.-B. Antioxidant Capacities and Total Phenolic Contents of 62 Fruits. Food Chem. 2011, 129, 345–350. [Google Scholar] [CrossRef]
- Manganaris, G.A.; Goulas, V.; Vicente, A.R.; Terry, L.A. Berry Antioxidants: Small Fruits Providing Large Benefits. J. Sci. Food Agric. 2014, 94, 825–833. [Google Scholar] [CrossRef]
- Fu, L.; Xu, B.-T.; Xu, X.-R.; Qin, X.-S.; Gan, R.-Y.; Li, H.-B. Antioxidant Capacities and Total Phenolic Contents of 56 Wild Fruits from South China. Molecules 2010, 15, 8602–8617. [Google Scholar] [CrossRef]
- Deng, G.-F.; Shen, C.; Xu, X.-R.; Kuang, R.-D.; Guo, Y.-J.; Zeng, L.-S.; Gao, L.-L.; Lin, X.; Xie, J.-F.; Xia, E.-Q.; et al. Potential of Fruit Wastes as Natural Resources of Bioactive Compounds. Int. J. Mol. Sci. 2012, 13, 8308–8323. [Google Scholar] [CrossRef]
- Marra, F.; Petrovicova, B.; Canino, F.; Maffia, A.; Mallamaci, C.; Muscolo, A. Pomegranate Wastes Are Rich in Bioactive Compounds with Potential Benefit on Human Health. Molecules 2022, 27, 5555. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.-F.; Lin, X.; Xu, X.-R.; Gao, L.-L.; Xie, J.-F.; Li, H.-B. Antioxidant Capacities and Total Phenolic Contents of 56 Vegetables. J. Funct. Foods 2013, 5, 260–266. [Google Scholar] [CrossRef]
- Panuccio, M.; Marra, F.; Maffia, A.; Mallamaci, C.; Muscolo, A. Recycling of Agricultural (Orange and Olive) Bio-Wastes into Ecofriendly Fertilizers for Improving Soil and Garlic Quality. Res. Cons. Rec. Adv. 2022, 15, 200083. [Google Scholar] [CrossRef]
- Deng, G.-F.; Xu, X.-R.; Zhang, Y.; Li, D.; Gan, R.-Y.; Li, H.-B. Phenolic Compounds and Bioactivities of Pigmented Rice. Crit. Rev. Food Sci. Nutr. 2013, 53, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Li, A.-N.; Li, S.; Li, H.-B.; Xu, D.-P.; Xu, X.-R.; Chen, F. Total Phenolic Contents and Antioxidant Capacities of 51 Edible and Wild Flowers. J. Funct. Foods 2014, 6, 319–330. [Google Scholar] [CrossRef]
- Zhang, Y.; Deng, G.; Xu, X.; Wu, S.; Li, S.; Li, H. Chemical Components and Bioactivities of Cape Gooseberry (Physalis Peruviana). Int. J. Food Nutr. Saf. 2013, 3, 15–24. [Google Scholar]
- Giampieri, F.; Tulipani, S.; Alvarez-Suarez, J.M.; Quiles, J.L.; Mezzetti, B.; Battino, M. The Strawberry: Composition, Nutritional Quality, and Impact on Human Health. Nutrition 2012, 28, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.G.V.; Garcia-Diaz, D.F.; Jimenez, P.; Silva, P.I. Bioactive Compounds and Health Benefits of Exotic Tropical Red–Black Berries. J. Funct. Foods 2013, 5, 539–549. [Google Scholar] [CrossRef]
- Sami, R.; Li, C.-J.; Zhao, Y.; Li, Y.; Sun, C.-H. Cabbage (Brassica oleracea L. Var. Capitata) Phytochemicals with Antioxidant and Anti-Inflammatory Potential. Asian Pac. J. Cancer APJCP 2013, 14, 6657–6662. [Google Scholar] [CrossRef]
- Hyson, D.A. A Comprehensive Review of Apples and Apple Components and Their Relationship to Human Health. Adv. Nutr. 2011, 2, 408–420. [Google Scholar] [CrossRef]
- Yang, J. Brazil Nuts and Associated Health Benefits: A Review. LWT Food Sci. Technol. 2009, 42, 1573–1580. [Google Scholar] [CrossRef]
- Sun, J.; Chu, Y.-F.; Wu, X.; Liu, R.H. Antioxidant and Antiproliferative Activities of Common Fruits. J. Agric. Food Chem. 2002, 50, 7449–7454. [Google Scholar] [CrossRef]
- Sung, J.; Lee, J. Antioxidant and Antiproliferative Activities of Grape Seeds from Different Cultivars. Food Sci. Biotechnol. 2010, 19, 321–326. [Google Scholar] [CrossRef]
- Ilari, S.; Giancotti, L.A.; Lauro, F.; Gliozzi, M.; Malafoglia, V.; Palma, E.; Tafani, M.; Russo, M.A.; Tomino, C.; Fini, M.; et al. Natural Antioxidant Control of Neuropathic Pain-Exploring the Role of Mitochondrial SIRT3 Pathway. Antioxidants 2020, 9, 1103. [Google Scholar] [CrossRef]
- Mohamed, M.T.; Zaitone, S.A.; Ahmed, A.; Mehanna, E.T.; El-Sayed, N.M. Raspberry Ketones Attenuate Cyclophosphamide-Induced Pulmonary Toxicity in Mice through Inhibition of Oxidative Stress and NF-ΚB Pathway. Antioxidants 2020, 9, 1168. [Google Scholar] [CrossRef]
- Kunnummal, S.P.; Khan, M. Diet–Gut Microbiome Interaction and Ferulic Acid Bioavailability: Implications on Neurodegenerative Disorders. Eur. J. Nutr. 2024, 63, 51–66. [Google Scholar] [CrossRef]
- Rawangkan, A.; Wongsirisin, P.; Namiki, K.; Iida, K.; Kobayashi, Y.; Shimizu, Y.; Fujiki, H.; Suganuma, M. Green Tea Catechin Is an Alternative Immune Checkpoint Inhibitor That Inhibits PD-L1 Expression and Lung Tumor Growth. Molecules 2018, 23, 2071. [Google Scholar] [CrossRef] [PubMed]
- Enkhbat, T.; Nishi, M.; Yoshikawa, K.; Jun, H.; Tokunaga, T.; Takasu, C.; Kashihara, H.; Ishikawa, D.; Tominaga, M.; Shimada, M. Epigallocatechin-3-Gallate Enhances Radiation Sensitivity in Colorectal Cancer Cells through Nrf2 Activation and Autophagy. Anticancer. Res. 2018, 38, 6247–6252. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chang, L.; Qu, Y.; Liang, J.; Jin, W.; Xia, X. Tea Polyphenols Inhibit the Proliferation, Migration, and Invasion of Melanoma Cells through the down-Regulation of TLR4. Int. J. Immunopathol. Pharmacol. 2018, 31, 0394632017739531. [Google Scholar] [CrossRef]
