β-Carotene Supplementation Improves Pancreas Function during Moderate Ethanol Consumption: Initial Characterization from a Morphological Overview
Abstract
1. Introduction
2. Results
2.1. Biochemical Evaluation
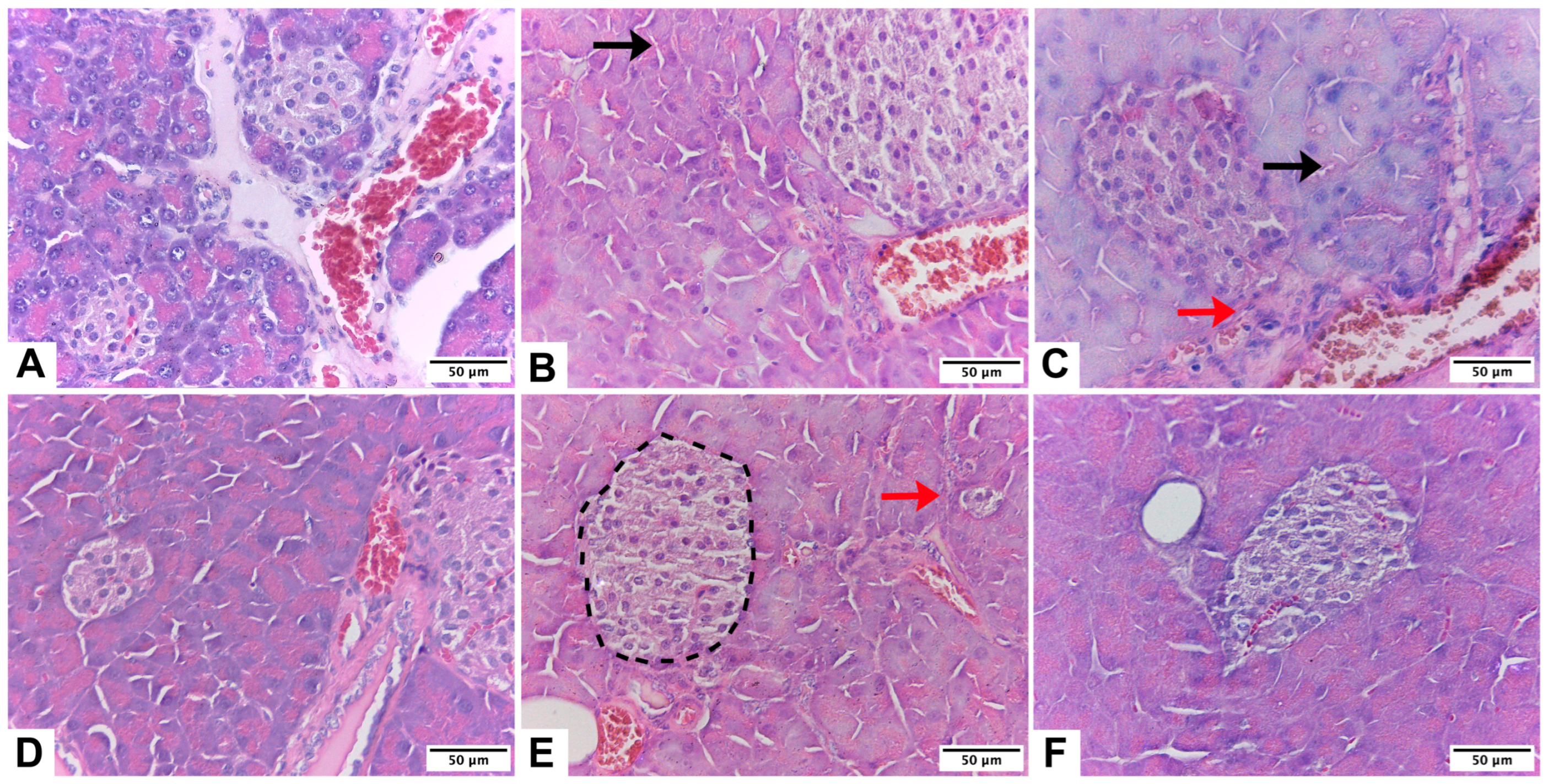
2.2. Histological Evaluation
2.3. Morphoquantitative Analysis of the Pancreas
3. Discussion
3.1. Summary of Key Findings and Interpretation
3.2. Biochemical Evaluation
3.3. Histological Evaluation
3.4. Morphoquantitative Analysis of the Pancreas
3.5. Scope and Limitations
4. Materials and Methods
4.1. Animals
4.2. Euthanasia
4.3. Biochemistry
4.4. Processing and Staining of Pancreas
4.5. Histological Evaluation
4.6. Morphoquantitative Analysis of the Pancreas
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anderson, P.; Dalziel, K.; Davies, E.; Fitzsimmons, D.; Hale, J.; Hughes, A.; Isaac, J.; Onishchenko, K.; Phillips, C.; Pockett, R. Survey of digestive health across Europe: Final report. Part 2: The economic impact and burden of digestive disorders. United Eur. Gastroenterol. J. 2014, 2, 544–546. [Google Scholar] [CrossRef]
- Żorniak, M.; Beyer, G.; Mayerle, J. Risk Stratification and Early Conservative Treatment of Acute Pancreatitis. Visc. Med. 2019, 35, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Conwell, D.L.; Banks, P.A.; Sandhu, B.S.; Sherman, S.; Al-Kaade, S.; Gardner, T.B.; Anderson, M.A.; Wilcox, C.M.; Lewis, M.D.; Muniraj, T.; et al. Validation of Demographics, Etiology, and Risk Factors for Chronic Pancreatitis in the USA: A Report of the North American Pancreas Study (NAPS) Group. Dig. Dis. Sci. 2017, 62, 2133–2140. [Google Scholar] [CrossRef]
- Lankisch, P.G.; Apte, M.; Banks, P.A. Acute pancreatitis. Lancet 2015, 386, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Whitcomb, D.C.; LaRusch, J.; Krasinskas, A.M.; Klei, L.; Smith, J.P.; Brand, R.E.; Neoptolemos, J.P.; Lerch, M.M.; Tector, M.; Sandhu, B.S.; et al. Alzheimer’s Disease Genetics Consortium. Common genetic variants in the CLDN2 and PRSS1-PRSS2 loci alter risk for alcohol-related and sporadic pancreatitis. Nat. Genet. 2012, 44, 1349–1354. [Google Scholar] [CrossRef] [PubMed]
- Pandol, S.J.; Lugea, A.; Mareninova, O.A.; Smoot, D.; Gorelick, F.S.; Gukovskaya, A.S.; Gukovsky, I. Investigating the pathobiology of alcoholic pancreatitis. Alcohol. Clin. Exp. Res. 2011, 35, 830–837. [Google Scholar] [CrossRef]
- Crockett, S.D.; Wani, S.; Gardner, T.B.; Falck-Ytter, Y.; Barkun, A.N.; American Gastroenterological Association Institute Clinical Guidelines Committee. American Gastroenterological Association Institute Guideline on Initial Management of Acute Pancreatitis. Gastroenterology 2018, 154, 1096–1101. [Google Scholar] [CrossRef]
- Xiao, A.Y.; Tan, M.L.Y.; Wu, L.M.; Asrani, V.M.; Windsor, J.A.; Yadav, D.; Petrov, M.S. Global incidence and mortality of pancreatic diseases: A systematic review, meta-analysis, and meta-regression of population-based cohort studies. Lancet Gastroenterol. Hepatol. 2016, 1, 45–55. [Google Scholar] [CrossRef]
