Effects of TiO2 Nanotubes and Reduced Graphene Oxide on Streptococcus mutans and Preosteoblastic Cells at an Early Stage
Abstract
1. Introduction
2. Results
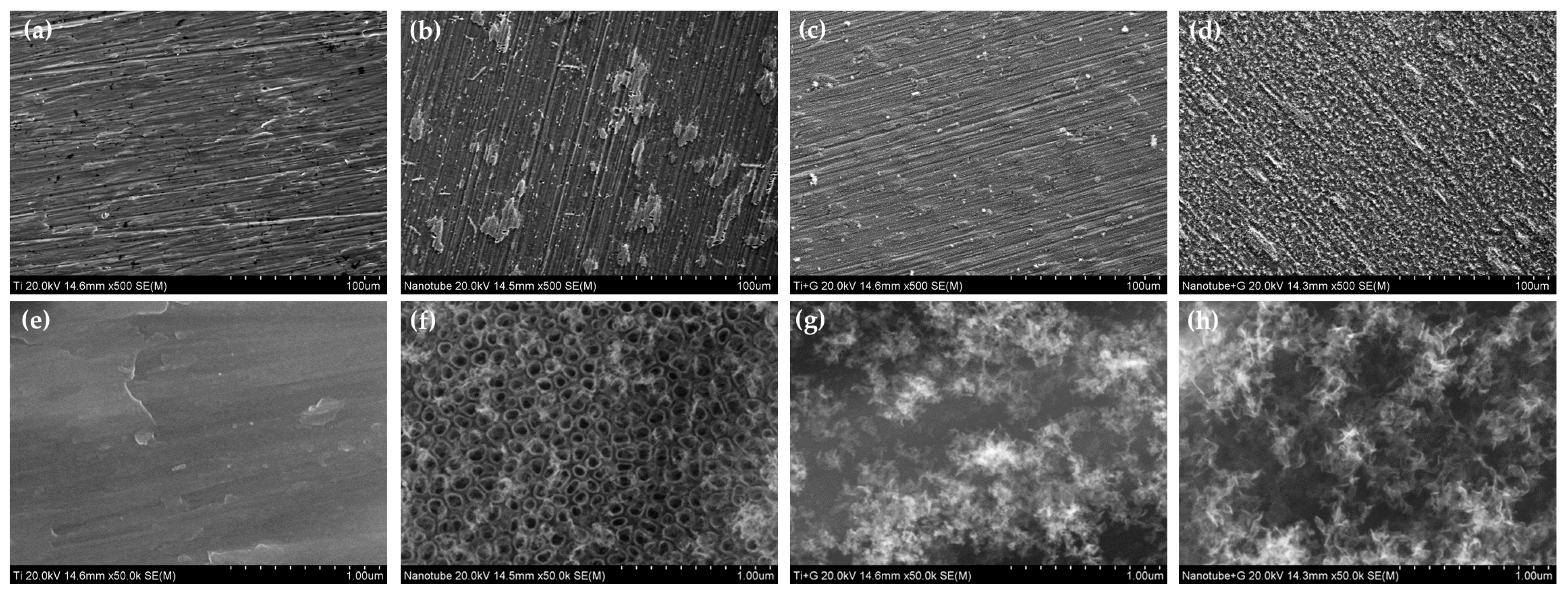
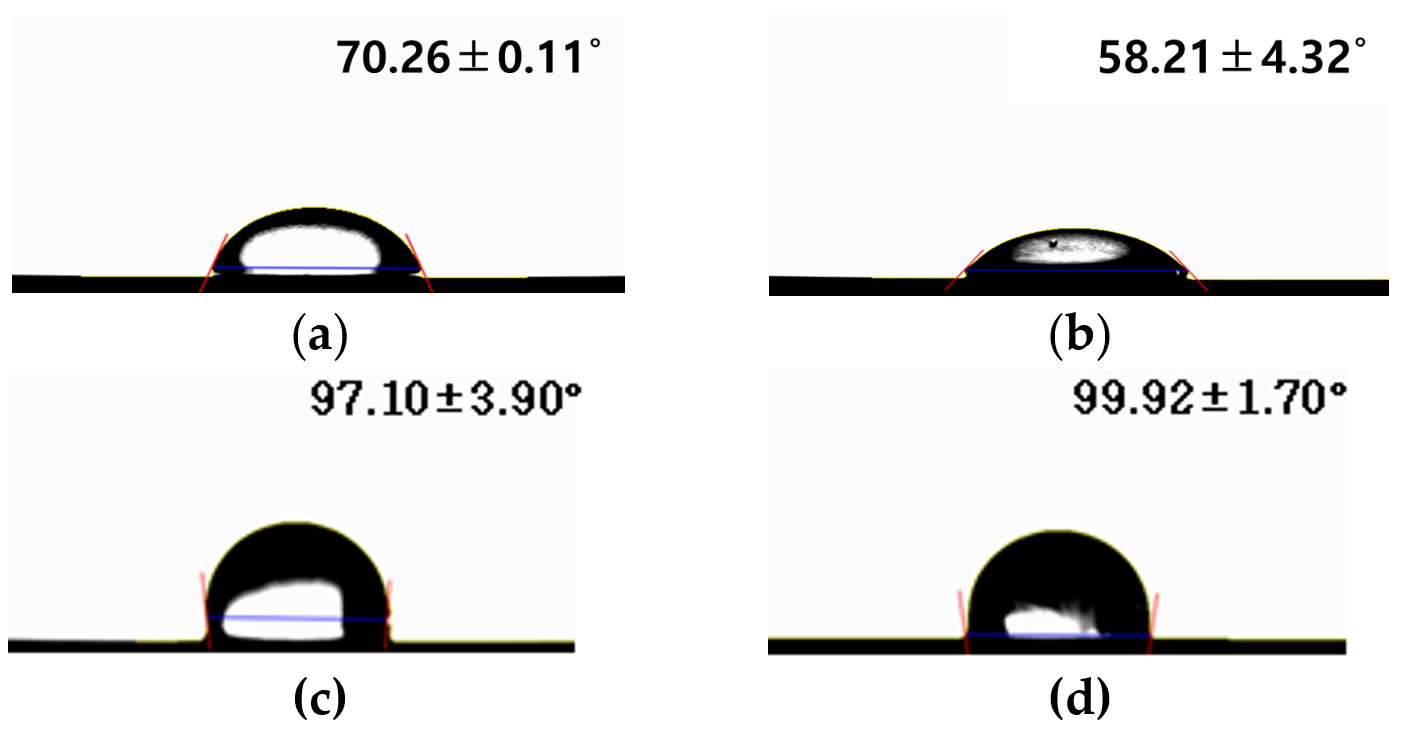
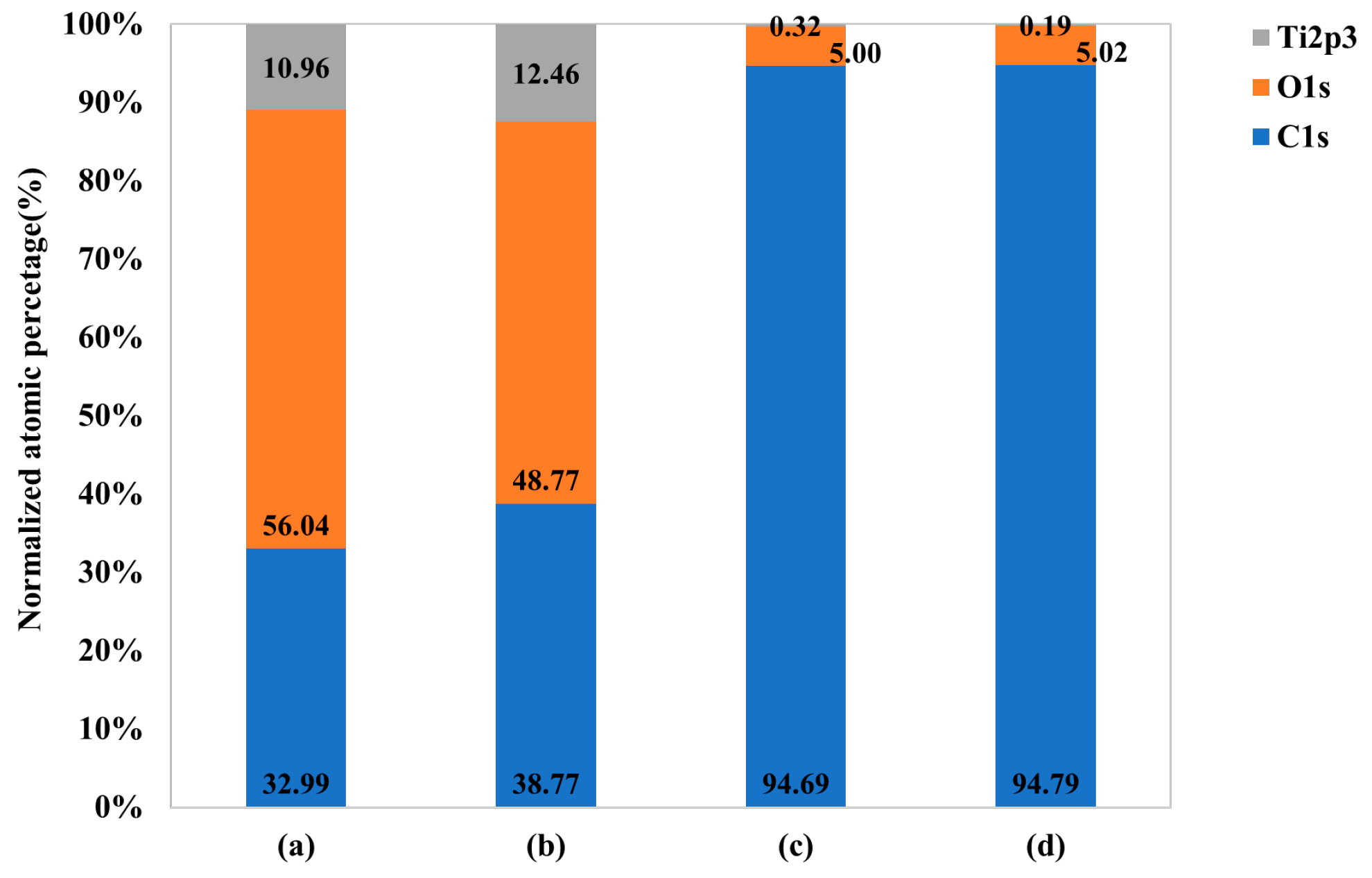
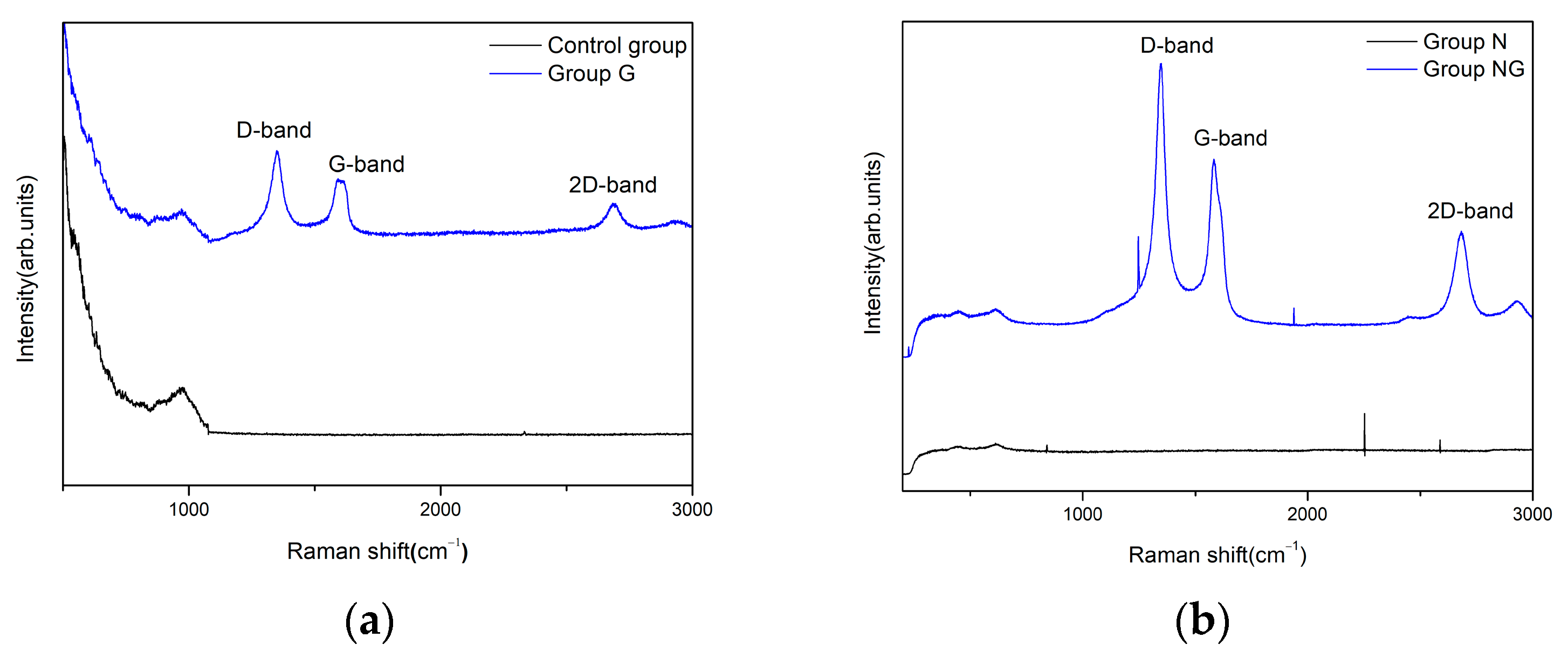
2.1. Surface Characteristics
2.2. Assessment of the Ability to Inhibit Biofilm Formation
2.3. Assessment of Osteoblastic Activity
3. Discussion
4. Materials and Methods
4.1. Samples
4.2. Surface Treatment
4.2.1. Anodic Oxidation
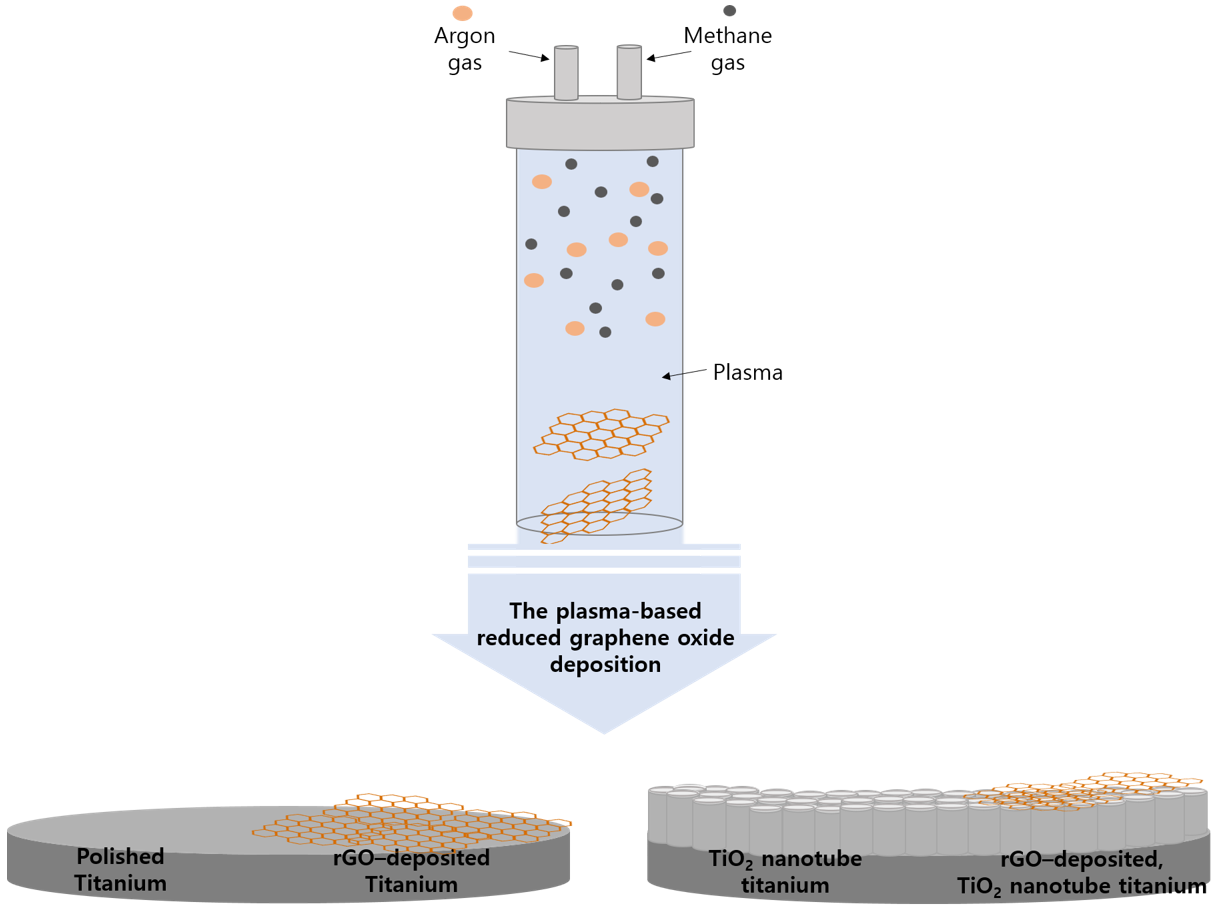
4.2.2. Reduced Graphene Oxide Deposition
4.3. Classification of Experimental Groups
4.4. Assessment of Surface Characteristics
4.5. Assessment of the Ability to Inhibit Biofilm Formation
4.6. Osteoblastic Activity
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Quirynen, M.; De Soete, M.; Van Steenberghe, D. Infectious risks for oral implants a review of the literature. Clin. Oral Implant. Res. Rev. Artic. 2002, 13, 1–19. [Google Scholar] [CrossRef]
- Mombelli, A.; Müller, N.; Cionca, N. The epidemiology of peri-implantitis. Clin. Oral Implant. Res. 2012, 23, 67–76. [Google Scholar] [CrossRef]
- Simonis, P.; Dufour, T.; Tenenbaum, H. Long-term implant survival and success: A 10–16-year follow-up of non-submerged dental implants. Clin. Oral Implant. Res. 2010, 21, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Garcés, M.; Gay Escoda, C. Periimplantitis. Med. Oral Patol. Oral Cir. Bucal 2004, 9, 63–74. [Google Scholar]
- Fürst, M.M.; Salvi, G.E.; Lang, N.P.; Persson, G.R. Bacterial colonization immediately after installation on oral titanium implants. Clin. Oral Implant. Res. 2007, 18, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Krzyściak, W.; Jurczak, A.; Kościelniak, D.; Bystrowska, B.; Skalniak, A. The virulence of Streptococcus mutans and the ability to form biofilms. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 499–515. [Google Scholar] [CrossRef]
