Identification of Sex-Specific Markers and Candidate Genes Using WGS Sequencing Reveals a ZW-Type Sex-Determination System in the Chinese Soft-Shell Turtle (Pelodiscus sinensis)
Abstract
1. Introduction
2. Results
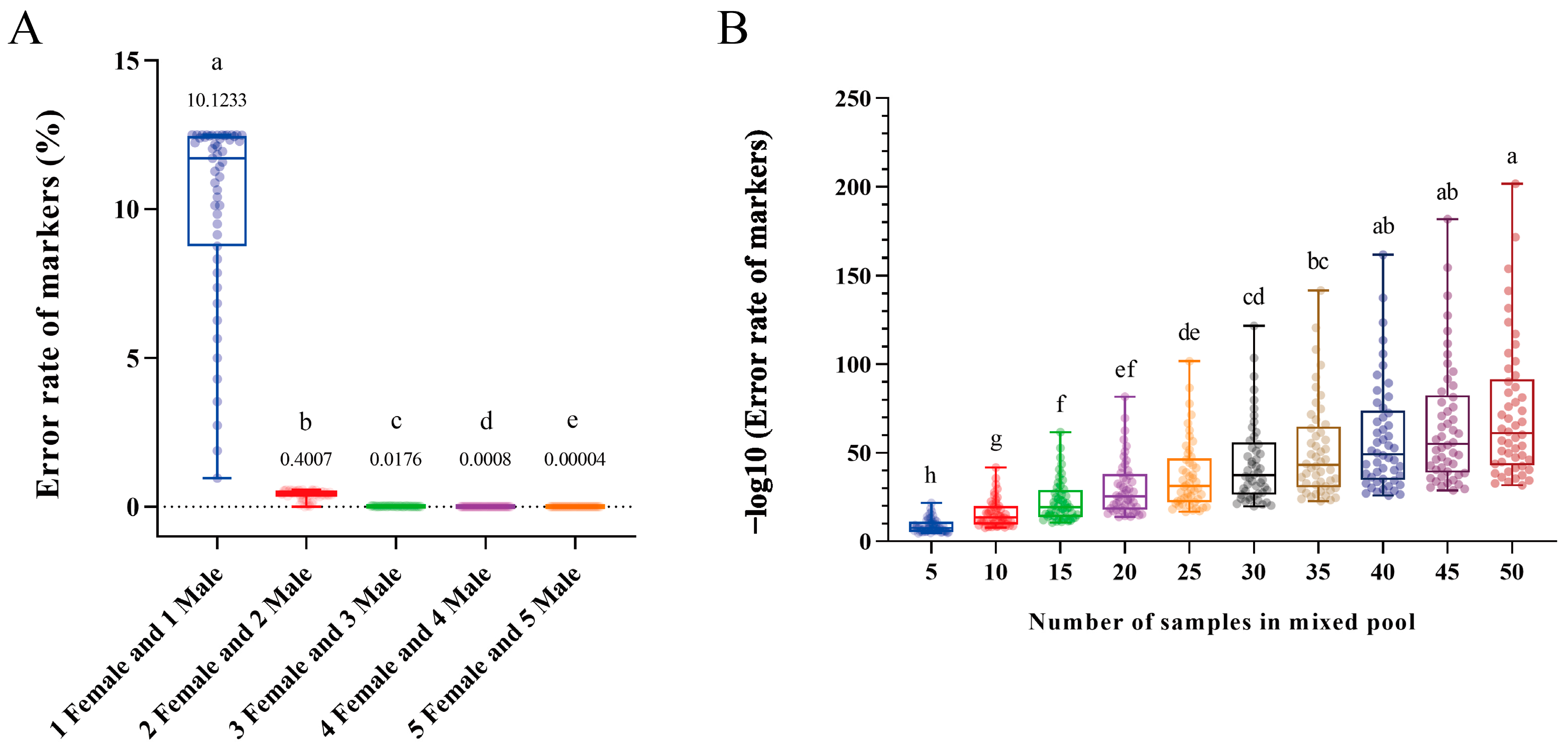
2.1. Results of the Mathematical Simulation
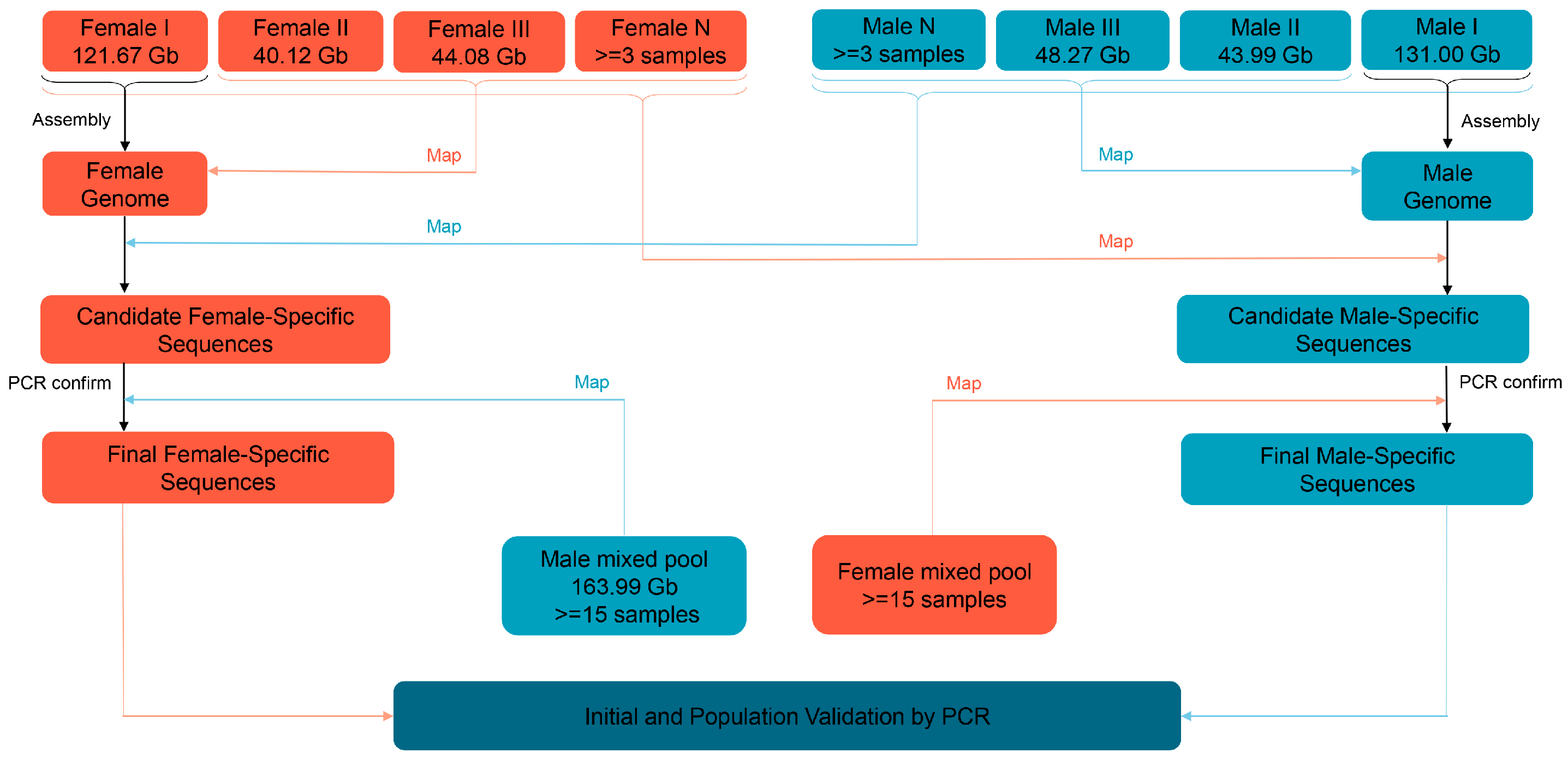
2.2. Genome Sequencing and Assembly
2.3. Screening the Sex-Specific Sequences
2.4. Validation of Female-Specific Markers in Adult P. sinensis
2.5. Validation of Female-Specific Markers during the Embryonic Stages of P. sinensis
2.6. Functional Annotation of Sex-Determining Candidate Genes
3. Discussion
4. Materials and Methods
4.1. Theories and Mathematical Models
4.2. Experimental Turtles and Eggs
4.3. DNA Sequencing and Genome Assembly
4.4. Sex-Specific Sequence Screening
4.5. Validation of Female Sex-Specific Sequences in P. sinensis
4.6. GO and KEGG Annotation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bureau of Fisheries, Ministry of Agriculture and Rural Affairs of PR China. China Fisheries Statistics Yearbook; China Agriculture Press: Beijing, China, 2023; p. 24.