- Iannuzzi, J.P.; King, J.A.; Leong, J.H.; Quan, J.; Windsor, J.W.; Tanyingoh, D.; Coward, S.; Forbes, N.; Heitman, S.J.; Shaheen, A.A.; et al. Global Incidence of Acute Pancreatitis Is Increasing Over Time: A Systematic Review and Meta-Analysis. Gastroenterology 2022, 162, 122–134. [Google Scholar] [CrossRef]
- Tenner, S.; Baillie, J.; DeWitt, J.; Vege, S.S. American College of Gastroenterology guideline: Management of acute pancreatitis. Am. J. Gastroenterol. 2013, 108, 1400–1415. [Google Scholar] [CrossRef]
- Yadav, D. Reassessing the Risk of Pancreatitis with Alcohol. Pancreas 2016, 45, 781–782. [Google Scholar] [CrossRef]
- Maléth, J.; Balázs, A.; Pallagi, P.; Balla, Z.; Kui, B.; Katona, M.; Judák, L.; Németh, I.; Kemény, L.V.; Rakonczay, Z., Jr.; et al. Alcohol disrupts levels and function of the cystic fibrosis transmembrane conductance regulator to promote development of pancreatitis. Gastroenterology 2015, 148, 427–439.e16. [Google Scholar] [CrossRef]
- Apte, M.V.; Wilson, J.S.; Lugea, A.; Pandol, S.J. A starring role for stellate cells in the pancreatic cancer microenvironment. Gastroenterology 2013, 144, 1210–1219. [Google Scholar] [CrossRef] [PubMed]
- Lugea, A.; Tischler, D.; Nguyen, J.; Gong, J.; Gukovsky, I.; French, S.W.; Gorelick, F.S.; Pandol, S.J. Adaptive unfolded protein response attenuates alcohol-induced pancreatic damage. Gastroenterology 2011, 140, 987–997. [Google Scholar] [CrossRef] [PubMed]
- Lugea, A.; Waldron, R.T.; Pandol, S.J. Pancreatic adaptive responses in alcohol abuse: Role of the unfolded protein response. Pancreatology 2015, 15 (Suppl. 4), S1–S5. [Google Scholar] [CrossRef]
- Sandoval, C.; Vásquez, B.; Mandarim-de-Lacerda, C.; del Sol, M. Ethanol intake and toxicity: In search of new treatments. Int. J. Morphol. 2017, 35, 942–949. [Google Scholar] [CrossRef]
- Sandoval, C.; Vásquez, B.; Souza-Mello, V.; Mandarim-de-Lacerda, C.; del Sol, M. Role of Alcohol Consumption and Antioxidants on Global Methylation of DNA and Cancer. Int. J. Morphol. 2018, 36, 367–372. [Google Scholar] [CrossRef]
- Wu, H.; Cai, P.; Clemens, D.L.; Jerrells, T.R.; Shakeel Ansari, G.A.; Kaphalia, B.S. Metabolic basis of ethanol-induced cytotoxicity in recombinant HepG2 Cells: Role of nonoxidative metabolism. Toxicol. Appl. Pharmacol. 2006, 216, 238–247. [Google Scholar] [CrossRef]
- Frenzer, A.; Butler, W.J.; Norton, I.D.; Wilson, J.S.; Apte, M.V.; Pirola, R.C.; Ryan, P.; Roberts-Thomson, I.C. Polymorphism in alcohol-metabolizing enzymes, glutathione S-transferases and apolipoprotein E and susceptibility to alcohol-induced cirrhosis and chronic pancreatitis. J. Gastroenterol. Hepatol. 2002, 17, 177–182. [Google Scholar] [CrossRef]
