Comprehensive Analysis of 1-Year-Old Female Apolipoprotein E-Deficient Mice Reveals Advanced Atherosclerosis with Vulnerable Plaque Characteristics
Abstract
:1. Introduction
2. Results
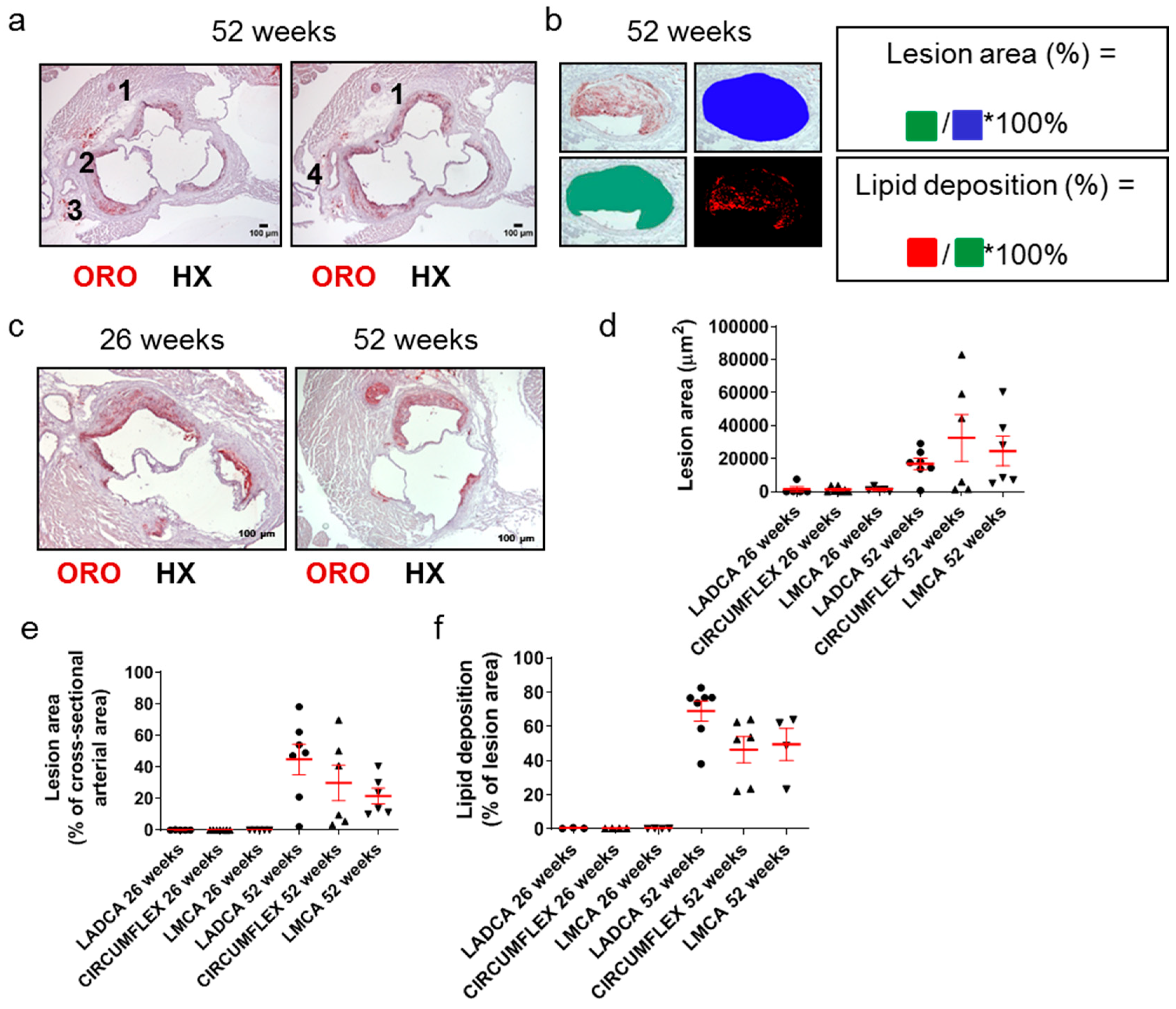
2.1. Atherosclerosis in Apoe-/- Mice Is a Highly Dynamic Process in Which Atherosclerotic Plaques Evolve over Time
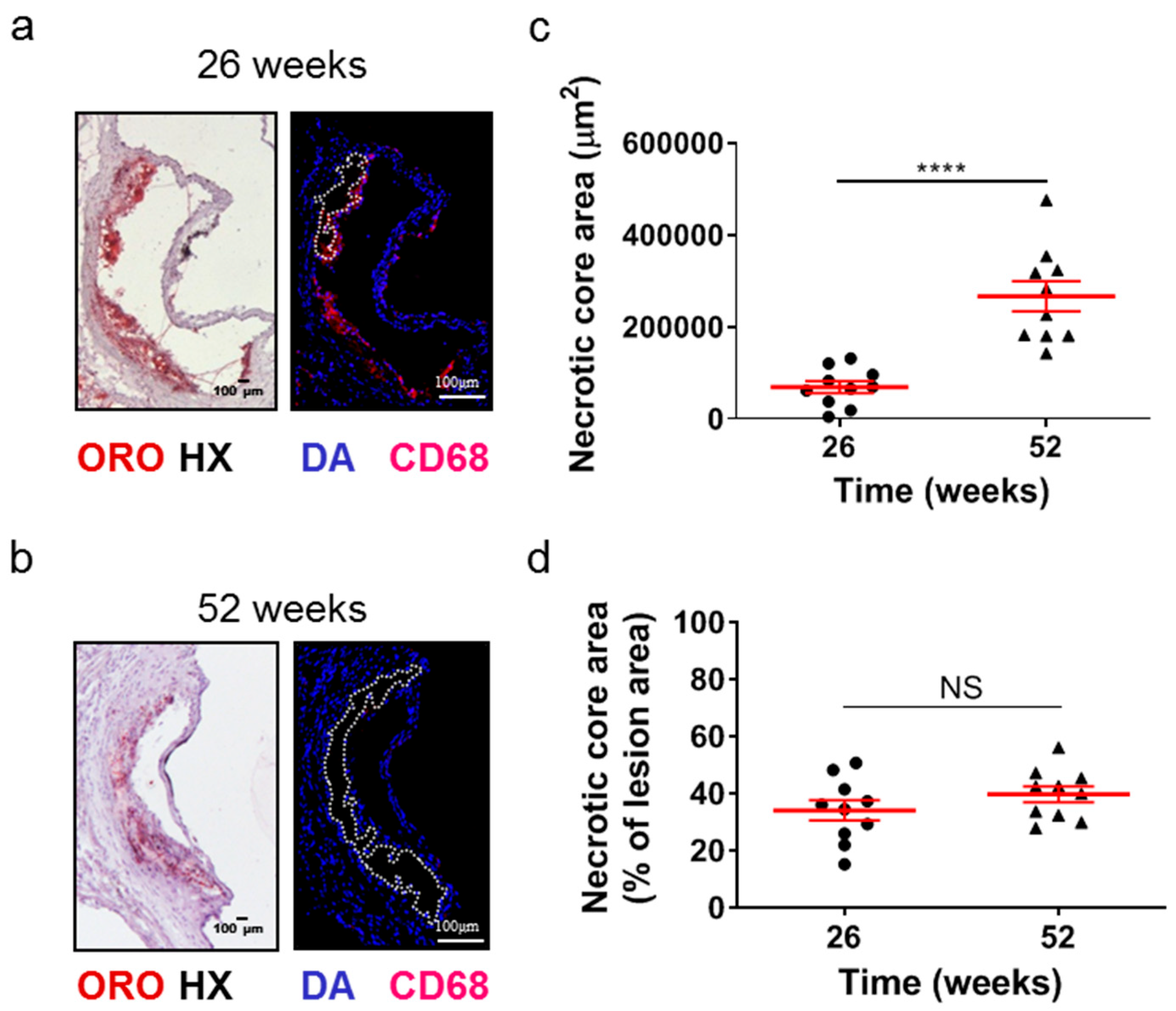
2.2. Atherosclerotic Lesions of Apoe-/- Mice Acquire Morphological Characteristics of a More “Vulnerable Phenotype” at Late Disease Stages
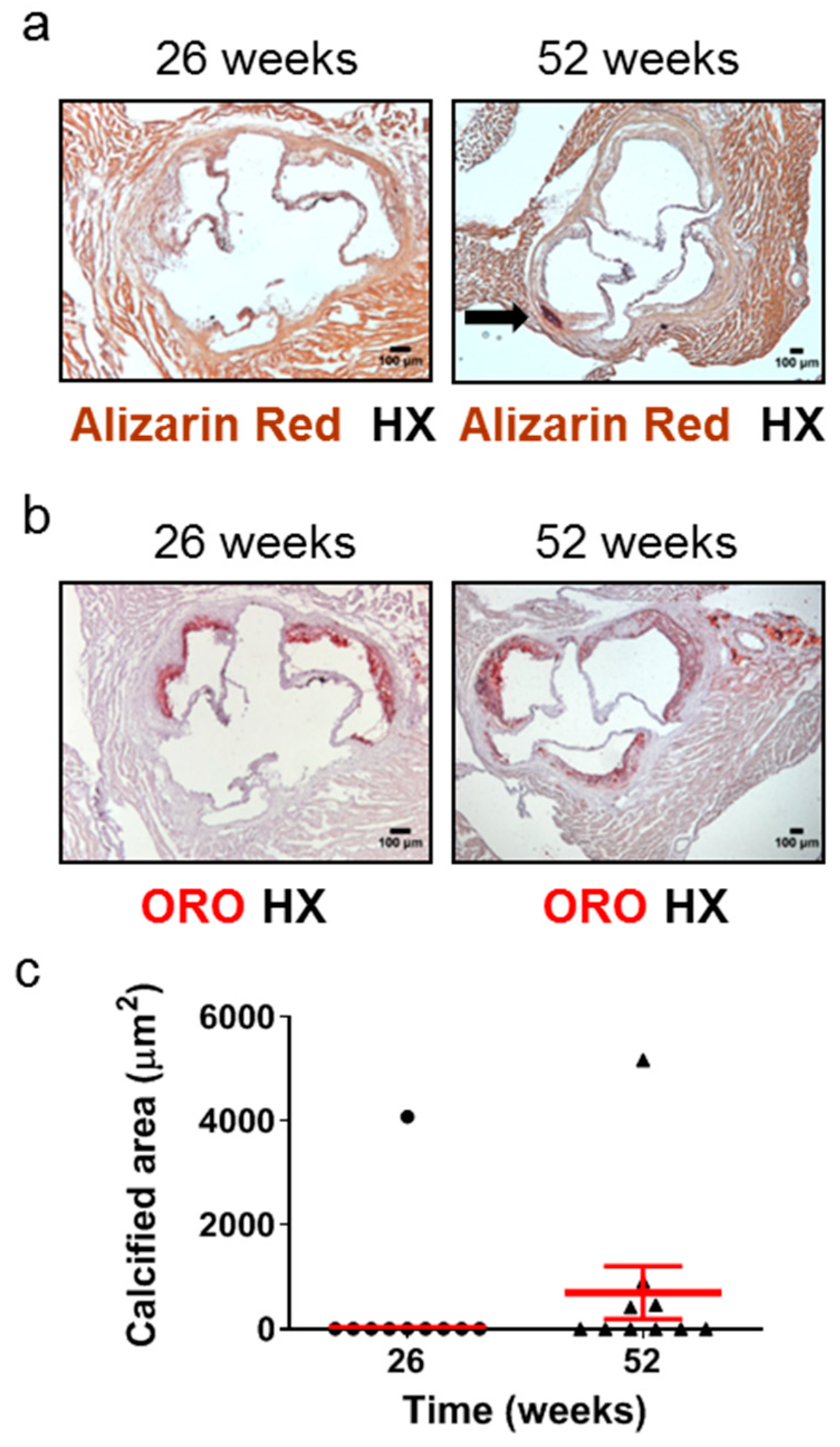
2.3. Aged Apoe-/- Mice Develop Plaque Calcification
2.4. Aged Apoe-/- Mice Develop Coronary Occlusion
2.5. One-Year Old Apoe-/- Mice Exhibit Higher Carotid Atherosclerosis, but Their Cardiac Function Is Not Affected
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Histological Analysis
4.3. Immunohistochemistry and Immunofluorescence Staining
4.4. Morphometry/Quantification of Aortic Lesion Area, Calcified Area, Macrophage Infiltration, Smooth Muscle Cell Accumulation, and Plaque Necrosis
4.5. Histology and Morphometry of Coronary Arteries
4.6. Serum Biochemistry
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Galkina, E.; Ley, K. Immune and inflammatory mechanisms of atherosclerosis (*). Annu. Rev. Immunol. 2009, 27, 165–197. [Google Scholar] [CrossRef]
- Golforoush, P.; Yellon, D.M.; Davidson, S.M. Mouse models of atherosclerosis and their suitability for the study of myocardial infarction. Basic Res. Cardiol. 2020, 115, 73. [Google Scholar] [CrossRef]
- Ross, R. Atherosclerosis—An inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, D. Atherogenesis in perspective: Hypercholesterolemia and inflammation as partners in crime. Nat. Med. 2002, 8, 1211–1217. [Google Scholar] [CrossRef]
- Virmani, R.; Burke, A.P.; Farb, A.; Kolodgie, F.D. Pathology of the vulnerable plaque. J. Am. Coll. Cardiol. 2006, 47 (Suppl. S8), C13–C188. [Google Scholar] [CrossRef]
- Virmani, R.; Kolodgie, F.D.; Burke, A.P.; Farb, A.; Schwartz, S.M. Lessons from sudden coronary death: A comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1262–1275. [Google Scholar] [CrossRef]