- Khan, N.; Adhami, V.M.; Mukhtar, H. Review: Green Tea Polyphenols in Chemoprevention of Prostate Cancer: Preclinical and Clinical Studies. Nutr. Cancer 2009, 61, 836–841. [Google Scholar] [CrossRef]
- Khan, N.; Bharali, D.J.; Adhami, V.M.; Siddiqui, I.A.; Cui, H.; Shabana, S.M.; Mousa, S.A.; Mukhtar, H. Oral Administration of Naturally Occurring Chitosan-Based Nanoformulated Green Tea Polyphenol EGCG Effectively Inhibits Prostate Cancer Cell Growth in a Xenograft Model. Carcinogenesis 2014, 35, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Feng, X.; Shi, Z.Q.; Ganapathy, A.; Mishra, M.K.; Atadja, P.; Morris, D.; Riabowol, K. ING1 and 5-Azacytidine Act Synergistically to Block Breast Cancer Cell Growth. PLoS ONE 2012, 7, e43671. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Kwak, J.; Choi, H.-K.; Choi, K.-C.; Kim, S.; Lee, J.; Jun, W.; Park, H.-J.; Yoon, H.-G. EGCG Suppresses Prostate Cancer Cell Growth Modulating Acetylation of Androgen Receptor by Anti-Histone Acetyltransferase Activity. Int. J. Mol. Med. 2012, 30, 69–74. [Google Scholar] [CrossRef]
- Alzahrani, A.M.; Shait Mohammed, M.R.; Alghamdi, R.A.; Ahmad, A.; Zamzami, M.A.; Choudhry, H.; Khan, M.I. Urolithin A and B Alter Cellular Metabolism and Induce Metabolites Associated with Apoptosis in Leukemic Cells. Int. J. Mol. Sci. 2021, 22, 5465. [Google Scholar] [CrossRef]
- Arumugam, A.; Razis, A.F.A. Apoptosis as a Mechanism of the Cancer Chemopreventive Activity of Glucosinolates: A Review. Asian Pac. J. Cancer Prev. 2018, 19, 1439–1448. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-Y.; Lansing, L.; Merillon, J.-M.; Davis, F.B.; Tang, H.-Y.; Shihi, A.; Vitrac, X.; Krisa, S.; Keating, T.; Cao, H.J.; et al. Integrin αVβ3 Contains a Receptor Site for Resveratrol. FASEB J. 2006, 20, 1742–1744. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.; Cai, L.; Udeani, G.O.; Slowing, K.V.; Thomas, C.F.; Beecher, C.W.W.; Fong, H.H.S.; Farnsworth, N.R.; Kinghorn, A.D.; Mehta, R.G.; et al. Cancer Chemopreventive Activity of Resveratrol, a Natural Product Derived from Grapes. Science 1997, 275, 218–220. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.; Wu, J.M. Differential Effects on Growth, Cell Cycle Arrest, and Induction of Apoptosis by Resveratrol in Human Prostate Cancer Cell Lines. Exp. Cell Res. 1999, 249, 109–115. [Google Scholar] [CrossRef]
- Ren, B.; Qin, W.; Wu, F.; Wang, S.; Pan, C.; Wang, L.; Zeng, B.; Ma, S.; Liang, J. Apigenin and Naringenin Regulate Glucose and Lipid Metabolism, and Ameliorate Vascular Dysfunction in Type 2 Diabetic Rats. Eur. J. Pharmacol. 2016, 773, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Kuban-Jankowska, A.; Kostrzewa, T.; Musial, C.; Barone, G.; Lo-Bosco, G.; Lo-Celso, F.; Gorska-Ponikowska, M. Green Tea Catechins Induce Inhibition of PTP1B Phosphatase in Breast Cancer Cells with Potent Anti-Cancer Properties: In Vitro Assay, Molecular Docking, and Dynamics Studies. Antioxidants 2020, 9, 1208. [Google Scholar] [CrossRef]
- Prasher, P.; Sharma, M.; Singh, S.K.; Gulati, M.; Chellappan, D.K.; Zacconi, F.; De Rubis, G.; Gupta, G.; Sharifi-Rad, J.; Cho, W.C.; et al. Luteolin: A Flavonoid with a Multifaceted Anticancer Potential. Cancer Cell Int. 2022, 22, 386. [Google Scholar] [CrossRef]
- Chang, Y.-H.; Chiang, Y.-F.; Chen, H.-Y.; Huang, Y.-J.; Wang, K.-L.; Hong, Y.-H.; Ali, M.; Shieh, T.-M.; Hsia, S.-M. Anti-Inflammatory and Anti-Hyperuricemic Effects of Chrysin on a High Fructose Corn Syrup-Induced Hyperuricemia Rat Model via the Amelioration of Urate Transporters and Inhibition of NLRP3 Inflammasome Signaling Pathway. Antioxidants 2021, 10, 564. [Google Scholar] [CrossRef]
- Cichon, N.; Saluk-Bijak, J.; Gorniak, L.; Przyslo, L.; Bijak, M. Flavonoids as a Natural Enhancer of Neuroplasticity—An Overview of the Mechanism of Neurorestorative Action. Antioxidants 2020, 9, 1035. [Google Scholar] [CrossRef]
- Ali, S.; Corbi, G.; Maes, M.; Scapagnini, G.; Davinelli, S. Exploring the Impact of Flavonoids on Symptoms of Depression: A Systematic Review and Meta-Analysis. Antioxidants 2021, 10, 1644. [Google Scholar] [CrossRef]
- García-Martínez, B.I.; Ruiz-Ramos, M.; Pedraza-Chaverri, J.; Santiago-Osorio, E.; Mendoza-Núñez, V.M. Hypoglycemic Effect of Resveratrol: A Systematic Review and Meta-Analysis. Antioxidants 2021, 10, 69. [Google Scholar] [CrossRef]
- Harandi-Zadeh, S.; Boycott, C.; Beetch, M.; Yang, T.; Martin, B.J.E.; Ren, K.; Kwasniak, A.; Dupuis, J.H.; Lubecka, K.; Yada, R.Y.; et al. Pterostilbene Changes Epigenetic Marks at Enhancer Regions of Oncogenes in Breast Cancer Cells. Antioxidants 2021, 10, 1232. [Google Scholar] [CrossRef]
