The Cellular Dysfunction of the Brain–Blood Barrier from Endothelial Cells to Astrocytes: The Pathway towards Neurotransmitter Impairment in Schizophrenia
Abstract
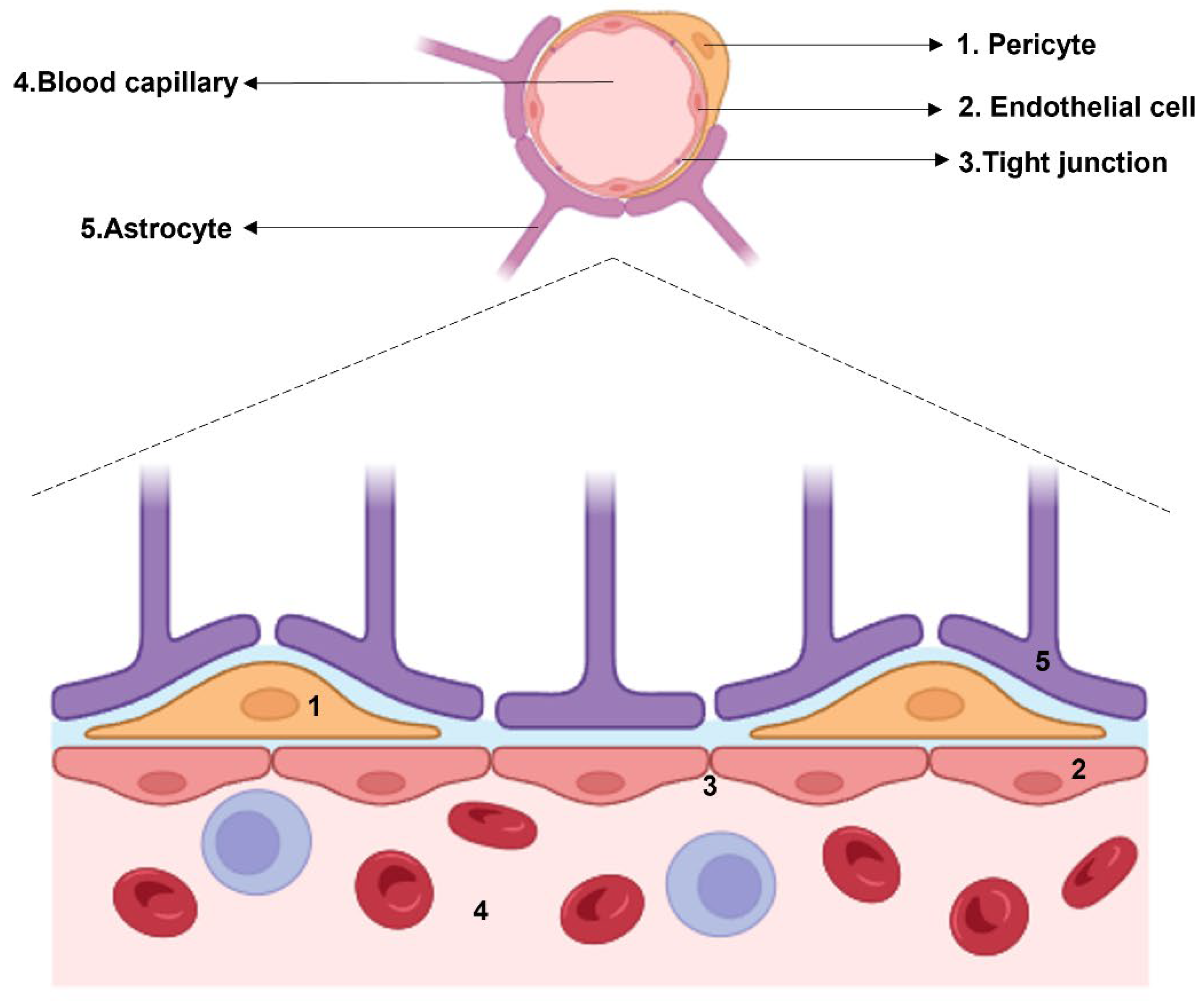
1. Introduction
2. Endothelial Cells in Schizophrenia: When the First Line of Defense Starts to Give Up
3. Pericytes: The Cellular Interface in the Crosstalk between Endothelial Cells and Astrocytes
4. Astrocytes, the Last Step before Neurons: A Cell Population between Vascularization and Cognition
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Crow, T.J. Positive and Negative Schizophrenic Symptoms and the Role of Dopamine. Br. J. Psychiatry 1980, 137, 383–386. [Google Scholar] [CrossRef]
- Buchwald, K.; Narayanan, A.; Siegert, R.J.; Vignes, M.; Arrowsmith, K.; Sandham, M. Centrality Statistics of Symptom Networks of Schizophrenia: A Systematic Review. Psychol. Med. 2024, 1–13. [Google Scholar] [CrossRef]
- Wolpe, N.; Vituri, A.; Jones, P.B.; Shahar, M.; Fernandez-Egea, E. The Longitudinal Structure of Negative Symptoms in Treatment Resistant Schizophrenia. Compr. Psychiatry 2024, 128, 152440. [Google Scholar] [CrossRef] [PubMed]
- Di Luzio, M.; Pontillo, M.; Villa, M.; Attardi, A.G.; Bellantoni, D.; Di Vincenzo, C.; Vicari, S. Clinical Features and Comorbidity in Very Early-Onset Schizophrenia: A Systematic Review. Front. Psychiatry 2023, 14, 799. [Google Scholar] [CrossRef] [PubMed]
- Kay, S.R.; Fiszbein, A.; Opler, L.A. The Positive and Negative Syndrome Scale (PANSS) for Schizophrenia. Schizophr. Bull. 1987, 13, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Stroup, T.S.; Gray, N. Management of Common Adverse Effects of Antipsychotic Medications. World Psychiatry 2018, 17, 341–356. [Google Scholar] [CrossRef] [PubMed]
- Arana, G.W. An Overview of Side Effects Caused by Typical Antipsychotics. J. Clin. Psychiatry 2000, 61 (Suppl. S8), 5–11, Discussion: J. Clin. Psychiatry 2000, 61 (Suppl. 8), 12–13.. [Google Scholar] [PubMed]
- Brisch, R.; Saniotis, A.; Wolf, R.; Bielau, H.; Bernstein, H.-G.; Steiner, J.; Bogerts, B.; Braun, A.K.; Jankowski, Z.; Kumaritlake, J.; et al. The Role of Dopamine in Schizophrenia from a Neurobiological and Evolutionary Perspective: Old Fashioned, but Still in Vogue. Front. Psychiatry 2014, 5, 47. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; MacDonald, M.L.; Elswick, D.E.; Sweet, R.A. The Glutamate Hypothesis of Schizophrenia: Evidence from Human Brain Tissue Studies. Ann. N. Y. Acad. Sci. 2015, 1338, 38–57. [Google Scholar] [CrossRef] [PubMed]
- Owen, M.J.; O’Donovan, M.C.; Thapar, A.; Craddock, N. Neurodevelopmental Hypothesis of Schizophrenia. Br. J. Psychiatry 2011, 198, 173–175. [Google Scholar] [CrossRef]
- Miller, B.J.; Goldsmith, D.R. Evaluating the Hypothesis That Schizophrenia Is an Inflammatory Disorder. Focus 2020, 18, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Stojanovic, A.; Martorell, L.; Montalvo, I.; Ortega, L.; Monseny, R.; Vilella, E.; Labad, J. Increased Serum Interleukin-6 Levels in Early Stages of Psychosis: Associations with at-Risk Mental States and the Severity of Psychotic Symptoms. Psychoneuroendocrinology 2014, 41, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, D.R.; Haroon, E.; Miller, A.H.; Strauss, G.P.; Buckley, P.F.; Miller, B.J. TNF-α and IL-6 Are Associated with the Deficit Syndrome and Negative Symptoms in Patients with Chronic Schizophrenia. Schizophr. Res. 2018, 199, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Müller, N.; Weidinger, E.; Leitner, B.; Schwarz, M.J. The Role of Inflammation in Schizophrenia. Front. Neurosci. 2015, 9, 372. [Google Scholar] [CrossRef]