- Gistera, A.; Hansson, G.K. The immunology of atherosclerosis. Nat. Rev. Nephrol. 2017, 13, 368–380. [Google Scholar] [CrossRef]
- Zernecke, A.; Winkels, H.; Cochain, C.; Williams, J.W.; Wolf, D.; Soehnlein, O.; Robbins, C.S.; Monaco, C.; Park, I.; McNamara, C.A.; et al. Meta-Analysis of Leukocyte Diversity in Atherosclerotic Mouse Aortas. Circ. Res. 2020, 127, 402–426. [Google Scholar] [CrossRef]
- Dobrescu, M.; Păun, D.; Grigorie, D.; Poiană, C. Hormonal mechanisms in atherosclerosis. Intern. Med. 2020, XVII, 19–35. [Google Scholar] [CrossRef]
- Mozaffarian, D. Global Scourge of Cardiovascular Disease: Time for Health Care Systems Reform and Precision Population Health. J. Am. Coll. Cardiol. 2017, 70, 26–28. [Google Scholar] [CrossRef]
- Ross, R.; Glomset, J.A. The pathogenesis of atherosclerosis (first of two parts). N. Engl. J. Med. 1976, 295, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.; Glomset, J.; Harker, L. Response to injury and atherogenesis. Am. J. Pathol. 1977, 86, 675–684. [Google Scholar]
- Bradley, A.; Evans, M.; Kaufman, M.H.; Robertson, E. Formation of germ-line chimaeras from embryo-derived teratocarcinoma cell lines. Nature 1984, 309, 255–256. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.J.; Kaufman, M.H. Establishment in culture of pluripotential cells from mouse embryos. Nature 1981, 292, 154–156. [Google Scholar] [CrossRef]
- Piedrahita, J.A.; Zhang, S.H.; Hagaman, J.R.; Oliver, P.M.; Maeda, N. Generation of mice carrying a mutant apolipoprotein E gene inactivated by gene targeting in embryonic stem cells. Proc. Natl. Acad. Sci. USA 1992, 89, 4471–4475. [Google Scholar] [CrossRef] [PubMed]
- Plump, A.S.; Smith, J.D.; Hayek, T.; Aalto-Setälä, K.; Walsh, A.; Verstuyft, J.G.; Rubin, E.M.; Breslow, J.L. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell 1992, 71, 343–353. [Google Scholar] [CrossRef]
- Zhang, S.H.; Reddick, R.L.; Piedrahita, J.A.; Maeda, N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science 1992, 258, 468–471. [Google Scholar] [CrossRef]
