Abstract
Vitellogenin (Vtg) is a precursor of yolk proteins in egg-laying vertebrates and invertebrates and plays an important role in vitellogenesis and embryonic development. However, the Vtg family remains poorly characterized in Exopalaemon carinicauda, a major commercial mariculture species found along the coasts of the Yellow and Bohai Seas. In this study, 10 Vtg genes from the genomes of E. carinicauda were identified and characterized. Phylogenetic analyses showed that the Vtg genes in crustaceans could be classified into four groups: Astacidea, Brachyra, Penaeidae, and Palaemonidae. EcVtg genes were unevenly distributed on the chromosomes of E. carinicauda, and a molecular evolutionary analysis showed that the EcVtg genes were primarily constrained by purifying selection during evolution. All putative EcVtg proteins were characterized by the presence of three conserved functional domains: a lipoprotein N-terminal domain (LPD_N), a domain of unknown function (DUF1943), and a von Willebrand factor type D domain (vWD). All EcVtg genes exhibited higher expression in the female hepatopancreas than in other tissues, and EcVtg gene expression during ovarian development suggested that the hepatopancreas is the main synthesis site in E. carinicauda. EcVtg1a, EcVtg2, and EcVtg3 play major roles in exogenous vitellogenesis, and EcVtg3 also plays a major role in endogenous vitellogenesis. Bilateral ablation of the eyestalk significantly upregulates EcVtg mRNA expression in the female hepatopancreas, indicating that the X-organ/sinus gland complex plays an important role in ovarian development, mostly by inducing Vtg synthesis. These results could improve our understanding of the function of multiple Vtg genes in crustaceans and aid future studies on the function of EcVtg genes during ovarian development in E. carinicauda.
1. Introduction
Vitellogenin (Vtg) is a major precursor of vitellin, which is a source of yolk nutrients for the development of ovaries and embryos in almost all oviparous organisms [1]. Vtg is generally present as a female-specific protein that binds to sugar, phosphorus, and lipids and acts as the major nutrient and energy source for early embryogenesis. In addition, an increasing number of studies have reported several non-nutritional roles of multiple Vtg genes, including in antioxidant stress responses and antibacterial activity [2,3,4]. The Vtg protein belongs to the lipid transporter superfamily and shares a conserved structural domain including a vitellogenin N-terminal domain, also known as the lipoprotein N-terminal domain (LPD_N), a domain of unknown function (DUF1943), and a von Willebrand factor type D domain (vWD) [5]. LPD_N is required for interaction with the Vtg receptor in Macrobrachium rosenbergii [6], which promotes the transport of Vg to oocytes, and also plays a conserved role in receptor recognition in other vertebrates and invertebrates [7]. DUF1943 and vWD play critical roles in pathogen recognition [8,9,10]. These studies indicate that Vtg, in addition to its involvement in yolk protein formation, also possibly plays non-nutritional roles for its conserved structural domain.
The process of yolk synthesis and accumulation in oocytes, known as vitellogenesis, plays a crucial role in the reproductive success of oviparous species. The Vtg gene family comprises various paralogs, exhibiting species–specific differences in both the structure and quantity of Vtg proteins among different oviparous vertebrates [1]. Most fish possess a tripartite Vtg system (VtgAa, VtgAb, and VtgC) in which all three forms of Vtg make a different contribution to the yolk. Different types of Vtg are essential and have disparate requisite functions at different times of development in fish [11]. The VtgAa is largely degraded into free amino acids during ovarian maturation. In contrast, the major yolks derived from VtgAb and VtgC remain largely intact and serve as yolk nutrients for later-stage embryos and larvae [12]. Genome editing reveals reproductive and developmental dependencies on specific types of vitellogenin in zebrafish (Danio rerio) [13]. Understanding the differences between variation of Vtg in mechanisms of yolk formation and processing is a benefit for the regulation of ovarian maturation and embryos and larvae development. Similar to in fish, previous studies on Metapenaeus ensis [14] and Pandalopsis japonica [15] have suggested the presence of multiple Vtg genes in these crustaceans, while most studies on the function of the Vtg gene in crustaceans have concentrated on a single Vtg gene. Meanwhile, the function of different vitellinogen genes in nutritional and non-nutritional roles in crustaceans has been little studied. Thus, considering their function in reproduction, the identification of the Vtg gene family in crustaceans and the analysis of their functions in ovarian development hold significant importance.