- Wilson, J.S.; Apte, M.V. Role of Alcohol Metabolism in Alcoholic Pancreatitis. Pancreas 2003, 27, 311–315. [Google Scholar] [CrossRef]
- Apte, M.V.; Wilson, J.S.; Korsten, M.A.; McCaughan, G.W.; Haber, P.S.; Pirola, R.C. Effects of ethanol and protein deficiency on pancreatic digestive and lysosomal enzymes. Gut 1995, 36, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, C.; Farías, J.; Zamorano, M.; Herrera, C. Vitamin Supplements as a Nutritional Strategy against Chronic Alcohol Consumption? An Updated Review. Antioxidants 2022, 11, 564. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, C.; Mella, L.; Godoy, K.; Adeli, K.; Farías, J. β-Carotene Increases Activity of Cytochrome P450 2E1 during Ethanol Consumption. Antioxidants 2022, 11, 1033. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, J.B.; Haigis, M.C. The multifaceted contributions of mitochondria to cellular metabolism. Nat. Cell Biol. 2018, 20, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Shalbueva, N.; Mareninova, O.A.; Gerloff, A.; Yuan, J.; Waldron, R.T.; Pandol, S.J.; Gukovskaya, A.S. Effects of oxidative alcohol metabolism on the mitochondrial permeability transition pore and necrosis in a mouse model of alcoholic pancreatitis. Gastroenterology 2013, 144, 437–446.e6. [Google Scholar] [CrossRef]
- Stolz, A.; Ernst, A.; Dikic, I. Cargo recognition and trafficking in selective autophagy. Nat. Cell Biol. 2014, 16, 495–501. [Google Scholar] [CrossRef]
- Yuan, J.; Liu, Y.; Tan, T.; Guha, S.; Gukovsky, I.; Gukovskaya, A.; Pandol, S.J. Protein kinase d regulates cell death pathways in experimental pancreatitis. Front. Physiol. 2012, 3, 60. [Google Scholar] [CrossRef]
- Fortunato, F.; Bürgers, H.; Bergmann, F.; Rieger, P.; Büchler, M.W.; Kroemer, G.; Werner, J. Impaired autolysosome formation correlates with Lamp-2 depletion: Role of apoptosis, autophagy, and necrosis in pancreatitis. Gastroenterology 2009, 137, 350–360. [Google Scholar] [CrossRef]
- Mareninova, O.A.; Sendler, M.; Malla, S.R.; Yakubov, I.; French, S.W.; Tokhtaeva, E.; Vagin, O.; Oorschot, V.; Lüllmann-Rauch, R.; Blanz, J.; et al. Lysosome associated membrane proteins maintain pancreatic acinar cell homeostasis: LAMP-2 deficient mice develop pancreatitis. Cell. Mol. Gastroenterol. Hepatol. 2015, 1, 678–694. [Google Scholar] [CrossRef]
- Cosen-Binker, L.I.; Lam, P.P.; Binker, M.G.; Reeve, J.; Pandol, S.; Gaisano, H.Y. Alcohol/cholecystokinin-evoked pancreatic acinar basolateral exocytosis is mediated by protein kinase C α phosphorylation of Munc18c. J. Biol. Chem. 2007, 282, 13047–13058. [Google Scholar] [CrossRef]
- Lerch, M.M.; Gorelick, F.S. Models of acute and chronic pancreatitis. Gastroenterology 2013, 144, 1180–1193. [Google Scholar] [CrossRef] [PubMed]