- Ishibashi, S.; Brown, M.S.; Goldstein, J.L.; Gerard, R.D.; Hammer, R.E.; Herz, J. Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J. Clin. Investig. 1993, 92, 883–893. [Google Scholar] [CrossRef]
- Wang, X.; Fu, Y.; Xie, Z.; Cao, M.; Qu, W.; Xi, X.; Zhong, S.; Piao, M.; Peng, X.; Jia, Y.; et al. Establishment of a Novel Mouse Model for Atherosclerotic Vulnerable Plaque. Front. Cardiovasc. Med. 2021, 8, 642751. [Google Scholar] [CrossRef]
- Jawien, J.; Nastalek, P.; Korbut, R. Mouse models of experimental atherosclerosis. J. Physiol. Pharmacol. 2004, 55, 503–517. [Google Scholar]
- Nakashima, Y.; Plump, A.S.; Raines, E.W.; Breslow, J.L.; Ross, R. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler. Thromb. 1994, 14, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Reddick, R.L.; Zhang, S.H.; Maeda, N. Atherosclerosis in mice lacking apo E. Evaluation of lesional development and progression. Arterioscler. Thromb. 1994, 14, 141–147. [Google Scholar] [CrossRef]
- Coleman, R.; Hayek, T.; Keidar, S.; Aviram, M. A mouse model for human atherosclerosis: Long-term histopathological study of lesion development in the aortic arch of apolipoprotein E-deficient (E0) mice. Acta Histochem. 2006, 108, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, M.E.; Polinsky, P.; Virmani, R.; Kauser, K.; Rubanyi, G.; Schwartz, S.M. Advanced atherosclerotic lesions in the innominate artery of the ApoE knockout mouse. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 2587–2592. [Google Scholar] [CrossRef]
- Matsui, Y.; Rittling, S.R.; Okamoto, H.; Inobe, M.; Jia, N.; Shimizu, T.; Akino, M.; Sugawara, T.; Morimoto, J.; Kimura, C.; et al. Osteopontin deficiency attenuates atherosclerosis in female apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Doddapattar, P.; Jain, M.; Dhanesha, N.; Lentz, S.R.; Chauhan, A.K. Fibronectin Containing Extra Domain a Induces Plaque Destabilization in the Innominate Artery of Aged Apolipoprotein E-Deficient Mice. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Queiroz, J.; Li, Y.; Jiang, X.-C.; Ron, D.; Hussain, M.M. Increased intestinal lipid absorption caused by Ire1beta deficiency contributes to hyperlipidemia and atherosclerosis in apolipoprotein E-deficient mice. Circ. Res. 2012, 110, 1575–1584. [Google Scholar] [CrossRef]