- Zhu, J.; Wang, Y.; Lei, L.; Chen, C.; Ji, L.; Li, J.; Wu, C.; Yu, W.; Luo, L.; Chen, W.; et al. Comparative genomic survey and functional analysis of DKKL1 during spermatogenesis in the Chinese soft-shelled turtle (Pelodiscus sinensis). Int. J. Biol. Macromol. 2023, 254, 127696. [Google Scholar] [CrossRef]
- Zhou, X.; Zhu, Y. Sex-specific growth characteristics of Chinese soft-shelled turtle, Pelodiscus sinensis. J. Aquacul. 2011, 32, 11–13. [Google Scholar]
- Zhu, J.; Lei, L.; Chen, C.; Wang, Y.; Liu, X.; Geng, L.; Li, R.; Chen, H.; Hong, X.; Yu, L.; et al. Whole-transcriptome analysis identifies gender dimorphic expressions of mRNAs and Non-Coding RNAs in Chinese Soft-Shell Turtle (Pelodiscus sinensis). Biology 2022, 11, 834. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yang, J.; Song, W.; Ren, J.; Fang, C.; Qian, G. Incubation temperature affects the development of calipash related traits and collagen deposition in embryos of soft-shelled turtle Pelodiscus sinensis. Aquaculture 2020, 528, 735546. [Google Scholar] [CrossRef]
- Lei, L.; Zhu, J.; Chen, C.; Wang, Y.; Wu, C.; Qi, M.; Wang, Y.; Liu, X.; Hong, X.; Yu, L.; et al. Genome-wide identification, evolution and expression analysis of bone morphogenetic protein (BMP) gene family in chinese soft-shell turtle (Pelodiscus sinensis). Front. Genet. 2023, 14, 1109478. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Wang, L.; Sha, H.; Zou, G. Development and validation of sex-specific markers in Pelodiscus sinensis using restriction site-associated DNA sequencing. Genes 2019, 10, 302. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Gui, J. Genetic basis and biotechnological manipulation of sexual dimorphism and sex determination in fish. Sci. China Life Sci. 2015, 58, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Mei, J.; Ge, C.; Liu, X.; Gui, J. Sex determination mechanisms and sex control approaches in aquaculture animals. Sci. China Life Sci. 2022, 65, 1091–1122. [Google Scholar] [CrossRef]
- Dan, Z.; Wang, X. Status and advances of genetics and breeding research for Pelodiscus sinensis. J. Nat. Sci. Hunan Norm. Univ. 2017, 40, 40–44. [Google Scholar]
- Nie, L.; Guo, C.; Wang, M.; Wang, Q. Sex determination mechanism of Trionyx sinensis. Chin. J. Appl. Environ. Biol. 2001, 3, 258–261. [Google Scholar]
- Zheng, J.; Zhu, M. Isolation and sequence analysis of the Sox-1, -2, -3 homologs in Trionyx sinensis and Alligator sinensis having temperature-dependent sex determination. Biochem. Genet. 2006, 44, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Sun, X. Sex determination in Trionyx sinensis. Chin. J. Zool. 2000, 6, 37–38. [Google Scholar]
- Kawagoshi, T.; Uno, Y.; Matsubara, K.; Matsuda, Y.; Nishida, C.; Research, G. The ZW micro-sex chromosomes of the Chinese soft-shelled turtle (Pelodiscus sinensis, Trionychidae, Testudines) have the same origin as chicken chromosome 15. Cytogenet. Genome Res. 2009, 125, 125–131. [Google Scholar] [CrossRef]
- Kawagoshi, T.; Nishida, C.; Matsuda, Y. The origin and differentiation process of X and Y chromosomes of the black marsh turtle (Siebenrockiella crassicollis, Geoemydidae, Testudines). Chromosome Res. 2012, 20, 95–110. [Google Scholar] [CrossRef][Green Version]
- Literman, R.; Badenhorst, D.; Valenzuela, N. qPCR-based molecular sexing by copy number variation in rRNA genes and its utility for sex identification in soft-shell turtles. Methods Ecol. Evol. 2015, 5, 872–880. [Google Scholar] [CrossRef]
- Rovatsos, M.; Praschag, P.; Fritz, U.; Kratochvšl, L. Stable Cretaceous sex chromosomes enable molecular sexing in softshell turtles (Testudines: Trionychidae). Sci. Rep. 2017, 7, 42150. [Google Scholar] [CrossRef] [PubMed]