- Murphy, C.E.; Walker, A.K.; Weickert, C.S. Neuroinflammation in Schizophrenia: The Role of Nuclear Factor Kappa B. Transl. Psychiatry 2021, 11, 528. [Google Scholar] [CrossRef] [PubMed]
- Vallée, A. Neuroinflammation in Schizophrenia: The Key Role of the WNT/β-Catenin Pathway. Int. J. Mol. Sci. 2022, 23, 2810. [Google Scholar] [CrossRef] [PubMed]
- Herberth, M.; Rahmoune, H.; Schwarz, E.; Koethe, D.; Harris, L.W.; Kranaster, L.; Witt, S.H.; Spain, M.; Barnes, A.; Schmolz, M.; et al. Identification of a Molecular Profile Associated with Immune Status in First-Onset Schizophrenia Patients. Clin. Schizophr. Relat. Psychoses 2014, 7, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Fond, G.; Godin, O.; Boyer, L.; Berna, F.; Andrianarisoa, M.; Coulon, N.; Brunel, L.; Bulzacka, E.; Aouizerate, B.; Capdevielle, D.; et al. Chronic Low-Grade Peripheral Inflammation Is Associated with Ultra Resistant Schizophrenia. Results from the FACE-SZ Cohort. Eur. Arch. Psychiatry Clin. Neurosci. 2019, 269, 985–992. [Google Scholar] [CrossRef]
- Comer, A.L.; Carrier, M.; Tremblay, M.-È.; Cruz-Martín, A. The Inflamed Brain in Schizophrenia: The Convergence of Genetic and Environmental Risk Factors That Lead to Uncontrolled Neuroinflammation. Front. Cell Neurosci. 2020, 14, 274. [Google Scholar] [CrossRef]
- Fond, G.; Lançon, C.; Korchia, T.; Auquier, P.; Boyer, L. The Role of Inflammation in the Treatment of Schizophrenia. Front. Psychiatry 2020, 11, 160. [Google Scholar] [CrossRef]
- Daneman, R.; Prat, A. The Blood–Brain Barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef] [PubMed]
- Akaishi, T.; Narikawa, K.; Suzuki, Y.; Mitsuzawa, S.; Tsukita, K.; Kuroda, H.; Nakashima, I.; Fujihara, K.; Aoki, M. Importance of the Quotient of Albumin, Quotient of Immunoglobulin G and Reibergram in Inflammatory Neurological Disorders with Disease-specific Patterns of Blood–Brain Barrier Permeability. Neurol. Clin. Neurosci. 2015, 3, 94–100. [Google Scholar] [CrossRef]
- Daneman, R. The Blood–Brain Barrier in Health and Disease. Ann. Neurol. 2012, 72, 648–672. [Google Scholar] [CrossRef] [PubMed]
- Kadry, H.; Noorani, B.; Cucullo, L. A Blood–Brain Barrier Overview on Structure, Function, Impairment, and Biomarkers of Integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef]
- Bechmann, I.; Galea, I.; Perry, V.H. What Is the Blood–Brain Barrier (Not)? Trends Immunol. 2007, 28, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Patabendige, A.A.K.; Dolman, D.E.M.; Yusof, S.R.; Begley, D.J. Structure and Function of the Blood–Brain Barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, B.; Liebner, S. Novel Insights into the Development and Maintenance of the Blood–Brain Barrier. Cell Tissue Res. 2014, 355, 687–699. [Google Scholar] [CrossRef]
- Dyrna, F.; Hanske, S.; Krueger, M.; Bechmann, I. The Blood-Brain Barrier. J. Neuroimmune Pharmacol. 2013, 8, 763–773. [Google Scholar] [CrossRef]
- Bentivoglio, M.; Kristensson, K. Tryps and Trips: Cell Trafficking across the 100-Year-Old Blood–Brain Barrier. Trends Neurosci. 2014, 37, 325–333. [Google Scholar] [CrossRef]
- Lewandowsky, M. Zur Lehre Der Cerebrospinalflussigkeit. Z. Klin. Med. 1909, 40, 480–494. [Google Scholar]
- Alluri, H.; Peddaboina, C.S.; Tharakan, B. Evaluation of Tight Junction Integrity in Brain Endothelial Cells Using Confocal Microscopy. In Vascular Hyperpermeability: Methods and Protocols; Springer: New York, NY, USA, 2024; pp. 257–262. [Google Scholar]