- Pratico, D.; Cyrus, T.; Li, H.; FitzGerald, G.A. Endogenous biosynthesis of thromboxane and prostacyclin in 2 distinct murine models of atherosclerosis. Blood 2000, 96, 3823–3826. [Google Scholar] [CrossRef]
- Salagianni, M.; Galani, I.E.; Lundberg, A.M.; Davos, C.H.; Varela, A.; Gavriil, A.; Lyytikäinen, L.-P.; Lehtimäki, T.; Sigala, F.; Folkersen, L.; et al. Toll-like receptor 7 protects from atherosclerosis by constraining “inflammatory” macrophage activation. Circulation 2012, 126, 952–962. [Google Scholar] [CrossRef]
- Aikawa, E.; Nahrendorf, M.; Figueiredo, J.-L.; Swirski, F.K.; Shtatland, T.; Kohler, R.H.; Jaffer, F.A.; Aikawa, M.; Weissleder, R. Osteogenesis associates with inflammation in early-stage atherosclerosis evaluated by molecular imaging in vivo. Circulation 2007, 116, 2841–2850. [Google Scholar] [CrossRef] [PubMed]
- Seimon, T.A.; Nadolski, M.J.; Liao, X.; Magallon, J.; Nguyen, M.; Feric, N.T.; Koschinsky, M.L.; Harkewicz, R.; Witztum, J.L.; Tsimikas, S.; et al. Atherogenic lipids and lipoproteins trigger CD36-TLR2-dependent apoptosis in macrophages undergoing endoplasmic reticulum stress. Cell Metab. 2010, 12, 467–482. [Google Scholar] [CrossRef] [PubMed]
- Tabas, I.; Seimon, T.; Timmins, J.; Li, G.; Lim, W. Macrophage apoptosis in advanced atherosclerosis. Ann. N. Y. Acad. Sci. 2009, 1173 (Suppl. 1), E40–E45. [Google Scholar] [CrossRef] [PubMed]
- Gronros, J.; Wikström, J.; Brandt-Eliasson, U.; Forsberg, G.B.; Behrendt, M.; Hansson, G.I.; Gan, L.M. Effects of rosuvastatin on cardiovascular morphology and function in an ApoE-knockout mouse model of atherosclerosis. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H2046–H2053. [Google Scholar] [CrossRef] [PubMed]