- Zengin, G.; Stojković, D.; Mahomoodally, M.F.; Jugreet, B.S.; Paksoy, M.Y.; Ivanov, M.; Gašić, U.; Gallo, M.; Montesano, D. Comprehensive Biological and Chemical Evaluation of Two Seseli Species (S. Gummiferum and S. Transcaucasicum). Antioxidants 2021, 10, 1510. [Google Scholar] [CrossRef]
- Omar, A.E.; Al-Khalaifah, H.S.; Osman, A.; Gouda, A.; Shalaby, S.I.; Roushdy, E.M.; Abdo, S.A.; Ali, S.A.; Hassan, A.M.; Amer, S.A. Modulating the Growth, Antioxidant Activity, and Immunoexpression of Proinflammatory Cytokines and Apoptotic Proteins in Broiler Chickens by Adding Dietary Spirulina Platensis Phycocyanin. Antioxidants 2022, 11, 991. [Google Scholar] [CrossRef]
- Ionescu, V.S.; Popa, A.; Alexandru, A.; Manole, E.; Neagu, M.; Pop, S. Dietary Phytoestrogens and Their Metabolites as Epigenetic Modulators with Impact on Human Health. Antioxidants 2021, 10, 1893. [Google Scholar] [CrossRef]
- Logie, E.; Vanden Berghe, W. Tackling Chronic Inflammation with Withanolide Phytochemicals—A Withaferin a Perspective. Antioxidants 2020, 9, 1107. [Google Scholar] [CrossRef] [PubMed]
- Samtiya, M.; Aluko, R.E.; Dhewa, T.; Moreno-Rojas, J.M. Potential Health Benefits of Plant Food-Derived Bioactive Components: An Overview. Foods 2021, 10, 839. [Google Scholar] [CrossRef]
- Paul, A.; Radhakrishnan, M. Pomegranate Seed Oil in Food Industry: Extraction, Characterization, and Applications. Trends Food Sci. Technol. 2020, 105, 273–283. [Google Scholar] [CrossRef]
- Magangana, T.P.; Makunga, N.P.; Fawole, O.A.; Opara, U.L. Processing Factors Affecting the Phytochemical and Nutritional Properties of Pomegranate (Punica granatum L.) Peel Waste: A Review. Molecules 2020, 25, 4690. [Google Scholar] [CrossRef] [PubMed]
- Neilson, A.P.; Goodrich, K.M.; Ferruzzi, M.G. Chapter 15—Bioavailability and Metabolism of Bioactive Compounds from Foods. In Nutrition in the Prevention and Treatment of Disease, 4th ed.; Coulston, A.M., Boushey, C.J., Ferruzzi, M.G., Delahanty, L.M., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 301–319. [Google Scholar] [CrossRef]
- Hu, Y.; Lin, Q.; Zhao, H.; Li, X.; Sang, S.; McClements, D.J.; Long, J.; Jin, Z.; Wang, J.; Qiu, C. Bioaccessibility and Bioavailability of Phytochemicals: Influencing Factors, Improvements, and Evaluations. Food Hydrocoll. 2023, 135, 108165. [Google Scholar] [CrossRef]
- Bohn, T.; Blackwood, M.; Francis, D.; Tian, Q.; Schwartz, S.J.; Clinton, S.K. Bioavailability of Phytochemical Constituents from a Novel Soy Fortified Lycopene Rich Tomato Juice Developed for Targeted Cancer Prevention Trials. Nutr. Cancer 2013, 65, 919–929. [Google Scholar] [CrossRef]
- Ozen, E.; Mihaylova, R.; Weech, M.; Kinsella, S.; Lovegrove, J.A.; Jackson, K.G. Association between Dietary Saturated Fat with Cardiovascular Disease Risk Markers and Body Composition in Healthy Adults: Findings from the Cross-Sectional BODYCON Study. Nutr. Met. 2022, 19, 15. [Google Scholar] [CrossRef]
- Aras, A.; Khokhar, A.R.; Qureshi, M.Z.; Silva, M.F.; Sobczak-Kupiec, A.; Pineda, E.A.G.; Hechenleitner, A.A.W.; Farooqi, A.A. Targeting Cancer with Nano-Bullets: Curcumin, EGCG, Resveratrol and Quercetin on Flying Carpets. Asian Pac. J. Cancer APJCP 2014, 15, 3865–3871. [Google Scholar] [CrossRef] [PubMed]
- Andreu Fernández, V.; Almeida Toledano, L.; Pizarro Lozano, N.; Navarro Tapia, E.; Gómez Roig, M.D.; De la Torre Fornell, R.; García Algar, Ó. Bioavailability of Epigallocatechin Gallate Administered with Different Nutritional Strategies in Healthy Volunteers. Antioxidants 2020, 9, 440. [Google Scholar] [CrossRef] [PubMed]
- Massi, A.; Bortolini, O.; Ragno, D.; Bernardi, T.; Sacchetti, G.; Tacchini, M.; De Risi, C. Research Progress in the Modification of Quercetin Leading to Anticancer Agents. Molecules 2017, 22, 1270. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Bruno, R.S. Endogenous and Exogenous Mediators of Quercetin Bioavailability. J. Nutr. Biochem. 2015, 26, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.L.; Hsu, C.H.; Lin, J.K.; Hsu, M.M.; Ho, Y.F.; Shen, T.S.; Ko, J.Y.; Lin, J.T.; Lin, B.R.; Ming-Shiang, W.; et al. Phase I Clinical Trial of Curcumin, a Chemopreventive Agent, in Patients with High-Risk or Pre-Malignant Lesions. Anticancer. Res. 2001, 21, 2895–2900. [Google Scholar]
- Prasad, S.; Tyagi, A.K.; Aggarwal, B.B. Recent Developments in Delivery, Bioavailability, Absorption and Metabolism of Curcumin: The Golden Pigment from Golden Spice. Cancer Res. Treat. 2014, 46, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Barani, M.; Sangiovanni, E.; Angarano, M.; Rajizadeh, M.A.; Mehrabani, M.; Piazza, S.; Gangadharappa, H.V.; Pardakhty, A.; Mehrbani, M.; Dell’Agli, M.; et al. Phytosomes as Innovative Delivery Systems for Phytochemicals: A Comprehensive Review of Literature. Int. J. Nanomed. 2021, 16, 6983–7022. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Tomas, M.; Ozdal, T.; Capanoglu, E. Effect of Food Matrix on the Content and Bioavailability of Flavonoids. Trends Food Sci. Technol. 2021, 117, 15–33. [Google Scholar] [CrossRef]