The ridgetail white shrimp, Exopalaemon carinicauda, belonging to the Palaemonidae family of crustaceans, is a major commercial mariculture species that is naturally distributed along the coasts of the Yellow Sea and Bohai Sea [16]. In addition to its economic value, E. carinicauda is a useful experimental organism in crustacean developmental biology because of its transparent body, large eggs, high reproductive capacity, and short reproductive cycle of only two to three months [17,18]. Female shrimps undergo at least three consecutive ovarian developmental stages during the reproductive cycle, and the ovaries of some shrimps gradually atrophy into cystic tubes after the first ovarian maturation stage. Several practical problems related to reproductive biology need to be solved in E. carinicauda. The precocious puberty usually occurred in the aquaculture of E. carinicauda, which affects its normal growth rate. The nonsynchronous ovarian development and spawning increase the consumption of manpower and financial resources of seeding. It is vital to better understand the regulatory mechanisms of vitellogenesis and ovarian development in E. carinicauda. The Vtg gene plays an important role in ovarian and embryo development in crustaceans [19], thus the Vtgs must be identified and clarified in E. carinicauda.
In recent years, many crustacean genomes have been sequenced and made available, providing a foundation for the identification and characterization of gene families at the whole-genome level [20,21]. However, only the Vtg-family genes of M. rosenbergii have been identified and characterized [22]. Selective regulation of multiple-Vtg uptake and processing during oocyte growth and ovarian maturation is critical to egg quality and developmental success. Differences in gene function and regulation amongst the different Vtg might have important physiological or biological effects [23]. There is significant variability in functioning of Vtg family members among the same tissue of different species, and in different tissues and developmental stages of the same species [12,24]. In E. carinicauda, only a single Vtg transcript has so far been identified [19]. The Vtg structure remains unknown, and as does whether multiple Vtgs are present in the E. carinicauda genome with contribution to vitellogenesis or not. Therefore, genome-wide identification, and investigation of the EcVtg-family genes in the E. carinicauda genome were performed in this study. The combination of long-read Pacific Biosciences and Hi-C technologies enabled the successful assembly of a high-quality genome sequence in E. carinicauda in our previous study. The final assembly covered 5.86 Gb, which was submitted to the NCBI genome database (PRJNA1055619). Firstly, the evolutionary relationships, gene structures, conserved domains, chromosomal locations, and motifs were analyzed to comprehensively understand the differences in the structure and potential function of Vtg family members using the genome sequences. In addition, we investigated gene expression patterns in different tissues and different embryo-development stages to identify their nutritional or non-nutritional roles. Finally, we performed the expression profile during ovarian development and expression changes after bilateral eyestalk ablation to evaluate their contribution to reproduction in E. carinicauda. This is the first systematic identification and functional investigation of EcVtgs in E. carinicauda. The results of this study will provide a basis for an improved understanding of the evolutionary and biological functions of Vtg genes in crustaceans, particularly in the context of ovarian development.
2. Results
2.1. Identification and Physicochemical Characterization of the EcVtg Gene Family
Ten Vtg genes were identified in the E. carinicauda genome using a combination of BLASTp and hidden Markov model (HMM) analyses and were confirmed using NCBI-CDD and SMART. The Vtg genes of E. carinicauda were named EcVtg1–8, according to the nomenclature of M. rosenbergii Vtgs. The results of BLASTX showed that EcVtg1–7 were the most homologous to Vtg1-7 in M. rosenbergii, whilst EcVtg8 showed approximately 47.07–54.51% similarity with other EcVtgs (Supplemental Table S1).