- Hartley, C.J.; Reddy, A.K.; Madala, S.; Martin-McNulty, B.; Vergona, R.; Sullivan, M.E.; Halks-Miller, M.; Taffet, G.E.; Michael, L.H.; Entman, M.L.; et al. Hemodynamic changes in apolipoprotein E-knockout mice. Am. J. Physiol. Heart Circ. Physiol. 2000, 279, H2326–H2334. [Google Scholar] [CrossRef] [PubMed]
- Hartley, C.J.; Reddy, A.K.; Michael, L.H.; Entman, M.L.; Taffet, G.E. Coronary flow reserve as an index of cardiac function in mice with cardiovascular abnormalities. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2009, 2009, 1094–1097. [Google Scholar]
- Johansson, M.E.; Hägg, U.; Wikström, J.; Wickman, A.; Bergström, G.; Gan, L.-M. Haemodynamically significant plaque formation and regional endothelial dysfunction in cholesterol-fed ApoE-/- mice. Clin. Sci. 2005, 108, 531–538. [Google Scholar] [CrossRef]
- Tomita, H.; Hagaman, J.; Friedman, M.H.; Maeda, N. Relationship between hemodynamics and atherosclerosis in aortic arches of apolipoprotein E-null mice on 129S6/SvEvTac and C57BL/6J genetic backgrounds. Atherosclerosis 2012, 220, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Finn, A.V.; Nakano, M.; Narula, J.; Kolodgie, F.D.; Virmani, R. Concept of vulnerable/unstable plaque. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1282–1292. [Google Scholar] [CrossRef]
- Hansson, G.K.; Libby, P.; Schönbeck, U.; Yan, Z.-Q. Innate and adaptive immunity in the pathogenesis of atherosclerosis. Circ. Res. 2002, 91, 281–291. [Google Scholar] [CrossRef]
- Virmani, R.; Burke, A.P.; Kolodgie, F.D.; Farb, A. Vulnerable plaque: The pathology of unstable coronary lesions. J. Interv. Cardiol. 2002, 15, 439–446. [Google Scholar] [CrossRef]
- Virmani, R.; Burke, A.P.; Kolodgie, F.D.; Farb, A. Pathology of the thin-cap fibroatheroma: A type of vulnerable plaque. J. Interv. Cardiol. 2003, 16, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Massy, Z.A.; Ivanovski, O.; Nguyen-Khoa, T.; Angulo, J.; Szumilak, D.; Mothu, N.; Phan, O.; Daudon, M.; Lacour, B.; Drüeke, T.B.; et al. Uremia accelerates both atherosclerosis and arterial calcification in apolipoprotein E knockout mice. J. Am. Soc. Nephrol. 