- Inoue, M.; Koga, T.; Sato, S.; Hamada, S. Synthesis of adherent insoluble glucan by the concerted action of the two glucosyltransferase components of Streptococcus mutans. FEBS Lett. 1982, 143, 101–104. [Google Scholar] [CrossRef]
- Badihi Hauslich, L.; Sela, M.N.; Steinberg, D.; Rosen, G.; Kohavi, D. The adhesion of oral bacteria to modified titanium surfaces: Role of plasma proteins and electrostatic forces. Clin. Oral Implant. Res. 2013, 24, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Anselme, K.; Bigerelle, M. Effect of a gold–palladium coating on the long-term adhesion of human osteoblasts on biocompatible metallic materials. Surf. Coat. Technol. 2006, 200, 6325–6330. [Google Scholar] [CrossRef]
- Le Guéhennec, L.; Soueidan, A.; Layrolle, P.; Amouriq, Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent. Mater. 2007, 23, 844–854. [Google Scholar] [CrossRef]
- Wennerberg, A.; Albrektsson, T. On implant surfaces: A review of current knowledge and opinions. Int. J. Oral Maxillofac. Implant. 2010, 25, 63–74. [Google Scholar]
- Park, S.; Min, D.; Lim, H.; Yoon, D.; Lee, K. Effect of heat treatment on phase transition of nanotubular titanium oxide arrays. J. Nanosci. Nanotechnol. 2011, 11, 1476–1479. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, X.; Chen, R.; Li, L. Fabrication of titanium oxide nanotube arrays by anodic oxidation. Solid State Commun. 2005, 134, 705–710. [Google Scholar] [CrossRef]
- Brammer, K.S.; Oh, S.; Frandsen, C.J.; Jin, S. Biomaterials and biotechnology schemes utilizing TiO2 nanotube arrays. Biomater. Sci. Eng. 2011, 9, 193–210. [Google Scholar]
- Lavenus, S.; Louarn, G.; Layrolle, P. Nanotechnology and dental implants. Int. J. Biomater. 2010, 2010, 915327. [Google Scholar] [CrossRef]
- Ellingsen, J.E.; Johansson, C.B.; Wennerberg, A.; Holmén, A. Improved retention and bone-to-implant contact with fluoride-modified titanium implants. Int. J. Oral Maxillofac. Implant. 2004, 19, 659–666. [Google Scholar]