- Pohl, P. What Do Metals Tell Us about Wine? TrAC Trends Anal. Chem. 2007, 26, 941–949. [Google Scholar] [CrossRef]
- Shahidi, N.; Pan, M.; Safaei, S.; Tran, K.; Crampin, E.J.; Nickerson, D.P. Hierarchical Semantic Composition of Biosimulation Models Using Bond Graphs. PLoS Comput. Biol. 2021, 17, e1008859. [Google Scholar] [CrossRef]
- Yao, K.; McClements, D.J.; Yan, C.; Xiao, J.; Liu, H.; Chen, Z.; Hou, X.; Cao, Y.; Xiao, H.; Liu, X. In Vitro and in Vivo Study of the Enhancement of Carotenoid Bioavailability in Vegetables Using Excipient Nanoemulsions: Impact of Lipid Content. Food Res. Int. 2021, 141, 110162. [Google Scholar] [CrossRef]
- Monfoulet, L.E.; Buffière, C.; Istas, G.; Dufour, C.; Bourvellec, C.L.; Mercier, S.; Bayle, D.; Boby, C.; Remond, D.; Borel, P.; et al. Effects of the Apple Matrix on the Postprandial Bioavailability of Flavan-3-Ols and Nutrigenomic Response of Apple Polyphenols in Minipigs Challenged with a High Fat Meal. Food Funct. 2020, 11, 5077–5090. [Google Scholar] [CrossRef]
- Wellala, C.K.D.; Bi, J.; Liu, X.; Wu, X.; Lyu, J.; Liu, J.; Liu, D.; Guo, C. Effect of High Pressure Homogenization on Water-Soluble Pectin Characteristics and Bioaccessibility of Carotenoids in Mixed Juice. Food Chem. 2022, 371, 131073. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Fernández, M.; Young Tie Yang, P.; Ludwig, I.A.; Clifford, M.N.; Cid, C.; Rodriguez-Mateos, A. In Vivo Study of the Bioavailability and Metabolic Profile of (Poly)Phenols after Sous-Vide Artichoke Consumption. Food Chem. 2022, 367, 130620. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.; Corbin, S.; Nunn, C.; Pottorff, M.; Kay, C.D.; Lila, M.A.; Iorrizo, M.; Ferruzzi, M.G. Influence of Simulated Food and Oral Processing on Carotenoid and Chlorophyll in Vitro Bioaccessibility among Six Spinach Genotypes. Food Funct. 2021, 12, 7001–7016. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, D.; Huang, M.; Huang, W.; Li, Y.; Feng, J. Interfacial Engineering Strategy to Improve the Stabilizing Effect of Curcumin-Loaded Nanostructured Lipid Carriers. Food Hydrocoll. 2022, 127, 107552. [Google Scholar] [CrossRef]
- Ramírez-Melo, L.M.; del Socorro Cruz-Cansino, N.; Delgado-Olivares, L.; Ramírez-Moreno, E.; Zafra-Rojas, Q.Y.; Hernández-Traspeña, J.L.; Suárez-Jacobo, Á. Optimization of Antioxidant Activity Properties of a Thermosonicated Beetroot (Beta vulgaris L.) Juice and Further In Vitro Bioaccessibility Comparison with Thermal Treatments. LWT 2022, 154, 112780. [Google Scholar] [CrossRef]
- Burca-Busaga, C.G.; Betoret, N.; Seguí, L.; García-Hernández, J.; Hernández, M.; Barrera, C. Antioxidants Bioaccessibility and Lactobacillus salivarius (CECT 4063) Survival Following the In Vitro Digestion of Vacuum Impregnated Apple Slices: Effect of the Drying Technique, the Addition of Trehalose, and High-Pressure Homogenization. Foods 2021, 10, 2155. [Google Scholar] [CrossRef]
- Sęczyk, Ł.; Ozdemir, F.A.; Kołodziej, B. In Vitro Bioaccessibility and Activity of Basil (Ocimum basilicum L.) Phytochemicals as Affected by Cultivar and Postharvest Preservation Method—Convection Drying, Freezing, and Freeze-Drying. Food Chem. 2022, 382, 132363. [Google Scholar] [CrossRef]
- Ribas-Agustí, A.; Martín-Belloso, O.; Soliva-Fortuny, R.; Elez-Martínez, P. Food Processing Strategies to Enhance Phenolic Compounds Bioaccessibility and Bioavailability in Plant-Based Foods. Crit. Rev. Food Sci. Nutr. 2018, 58, 2531–2548. [Google Scholar] [CrossRef]
- Tomé-Sánchez, I.; Martín-Diana, A.B.; Peñas, E.; Frias, J.; Rico, D.; Jiménez-Pulido, I.; Martínez-Villaluenga, C. Bioprocessed Wheat Ingredients: Characterization, Bioaccessibility of Phenolic Compounds, and Bioactivity During In Vitro Digestion. Front. Plant Sci. 2021, 12, 790898. [Google Scholar] [CrossRef]
- Hu, Y.; Julian McClements, D.; Li, X.; Chen, L.; Long, J.; Jiao, A.; Xie, F.; Wang, J.; Jin, Z.; Qiu, C. Improved Art Bioactivity by Encapsulation within Cyclodextrin Carboxylate. Food Chem. 2022, 384, 132429. [Google Scholar] [CrossRef]
- Hao, J.; Xu, J.; Zhang, W.; Li, X.; Liang, D.; Xu, D.; Cao, Y.; Sun, B. The Improvement of the Physicochemical Properties and Bioaccessibility of Lutein Microparticles by Electrostatic Complexation. Food Hydrocoll. 2022, 125, 107381. [Google Scholar] [CrossRef]
- He, J.-R.; Zhu, J.-J.; Yin, S.-W.; Yang, X.-Q. Bioaccessibility and Intracellular Antioxidant Activity of Phloretin Embodied by Gliadin/Sodium Carboxymethyl Cellulose Nanoparticles. Food Hydrocoll. 2022, 122, 107076. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST Static in Vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]