2005, 16, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Nikolov, I.G.; Joki, N.; Nguyen-Khoa, T.; Ivanovski, O.; Phan, O.; Lacour, B.; Drüeke, T.B.; Massy, Z.A.; Dos Reis, L.M.; Jorgetti, V.; et al. Chronic kidney disease bone and mineral disorder (CKD-MBD) in apolipoprotein E-deficient mice with chronic renal failure. Bone 2010, 47, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Stary, H.C.; Chandler, A.B.; Dinsmore, R.E.; Fuster, V.; Glagov, S.; Insull, W., Jr.; Rosenfeld, M.E.; Schwartz, C.J.; Wagner, W.D.; Wissler, R.W. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation 1995, 92, 1355–1374. [Google Scholar] [CrossRef]
- Stary, H.C.; Chandler, A.B.; Glagov, S.; Guyton, J.R.; Insull, W., Jr.; Rosenfeld, M.E.; Schaffer, S.A.; Schwartz, C.J.; Wagner, W.D.; Wissler, R.W. A definition of initial, fatty streak, and intermediate lesions of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler. Thromb. 1994, 14, 840–856. [Google Scholar] [CrossRef]
- Li, H.; Cao, Z.; Wang, L.; Liu, C.; Lin, H.; Tang, Y.; Yao, P. Macrophage Subsets and Death Are Responsible for Atherosclerotic Plaque Formation. Front. Immunol. 2022, 13, 843712. [Google Scholar] [CrossRef]
- Siouti, E.; Andreakos, E. The many facets of macrophages in rheumatoid arthritis. Biochem. Pharmacol. 2019, 165, 152–169. [Google Scholar] [CrossRef]
- Feng, B.; Tabas, I. ABCA1-mediated cholesterol efflux is defective in free cholesterol-loaded macrophages. Mechanism involves enhanced ABCA1 degradation in a process requiring full NPC1 activity. J. Biol. Chem. 2002, 277, 43271–43280. [Google Scholar] [CrossRef]
- McRobb, L.; Handelsman, D.J.; Heather, A.K. Androgen-induced progression of arterial calcification in apolipoprotein E-null mice is uncoupled from plaque growth and lipid levels. Endocrinology 2009, 150, 841–848. [Google Scholar] [CrossRef]
- Davies, M.J.; Thomas, A.C. Plaque fissuring--the cause of acute myocardial infarction, sudden ischaemic death, and crescendo angina. Br. Heart J. 1985, 53, 363–373. [Google Scholar] [CrossRef]