- Zhang, Y.; Nayak, T.R.; Hong, H.; Cai, W. Graphene: A versatile nanoplatform for biomedical applications. Nanoscale 2012, 4, 3833–3842. [Google Scholar] [CrossRef]
- Feng, L.; Wu, L.; Qu, X. New horizons for diagnostics and therapeutic applications of graphene and graphene oxide. Adv. Mater. 2013, 25, 168–186. [Google Scholar] [CrossRef]
- Nayak, T.R.; Andersen, H.; Makam, V.S.; Khaw, C.; Bae, S.; Xu, X.; Ee, P.-L.R.; Ahn, J.-H.; Hong, B.H.; Pastorin, G. Graphene for controlled and accelerated osteogenic differentiation of human mesenchymal stem cells. ACS Nano 2011, 5, 4670–4678. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef]
- Loh, K.P.; Bao, Q.; Ang, P.K.; Yang, J. The chemistry of graphene. J. Mater. Chem. 2010, 20, 2277–2289. [Google Scholar] [CrossRef]
- Zhou, D.; Cheng, Q.-Y.; Han, B.-H. Solvothermal synthesis of homogeneous graphene dispersion with high concentration. Carbon 2011, 49, 3920–3927. [Google Scholar] [CrossRef]
- Yi, M.; Shen, Z. A review on mechanical exfoliation for the scalable production of graphene. J. Mater. Chem. A 2015, 3, 11700–11715. [Google Scholar] [CrossRef]
- Brownson, D.A.; Banks, C.E. The electrochemistry of CVD graphene: Progress and prospects. Phys. Chem. Chem. Phys. 2012, 14, 8264–8281. [Google Scholar] [CrossRef]
- Sali, S.; Mackey, H.R.; Abdala, A.A. Effect of graphene oxide synthesis method on properties and performance of polysulfone-graphene oxide mixed matrix membranes. Nanomaterials 2019, 9, 769. [Google Scholar] [CrossRef] [PubMed]
- Brownson, D.A.; Metters, J.P.; Kampouris, D.K.; Banks, C.E. Graphene electrochemistry: Surfactants inherent to graphene can dramatically effect electrochemical processes. Electroanalysis 2011, 23, 894–899. [Google Scholar] [CrossRef]
- Brownson, D.A.; Banks, C.E. Graphene electrochemistry: Surfactants inherent to graphene inhibit metal analysis. Electrochem. Commun. 2011, 13, 111–113. [Google Scholar] [CrossRef]
- Brownson, D.A.; Banks, C.E. Fabricating graphene supercapacitors: Highlighting the impact of surfactants and moieties. Chem. Commun. 2012, 48, 1425–1427. [Google Scholar] [CrossRef]