- Makran, M.; Faubel, N.; López-García, G.; Cilla, A.; Barberá, R.; Alegría, A.; Garcia-Llatas, G. Sterol Bioaccessibility in a Plant Sterol-Enriched Beverage Using the INFOGEST Digestion Method: Influence of Gastric Lipase, Bile Salts and Cholesterol Esterase. Food Chem. 2022, 382, 132305. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Jung, K.-J.; Zhang, R.; Muriel Mundo, J.L.; McClements, D.J. In Situ Monitoring of Lipid Droplet Release from Biopolymer Microgels under Simulated Gastric Conditions Using Magnetic Resonance Imaging and Spectroscopy. Food Res. Int. 2019, 123, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, M.P.; Moriwaki, M.; Uguri, K.; Timm, D.; Kuroiwa, Y. Bioavailability of Dietary Isoquercitrin-γ-Cyclodextrin Molecular Inclusion Complex in Sprague–Dawley Rats and Healthy Humans. J. Funct. Foods 2021, 85, 104663. [Google Scholar] [CrossRef]
- Zou, L.; Wu, D.; Ren, G.; Hu, Y.; Peng, L.; Zhao, J.; Garcia-Perez, P.; Carpena, M.; Prieto, M.A.; Cao, H.; et al. Bioactive Compounds, Health Benefits, and Industrial Applications of Tartary Buckwheat (Fagopyrum tataricum). Crit. Rev. Food Sci. Nutr. 2023, 63, 657–673. [Google Scholar] [CrossRef] [PubMed]
- Giftson, J.S.; Jayanthi, S.; Nalini, N. Chemopreventive Efficacy of Gallic Acid, an Antioxidant and Anticarcinogenic Polyphenol, against 1,2-Dimethyl Hydrazine Induced Rat Colon Carcinogenesis. Investig. New Drugs 2010, 28, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Jiang, B.; Wang, H.; Shen, C.; Chen, H.; Zeng, L. Curcumin Suppresses Intestinal Fibrosis by Inhibition of PPARγ-Mediated Epithelial-Mesenchymal Transition. Evid. -Based Complement. Altern. Med. 2017, 2017, 7876064. [Google Scholar] [CrossRef]
- Keyvani-Ghamsari, S.; Rahimi, M.; Khorsandi, K. An Update on the Potential Mechanism of Gallic Acid as an Antibacterial and Anticancer Agent. Food Sci. Nutr. 2023, 11, 5856–5872. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Pezzani, R.; Redaelli, M.; Zorzan, M.; Imran, M.; Ahmed Khalil, A.; Salehi, B.; Sharopov, F.; Cho, W.C.; Sharifi-Rad, J. Preclinical Activities of Epigallocatechin Gallate in Signaling Pathways in Cancer. Molecules 2020, 25, 467. [Google Scholar] [CrossRef]
- Chen, B.-H.; Hsieh, C.-H.; Tsai, S.-Y.; Wang, C.-Y.; Wang, C.-C. Anticancer effects of epigallocatechin-3-gallate nanoemulsion on lung cancer cells through the activation of AMP-activated protein kinase signaling pathway. Sci. Rep. 2020, 10, 5163. [Google Scholar] [CrossRef] [PubMed]
- Almatroodi, S.A.; Almatroudi, A.; Khan, A.A.; Alhumaydhi, F.A.; Alsahli, M.A.; Rahmani, A.H. Potential Therapeutic Targets of Epigallocatechin Gallate (EGCG), the Most Abundant Catechin in Green Tea, and Its Role in the Therapy of Various Types of Cancer. Molecules 2020, 25, 3146. [Google Scholar] [CrossRef]
- Kim, E.; Hwang, K.; Lee, J.; Han, S.Y.; Kim, E.-M.; Park, J.; Cho, J.Y. Skin Protective Effect of Epigallocatechin Gallate. Int. J. Mol. Sci. 2018, 19, 173. [Google Scholar] [CrossRef]
- Naponelli, V.; Ramazzina, I.; Lenzi, C.; Bettuzzi, S.; Rizzi, F. Green Tea Catechins for Prostate Cancer Prevention: Present Achievements and Future Challenges. Antioxidants 2017, 6, 26. [Google Scholar] [CrossRef]
- Choudhari, A.S.; Mandave, P.C.; Deshpande, M.; Ranjekar, P.; Prakash, O. Phytochemicals in Cancer Treatment: From Preclinical Studies to Clinical Practice. Front. Pharmacol. 2020, 10, 175. [Google Scholar] [CrossRef] [PubMed]
- Zan, L.; Chen, Q.; Zhang, L.; Li, X. Epigallocatechin Gallate (EGCG) Suppresses Growth and Tumorigenicity in Breast Cancer Cells by Downregulation of miR-25. Bioengineered 2019, 10, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Marín, V.; Burgos, V.; Pérez, R.; Maria, D.A.; Pardi, P.; Paz, C. The Potential Role of Epigallocatechin-3-Gallate (EGCG) in Breast Cancer Treatment. Int. J. Mol. Sci. 2023, 24, 10737. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Liberona, N.; González-Domínguez, R.; Vegas, E.; Riso, P.; Del Bo’, C.; Bernardi, S.; Peron, G.; Guglielmetti, S.; Gargari, G.; Kroon, P.A.; et al. Increased Intestinal Permeability in Older Subjects Impacts the Beneficial Effects of Dietary Polyphenols by Modulating Their Bioavailability. J. Agric. Food Chem. 2020, 68, 12476–12484. [Google Scholar] [CrossRef] [PubMed]
- Dima, C.; Assadpour, E.; Dima, S.; Jafari, S.M. Bioavailability of Nutraceuticals: Role of the Food Matrix, Processing Conditions, the Gastrointestinal Tract, and Nanodelivery Systems. Compr. Rev. Food Sci. Food Saf. 2020, 19, 954–994. [Google Scholar] [CrossRef] [PubMed]
- Thakur, N.; Raigond, P.; Singh, Y.; Mishra, T.; Singh, B.; Lal, M.K.; Dutt, S. Recent Updates on Bioaccessibility of Phytonutrients. Trends Food Sci. Technol. 2020, 97, 366–380. [Google Scholar] [CrossRef]
- Nicolescu, A.; Babotă, M.; Barros, L.; Rocchetti, G.; Lucini, L.; Tanase, C.; Mocan, A.; Bunea, C.I.; Crișan, G. Bioaccessibility and Bioactive Potential of Different Phytoche mical Classes from Nutraceuticals and Functional Foods. Front. Nutr. 2023, 10, 1184535. [Google Scholar] [CrossRef]