The basic physicochemical properties of the proteins encoded with the EcVtg genes in E. carinicauda using he ExPASy tool (http://www.expasy.ch/tools/pi_tool.html, Swiss Institute of Bioinformatics, Lausanne, Switzerland) are shown in Table 1 and Supplemental Table S2. Bioinformatic analysis showed that the 10 EcVtg proteins contained 2421 (EcVtg5) to 2581 (EcVtg8) amino acids; the predicted molecular weights (MWs) ranged from 275.06 kDa (EcVtg5) to 291.37 kDa (EcVtg2); and the theoretical isoelectric point (pI) ranged from 7.27 (EcVtg6b) to 9.14 (EcVtg1a). The grand-average hydropathicity values of EcVtg proteins were all negative, indicating that all proteins were hydrophobic. The predicted protein-instability index (II) showed that all EcVtg proteins were unstable. In addition, the signal peptide prediction results demonstrated that all EcVtgs encoded proteins with Sec/SPI signal peptides had a likelihood of 0.9773–0.9983, and these gene products were preliminarily identified as secretory proteins.

Table 1.
Summary of sequence characteristics of Vtg genes in E. carinicauda.
The prediction of subcellular localization (using the online Wolf PSORT tool, https://www.genscript.com/wolf-psort.html, GenScript Biotech Corporation, Nanjing, China) suggested that most EcVtg proteins in E. carinicauda resided in the extracellular space. However, proteins encoded with EcVtg1b, EcVtg4, and EcVtg7 were found to