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte–Endothelial Interactions at the Blood–Brain Barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Friedman, A.; Kaufer, D. Blood-Brain Barrier Breakdown and Blood-Brain Communication in Neurological and Psychiatric Diseases. Cardiovasc. Psychiatry Neurol. 2011, 2011, 431470. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C. The Neurovascular Unit Coming of Age: A Journey through Neurovascular Coupling in Health and Disease. Neuron 2017, 96, 17–42. [Google Scholar] [CrossRef] [PubMed]
- Abdullahi, W.; Tripathi, D.; Ronaldson, P.T. Blood-Brain Barrier Dysfunction in Ischemic Stroke: Targeting Tight Junctions and Transporters for Vascular Protection. Am. J. Physiol.-Cell Physiol. 2018, 315, C343–C356. [Google Scholar] [CrossRef]
- Stamatovic, S.M.; Johnson, A.M.; Keep, R.F.; Andjelkovic, A. V Junctional Proteins of the Blood-Brain Barrier: New Insights into Function and Dysfunction. Tissue Barriers 2016, 4, e1154641. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuki, S.; Yamaguchi, H.; Katsukura, Y.; Asashima, T.; Terasaki, T. MRNA Expression Levels of Tight Junction Protein Genes in Mouse Brain Capillary Endothelial Cells Highly Purified by Magnetic Cell Sorting. J. Neurochem. 2008, 104, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Zihni, C.; Mills, C.; Matter, K.; Balda, M.S. Tight Junctions: From Simple Barriers to Multifunctional Molecular Gates. Nat. Rev. Mol. Cell Biol. 2016, 17, 564–580. [Google Scholar] [CrossRef]
- Martìn-Padura, I.; Lostaglio, S.; Schneemann, M.; Williams, L.; Romano, M.; Fruscella, P.; Panzeri, C.; Stoppacciaro, A.; Ruco, L.; Villa, A.; et al. Junctional Adhesion Molecule, a Novel Member of the Immunoglobulin Superfamily That Distributes at Intercellular Junctions and Modulates Monocyte Transmigration. J. Cell Biol. 1998, 142, 117–127. [Google Scholar] [CrossRef]
- Poliak, S.; Matlis, S.; Ullmer, C.; Scherer, S.S.; Peles, E. Distinct Claudins and Associated PDZ Proteins Form Different Autotypic Tight Junctions in Myelinating Schwann Cells. J. Cell Biol. 2002, 159, 361–372. [Google Scholar] [CrossRef]
- Kaya, M.; Ahishali, B. Basic Physiology of the Blood-Brain Barrier in Health and Disease: A Brief Overview. Tissue Barriers 2021, 9, 1840913. [Google Scholar] [CrossRef]
- Yuan, S.; Liu, K.; Qi, Z. Occludin Regulation of Blood–Brain Barrier and Potential Therapeutic Target in Ischemic Stroke. Brain Circ. 2020, 6, 152. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Teng, T.; Li, R.; Simonyi, A.; Sun, G.Y.; Lee, J.C. TNFα Alters Occludin and Cerebral Endothelial Permeability: Role of P38MAPK. PLoS ONE 2017, 12, e0170346. [Google Scholar] [CrossRef] [PubMed]
- Utech, M.; Bruewer, M.; Parkos, C.; Hopkins, A.; Ivanov, A.; Nusrat, A. IFN-Gamma Induces Endocytosis of Epithelial Tight Junction Transmembrane Proteins into a Vacuolar Apical Compartment. J. Am. Coll. Surg. 2004, 199, 20. [Google Scholar] [CrossRef]
- Ni, C.; Wang, C.; Zhang, J.; Qu, L.; Liu, X.; Lu, Y.; Yang, W.; Deng, J.; Lorenz, D.; Gao, P.; et al. Interferon-γ Safeguards Blood-Brain Barrier during Experimental Autoimmune Encephalomyelitis. Am. J. Pathol. 2014, 184, 3308–3320. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jin, S.; Sonobe, Y.; Cheng, Y.; Horiuchi, H.; Parajuli, B.; Kawanokuchi, J.; Mizuno, T.; Takeuchi, H.; Suzumura, A. Interleukin-1β Induces Blood–Brain Barrier Disruption by Downregulating Sonic Hedgehog in Astrocytes. PLoS ONE 2014, 9, e110024. [Google Scholar] [CrossRef]
- Hartmann, C.; Schwietzer, Y.A.; Otani, T.; Furuse, M.; Ebnet, K. Physiological Functions of Junctional Adhesion Molecules (JAMs) in Tight Junctions. Biochim. Biophys. Acta (BBA)—Biomembr. 2020, 1862, 183299. [Google Scholar] [CrossRef]