- Ting, Y.; Zhao, Q.; Xia, C.; Huang, Q. Using in Vitro and in Vivo Models to Evaluate the Oral Bioavailability of Nutraceuticals. J. Agric. Food Chem. 2015, 63, 1332–1338. [Google Scholar] [CrossRef]
- Rein, M.J.; Renouf, M.; Cruz-Hernandez, C.; Actis-Goretta, L.; Thakkar, S.K.; da Silva Pinto, M. Bioavailability of Bioactive Food Compounds: A Challenging Journey to Bioefficacy. Br. J. Clin. Pharmacol. 2013, 75, 588–602. [Google Scholar] [CrossRef]
- López-Gámez, G.; Elez-Martínez, P.; Martín-Belloso, O.; Soliva-Fortuny, R. Recent Advances toward the Application of Non-Thermal Technologies in Food Processing: An Insight on the Bioaccessibility of Health-Related Constituents in Plant-Based Products. Foods 2021, 10, 1538. [Google Scholar] [CrossRef]
- Barba, F.J.; Mariutti, L.R.B.; Bragagnolo, N.; Mercadante, A.Z.; Barbosa-Cánovas, G.V.; Orlien, V. Bioaccessibility of Bioactive Compounds from Fruits and Vegetables after Thermal and Nonthermal Processing. Trends Food Sci. Technol. 2017, 67, 195–206. [Google Scholar] [CrossRef]
- Rousseau, S.; Kyomugasho, C.; Celus, M.; Hendrickx, M.E.G.; Grauwet, T. Barriers Impairing Mineral Bioaccessibility and Bioavailability in Plant-Based Foods and the Perspectives for Food Processing. Crit. Rev. Food Sci. Nutr. 2020, 60, 826–843. [Google Scholar] [CrossRef]
- Yin, R.; Kuo, H.C.; Hudlikar, R.; Sargsyan, D.; Li, S.; Wang, L.; Wu, R.; Kong, A.N. Gut microbiota, dietary phytochemicals and benefits to human health. Curr. Pharmacol. Rep. 2019, 5, 332–344. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Sandhu, K.; Peterson, V.; Dinan, T.G. The Gut Microbiome in Neurological Disorders. Lancet Neurol. 2020, 19, 179–194. [Google Scholar] [CrossRef]
- Rodríguez-Daza, M.C.; Pulido-Mateos, E.C.; Lupien-Meilleur, J.; Guyonnet, D.; Desjardins, Y.; Roy, D. Polyphenol-Mediated Gut Microbiota Modulation: Toward Prebiotics and Further. Front. Nutr. 2021, 8, 689456. [Google Scholar] [CrossRef]
- Beltrán, D.; Romo-Vaquero, M.; Espín, J.C.; Tomás-Barberán, F.A.; Selma, M.V. Ellagibacter Isourolithinifaciens Gen. Nov., Sp. Nov., a New Member of the Family Eggerthellaceae, Isolated from Human Gut. Int. J. Syst. Evol. Microbiol. 2018, 68, 1707–1712. [Google Scholar] [CrossRef] [PubMed]
- Rowland, I.; Faughnan, M.; Hoey, L.; Wähälä, K.; Williamson, G.; Cassidy, A. Bioavailability of Phyto-Oestrogens. Br. J. Nutr. 2003, 89, S45–S58. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Yeo, J. Bioactivities of Phenolics by Focusing on Suppression of Chronic Diseases: A Review. Int. J. Mol. Sci. 2018, 19, 1573. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-J.; Gan, R.-Y.; Li, S.; Zhou, Y.; Li, A.-N.; Xu, D.-P.; Li, H.-B. Antioxidant Phytochemicals for the Prevention and Treatment of Chronic Diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef]
- Saeidnia, S.; Abdollahi, M. Antioxidants: Friends or Foe in Prevention or Treatment of Cancer: The Debate of the Century. Toxicol. Appl. Pharmacol. 2013, 271, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tsao, R. Dietary Polyphenols, Oxidative Stress and Antioxidant and Anti-Inflammatory Effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). Risk Assessment for Peri- and Post-Menopausal Women Taking Food Supplements Containing Isolated Isoflavones. EFSA J. 2015, 13, 4246. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; de Oliveira, M.C.; Attia, Y.A.; Kamal, M.; Almohmadi, N.H.; Youssef, I.M.; Khalifa, N.E.; Moustafa, M.; Al-Shehri, M.; Taha, A.E. The Efficacy of Polyphenols as an Antioxidant Agent: An Updated Review. Int. J. Biol. Macromol. 2023, 250, 126525. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef]
- Conti, P.; Caraffa, A.; Gallenga, C.E.; Ross, R.; Kritas, S.K.; Frydas, I.; Younes, A.; Di Emidio, P.; Ronconi, G.; Pandolfi, F. Powerful Anti-Inflammatory Action of Luteolin: Potential Increase with IL-38. Biofactors 2021, 47, 165–169. [Google Scholar] [CrossRef]
- Vauzour, D.; Vafeiadou, K.; Rodriguez-Mateos, A.; Rendeiro, C.; Spencer, J.P.E. The Neuroprotective Potential of Flavonoids: A Multiplicity of Effects. Genes Nutr. 2008, 3, 115–126. [Google Scholar] [CrossRef]
- Benavente-García, O.; Castillo, J. Update on Uses and Properties of Citrus Flavonoids: New Findings in Anticancer, Cardiovascular, and Anti-Inflammatory Activity. J. Agric. Food Chem. 2008, 56, 6185–6205. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; He, C.; Wang, M.; Ma, X.; Mo, F.; Yang, S.; Han, J.; Wei, X. Targeting Epigenetic Regulators for Cancer Therapy: Mechanisms and Advances in Clinical Trials. Signal Transduct. Target. Ther. 2019, 4, 62. [Google Scholar] [CrossRef] [PubMed]