- Meyrelles, S.S.; Peotta, V.A.; Pereira, T.M.C.; Vasquez, E.C. Endothelial dysfunction in the apolipoprotein E-deficient mouse: Insights into the influence of diet, gender and aging. Lipids Health Dis. 2011, 10, 211. [Google Scholar] [CrossRef] [PubMed]
- Tangirala, R.K.; Rubin, E.M.; Palinski, W. Quantitation of atherosclerosis in murine models: Correlation between lesions in the aortic origin and in the entire aorta, and differences in the extent of lesions between sexes in LDL receptor-deficient and apolipoprotein E-deficient mice. J. Lipid Res. 1995, 36, 2320–2328. [Google Scholar] [CrossRef] [PubMed]
- Pereira, T.M.; Nogueira, B.V.; Lima, L.C.F.; Porto, M.L.; Arruda, J.A.; Vasquez, E.C.; Meyrelles, S.S. Cardiac and vascular changes in elderly atherosclerotic mice: The influence of gender. Lipids Health Dis. 2010, 9, 87. [Google Scholar] [CrossRef] [PubMed]
- Bourassa, P.A.; Milos, P.M.; Gaynor, B.J.; Breslow, J.L.; Aiello, R.J. Estrogen reduces atherosclerotic lesion development in apolipoprotein E-deficient mice. Proc. Natl. Acad. Sci. USA 1996, 93, 10022–10027. [Google Scholar] [CrossRef]
- Surra, J.C.; Guillén, N.; Arbonés-Mainar, J.M.; Barranquero, C.; Navarro, M.A.; Arnal, C.; Orman, I.; Segovia, J.C.; Osada, J. Sex as a profound modifier of atherosclerotic lesion development in apolipoprotein E-deficient mice with different genetic backgrounds. J. Atheroscler. Thromb. 2010, 17, 712–721. [Google Scholar] [CrossRef]
- Smith, D.D.; Tan, X.; Tawfik, O.; Milne, G.; Stechschulte, D.J.; Dileepan, K.N.; Tawfik, O.; Milne, G.; Stechschulte, D.J.; Dileepan, K.N. Increased aortic atherosclerotic plaque development in female apolipoprotein E-null mice is associated with elevated thromboxane A2 and decreased prostacyclin production. J. Physiol. Pharmacol. 2010, 61, 309–316. [Google Scholar]


| Parameter | Apoe-/- Mice 26 Weeks (n = 21) | Apoe-/- Mice 52 Weeks (n = 34) | p Value |
|---|---|---|---|
| Heart rate, beats/min | 536.28 ± 6.41 | 568.61 ± 7.23 | 0.003 (**) |
| EDD, mm | 3.04 ± 0.045 | 3.01 ± 0.05 | 0.695 (NS) |