- Worzfeld, T.; Schwaninger, M. Apicobasal Polarity of Brain Endothelial Cells. J. Cereb. Blood Flow Metab. 2016, 36, 340–362. [Google Scholar] [CrossRef]
- Cruz-Orengo, L.; Daniels, B.P.; Dorsey, D.; Basak, S.A.; Grajales-Reyes, J.G.; McCandless, E.E.; Piccio, L.; Schmidt, R.E.; Cross, A.H.; Crosby, S.D.; et al. Enhanced Sphingosine-1-Phosphate Receptor 2 Expression Underlies Female CNS Autoimmunity Susceptibility. J. Clin. Investig. 2014, 124, 2571–2584. [Google Scholar] [CrossRef]
- Uranova, N.A.; Vikhreva, O.V.; Rachmanova, V.I.; Orlovskaya, D.D. Ultrastructural Alterations of Myelinated Fibers and Oligodendrocytes in the Prefrontal Cortex in Schizophrenia: A Postmortem Morphometric Study. Schizophr. Res. Treat. 2011, 2011, 325789. [Google Scholar] [CrossRef]
- De Picker, L.J.; Victoriano, G.M.; Richards, R.; Gorvett, A.J.; Lyons, S.; Buckland, G.R.; Tofani, T.; Norman, J.L.; Chatelet, D.S.; Nicoll, J.A.R.; et al. Immune Environment of the Brain in Schizophrenia and during the Psychotic Episode: A Human Post-Mortem Study. Brain Behav. Immun. 2021, 97, 319–327. [Google Scholar] [CrossRef]
- Cai, H.Q.; Weickert, T.W.; Catts, V.S.; Balzan, R.; Galletly, C.; Liu, D.; O’Donnell, M.; Shannon Weickert, C. Altered Levels of Immune Cell Adhesion Molecules Are Associated with Memory Impairment in Schizophrenia and Healthy Controls. Brain Behav. Immun. 2020, 89, 200–208. [Google Scholar] [CrossRef]
- Weickert, T.; Cai, H.; O’Donnell, M.; Balzan, R.; Wells, R.; Liu, D.; Galletly, C.; Weickert, C.S. O1.5. ICAM-1 is increased in brain and peripheral levels of soluble ICAM-1 is related to cognitive deficits in schizophrenia. Schizophr. Bull. 2018, 44, S73–S74. [Google Scholar] [CrossRef]
- Nakagawa, S.; Deli, M.A.; Kawaguchi, H.; Shimizudani, T.; Shimono, T.; Kittel, Á.; Tanaka, K.; Niwa, M. A New Blood–Brain Barrier Model Using Primary Rat Brain Endothelial Cells, Pericytes and Astrocytes. Neurochem. Int. 2009, 54, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Armulik, A.; Genové, G.; Mäe, M.; Nisancioglu, M.H.; Wallgard, E.; Niaudet, C.; He, L.; Norlin, J.; Lindblom, P.; Strittmatter, K.; et al. Pericytes Regulate the Blood–Brain Barrier. Nature 2010, 468, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Lacombe, R.J.S.; Chouinard-Watkins, R.; Bazinet, R.P. Brain Docosahexaenoic Acid Uptake and Metabolism. Mol. Asp. Med. 2018, 64, 109–134. [Google Scholar] [CrossRef] [PubMed]
- Ahishali, B.; Kaya, M. Evaluation of Blood-Brain Barrier Integrity Using Vascular Permeability Markers: Evans Blue, Sodium Fluorescein, Albumin-Alexa Fluor Conjugates, and Horseradish Peroxidase. In Permeability Barrier: Methods and Protocols; Humana: New York, NY, USA, 2020; pp. 87–103. [Google Scholar]
- Sharma, K.; Zhang, Y.; Paudel, K.R.; Kachelmeier, A.; Hansbro, P.M.; Shi, X. The Emerging Role of Pericyte-Derived Extracellular Vesicles in Vascular and Neurological Health. Cells 2022, 11, 3108. [Google Scholar] [CrossRef] [PubMed]
- Najjar, S.; Pahlajani, S.; De Sanctis, V.; Stern, J.N.H.; Najjar, A.; Chong, D. Neurovascular Unit Dysfunction and Blood–Brain Barrier Hyperpermeability Contribute to Schizophrenia Neurobiology: A Theoretical Integration of Clinical and Experimental Evidence. Front. Psychiatry 2017, 8, 83. [Google Scholar] [CrossRef] [PubMed]
- Wilson, L.; Szigeti, A.; Kearney, A.; Clarke, M. Clinical Characteristics of Primary Psychotic Disorders with Concurrent Substance Abuse and Substance-Induced Psychotic Disorders: A Systematic Review. Schizophr. Res. 2018, 197, 78–86. [Google Scholar] [CrossRef]