- Sendler, M.; Dummer, A.; Weiss, F.U.; Krüger, B.; Wartmann, T.; Scharffetter-Kochanek, K.; van Rooijen, N.; Malla, S.R.; Aghdassi, A.; Halangk, W.; et al. Tumour necrosis factor α secretion induces protease activation and acinar cell necrosis in acute experimental pancreatitis in mice. Gut 2013, 62, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Maisonneuve, P.; Lowenfels, A.B.; Müllhaupt, B.; Cavallini, G.; Lankisch, P.G.; Andersen, J.R.; Dimagno, E.P.; Andrén-Sandberg, A.; Domellöf, L.; Frulloni, L.; et al. Cigarette smoking accelerates progression of alcoholic chronic pancreatitis. Gut 2005, 54, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Ammann, R.W. The natural history of alcoholic chronic pancreatitis. Intern. Med. 2001, 40, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Etemad, B.; Whitcomb, D.C. Chronic pancreatitis: Diagnosis, classification, and new genetic developments. Gastroenterology 2001, 120, 682–707. [Google Scholar] [CrossRef] [PubMed]
- Sáez, J.; Martínez, J.; Trigo, C.; Sánchez-Payá, J.; Compañy, L.; Laveda, R.; Griñó, P.; García, C.; Pérez-Mateo, M. Clinical value of rapid urine trypsinogen-2 test strip, urinary trypsinogen activation peptide, and serum and urinary activation peptide of carboxypeptidase B in acute pancreatitis. World J. Gastroenterol. 2005, 11, 7261–7265. [Google Scholar] [CrossRef]
- Ismail, O.Z.; Bhayana, V. Lipase or amylase for the diagnosis of acute pancreatitis? Clin. Biochem. 2017, 50, 1275–1280. [Google Scholar] [CrossRef]
- Matull, W.R.; Pereira, S.P.; O’Donohue, J.W. Biochemical markers of acute pancreatitis. J. Clin. Pathol. 2006, 59, 340–344. [Google Scholar] [CrossRef]
- Chan, K.S.; Shelat, V.G. Diagnosis, severity stratification and management of adult acute pancreatitis-current evidence and controversies. World J. Gastrointest. Surg. 2022, 14, 1179–1197. [Google Scholar] [CrossRef]
- Forsmark, C.E.; Baillie, J.; AGA Institute Clinical Practice and Economics Committee; AGA Institute Governing Board. AGA Institute technical review on acute pancreatitis. Gastroenterology 2007, 132, 2022–2044. [Google Scholar] [CrossRef]
- Ekka, N.M.; Kujur, A.D.; Guria, R.; Mundu, M.; Mishra, B.; Sekhar, S.; Kumar, A.; Prakash, J.; Birua, H. Serum Lipase Amylase Ratio as an Indicator to Differentiate Alcoholic From Non-alcoholic Acute Pancreatitis: A Systematic Review and Meta-Analysis. Cureus 2023, 15, e35618. [Google Scholar] [CrossRef] [PubMed]
- Gumaste, V.V.; Dave, P.B.; Weissman, D.; Messer, J. Lipase/amylase ratio. A new index that distinguishes acute episodes of alcoholic from nonalcoholic acute pancreatitis. Gastroenterology 1991, 101, 1361–1366. [Google Scholar] [CrossRef] [PubMed]