- Arora, V.; Nikhil, V.; Suri, N.; Arora, P. Cold atmospheric plasma (CAP) in dentistry. Dentistry 2014, 4, 1. [Google Scholar] [CrossRef]
- Lambert, T.N.; Luhrs, C.C.; Chavez, C.A.; Wakeland, S.; Brumbach, M.T.; Alam, T.M. Graphite oxide as a precursor for the synthesis of disordered graphenes using the aerosol-through-plasma method. Carbon 2010, 48, 4081–4089. [Google Scholar] [CrossRef]
- Phillips, J.; Luhrs, C.C.; Richard, M. Engineering particles using the aerosol-through-plasma method. IEEE Trans. Plasma Sci. 2009, 37, 726–739. [Google Scholar] [CrossRef]
- Rho, K.; Park, C.; Alam, K.; Kim, D.; Ji, M.-K.; Lim, H.-P.; Cho, H. Biological effects of plasma-based graphene oxide deposition on titanium. J. Nanomater. 2019, 2019, 9124989. [Google Scholar] [CrossRef]
- Alam, K.; Jo, Y.Y.; Park, C.-K.; Cho, H. Synthesis of graphene oxide using atmospheric plasma for prospective biological applications. Int. J. Nanomed. 2020, 15, 5813–5824. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, V.C.; Jachak, A.; Hurt, R.H.; Kane, A.B. Biological interactions of graphene-family nanomaterials: An interdisciplinary review. Chem. Res. Toxicol. 2012, 25, 15–34. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A. Graphene: Safe or toxic? The two faces of the medal. Angew. Chem. Int. Ed. 2013, 52, 4986–4997. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Shin, Y.C.; Lee, S.-M.; Jin, O.S.; Kang, S.H.; Hong, S.W.; Jeong, C.-M.; Huh, J.B.; Han, D.-W. Enhanced osteogenesis by reduced graphene oxide/hydroxyapatite nanocomposites. Sci. Rep. 2015, 5, 18833. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.; Moore, E.; Thomas, A.; Johnson, J. Graphene-Based Materials in Dental Applications: Antibacterial, Biocompatible, and Bone Regenerative Properties. Int. J. Biomater. 2023, 2023, 8803283. [Google Scholar] [CrossRef]