- Misiak, B.; Stramecki, F.; Gawęda, Ł.; Prochwicz, K.; Sąsiadek, M.M.; Moustafa, A.A.; Frydecka, D. Interactions Between Variation in Candidate Genes and Environmental Factors in the Etiology of Schizophrenia and Bipolar Disorder: A Systematic Review. Mol. Neurobiol. 2018, 55, 5075–5100. [Google Scholar] [CrossRef]
- Khandaker, G.M.; Cousins, L.; Deakin, J.; Lennox, B.R.; Yolken, R.; Jones, P.B. Inflammation and Immunity in Schizophrenia: Implications for Pathophysiology and Treatment. Lancet Psychiatry 2015, 2, 258–270. [Google Scholar] [CrossRef]
- Ermakov, E.A.; Melamud, M.M.; Buneva, V.N.; Ivanova, S.A. Immune System Abnormalities in Schizophrenia: An Integrative View and Translational Perspectives. Front. Psychiatry 2022, 13, 568. [Google Scholar] [CrossRef] [PubMed]
- Najjar, S.; Pearlman, D.M.; Alper, K.; Najjar, A.; Devinsky, O. Neuroinflammation and Psychiatric Illness. J. Neuroinflamm. 2013, 10, 816. [Google Scholar] [CrossRef] [PubMed]
- Borovcanin, M.M.; Jovanovic, I.; Radosavljevic, G.; Pantic, J.; Minic Janicijevic, S.; Arsenijevic, N.; Lukic, M.L. Interleukin-6 in Schizophrenia—Is There a Therapeutic Relevance? Front. Psychiatry 2017, 8, 221. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro-Santos, R.; de Campos-Carli, S.M.; Ferretjans, R.; Teixeira-Carvalho, A.; Martins-Filho, O.A.; Teixeira, A.L.; Salgado, J.V. The Association of Cognitive Performance and IL-6 Levels in Schizophrenia Is Influenced by Age and Antipsychotic Treatment. Nord. J. Psychiatry 2020, 74, 187–193. [Google Scholar] [CrossRef]
- Patlola, S.R.; Donohoe, G.; McKernan, D.P. The Relationship between Inflammatory Biomarkers and Cognitive Dysfunction in Patients with Schizophrenia: A Systematic Review and Meta-Analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry 2023, 121, 110668. [Google Scholar] [CrossRef]
- Efffendy, E.; Amin, M.M.; Utami, N. Role of Tumor Necrosis Factor-Alpha in Schizophrenia and Cognitive Impairment. Open Access Maced. J. Med. Sci. 2021, 9, 160–163. [Google Scholar] [CrossRef]
- Pollak, T.A.; Drndarski, S.; Stone, J.M.; David, A.S.; McGuire, P.; Abbott, N.J. The Blood–Brain Barrier in Psychosis. Lancet Psychiatry 2018, 5, 79–92. [Google Scholar] [CrossRef]
- Scholz, C.; Jacob, C.P.; Buttenschon, H.N.; Kittel-Schneider, S.; Boreatti-Hümmer, A.; Zimmer, M.; Walter, U.; Lesch, K.; Mors, O.; Kneitz, S.; et al. Functional Variants of TSPAN8 Are Associated with Bipolar Disorder and Schizophrenia. Am. J. Med. Genet. Part. B Neuropsychiatr. Genet. 2010, 153B, 967–972. [Google Scholar] [CrossRef]
- Xia, X.; Wang, Y.; Huang, Y.; Zhang, H.; Lu, H.; Zheng, J.C. Exosomal MiRNAs in Central Nervous System Diseases: Biomarkers, Pathological Mediators, Protective Factors and Therapeutic Agents. Prog. Neurobiol. 2019, 183, 101694. [Google Scholar] [CrossRef]
- Mingardi, J.; La Via, L.; Tornese, P.; Carini, G.; Trontti, K.; Seguini, M.; Tardito, D.; Bono, F.; Fiorentini, C.; Elia, L.; et al. MiR-9-5p Is Involved in the Rescue of Stress-Dependent Dendritic Shortening of Hippocampal Pyramidal Neurons Induced by Acute Antidepressant Treatment with Ketamine. Neurobiol. Stress 2021, 15, 100381. [Google Scholar] [CrossRef]
- Ferrucci, L.; Cantando, I.; Cordella, F.; Di Angelantonio, S.; Ragozzino, D.; Bezzi, P. Microglia at the Tripartite Synapse during Postnatal Development: Implications for Autism Spectrum Disorders and Schizophrenia. Cells 2023, 12, 2827. [Google Scholar] [CrossRef]
- Chung, W.-S.; Allen, N.J.; Eroglu, C. Astrocytes Control Synapse Formation, Function, and Elimination. Cold Spring Harb. Perspect. Biol. 2015, 7, a020370. [Google Scholar] [CrossRef] [PubMed]