localize in the endoplasmic reticulum, while EcVtg8 was observed in the nucleus (Table 2). Additionally, the secondary structures of the EcVtg proteins were predominantly composed of alpha helices (38.00–40.09%), followed by random coils (35.35–37.26%). Extended strands and beta turns collectively accounted for 19.54–20.25% and 4.44–5.25% of the structural composition, respectively (Table 2).

Table 2.
The predicted secondary structure and subcellular-location prediction of EcVtg proteins in E. carinicauda.
2.2. Chromosome Location and Phylogenetics of the EcVtg Gene Family
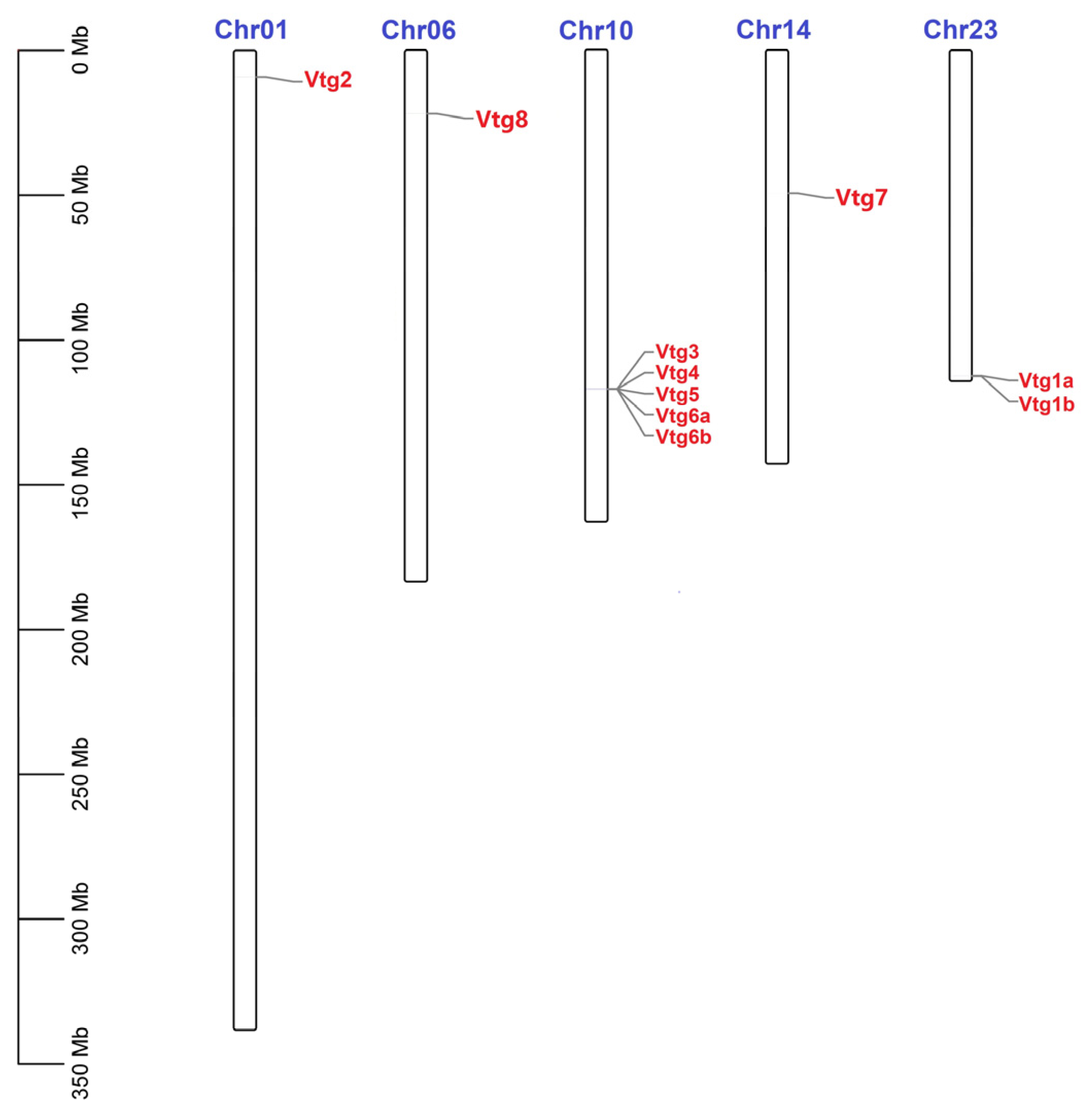
To better understand the mechanism underlying the genomic distribution of EcVtgs on E. carinicauda chromosomes, a chromosome map of EcVtgs was constructed based on the genomic sequences of E. carinicauda (Figure 1). The results showed that all EcVtg genes were anchored on five chromosomes of the E. carinicauda genome, and the number of EcVtgs on the chromosomes ranged from one to seven. Chromosome 10 contained the largest number of genes (five), followed by chromosome 23 with two EcVtg genes.
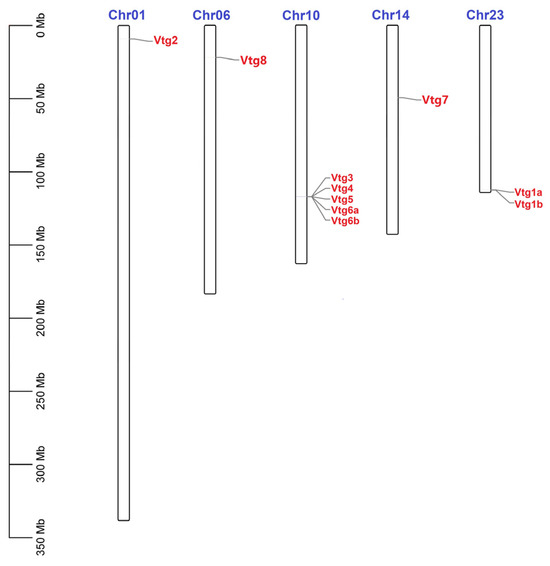
Figure 1.
Chromosomal distribution of EcVtgs in E. carinicauda. The scale on the left was used to indicate the length of the chromosome. The bars refer to a total of five chromosomes. The corresponding gene names are written on the right side of the chromosomes.