- Farkhondeh, T.; Folgado, S.L.; Pourbagher-Shahri, A.M.; Ashrafizadeh, M.; Samarghandian, S. The Therapeutic Effect of Resveratrol: Focusing on the Nrf2 Signaling Pathway. Biomed. Pharmacother. 2020, 127, 110234. [Google Scholar] [CrossRef]
- Li, X.-M.; Li, X.; Wu, Z.; Wang, Y.; Cheng, J.-S.; Wang, T.; Zhang, B. Chitosan Hydrochloride/Carboxymethyl Starch Complex Nanogels Stabilized Pickering Emulsions for Oral Delivery of β-Carotene: Protection Effect and In Vitro Digestion Study. Food Chem. 2020, 315, 126288. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Fei, Y.; Zhang, S.; Qiu, B.; Zhu, L.; Li, F.; Berglund, B.; Xiao, H.; Li, L. Gut Microbiota Composition in Relation to the Metabolism of Oral Administrated Resveratrol. Nutrients 2022, 14, 1013. [Google Scholar] [CrossRef] [PubMed]
- Konstantinopoulos, P.A.; Matulonis, U.A. Clinical and Translational Advances in Ovarian Cancer Therapy. Nat. Cancer 2023, 4, 1239–1257. [Google Scholar] [CrossRef] [PubMed]
- Moussavi, M. Insight into the Mechanisms by Which Apigenin, Curcumin and Sulfasalazine Induce Apoptosis in Colon Cancer Cells. Ph.D. Thesis, University of British Columbia, Vancouver, BC, Canada, 2003. [Google Scholar] [CrossRef]
- Messina, M. Soy and Health Update: Evaluation of the Clinical and Epidemiologic Literature. Nutrients 2016, 8, 754. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.-R.; Mukherjee, P.; Gugger, E.T.; Tanaka, T.; Blackburn, G.L.; Clinton, S.K. Inhibition of Murine Bladder Tumorigenesis by Soy Isoflavones via Alterations in the Cell Cycle, Apoptosis, and Angiogenesis1. Cancer Res. 1998, 58, 5231–5238. [Google Scholar]
- Mithul Aravind, S.; Wichienchot, S.; Tsao, R.; Ramakrishnan, S.; Chakkaravarthi, S. Role of Dietary Polyphenols on Gut Microbiota, Their Metabolites and Health Benefits. Food Res. Int. 2021, 142, 110189. [Google Scholar] [CrossRef]
- Abdallah, A.; Elemba, E.; Zhong, Q.; Sun, Z. Gastrointestinal Interaction between Dietary Amino Acids and Gut Microbiota: With Special Emphasis on Host Nutrition. Curr. Protein Pept. Sci. 2020, 21, 785–798. [Google Scholar] [CrossRef]
- Saha, P.; Yeoh, B.S.; Singh, R.; Chandrasekar, B.; Vemula, P.K.; Haribabu, B.; Vijay-Kumar, M.; Jala, V.R. Gut Microbiota Conversion of Dietary Ellagic Acid into Bioactive Phytoceutical Urolithin a Inhibits Heme Peroxidases. PLoS ONE 2016, 11, e0156811. [Google Scholar] [CrossRef]
- Mendez-Encinas, M.A.; Carvajal-Millan, E.; Rascon-Chu, A.; Astiazaran-Garcia, H.F.; Valencia-Rivera, D.E. Ferulated Arabinoxylans and Their Gels: Functional Properties and Potential Application as Antioxidant and Anticancer Agent. Oxid. Med. Cell Longev. 2018, 2018, e2314759. [Google Scholar] [CrossRef] [PubMed]
- Odriozola-Serrano, I.; Nogueira, D.P.; Esparza, I.; Vaz, A.A.; Jiménez-Moreno, N.; Martín-Belloso, O.; Ancín-Azpilicueta, C. Stability and Bioaccessibility of Phenolic Compounds in Rosehip Extracts during In Vitro Digestion. Antioxidants 2023, 12, 1035. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, B.; Xia, Y.; Li, H.; Shi, X.; Wang, J.; Deng, Z. Bioaccessibility and transformation pathways of phenolic compounds in processed mulberry (Morus alba L.) leaves after in vitro gastrointestinal digestion and faecal fermentation. J. Funct. Foods 2019, 60, 103406. [Google Scholar] [CrossRef]


| Type of Polyphenol | Target | Mechanism of Action | Modification Types | Reference |
|---|---|---|---|---|
| Epigallocatechin gallate | Lung Cancer | Causes a decrease of Hepatoma-derived growth factor (HDGF) | Decreased tumour multiplicity in mice | [42] |
| Epigallocatechin gallate | Colorectal Cancer | Causes inhibition of cell proliferation induction of Nrf2 nuclear translocation and autophagy, expression of LC3 and caspase-9 mRNA | Reduced tumour multiplicity, tumour size | [43] |
| Epigallocatechin gallate | Skin Cancer | Causes inhibition of the proliferation, inhibition of NF-κB activity, IL-1β secretion related with downregulation of NLRP1 | Inhibited melanoma tumour growth | [44] |
| Epigallocatechin gallate | Prostate Cancer | Causes inhibition of class I HDACs (HDAC1, 2, 3, and 8), induction of tumour cell apoptosis | Inhibited tumour growth | [45] |
| Epigallocatechin gallate | Breast Cancer | Causes inhibition of cell growth | Inhibited tumour growth | [46] |
| Epigallocatechin gallate | Breast Cancer | Modifies activation of caspases-3, -8, and -9 | Inhibited tumour growth | [47] |
| Epigallocatechin gallate | Breast Cancer | Causes decrease of AKT and increase Bax/Bcl-2 ratio, comparable to tamoxifen | Inhibited tumour growth | [48] |
| Ellagitannin | Leukaemia | Causes apoptosis | Inhibited proliferation of leukemic cells | [49] |
| Glucosinolate | Liver Cancer | Causes apoptosis | Reduced tumour growth | [50] |
| Resveratrol | αVβ3 integrin receptor in MCF-7 cells | Causes apoptosis of breast cancer cells | Reduced tumour growth | [51] |