- Badenhorst, D.; Stanyon, R.; Engstrom, T.; Valenzuela, N. A ZZ/ZW microchromosome system in the spiny softshell turtle, Apalone spinifera, reveals an intriguing sex chromosome conservation in Trionychidae. Chromosome Res. 2013, 21, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Zhao, B.; Tang, W.; Sun, B.; Zeng, Z.; Valenzuela, N.; Du, W. Temperature-dependent sex determination ruled out in the Chinese soft-shelled turtle (Pelodiscus sinensis) via molecular cytogenetics and incubation experiments across populations. Sex. Dev. 2015, 9, 111–117. [Google Scholar] [CrossRef]
- Liao, X.; Xu, G.; Chen, S. Molecular method for sex identification of half-smooth tongue sole (Cynoglossus semilaevis) using a novel sex-linked microsatellite marker. Int. J. Mol. Sci. 2014, 15, 12952–12958. [Google Scholar] [CrossRef]
- Gamble, T. Using RAD-seq to recognize sex-specific markers and sex chromosome systems. Mol. Ecol. 2016, 25, 2114–2116. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, Y.; Wang, Y.; Jiang, S.; Zhang, C.; Li, B. GWAS reveal novel sex-related markers and candidate genes in sea urchin Mesocentrotus nudus. Mar. Biotechnol. 2022, 24, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.; Qiang, J.; Tao, Y.; Li, Y.; Lu, S.; Bing, X. Development and validation of a PCR-RFLP/TaqMan MGB probe method for rapid sex identification of largemouth bass (Micropterus salmoides). Aquacult. Rep. 2023, 30, 101593. [Google Scholar] [CrossRef]
- Han, J.; Jang, H.; Cheong, S.; Kim, S.; Park, S.; Na, K. Sex determination by PCR-RFLP in the oriental white stork Ciconia boyciana. Zool. Stud. 2009, 48, 619–624. [Google Scholar]
- Kovács, B.; Egedi, S.; Bártfai, R.; Orbán, L. Male-specific DNA markers from African catfish (Clarias gariepinus). Genetica 2000, 110, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Vale, L.; Dieguez, R.; Sánchez, L.; Martínez, P.; Viñas, A. A sex-associated sequence identified by RAPD screening in gynogenetic individuals of turbot (Scophthalmus maximus). Mol. Biol. Rep. 2014, 41, 1501–1509. [Google Scholar] [CrossRef]
- Lee, B.; Coutanceau, J.; Ozouf-Costaz, C.; D’Cotta, H.; Baroiller, J.; Kocher, T. Genetic and physical mapping of sex-linked AFLP markers in Nile tilapia (Oreochromis niloticus). Mar. Biotechnol. 2011, 13, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Felip, A.; Martínez-Rodríguez, G.; Piferrer, F.; Carrillo, M.; Zanuy, S. AFLP analysis confirms exclusive maternal genomic contribution of meiogynogenetic sea bass (Dicentrarchus labrax L.). Mar. Biotechnol. 2000, 2, 301–306. [Google Scholar] [CrossRef]
- Cui, Z.; Zhang, J.; Sun, Z.; Liu, B.; Zhao, C.; Chang, Y. Identification of sex-specific markers through 2b-RAD sequencing in the sea urchin (Mesocentrotus nudus). Front. Genet. 2021, 12, 717538. [Google Scholar] [CrossRef]
- Flachowsky, H.; Schumann, E.; Weber, W.; Peil, A. Application of AFLP for the detection of sex-specific markers in hemp. Plant Breed. 2001, 120, 305–309. [Google Scholar] [CrossRef]
- Gamble, T.; Zarkower, D. Identification of sex-specific molecular markers using restriction site-associated DNA sequencing. Mol. Ecol. Resour. 2014, 14, 902–913. [Google Scholar] [CrossRef]
- Hu, Q.; Chang, C.; Wang, Q.; Tian, H.; Qiao, Z.; Wang, L.; Meng, Y.; Xu, C.; Xiao, H. Genome-wide RAD sequencing to identify a sex-specific marker in Chinese giant salamander Andrias davidianus. BMC Genom. 2019, 20, 415. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wu, W.; Ning, Z.; Zhou, R. Identification and characterization of sex-specific markers in the milky mangrove Excoecaria agallocha using double digest restriction site-associated DNA sequencing. Aquat. Bot. 2018, 144, 54–60. [Google Scholar] [CrossRef]