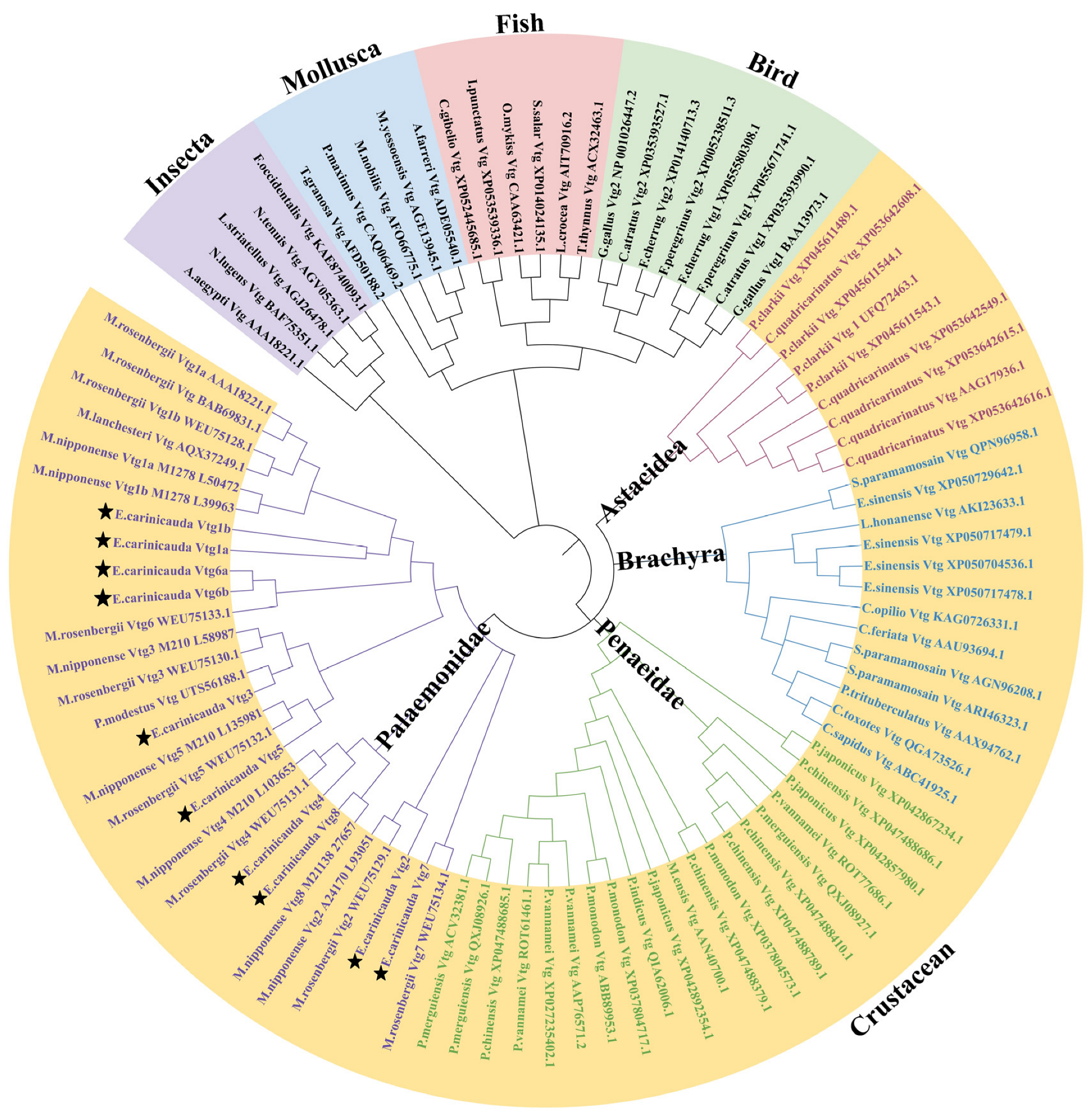
To clarify the phylogenetic relationships and classification of Vtg genes among different species, a phylogenetic tree was constructed using Vtg protein sequences from E. carinicauda and other representative species (Figure 2 and Supplementary Table S4). According to the results, 94 Vtg genes were classified into five distinct groups: insecta, mollusks, fish, birds, and crustaceans, in accordance with the classic taxonomic structure. The crustacean group was subdivided into four distinct clusters: Astacidea, Brachyra, Penaeidae, and Palaemonidae. All EcVtg proteins converged with the Palaemonidae clade. The names of the members of the E. carinicauda EcVtg gene family were confirmed according to the branches of the phylogenetic tree and multiple sequence alignment analysis. The number of the EcVtg-gene-family members in E. carinicauda was similar to that in M. rosenbergii and Macrobrachium nipponensis. The EcVtg family of E. carinicauda contained eight members; that of M. rosenbergii contained seven members, except Vtg8; and that of M. nipponensis contained six members, except Vtg5 and Vtg6.