- Tarasov, V.V.; Svistunov, A.A.; Chubarev, V.N.; Sologova, S.S.; Mukhortova, P.; Levushkin, D.; Somasundaram, S.G.; Kirkland, C.E.; Bachurin, S.O.; Aliev, G. Alterations of Astrocytes in the Context of Schizophrenic Dementia. Front. Pharmacol. 2020, 10, 1612. [Google Scholar] [CrossRef] [PubMed]
- Mısır, E.; Akay, G.G. Synaptic Dysfunction in Schizophrenia. Synapse 2023, 77, e22276. [Google Scholar] [CrossRef] [PubMed]
- Notter, T. Astrocytes in Schizophrenia. Brain Neurosci. Adv. 2021, 5, 239821282110091. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Figueiredo, E.C.; Calì, C.; Petrelli, F.; Bezzi, P. Emerging Evidence for Astrocyte Dysfunction in Schizophrenia. Glia 2022, 70, 1585–1604. [Google Scholar] [CrossRef]
- Chang, C.-Y.; Luo, D.-Z.; Pei, J.-C.; Kuo, M.-C.; Hsieh, Y.-C.; Lai, W.-S. Not Just a Bystander: The Emerging Role of Astrocytes and Research Tools in Studying Cognitive Dysfunctions in Schizophrenia. Int. J. Mol. Sci. 2021, 22, 5343. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, H.-G.; Steiner, J.; Bogerts, B. Glial Cells in Schizophrenia: Pathophysiological Significance and Possible Consequences for Therapy. Expert. Rev. Neurother. 2009, 9, 1059–1071. [Google Scholar] [CrossRef]
- Hohoff, C.; Ponath, G.; Freitag, C.M.; Kästner, F.; Krakowitzky, P.; Domschke, K.; Koelkebeck, K.; Kipp, F.; von Eiff, C.; Deckert, J.; et al. Risk Variants in the S100B Gene Predict Elevated S100B Serum Concentrations in Healthy Individuals. Am. J. Med. Genet. Part. B Neuropsychiatr. Genet. 2010, 153B, 291–297. [Google Scholar] [CrossRef]
- Hong, S.; Lee, E.E.; Martin, A.S.; Soontornniyomkij, B.; Soontornniyomkij, V.; Achim, C.L.; Reuter, C.; Irwin, M.R.; Eyler, L.T.; Jeste, D.V. Abnormalities in Chemokine Levels in Schizophrenia and Their Clinical Correlates. Schizophr. Res. 2017, 181, 63–69. [Google Scholar] [CrossRef]
- Yelmo-Cruz, S.; Morera-Fumero, A.L.; Abreu-González, P. S100B and Schizophrenia. Psychiatry Clin. Neurosci. 2013, 67, 67–75. [Google Scholar] [CrossRef]
- Park, H.J.; Kim, S.K.; Kim, J.W.; Kang, W.S.; Chung, J.-H. Association of Thrombospondin 1 Gene with Schizophrenia in Korean Population. Mol. Biol. Rep. 2012, 39, 6875–6880. [Google Scholar] [CrossRef] [PubMed]
- Morita, Y.; Ujike, H.; Tanaka, Y.; Otani, K.; Kishimoto, M.; Morio, A.; Kotaka, T.; Okahisa, Y.; Matsushita, M.; Morikawa, A.; et al. A Genetic Variant of the Serine Racemase Gene Is Associated with Schizophrenia. Biol. Psychiatry 2007, 61, 1200–1203. [Google Scholar] [CrossRef] [PubMed]
- Labrie, V.; Roder, J.C. The Involvement of the NMDA Receptor D-Serine/Glycine Site in the Pathophysiology and Treatment of Schizophrenia. Neurosci. Biobehav. Rev. 2010, 34, 351–372. [Google Scholar] [CrossRef] [PubMed]
- Catts, V.S.; Wong, J.; Fillman, S.G.; Fung, S.J.; Shannon Weickert, C. Increased Expression of Astrocyte Markers in Schizophrenia: Association with Neuroinflammation. Aust. N. Z. J. Psychiatry 2014, 48, 722–734. [Google Scholar] [CrossRef] [PubMed]
- Fritschi, L.; Lindmar, J.H.; Scheidl, F.; Lenk, K. Neuronal and Astrocytic Regulations in Schizophrenia: A Computational Modelling Study. Front. Cell Neurosci. 2021, 15, 718459. [Google Scholar] [CrossRef]
- Kim, J.; Iwata, Y.; Plitman, E.; Caravaggio, F.; Chung, J.K.; Shah, P.; Blumberger, D.M.; Pollock, B.G.; Remington, G.; Graff-Guerrero, A.; et al. A Meta-Analysis of Transcranial Direct Current Stimulation for Schizophrenia: “Is More Better?”. J. Psychiatr. Res. 2019, 110, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Kwak, T.H.; Kang, J.H.; Hali, S.; Kim, J.; Kim, K.-P.; Park, C.; Lee, J.-H.; Ryu, H.K.; Na, J.E.; Jo, J.; et al. Generation of Homogeneous Midbrain Organoids with in Vivo—Like Cellular Composition Facilitates Neurotoxin-Based Parkinson’s Disease Modeling. Stem Cells 2020, 38, 727–740. [Google Scholar] [CrossRef]