- Fowler, B.; Buonaccorsi, V. Genomic characterization of sex-identification markers in Sebastes carnatus and Sebastes chrysomelas rockfishes. Mol. Ecol. 2016, 25, 2165–2175. [Google Scholar] [CrossRef]
- Wang, T.; Li, Z.; Yu, Z.; Wang, Z.; Lian, Z.; Du, W.; Zhao, X.; Wang, M.; Miao, C.; Ding, M.; et al. Production of YY males through self-fertilization of an occasional hermaphrodite in Lanzhou catfish (Silurus lanzhouensis). Aquaculture 2021, 539, 736622. [Google Scholar] [CrossRef]
- Li, S.; Xu, L.; Shi, Y.; Chen, J. Male-specific markers developed by next-generation sequencing confirmed an XX/XY sex-determination system in farmed ayu (Plecoglossus altivelis). Aquaculture 2021, 541, 736822. [Google Scholar] [CrossRef]
- Lin, A.; Xiao, S.; Xu, S.; Ye, K.; Lin, X.; Sun, S.; Wang, Z. Identification of a male-specific DNA marker in the large yellow croaker (Larimichthys crocea). Aquaculture 2017, 480, 116–122. [Google Scholar] [CrossRef]
- Suda, A.; Nishiki, I.; Iwasaki, Y.; Matsuura, A.; Akita, T.; Suzuki, N.; Fujiwara, A. Improvement of the Pacific bluefin tuna (Thunnus orientalis) reference genome and development of male-specific DNA markers. Sci. Rep. 2019, 9, 14450. [Google Scholar] [CrossRef]
- Han, C.; Zhu, Q.; Zhou, X.; Ouyang, H.; Zhang, Y. A PCR-based genetic sex identification method in spotted mandarin fish (Siniperca scherzeri) and big eye mandarin fish (Siniperca kneri). Aquacult. Rep. 2020, 18, 100552. [Google Scholar] [CrossRef]
- Li, X.; Gui, J. Diverse and variable sex determination mechanisms in vertebrates. Sci. China Life Sci. 2018, 61, 1503–1514. [Google Scholar] [CrossRef]
- Han, C.; Zhou, X.; Lu, H.; Zhu, Q.; Han, L.; Li, S.; Lin, H.; Zhang, Y. A simple PCR-based genetic sex identification method in the blotched snakehead (Channa maculata) developed by high-throughput sequencing. Aquaculture 2021, 538, 736579. [Google Scholar] [CrossRef]
- Grover, A.; Sharma, P. Development and use of molecular markers: Past and present. Crit. Rev. Biotechnol. 2016, 36, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Azevedo, L. Molecular markers: An overview of data published for Fungi over the last ten years. J. Fungi 2022, 8, 803. [Google Scholar] [CrossRef] [PubMed]
- Ou, M.; Yang, C.; Luo, Q.; Huang, R.; Zhang, A.; Liao, L.; Li, Y.; He, L.; Zhu, Z.; Chen, K.; et al. An NGS-based approach for the identification of sex-specific markers in snakehead (Channa argus). Oncotarget 2017, 8, 98733–98744. [Google Scholar] [CrossRef] [PubMed]
- Ou, M.; Chen, K.; Gao, D.; Wu, Y.; Chen, Z.; Luo, Q.; Liu, H.; Zhao, J. Comparative transcriptome analysis on four types of gonadal tissues of blotched snakehead (Channa maculata). Comp. Biochem. Physiol. Part D Genom. Proteom. 2020, 35, 100708. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Wang, Y.; Su, B.; Zhao, C.; Yin, D.; Chen, C.; Yang, Y.; Wang, C.; Luo, B.; Wang, H.; et al. Gap-free genome assembly of anadromous Coilia nasus. Sci. Data 2023, 10, 360. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Liu, H.; Wang, Y.; Li, M.; Ji, L.; Wang, K.; Wei, C.; Li, W.; Chen, C.; Yu, L.; et al. A chromosome-level genome assembly of the Asian giant softshell turtle Pelochelys cantorii. Sci. Data 2023, 10, 754. [Google Scholar] [CrossRef] [PubMed]
- Star, B.; Tørresen, O.; Nederbragt, A.; Jakobsen, K.; Pampoulie, C.; Jentoft, S. Genomic characterization of the Atlantic cod sex-locus. Sci. Rep. 2016, 6, 31235. [Google Scholar] [CrossRef]