- Esposito, I.; Hruban, R.H.; Verbeke, C.; Terris, B.; Zamboni, G.; Scarpa, A.; Morohoshi, T.; Suda, K.; Luchini, C.; Klimstra, D.S.; et al. Working group for the International (IAP—APA—JPS—EPC) Consensus Guidelines for Chronic Pancreatitis. Guidelines on the histopathology of chronic pancreatitis. Recommendations from the working group for the international consensus guidelines for chronic pancreatitis in collaboration with the International Association of Pancreatology, the American Pancreatic Association, the Japan Pancreas Society, and the European Pancreatic Club. Pancreatology 2020, 20, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Haber, P.S.; Apte, M.V.; Moran, C.; Applegate, T.L.; Pirola, R.C.; Korsten, M.A.; McCaughan, G.W.; Wilson, J.S. Non-oxidative metabolism of ethanol by rat pancreatic acini. Pancreatology 2004, 4, 82–89. [Google Scholar] [CrossRef]
- Haber, P.S.; Apte, M.V.; Applegate, T.L.; Norton, I.D.; Korsten, M.A.; Pirola, R.C.; Wilson, J.S. Metabolism of ethanol by rat pancreatic acinar cells. J. Lab. Clin. Med. 1998, 132, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Gukovskaya, A.S.; Mouria, M.; Gukovsky, I.; Reyes, C.N.; Kasho, V.N.; Faller, L.D.; Pandol, S.J. Ethanol metabolism and transcription factor activation in pancreatic acinar cells in rats. Gastroenterology 2002, 122, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Vonlaufen, A.; Wilson, J.S.; Pirola, R.C.; Apte, M.V. Role of alcohol metabolism in chronic pancreatitis. Alcohol Res. Health 2007, 30, 48–54. [Google Scholar]
- Panes, J.; Caballeria, J.; Guitart, R.; Pares, A.; Soler, X.; Rodamilans, M.; Navasa, M.; Pares, X.; Bosch, J.; Rodes, J. Determinants of ethanol and acetaldehyde metabolism in chronic alcoholics. Alcohol. Clin. Exp. Res. 1993, 17, 48–53. [Google Scholar] [CrossRef]
- Sandoval, C.; Vásquez, B.; Vasconcellos, A.; Souza-Mello, V.; Adeli, K.; Mandarim-De-Lacerda, C.; del Sol, M. Oral supplementation of β-carotene benefits the hepatic structure and metabolism in mice exposed to chronic ethanol consumption. Sains Malays. 2022, 51, 285–296. [Google Scholar] [CrossRef]
- Vidal, F.; Toda, R.; Gutiérrez, C.; Broch, M.; Fernández-Muixí, F.; Lorenzo, A.; Richart, C. Influence of chronic alcohol abuse and liver disease on hepatic aldehyde dehydrogenase activity. Alcohol 1998, 15, 3–8. [Google Scholar] [CrossRef]
- Ammann, R.W.; Heitz, P.U.; Kloppel, G. Course of alcoholic chronic pancreatitis: A prospective clinicomorphological long-term study. Gastroenterology 1996, 111, 224–231. [Google Scholar] [CrossRef] [PubMed]
- DiMagno, M.J. Oktoberfest binge drinking and acute pancreatitis: Is there really no relationship? Clin. Gastroenterol. Hepatol. 2011, 9, 920–922. [Google Scholar] [CrossRef] [PubMed]
- Sozio, M.; Crabb, D.W. Alcohol and lipid metabolism. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E10–E16. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, C.; Vásquez, B.; Souza-Mello, V.; Adeli, K.; Mandarim-de-Lacerda, C.; del Sol, M. Morphoquantitative effects of oral β-carotene supplementation on liver of C57BL/6 mice exposed to ethanol consumption. Int. J. Clin. Exp. Pathol. 2019, 12, 1713–1722. [Google Scholar] [PubMed]