- Chehroudi, B.; Gould, T.; Brunette, D. Effects of a grooved titanium-coated implant surface on epithelial cell behavior in vitro and in vivo. J. Biomed. Mater. Res. 1989, 23, 1067–1085. [Google Scholar] [CrossRef]
- Ricci, J.; Spivak, J.; Blumenthal, N.; Alexander, H. Modulation of bone ingrowth by surface chemistry and roughness. In The Bone-Biomaterial Interface; University of Toronto Press: Toronto, ON, Canada, 1991; pp. 334–349. [Google Scholar]
- Quirynen, M.; Bollen, C. The influence of surface roughness and surface-free energy on supra-and subgingival plaque formation in man: A review of the literature. J. Clin. Periodontol. 1995, 22, 1–14. [Google Scholar] [CrossRef]
- Rimondini, L.; Farè, S.; Brambilla, E.; Felloni, A.; Consonni, C.; Brossa, F.; Carrassi, A. The effect of surface roughness on early in vivo plaque colonization on titanium. J. Periodontol. 1997, 68, 556–562. [Google Scholar] [CrossRef]
- Shi, X.; Xu, Q.; Tian, A.; Tian, Y.; Xue, X.; Sun, H.; Yang, H.; Dong, C. Antibacterial activities of TiO2 nanotubes on Porphyromonas gingivalis. RSC Adv. 2015, 5, 34237–34242. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Toxicity of graphene and graphene oxide nanowalls against bacteria. ACS Nano 2010, 4, 5731–5736. [Google Scholar] [CrossRef] [PubMed]
- Dutta, T.; Sarkar, R.; Pakhira, B.; Ghosh, S.; Sarkar, R.; Barui, A.; Sarkar, S. ROS generation by reduced graphene oxide (rGO) induced by visible light showing antibacterial activity: Comparison with graphene oxide (GO). RSC Adv. 2015, 5, 80192–80195. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E.; Esfandiar, A. Wrapping bacteria by graphene nanosheets for isolation from environment, reactivation by sonication, and inactivation by near-infrared irradiation. J. Phys. Chem. B 2011, 115, 6279–6288. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: Membrane and oxidative stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef] [PubMed]