- Xing, T.; Li, Y.; Liu, J. Female-specific genomic regions and molecular sex identification of the clearhead icefish (Protosalanx hyalocranius). BMC Genom. 2021, 22, 495. [Google Scholar] [CrossRef]
- Weng, X.; Xu, Y.; Dong, X.; Luo, X.; You, W.; Ke, C.; Cai, M. Sex-specific markers developed by next-generation sequencing confirmed a male heterogametic sex determination in small abalone, Haliotis diversicolor. Aquaculture 2022, 555, 738256. [Google Scholar] [CrossRef]
- Du, J.; Liu, Q.; Zheng, Y. Screening and characterization of sex-specific sequences through 2b-RAD sequencing in American shad (Alosa sapidissima). PLoS ONE 2023, 18, e0282165. [Google Scholar] [CrossRef]
- Brown, J.; Taggart, J.; Bekaert, M.; Wehner, S.; Palaiokostas, C.; Setiawan, A.; Symonds, J.; Penman, D. Mapping the sex determination locus in the hāpuku (Polyprion oxygeneios) using ddRAD sequencing. BMC Genom. 2016, 17, 448. [Google Scholar] [CrossRef]
- Augstenová, B.; Johnson Pokorná, M.; Altmanová, M.; Frynta, D.; Rovatsos, M.; Kratochvíl, L. ZW, XY, and yet ZW: Sex chromosome evolution in snakes even more complicated. Evolution 2018, 72, 1701–1707. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Pascual-Anaya, J.; Zadissa, A.; Li, W.; Niimura, Y.; Huang, Z.; Li, C.; White, S.; Xiong, Z.; Fang, D.; et al. The draft genomes of soft-shell turtle and green sea turtle yield insights into the development and evolution of the turtle-specific body plan. Nat. Genet. 2013, 45, 701–706. [Google Scholar] [CrossRef]
- Sun, W.; Cai, H.; Zhang, G.; Zhang, H.; Bao, H.; Wang, L.; Ye, J.; Qian, G.; Ge, C. Dmrt1 is required for primary male sexual differentiation in Chinese soft-shelled turtle Pelodiscus sinensis. Sci. Rep. 2017, 7, 4433. [Google Scholar] [CrossRef] [PubMed]
- Zuo, F.; Shen, Y.; Wang, W. A minimally-invasive protocol for molecular sex identification of the Chinese soft-shelled turtle: Pelodiscus sinensis. Freshw. Sci. 2020, 39, 147–155. [Google Scholar] [CrossRef]
- Montiel, E.; Badenhorst, D.; Tamplin, J.; Burke, R.; Valenzuela, N. Discovery of the youngest sex chromosomes reveals first case of convergent co-option of ancestral autosomes in turtles. Chromosoma 2017, 126, 105–113. [Google Scholar] [CrossRef]
- Kawai, A.; Nishida-Umehara, C.; Ishijima, J.; Tsuda, Y.; Ota, H.; Matsuda, Y. Different origins of bird and reptile sex chromosomes inferred from comparative mapping of chicken Z-linked genes. Cytogenet. Genome Res. 2007, 117, 92–102. [Google Scholar] [CrossRef]
- Jin, L.; Sun, W.; Bao, H.; Liang, X.; Li, P.; Shi, S.; Wang, Z.; Qian, G.; Ge, C. The forkhead factor Foxl2 participates in the ovarian differentiation of Chinese soft-shelled turtle Pelodiscus sinensis. Dev. Biol. 2022, 492, 101–110. [Google Scholar] [CrossRef]
- Zhou, Y.; Sun, W.; Cai, H.; Bao, H.; Zhang, Y.; Qian, G.; Ge, C. The role of anti-mullerian hormone in testis differentiation reveals the significance of the TGF-beta pathway in reptilian sex determination. Genetics 2019, 213, 1317–1327. [Google Scholar] [CrossRef]
- Hsiao, P.; Lin, D.; Nakao, R.; Chang, C. The linkage of Kennedy’s neuron disease to ARA24, the first identified androgen receptor polyglutamine region-associated coactivator. J. Biol. Chem. 1999, 274, 20229–20234. [Google Scholar] [CrossRef]
- Harada, N.; Ohmori, Y.; Yamaji, R.; Higashimura, Y.; Okamoto, K.; Isohashi, F.; Nakano, Y.; Inui, H. ARA24/Ran enhances the androgen-dependent NH2- and COOH-terminal interaction of the androgen receptor. Biochem. Biophys. Res. Commun. 2008, 373, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Jans, D.A. Dual nuclear import mechanisms of sex determining factor SRY: Intracellular Ca2+ as a switch. FASEB J. 2011, 25, 665–675. [Google Scholar] [CrossRef]