- Yang, B.C.; Wu, S.Y.; Leung, P.S. Alcohol ingestion induces pancreatic islet dysfunction and apoptosis via mediation of FGF21 resistance. Ann. Transl. Med. 2020, 8, 310. [Google Scholar] [CrossRef]
- Hausmann, S.; Kong, B.; Michalski, C.; Erkan, M.; Friess, H. The Role of Inflammation in Pancreatic Cancer. In Inflammation and Cancer; Aggarwal, B.B., Sung, B., Gupta, S.C., Eds.; Springer: Basel, Switzerland, 2014; pp. 129–151. [Google Scholar]
- Zhang, J.; Fan, H.; Gross, M.; Liu, N.; Carlson, H.; Wood, A.; Hoffman, K.; Petrosino, J.; Pankratz, N.; Thyagarajan, B.; et al. Progressive reduction in circulating levels of carotenoids and other micronutrients in patients with chronic pancreatitis. Pancreatology 2022, 22, 1126–1133. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jiang, W.; Shao, L.; Zhong, D.; Wu, Y.; Cai, J. Association between intake of antioxidants pancreatic cancer risk: A meta-analysis. Int. J. Food Sci. Nutr. 2016, 67, 744. [Google Scholar] [CrossRef]
- Curran, F.J.; Sattar, N.; Talwar, D.; Baxter, J.N.; Imrie, C.W. Relationship of carotenoid and vitamins A and E with the acute inflammatory response in acute pancreatitis. Br. J. Surg. 2000, 87, 301–305. [Google Scholar] [CrossRef]
- Lavy, A.; Karban, A.; Suissa, A.; Yassin, K.; Hermesh, I.; Ben-Amotz, A. Natural beta-carotene for the prevention of post-ERCP pancreatitis. Pancreas 2004, 29, e45–e50. [Google Scholar] [CrossRef]
- Pham, A.; Forsmark, C. Chronic pancreatitis: Review and update of etiology, risk factors, and management. F1000Research 2018, 7, F1000. [Google Scholar] [CrossRef]
- Xu, M.; Cai, J.; Wei, H.; Zhou, M.; Xu, P.; Huang, H.; Peng, W.; Du, F.; Gong, A.; Zhang, Y. Scoparone protects against pancreatic fibrosis via TGF-β/Smad signaling in rats. Cell. Physiol. Biochem. 2016, 40, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Tsang, S.W.; Zhang, H.J.; Chen, Y.G.; Auyeung, K.K.; Bian, Z.X. Eruberin A, a natural flavanol glycoside, exerts anti-fibrotic action on pancreatic stellate cells. Cell. Physiol. Biochem. 2015, 36, 2433–2446. [Google Scholar] [CrossRef]
- Jiang, F.; Liao, Z.; Hu, L.H.; Du, Y.Q.; Man, X.H.; Gu, J.J.; Gao, J.; Gong, Y.F.; Li, Z.S. Comparison of antioxidative and antifibrotic effects of alpha-tocopherol with those of tocotrienol-rich fraction in a rat model of chronic pancreatitis. Pancreas 2011, 40, 1091–1096. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Fang, Y.Z.; Yang, S.; Lupton, J.R.; Turner, N.D. Glutathione metabolism and its implications for health. J. Nutr. 2004, 134, 489–492. [Google Scholar] [CrossRef]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C.J. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef]
- Committee for the update of the guide for the care and use of laboratory animals, institute for laboratory animal research, division on earth and life studies, national research council. In Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies Press: Washington, DC, USA, 2011.