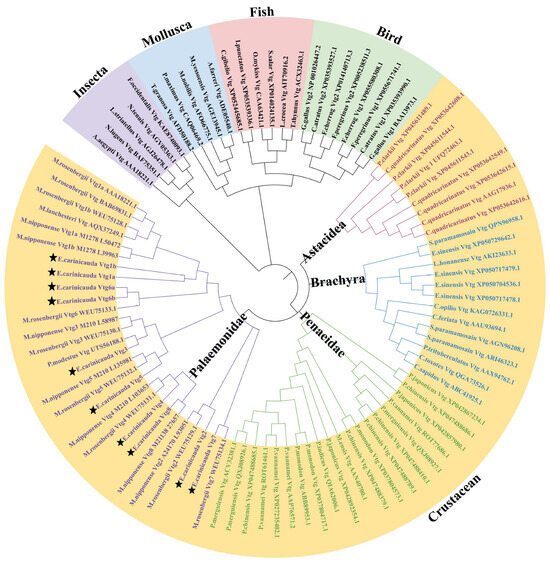
Figure 2.
Phylogenetic analysis of Vtgs in representative species. The different colors represent five groups: purple, insecta; blue, mollusca; pink, fish; green, bird; yellow, crustacean. Different font colors represent four subgroups in crustacean; purple, Palaemonidae; green, Penaeidae; blue, Brachyra; red, Astacidea. The Vtgs of E. carinicauda were marked by star. Gene IDs and the full sequence of 94 Vtg proteins are listed in Supplemental Table S3.
2.3. Gene Structures and Conserved Motifs of the EcVtg Gene Family
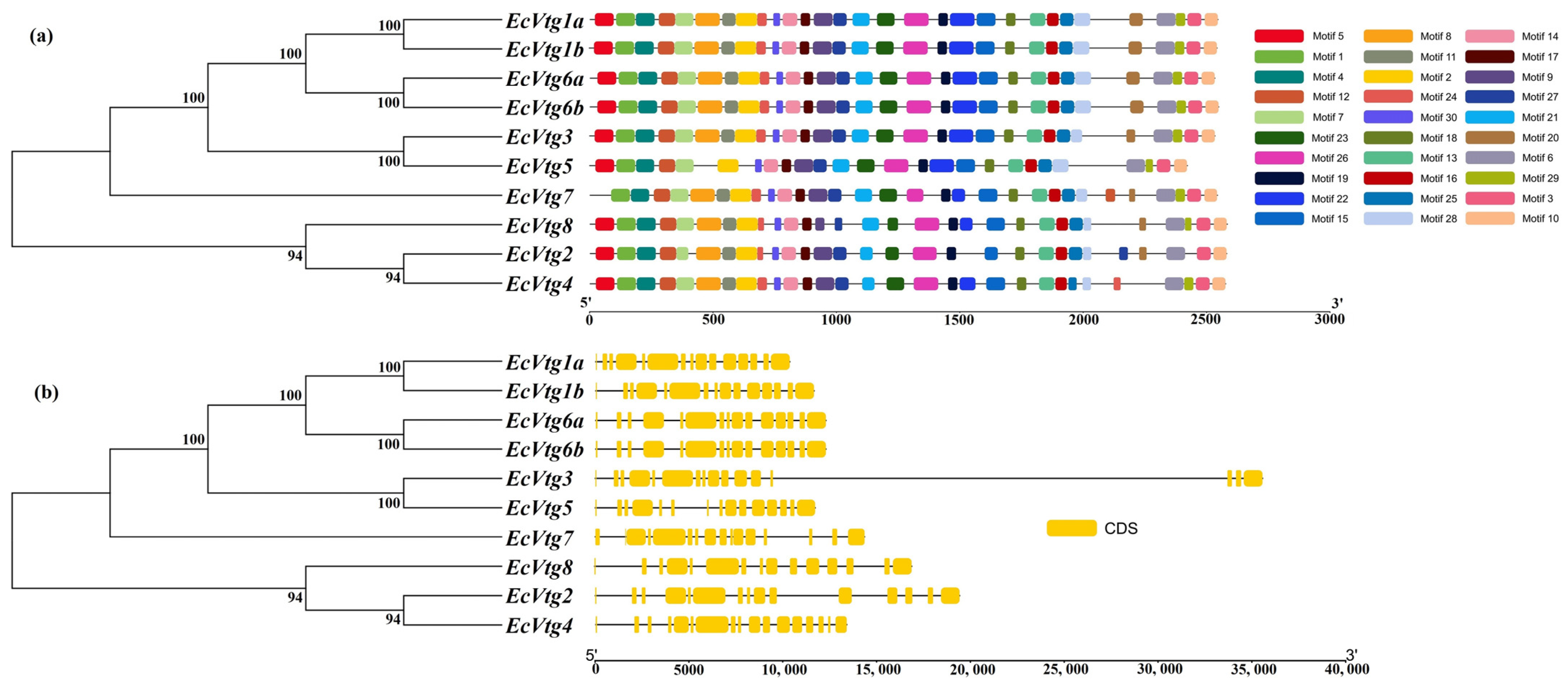
The investigation into the EcVtg conserved motifs and exon–intron structures aimed to deepen our understanding of the conservation and diversification within the EcVtg genes using TBtools. As depicted in Figure 3, the 10 EcVtg proteins were classified into two subfamilies, aligning with the outcomes of the unrooted phylogenetic tree (Figure 3a). Genes with closer relationships exhibited significant structural conservation; however, variations were noted among different EcVtg proteins. For instance, EcVtg1a, EcVtg1b, EcVtg3, EcVtg6a, EcVtg6b, and EcVtg8 contained 30 motifs, while EcVtg4 and EcVtg7 contained 9 motifs, excluding motifs 20 and 5, respectively. Moreover, EcVtg5 displayed the fewest number of motifs. Specifically, EcVtg2 contained 28 motifs, excluding 22 and 29.