- Hanover, J.; Love, D.; Prinz, W. Calmodulin-driven nuclear entry: Trigger for sex determination and terminal differentiation. J. Biol. Chem. 2009, 284, 12593–12597. [Google Scholar] [CrossRef] [PubMed]
- Dostie, J.; Ferraiuolo, M.; Pause, A.; Adam, S.; Sonenberg, N. A novel shuttling protein, 4E-T, mediates the nuclear import of the mRNA 5’ cap-binding protein, eIF4E. EMBO J. 2000, 19, 3142–3156. [Google Scholar] [CrossRef] [PubMed]
- Penalva, L.; Sánchez, L. RNA binding protein sex-lethal (Sxl) and control of Drosophila sex determination and dosage compensation. Microbiol. Mol. Biol. Rev. 2003, 67, 343–359. [Google Scholar] [CrossRef] [PubMed]
- Graham, P.; Yanowitz, J.; Penn, J.; Deshpande, G.; Schedl, P. The translation initiation factor eIF4E regulates the sex-specific expression of the master switch gene Sxl in Drosophila melanogaster. PLoS Genet. 2011, 7, e1002185. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Che, Y.; Li, G.; Liu, B.; Shen, T.; Wang, H.; Linghu, H. Crk and CrkL present with different expression and significance in epithelial ovarian carcinoma. Mol. Carcinog. 2011, 50, 506–515. [Google Scholar] [CrossRef]
- Wang, S.; Tang, W.; Ma, L.; Yang, J.; Huang, K.; Du, X.; Luo, A.; Shen, W.; Ding, T.; Ye, S.; et al. MiR-145 regulates steroidogenesis in mouse primary granulosa cells through targeting Crkl. Life Sci. 2021, 282, 119820. [Google Scholar] [CrossRef]
- Jiang, Q.; Zheng, H.; Zheng, L.; Wang, Y.; Wang, M.; Xie, X.; Zhu, D. Molecular characterization of the insulin-like androgenic gland hormone in the swimming crab, Portunus trituberculatus, and its involvement in the insulin signaling system. Front. Endocrinol. 2020, 11, 585. [Google Scholar] [CrossRef]
- Nef, S.; Verma-Kurvari, S.; Merenmies, J.; Vassalli, J.; Efstratiadis, A.; Accili, D.; Parada, L. Testis determination requires insulin receptor family function in mice. Nature 2003, 426, 291–295. [Google Scholar] [CrossRef]
- Neirijnck, Y.; Papaioannou, M.; Nef, S. The Insulin/IGF system in mammalian sexual development and reproduction. Int. J. Mol. Sci. 2019, 20, 4440. [Google Scholar] [CrossRef] [PubMed]
- Pitetti, J.; Calvel, P.; Romero, Y.; Conne, B.; Truong, V.; Papaioannou, M.; Schaad, O.; Docquier, M.; Herrera, P.; Wilhelm, D.; et al. Insulin and IGF1 receptors are essential for XX and XY gonadal differentiation and adrenal development in mice. PLoS Genet. 2013, 9, e1003160. [Google Scholar] [CrossRef]
- Rosner, W.; Hryb, D.J.; Khan, M.; Nakhla, A.; Romas, N. Androgen and estrogen signaling at the cell membrane via G-proteins and cyclic adenosine monophosphate. Steroids 1999, 64, 100–106. [Google Scholar] [CrossRef]
- Roy, L.; McDonald, C.; Jiang, C.; Maroni, D.; Zeleznik, A.; Wyatt, T.; Hou, X.; Davis, J. Convergence of 3′,5′-cyclic adenosine 5′-monophosphate/protein kinase A and glycogen synthase kinase-3beta/beta-catenin signaling in corpus luteum progesterone synthesis. Endocrinology 2009, 150, 5036–5045. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wei, C.; Zhao, W.; Du, H.; Chen, Y. Effects of incubation temperatures on embryonic development in the Asian yellow pond turtle. Aquaculture 2006, 259, 243–248. [Google Scholar] [CrossRef]
- Zhu, J.; Lei, L.; Chen, C.; Li, J.; Wang, Y.; Wu, C.; Wang, Y.; Hong, X.; Liu, X.; Yu, L.; et al. Development and evaluation of a medium-free incubation method for hatching Chinese soft-shelled turtle (Pelodiscus sinensis) eggs. Aquacult. Rep. 2023, 31, 101643. [Google Scholar] [CrossRef]