- Pulingam, T.; Thong, K.L.; Appaturi, J.N.; Lai, C.W.; Leo, B.F. Mechanistic actions and contributing factors affecting the antibacterial property and cytotoxicity of graphene oxide. Chemosphere 2021, 281, 130739. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-W.; Cao, A.; Jiang, Y.; Zhang, X.; Liu, J.-H.; Liu, Y.; Wang, H. Superior antibacterial activity of zinc oxide/graphene oxide composites originating from high zinc concentration localized around bacteria. ACS Appl. Mater. Interfaces 2014, 6, 2791–2798. [Google Scholar] [CrossRef]
- Jannesari, M.; Akhavan, O.; Hosseini, H.R.M.; Bakhshi, B. Oxygen-rich graphene/ZnO2-Ag nanoframeworks with pH-switchable catalase/peroxidase activity as O2 nanobubble-self generator for bacterial inactivation. J. Colloid Interface Sci. 2023, 637, 237–250. [Google Scholar] [CrossRef]
- Liu, Z.; Robinson, J.T.; Sun, X.; Dai, H. PEGylated nanographene oxide for delivery of water-insoluble cancer drugs. J. Am. Chem. Soc. 2008, 130, 10876–10877. [Google Scholar] [CrossRef]
- Nostro, A.; Cannatelli, M.; Crisafi, G.; Musolino, A.; Procopio, F.; Alonzo, V. Modifications of hydrophobicity, in vitro adherence and cellular aggregation of Streptococcus mutans by Helichrysum italicum extract. Lett. Appl. Microbiol. 2004, 38, 423–427. [Google Scholar] [CrossRef]
- Sobon, G.; Sotor, J.; Jagiello, J.; Kozinski, R.; Zdrojek, M.; Holdynski, M.; Paletko, P.; Boguslawski, J.; Lipinska, L.; Abramski, K.M. Graphene oxide vs. reduced graphene oxide as saturable absorbers for Er-doped passively mode-locked fiber laser. Opt. Express 2012, 20, 19463–19473. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Lua, A.C. A facile method for the large-scale continuous synthesis of graphene sheets using a novel catalyst. Sci. Rep. 2013, 3, 3037. [Google Scholar] [CrossRef] [PubMed]
- Silva Filho, J.; Venancio, E.; Silva, S.; Takiishi, H.; Martinez, L.; Antunes, R. A thermal method for obtention of 2 to 3 reduced graphene oxide layers from graphene oxide. SN Appl. Sci. 2020, 2, 1450. [Google Scholar] [CrossRef]