- Furuya, D.T.; Binsack, R.; Machado, U.F. Low ethanol consumption increases insulin sensitivity in Wistar rats. Braz. J. Med. Biol. Res. 2003, 36, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.C.; Chen, Y.L.; Yang, S.Y.; Ho, P.Y.; Yang, S.S.; Hu, J.T.; Yang, S.C. The antiapoptotic effects of different doses of β-carotene in chronic ethanol-fed rats. Hepatobiliary Surg. Nutr. 2013, 2, 132–141. [Google Scholar] [CrossRef]
- Diao, Y.; Nie, J.; Tan, P.; Zhao, Y.; Zhao, T.; Tu, J.; Ji, H.; Cao, Y.; Wu, Z.; Liang, H.; et al. Long-term low-dose ethanol intake improves healthspan and resists high-fat diet-induced obesity in mice. Aging 2020, 12, 13128–13146. [Google Scholar] [CrossRef]
- Scherle, W. A simple method for volumetry of organs in quantitative stereology. Mikroskopie 1970, 26, 57–60. [Google Scholar]
- Mandarim-de-Lacerda, C.A.; del Sol, M. Tips for studies with quantitative morphology (morphometry and stereology). Int. J. Morphol. 2017, 35, 1482–1494. [Google Scholar] [CrossRef]
- Gundersen, H.J. Stereology of arbitrary particles. A review of unbiased number and size estimators and the presentation of some new ones, in memory of William R. Thompson. J. Microsc. 1986, 143 Pt 1, 3–45. [Google Scholar] [CrossRef] [PubMed]
| Media ± SD | |||||||
|---|---|---|---|---|---|---|---|
| C | LA | MA | B | LA + B | MA + B | p | |
| Lipase (U/L) | 51.315 ± 7.230 | 43.363 ± 3.377 a | 55.975 ± 14.098 | 65.383 ± 6.679 ab | 64.315 ± 9.555 ab | 47.139 ± 6.099 de | 0.002 |
| Uric acid (μmol/L) | 99.511 ± 29.729 | 124.666 ± 24.118 | 59.983 ± 18.445 ab | 104.487 ± 28.909 c | 91.219 ± 26.863 bc | 113.609 ± 14.520 ce | <0.001 |
| Amylase (U/L) | 658.420 ± 195.988 | 705.070 ± 95.701 | 712.886 ± 134.047 | 709.438 ± 125.675 | 735.407 ± 123.837 | 825.953 ± 193.412 | 0.155 |
| Lipase/Amylase ratio | 0.081 ± 0.018 | 0.062 ± 0.011 | 0.085 ± 0.041 | 0.096 ± 0.027 b | 0.096 ± 0.021 b | 0.058 ± 0.011 de | <0.001 |
| Parameters | Score | Fibrosis Grade | Group Frequency (%) | |||||
|---|---|---|---|---|---|---|---|---|
| C | LA | MA | B | LA + B | MA + B | |||
| Peri-lobular parenchyma | 1 | Mild | 80 | 0 | 0 | 16 | 0 | 20 |
| 2 | Moderate | 20 | 0 | 0 | 76 | 0 | 76 | |
| 3 | Marked | 0 | 80 | 0 | 8 | 20 | 4 | |
| 4 | Mild | 0 | 20 | 0 | 0 | 64 | 0 | |
| 5 | Moderate | 0 | 0 | 72 | 0 | 16 | 0 | |
| 6 | Marked | 0 | 0 | 28 | 0 | 0 | 0 | |
| Intralobular parenchyma | 1 | Mild | 84 | 0 | 0 | 20 | 0 | 16 |
| 2 | Moderate | 16 | 0 | 0 | 68 | 0 | 64 | |
| 3 | Marked | 0 | 76 | 0 | 12 | 20 | 20 | |
| 4 | Mild | 0 | 24 | 0 | 0 | 52 | 0 | |
| 5 | Moderate | 0 | 0 | 76 | 0 | 28 | 0 | |
| 6 | Marked | 0 | 0 | 24 | 0 | 0 | 0 | |
| Media ± SD | |||||||
|---|---|---|---|---|---|---|---|
| C | LA | MA | B | LA + B | MA + B | p | |
| Peri-lobular parenchyma | 1.200 ± 0.408 | 3.200 ± 0.408 a | 5.280 ± 0.458 ab | 1.920 ± 0.493 abc | 3.960 ± 0.611 abcd | 1.840 ± 0.472 abce | <0.001 |
| Intralobular parenchyma | 1.160 ± 0.374 | 3.240 ± 0.435 a | 5.240 ± 0.435 ab | 1.920 ± 0.571 abc | 4.080 ± 0.702 abcd | 2.040 ± 0.611 abce | <0.001 |
| Score | 2.360 ± 0.568 | 6.440 ± 0.711 a | 10.520 ± 0.714 ab | 3.840 ± 0.850 abc | 8.040 ± 1.01 abcd | 3.880 ± 0.781 abce | <0.001 |
| Media ± SD | |||||||
|---|---|---|---|---|---|---|---|
| C | LA | MA | B | LA + B | MA + B | p | |
| NV islets (mm−3) | 429.183 ± 52.588 | 604.177 ± 20.168 a | 651.080 ± 51.059 a | 672.110 ± 85.790 a | 715.472 ± 69.053 a | 707.522 ± 31.115 a | <0.001 |
| VV islets (%) | 23.053 ± 7.539 | 28.307 ± 7.251 | 36.167 ± 8.927 a | 27.595 ± 2.455 | 28.547 ± 4.488 | 38.386 ± 8.445 a | 0.001 |