- Tokita, M.; Kuratani, S. Normal embryonic stages of the Chinese Soft-shelled turtle Pelodiscus sinensis (Trionychidae). Zoolog. Sci. 2001, 18, 705–715. [Google Scholar] [CrossRef]
- Luo, R.; Liu, B.; Xie, Y.; Li, Z.; Huang, W.; Yuan, J.; He, G.; Chen, Y.; Pan, Q.; Liu, Y.; et al. SOAPdenovo2: An empirically improved memory-efficient short-read de novo assembler. Gigascience 2012, 1, 18. [Google Scholar] [CrossRef]
- Marçais, G.; Kingsford, C. A fast, lock-free approach for efficient parallel counting of occurrences of k-mers. Bioinformatics 2011, 27, 764–770. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.; Remm, M.; Rozen, S. Primer 3-new capabilities and interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Trapnell, C.; Pop, M.; Salzberg, S. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009, 10, R25. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Chen, C.; Liu, X.; Wang, Y.; Lei, L.; Hong, X.; Yu, L.; Xu, H.; Li, W.; Zhu, X. Cloning and expression analysis of histone H2A variant in oocytes of Chinese soft-shelled turtle (Pelodiscus sinensis). Acta Hydrobiol. Sin. 2021, 45, 1207–1213. [Google Scholar]
- Ashburner, M.; Ball, C.; Blake, J.; Botstein, D.; Butler, H.; Cherry, J.; Davis, A.; Dolinski, K.; Dwight, S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]





| Name | Sequence (5′-3′) | TM (°C) |
|---|---|---|
| P44-F | GTTTCGAGTTTAGGGTTC | 56 |
| P44-R | GTGCCAATCCCTCTGTAT | |
| P45-F | TCTTGTAGTCGGATAGGC | 58 |
| P45-R | CAATAGACGGTTGTACTGAA | |
| P66-F | GCCTCGTACATTGTGATT | 56 |
| P66-R | TGTAGACAAGCCAAATCC | |
| P67-F | CCACCATCACCAGCACAT | 60 |
| P67-R | AAATGAGCTGGTAGTCTGG | |
| P68-F | CTCAAATACAGAATGGGATG | 56 |
| P68-R | CCATCCTTCGGACACTACA | |
| P69-F | TCCCTAAGGAGGTCTTCACG | 60 |
| P69-R | CATTCGGCTGCTTGGTGA | |
| PB1-F | GGATCTCATTTGTGAGCCTACATGT | 62 |
| PB1-R | CCCACAGCTTGCTTTCCWTGTTTAG |
| GSD | Female (Aa) | Male (AA/aa) | Female (AA/aa) | Male (Aa) |
|---|---|---|---|---|
| ZW/XY |
| GSD | Mathematical Model |
|---|---|
| ZW | |
| XY | |
| ZW or XY |
| GSD | Mathematical Model |
|---|---|
| ZW | |
| XY |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Wang, Y.; Chen, C.; Ji, L.; Hong, X.; Liu, X.; Chen, H.; Wei, C.; Zhu, X.; Li, W. Identification of Sex-Specific Markers and Candidate Genes Using WGS Sequencing Reveals a ZW-Type Sex-Determination System in the Chinese Soft-Shell Turtle (Pelodiscus sinensis). Int. J. Mol. Sci. 2024, 25, 819. https://doi.org/10.3390/ijms25020819
Zhu J, Wang Y, Chen C, Ji L, Hong X, Liu X, Chen H, Wei C, Zhu X, Li W. Identification of Sex-Specific Markers and Candidate Genes Using WGS Sequencing Reveals a ZW-Type Sex-Determination System in the Chinese Soft-Shell Turtle (Pelodiscus sinensis). International Journal of Molecular Sciences. 2024; 25(2):819. https://doi.org/10.3390/ijms25020819
Chicago/Turabian StyleZhu, Junxian, Yongchang Wang, Chen Chen, Liqin Ji, Xiaoyou Hong, Xiaoli Liu, Haigang Chen, Chengqing Wei, Xinping Zhu, and Wei Li. 2024. "Identification of Sex-Specific Markers and Candidate Genes Using WGS Sequencing Reveals a ZW-Type Sex-Determination System in the Chinese Soft-Shell Turtle (Pelodiscus sinensis)" International Journal of Molecular Sciences 25, no. 2: 819. https://doi.org/10.3390/ijms25020819
APA StyleZhu, J., Wang, Y., Chen, C., Ji, L., Hong, X., Liu, X., Chen, H., Wei, C., Zhu, X., & Li, W. (2024). Identification of Sex-Specific Markers and Candidate Genes Using WGS Sequencing Reveals a ZW-Type Sex-Determination System in the Chinese Soft-Shell Turtle (Pelodiscus sinensis). International Journal of Molecular Sciences, 25(2), 819. https://doi.org/10.3390/ijms25020819