| ESD, mm | 1.60 ± 0.03 | 1.63 ± 0.03 | 0.465 (NS) |
| FS, % | 47.05 ± 0.91 | 45.46 ± 0.66 | 0.157 (NS) |
| PWT, mm | 0.71 ± 0.01 | 0.75 ± 0.01 | 0.009 (**) |
| r/h | 2.12 ± 0.03 | 2.01 ± 0.04 | 0.063(NS) |
| Peak aortic velocity, cm/s | 76.15 ± 2.40 | 86.09 ± 3.78 | 0.649 (NS) |
| Mean aortic velocity, cm/s | 38.26 ± 1.50 | 43.78 ± 2.07 | 0.067(NS) |
| Peak aortic acceleration, m/s2 | 104.93 ± 2.55 | 105.77 ± 2.07 | 0.809 (NS) |
| Peak carotid velocity, cm/s | 70.23 ± 3.06 | 74.05 ± 2.22 | 0.308 (NS) |
| Mean carotid velocity, cm/s | 27.81 ± 1.33 | 31.67 ± 1.05 | 0.027 (*) |
| Carotid pulsatility index | 2.69 ± 0.13 | 2.27 ± 0.11 | 0.019 (*) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotsovilis, S.; Salagianni, M.; Varela, A.; Davos, C.H.; Galani, I.E.; Andreakos, E. Comprehensive Analysis of 1-Year-Old Female Apolipoprotein E-Deficient Mice Reveals Advanced Atherosclerosis with Vulnerable Plaque Characteristics. Int. J. Mol. Sci. 2024, 25, 1355. https://doi.org/10.3390/ijms25021355
Kotsovilis S, Salagianni M, Varela A, Davos CH, Galani IE, Andreakos E. Comprehensive Analysis of 1-Year-Old Female Apolipoprotein E-Deficient Mice Reveals Advanced Atherosclerosis with Vulnerable Plaque Characteristics. International Journal of Molecular Sciences. 2024; 25(2):1355. https://doi.org/10.3390/ijms25021355
Chicago/Turabian StyleKotsovilis, Sotirios, Maria Salagianni, Aimilia Varela, Constantinos H. Davos, Ioanna E. Galani, and Evangelos Andreakos. 2024. "Comprehensive Analysis of 1-Year-Old Female Apolipoprotein E-Deficient Mice Reveals Advanced Atherosclerosis with Vulnerable Plaque Characteristics" International Journal of Molecular Sciences 25, no. 2: 1355. https://doi.org/10.3390/ijms25021355
APA StyleKotsovilis, S., Salagianni, M., Varela, A., Davos, C. H., Galani, I. E., & Andreakos, E. (2024). Comprehensive Analysis of 1-Year-Old Female Apolipoprotein E-Deficient Mice Reveals Advanced Atherosclerosis with Vulnerable Plaque Characteristics. International Journal of Molecular Sciences, 25(2), 1355. https://doi.org/10.3390/ijms25021355