- Orzylowski, M.; Fujiwara, E.; Mousseau, D.D.; Baker, G.B. An Overview of the Involvement of D-Serine in Cognitive Impairment in Normal Aging and Dementia. Front. Psychiatry 2021, 12, 754032. [Google Scholar] [CrossRef]
- Kim, J.-W.; Kim, H.-J.; Ban, J.Y.; Park, H.J.; Kim, S.K.; Kang, S.W.; Chung, J.-H.; Park, J.K.; Kim, J.W. Assessment between Phosphoglycerate Dehydrogenase Gene and Schizophrenia in Korean Population. Psychiatr. Genet. 2009, 19, 161. [Google Scholar] [CrossRef]
- Eulenburg, V.; Armsen, W.; Betz, H.; Gomeza, J. Glycine Transporters: Essential Regulators of Neurotransmission. Trends Biochem. Sci. 2005, 30, 325–333. [Google Scholar] [CrossRef] [PubMed]
- MacKay, M.-A.B.; Kravtsenyuk, M.; Thomas, R.; Mitchell, N.D.; Dursun, S.M.; Baker, G.B. D-Serine: Potential Therapeutic Agent and/or Biomarker in Schizophrenia and Depression? Front. Psychiatry 2019, 10, 25. [Google Scholar] [CrossRef]
- Zhang, H.X.; Lyons-Warren, A.; Thio, L.L. The Glycine Transport Inhibitor Sarcosine Is an Inhibitory Glycine Receptor Agonist. Neuropharmacology 2009, 57, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K. Glycine Transporter-1: A New Potential Therapeutic Target for Schizophrenia. Curr. Pharm. Des. 2011, 17, 112–120. [Google Scholar] [CrossRef]
- Petrelli, F.; Dallérac, G.; Pucci, L.; Calì, C.; Zehnder, T.; Sultan, S.; Lecca, S.; Chicca, A.; Ivanov, A.; Asensio, C.S.; et al. Dysfunction of Homeostatic Control of Dopamine by Astrocytes in the Developing Prefrontal Cortex Leads to Cognitive Impairments. Mol. Psychiatry 2020, 25, 732–749. [Google Scholar] [CrossRef]
- Corkrum, M.; Araque, A. Astrocyte-Neuron Signaling in the Mesolimbic Dopamine System: The Hidden Stars of Dopamine Signaling. Neuropsychopharmacology 2021, 46, 1864–1872. [Google Scholar] [CrossRef]
- Corkrum, M.; Covelo, A.; Lines, J.; Bellocchio, L.; Pisansky, M.; Loke, K.; Quintana, R.; Rothwell, P.E.; Lujan, R.; Marsicano, G.; et al. Dopamine-Evoked Synaptic Regulation in the Nucleus Accumbens Requires Astrocyte Activity. Neuron 2020, 105, 1036–1047.e5. [Google Scholar] [CrossRef] [PubMed]
- Pittolo, S.; Yokoyama, S.; Willoughby, D.D.; Taylor, C.R.; Reitman, M.E.; Tse, V.; Wu, Z.; Etchenique, R.; Li, Y.; Poskanzer, K.E. Dopamine Activates Astrocytes in Prefrontal Cortex via A1-Adrenergic Receptors. Cell Rep. 2022, 40, 111426. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Liu, Y.; Yan, J.-W.; Hu, X.-L.; Zhu, D.-M.; Xu, X.-T.; Li, X.-S. Gene Polymorphisms of DISC1 Is Associated with Schizophrenia: Evidence from a Meta-Analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 81, 64–73. [Google Scholar] [CrossRef]
- Mastrogiacomo, R.; Trigilio, G.; Devroye, C.; Dautan, D.; Ferretti, V.; Losi, G.; Caffino, L.; Orso, G.; Marotta, R.; Maltese, F.; et al. Dysbindin-1A Modulation of Astrocytic Dopamine and Basal Ganglia Dependent Behaviors Relevant to Schizophrenia. Mol. Psychiatry 2022, 27, 4201–4217. [Google Scholar] [CrossRef]
- Cabezas, R.; Ãvila, M.; Gonzalez, J.; El-Bachã¡, R.S.; Bã¡Ez, E.; Garcã a-Segura, L.M.; Coronel, J.C.J.; Capani, F.; Cardona-Gomez, G.P.; Barreto, G.E. Astrocytic Modulation of Blood Brain Barrier: Perspectives on Parkinson’s Disease. Front. Cell Neurosci. 2014, 8, 211. [Google Scholar] [CrossRef]
- Filosa, J.A.; Morrison, H.W.; Iddings, J.A.; Du, W.; Kim, K.J. Beyond Neurovascular Coupling, Role of Astrocytes in the Regulation of Vascular Tone. Neuroscience 2016, 323, 96–109. [Google Scholar] [CrossRef] [PubMed]