| Resveratrol | Liver Cancer | Enhances autophagic flux and apoptosis simultaneously in a dose- and time-dependent manner in HL-60 cells and Hepa 1c1c7 cells | Cancer chemo-preventive agent | [52] |
| Resveratrol | Human Prostate Cancer | Causes a decrease in DU-145, PC-3, and JCA- levels | Decreased prostate cancer cell growth | [53] |
| Apigenin | Glycaemia | Causes an increase in the activity of cellular antioxidants, catalase, superoxide dismutase and glutathione. | Decreased and prevented hyperglycaemia | [54] |
| (–)-Epicatechin | Breast Cancer | Causes inhibition of the MCF-7 cell viability | Decreased tumour cell growth | [55] |
| Luteolin | Breast Cancer | Causes decrease in the viability of MCF-7 breast cancer cells | Decreased breast cancer cell growth | [56] |
| Gallic acid | Colon Cancer (rats) | Causes an increase in superoxide dismutase, catalase, glutathione reductase, and glutathione peroxidase activities | Decreased widespread cancer | [57] |
| Type of Polyphenol | Target | Mechanism of Action | Modification Types | Reference |
|---|---|---|---|---|
| Curcumin | Intestine Crohn’s Disease (CD) | Causes repression of * TGF-β1 | Reduction of intestinal fibrotic stricture in Crohn’s disease | [103] |
| Epigallocatechin gallate | Lung Cancer | Acts as an alternative immune checkpoint inhibitor | Decrement in tumour multiplicity | [104] |
| Epigallocatechin gallate | Lung Cancer | Modulates Akt, NF-κB, MAP kinases and cell cycle pathways | Reduction in tumour multiplicity, tumour size | [105] |
| Epigallocatechin gallate | Colon Cancer | Causes decrease in the levels of proinflammatory eicosanoids, prostaglandin E2, and leukotriene B4 | Reduction in tumour growth | [106] |
| Epigallocatechin gallate | Colon Cancer | Causes apoptosis and augmented expression levels of RXR α, β, and γ in the adenocarcinomas | Reduction in tumour growth | [107] |
| Epigallocatechin gallate | Skin Cancer | Causes inhibition of the proliferation, inhibition of NF-κB activity, IL-1β secretion related with downregulation of NLRP1 | Inhibition of melanoma tumour growth | [108] |
| Epigallocatechin gallate | Skin Cancer | Induces photoprotective effect against acute UVB | Reduction in tumour size and tumour volume | [109] |
| Epigallocatechin gallate | Prostate Cancer | Causes inhibition of agonist-dependent AR activation and AR-regulated gene transcription | Reduction in tumour growth | [110] |
| Allicin | Cholangiocarcinoma | Causes reduction in the activity of the PI3K/AKt signalling pathway | Suppression of the growth of human liver bile duct carcinoma | [111] |
| Epigallocatechin gallate | Breast Cancer | Causes downregulation of miR-25 | Reduction in tumour growth | [112] |
| Epigallocatechin gallate | Breast Cancer | Causes decrease in AKt and increase in Bax/Bcl-2 ratio, comparable to tamoxifen | Reduction in tumour growth | [113] |
| ID | Bioavailability Index (%) |
|---|---|
| Caffeic acid | 13.4 |
| Chlorogenic acid | 148 |
| p-Coumaric acid | 2.2 |
| 3,5-Dicaffeoylquinic acid | - |
| Ellagic acid | 93.4 |
| Ferulic acid | - |
| Gallic acid | 0.68 |
| Protocatechuic acid | 2.5 |
| Rosmarinic acid | - |
| p-Salicylic acid | - |
| o-Salicylic acid | - |
| Syringic acid | 7.6 |
| Vanillic acid | - |
| Quercetin | 43.2 |
| Taxifolin | 61.1 |
| Isoquercetin | 86.8 |
| Hyperoside | 95.2 |
| Quercitrin | 107 |
| Kaempferol | - |
| Rutin | 138 |
| Narcissoside | 121 |
| Isorhamnetin-3-O-glucoside | 83.3 |
| Catechin | 41.9 |
| Epicatechin | 15.2 |
| Epicatechin gallate | 92.3 |
| Procyanidin A2 | 104 |
| Procyanidin B1 | 213 |
| Procyanidin B2 | 40.1 |
| Procyanidin C1 | - |
| Total flavanols | 72 |
| Luteolin | - |
| Luteolin-7-O-glucoside | 92.6 |
| Tangeretin | 39.4 |
| Fisetin | - |
| Phlorizin | 153.1 |
| Methyl gallate | 97.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muscolo, A.; Mariateresa, O.; Giulio, T.; Mariateresa, R. Oxidative Stress: The Role of Antioxidant Phytochemicals in the Prevention and Treatment of Diseases. Int. J. Mol. Sci. 2024, 25, 3264. https://doi.org/10.3390/ijms25063264
Muscolo A, Mariateresa O, Giulio T, Mariateresa R. Oxidative Stress: The Role of Antioxidant Phytochemicals in the Prevention and Treatment of Diseases. International Journal of Molecular Sciences. 2024; 25(6):3264. https://doi.org/10.3390/ijms25063264
Chicago/Turabian StyleMuscolo, Adele, Oliva Mariateresa, Torello Giulio, and Russo Mariateresa. 2024. "Oxidative Stress: The Role of Antioxidant Phytochemicals in the Prevention and Treatment of Diseases" International Journal of Molecular Sciences 25, no. 6: 3264. https://doi.org/10.3390/ijms25063264
APA StyleMuscolo, A., Mariateresa, O., Giulio, T., & Mariateresa, R. (2024). Oxidative Stress: The Role of Antioxidant Phytochemicals in the Prevention and Treatment of Diseases. International Journal of Molecular Sciences, 25(6), 3264. https://doi.org/10.3390/ijms25063264