- Oh, S.; Daraio, C.; Chen, L.H.; Pisanic, T.R.; Fiñones, R.R.; Jin, S. Significantly accelerated osteoblast cell growth on aligned TiO2 nanotubes. J. Biomed. Mater. Res. Part A 2006, 78, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Popat, K.C.; Daniels, R.H.; Dubrow, R.S.; Hardev, V.; Desai, T.A. Nanostructured surfaces for bone biotemplating applications. J. Orthop. Res. 2006, 24, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Gan, X.; Wang, J.; Zhu, X.; Li, G. Hemoglobin-based hydrogen peroxide biosensor tuned by the photovoltaic effect of nano titanium dioxide. Anal. Chem. 2005, 77, 6102–6104. [Google Scholar] [CrossRef] [PubMed]
- Puckett, S.D.; Taylor, E.; Raimondo, T.; Webster, T.J. The relationship between the nanostructure of titanium surfaces and bacterial attachment. Biomaterials 2010, 31, 706–713. [Google Scholar] [CrossRef]
- MacDonald, D.; Deo, N.; Markovic, B.; Stranick, M.; Somasundaran, P. Adsorption and dissolution behavior of human plasma fibronectin on thermally and chemically modified titanium dioxide particles. Biomaterials 2002, 23, 1269–1279. [Google Scholar] [CrossRef]
- Colon, G.; Ward, B.C.; Webster, T.J. Increased osteoblast and decreased Staphylococcus epidermidis functions on nanophase ZnO and TiO2. J. Biomed. Mater. Res. Part A 2006, 78, 595–604. [Google Scholar] [CrossRef]
- Ji, J.; Zhang, W. Bacterial behaviors on polymer surfaces with organic and inorganic antimicrobial compounds. J. Biomed. Mater. Res. Part A 2009, 88, 448–453. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, S.J.; Khang, G.; Lee, H.B. Interaction of fibroblasts on polycarbonate membrane surfaces with different micropore sizes and hydrophilicity. J. Biomater. Sci. Polym. Ed. 1999, 10, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Turu, I.C.; Bayraktar, S.; Akgul, B.; Ilhan-Sungur, E.; Abamor, E.S.; Cansever, N. Formation of TiO2 nanotubes and deposition of silver nanoparticle and reduced graphene oxide: Antibacterial and biocompatibility behavior. Surf. Coat. Technol. 2023, 470, 129866. [Google Scholar] [CrossRef]