- Booth, S.; Ramadan, A.; Zhang, D.; Lu, L.; Kirouac, G.; Jackson, M.F.; Anderson, C.; Ko, J.H. The Vasomotor Response to Dopamine Is Altered in the Rat Model of l-dopa-Induced Dyskinesia. Mov. Disord. 2021, 36, 938–947. [Google Scholar] [CrossRef] [PubMed]
- Gallo, V.; Ghiani, C.A. Glutamate Receptors in Glia: New Cells, New Inputs and New Functions. Trends Pharmacol. Sci. 2000, 21, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Cieślik, P.; Radulska, A.; Burnat, G.; Kalinowski, L.; Wierońska, J.M. Serotonergic–Muscarinic Interaction within the Prefrontal Cortex as a Novel Target to Reverse Schizophrenia-Related Cognitive Symptoms. Int. J. Mol. Sci. 2021, 22, 8612. [Google Scholar] [CrossRef] [PubMed]
- Hanson, D.R.; Gottesman, I.I. Theories of Schizophrenia: A Genetic-Inflammatory-Vascular Synthesis. BMC Med. Genet. 2005, 6, 7. [Google Scholar] [CrossRef]
- Luissint, A.-C.; Artus, C.; Glacial, F.; Ganeshamoorthy, K.; Couraud, P.-O. Tight Junctions at the Blood Brain Barrier: Physiological Architecture and Disease-Associated Dysregulation. Fluids Barriers CNS 2012, 9, 23. [Google Scholar] [CrossRef]
- Dejana, E. Endothelial Cell–Cell Junctions: Happy Together. Nat. Rev. Mol. Cell Biol. 2004, 5, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Stamatovic, S.; Keep, R.; Andjelkovic, A. Brain Endothelial Cell-Cell Junctions: How to “Open” the Blood Brain Barrier. Curr. Neuropharmacol. 2008, 6, 179–192. [Google Scholar] [CrossRef]
- Nag, S. Morphology and Properties of Astrocytes. In The Blood-Brain and Other Neural Barriers: Reviews and Protocols; Humana: New York, NY, USA, 2011; pp. 69–100. [Google Scholar]
- MacVicar, B.A.; Newman, E.A. Astrocyte Regulation of Blood Flow in the Brain. Cold Spring Harb. Perspect. Biol. 2015, 7, a020388. [Google Scholar] [CrossRef]
- Grammas, P.; Sanchez, A.; Tripathy, D.; Luo, E.; Martinez, J. Vascular Signaling Abnormalities in Alzheimer Disease. Cleve Clin. J. Med. 2011, 78, S50–S53. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Trindade, P.; Nascimento, J.M.; Casas, B.S.; Monteverde, T.; Gasparotto, J.; Ribeiro, C.T.; Devalle, S.; Sauma, D.; Moreira, J.C.F.; Gelain, D.P.; et al. Induced Pluripotent Stem Cell-Derived Astrocytes from Patients with Schizophrenia Exhibit an Inflammatory Phenotype That Affects Vascularization. Mol. Psychiatry 2023, 28, 871–882. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanca, S.; Rossetti, M.; Bokulic Panichi, L.; Bongioanni, P. The Cellular Dysfunction of the Brain–Blood Barrier from Endothelial Cells to Astrocytes: The Pathway towards Neurotransmitter Impairment in Schizophrenia. Int. J. Mol. Sci. 2024, 25, 1250. https://doi.org/10.3390/ijms25021250
Stanca S, Rossetti M, Bokulic Panichi L, Bongioanni P. The Cellular Dysfunction of the Brain–Blood Barrier from Endothelial Cells to Astrocytes: The Pathway towards Neurotransmitter Impairment in Schizophrenia. International Journal of Molecular Sciences. 2024; 25(2):1250. https://doi.org/10.3390/ijms25021250
Chicago/Turabian StyleStanca, Stefano, Martina Rossetti, Leona Bokulic Panichi, and Paolo Bongioanni. 2024. "The Cellular Dysfunction of the Brain–Blood Barrier from Endothelial Cells to Astrocytes: The Pathway towards Neurotransmitter Impairment in Schizophrenia" International Journal of Molecular Sciences 25, no. 2: 1250. https://doi.org/10.3390/ijms25021250
APA StyleStanca, S., Rossetti, M., Bokulic Panichi, L., & Bongioanni, P. (2024). The Cellular Dysfunction of the Brain–Blood Barrier from Endothelial Cells to Astrocytes: The Pathway towards Neurotransmitter Impairment in Schizophrenia. International Journal of Molecular Sciences, 25(2), 1250. https://doi.org/10.3390/ijms25021250