| SV islets (mm−1) | 7.011 ± 0.219 | 9.626 ± 1.093 | 10.077 ± 1.122 | 9.370 ± 3.840 | 9.370 ± 3.511 | 9.182 ± 6.373 | 0.144 |
| TM islets | 3.270 ± 0.028 | 4.092 ± 0.052 | 6.862 ± 0.639 ab | 6.208 ± 0.701 a | 4.984 ± 0.460 | 8.398 ± 0.633 abd | <0.001 |
| NV acinar cells (mm−3) | 2476.634 ± 854.875 | 2529.968 ± 890.316 | 3030.235 ± 283.025 | 2668.711 ± 907.537 | 2710.377 ± 466.376 | 2296.665 ± 756.358 | 0.245 |
| VV acinar cells (%) | 4.872 ± 1.052 | 5.228 ± 1.988 | 4.537 ± 0.295 | 3.904 ± 0.267 b | 5.014 ± 0.340 | 5.452 ± 0.380 c | 0.014 |
| SV acinar cells (mm−1) | 15.266 ± 6.842 | 15.994 ± 2.595 | 13.589 ± 3.246 | 12.043 ± 3.546 b | 13.698 ± 1.666 | 14.439 ± 1.017 | 0.014 |
| Score | Fibrosis Grade | Peri-Lobular Parenchyma | Intralobular Parenchyma |
|---|---|---|---|
| 1 | Mild | Lobules are separated by fibrous tissue without any changes in structure or atrophy. | Thin fibrous threads that separate the acini within the lobules, but without any substantial changes to the overall structure. |
| 2 | Moderate | Lobules are separated by fibrous tissue with changes in structure or atrophy (between 0 and 20%). | Fibrous threads that separate the acini within the lobules, with substantial changes to the overall structure (between 0 and 20%). |
| 3 | Marked | Lobules are separated by fibrous tissue with changes in structure or atrophy (between 20 and 40%). | Fibrous threads that separate the acini within the lobules, with substantial changes to the overall structure (between 20 and 40%). |
| 4 | Mild | Lobules are separated by fibrous tissue with changes in structure or atrophy (between 40 and 60%). | Fibrous threads that separate the acini within the lobules, with substantial changes to the overall structure (between 40 and 60%). |
| 5 | Moderate | Lobules are separated by fibrous tissue with changes in structure or atrophy (between 60 and 80%). | Fibrous threads that separate the acini within the lobules, with substantial changes to the overall structure (between 60 and 80%). |
| 6 | Marked | Lobules are separated by fibrous tissue with changes in structure or atrophy (between 80 and 100%). | Fibrous threads that separate the acini within the lobules, with substantial changes to the overall structure (between 80 and 100%). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandoval, C.; Vera, A.; Birditt, K.; Godoy, K.; Carmine, F.; Caamaño, J.; Farías, J. β-Carotene Supplementation Improves Pancreas Function during Moderate Ethanol Consumption: Initial Characterization from a Morphological Overview. Int. J. Mol. Sci. 2024, 25, 1219. https://doi.org/10.3390/ijms25021219
Sandoval C, Vera A, Birditt K, Godoy K, Carmine F, Caamaño J, Farías J. β-Carotene Supplementation Improves Pancreas Function during Moderate Ethanol Consumption: Initial Characterization from a Morphological Overview. International Journal of Molecular Sciences. 2024; 25(2):1219. https://doi.org/10.3390/ijms25021219
Chicago/Turabian StyleSandoval, Cristian, Angeles Vera, Katherine Birditt, Karina Godoy, Florencia Carmine, José Caamaño, and Jorge Farías. 2024. "β-Carotene Supplementation Improves Pancreas Function during Moderate Ethanol Consumption: Initial Characterization from a Morphological Overview" International Journal of Molecular Sciences 25, no. 2: 1219. https://doi.org/10.3390/ijms25021219
APA StyleSandoval, C., Vera, A., Birditt, K., Godoy, K., Carmine, F., Caamaño, J., & Farías, J. (2024). β-Carotene Supplementation Improves Pancreas Function during Moderate Ethanol Consumption: Initial Characterization from a Morphological Overview. International Journal of Molecular Sciences, 25(2), 1219. https://doi.org/10.3390/ijms25021219