- Murakami, A.; Arimoto, T.; Suzuki, D.; Iwai-Yoshida, M.; Otsuka, F.; Shibata, Y.; Igarashi, T.; Kamijo, R.; Miyazaki, T. Antimicrobial and osteogenic properties of a hydrophilic-modified nanoscale hydroxyapatite coating on titanium. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 374–382. [Google Scholar] [CrossRef] [PubMed]





| Parameter | Value |
|---|---|
| Average working power (W) | 300 |
| Voltage (V) | 27 |
| Frequency (MHz) | 900 |
| Atmospheric pressure (Torr) | 760 |
| Plasma density (cm3) | 1015 |
| Group | Coating Condition |
|---|---|
| Control | Polished titanium |
| N | TiO2 nanotube titanium |
| G | Reduced graphene oxide-deposited titanium |
| NG | Reduced graphene oxide-deposited TiO2 nanotube titanium |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, M.-K.; Kim, H.; Jeong, G.; Kim, W.-J.; Ryu, J.-H.; Cho, H.; Lim, H.-P. Effects of TiO2 Nanotubes and Reduced Graphene Oxide on Streptococcus mutans and Preosteoblastic Cells at an Early Stage. Int. J. Mol. Sci. 2024, 25, 1351. https://doi.org/10.3390/ijms25021351
Ji M-K, Kim H, Jeong G, Kim W-J, Ryu J-H, Cho H, Lim H-P. Effects of TiO2 Nanotubes and Reduced Graphene Oxide on Streptococcus mutans and Preosteoblastic Cells at an Early Stage. International Journal of Molecular Sciences. 2024; 25(2):1351. https://doi.org/10.3390/ijms25021351
Chicago/Turabian StyleJi, Min-Kyung, Hyeonji Kim, Geonwoo Jeong, Won-Jae Kim, Je-Hwang Ryu, Hoonsung Cho, and Hyun-Pil Lim. 2024. "Effects of TiO2 Nanotubes and Reduced Graphene Oxide on Streptococcus mutans and Preosteoblastic Cells at an Early Stage" International Journal of Molecular Sciences 25, no. 2: 1351. https://doi.org/10.3390/ijms25021351
APA StyleJi, M.-K., Kim, H., Jeong, G., Kim, W.-J., Ryu, J.-H., Cho, H., & Lim, H.-P. (2024). Effects of TiO2 Nanotubes and Reduced Graphene Oxide on Streptococcus mutans and Preosteoblastic Cells at an Early Stage. International Journal of Molecular Sciences, 25(2), 1351. https://doi.org/10.3390/ijms25021351