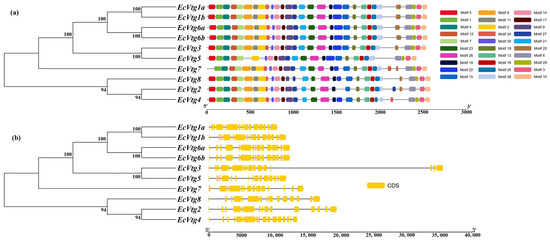
Figure 3.
A schematic diagram of gene structure and conserved motif of the EcVtg gene family. (a) The conserved motifs of EcVtgs. The thirty conserved motifs are displayed in different colors and correspond one to one in the structural diagram. (b) The gene structures of EcVtgs. The yellow box represents the coding sequence; the black line represents the intron.
The count of exons within the EcVtg family ranged from 15 to 17 (Figure 3b). EcVtg4 possessed the highest number of exons, whereas EcVtg3 and EcVtg7 contained 16 exons. In contrast, the remaining EcVtg members consisted of 15 exons each.
2.4. Gene Duplication and Synteny Analysis
The Ka/Ks ratios were computed to explore the evolutionary constraints and potential selective pressures on the identified duplicated gene pairs (Supplementary Table S4) using KaKs_Calculator 2.0. The findings indicated that the Ka/Ks ratio values for the tandemly and segmentally duplicated EcVtg gene pairs ranged from 0.1981 to 0.4303, all of which were less than 1.0. This suggests that repetitive EcVtg genes in E. carinicauda were predominantly subjected to purifying selection during their evolutionary course.
2.5. Expression of EcVtg Genes in Different Tissues and Developmental Stages
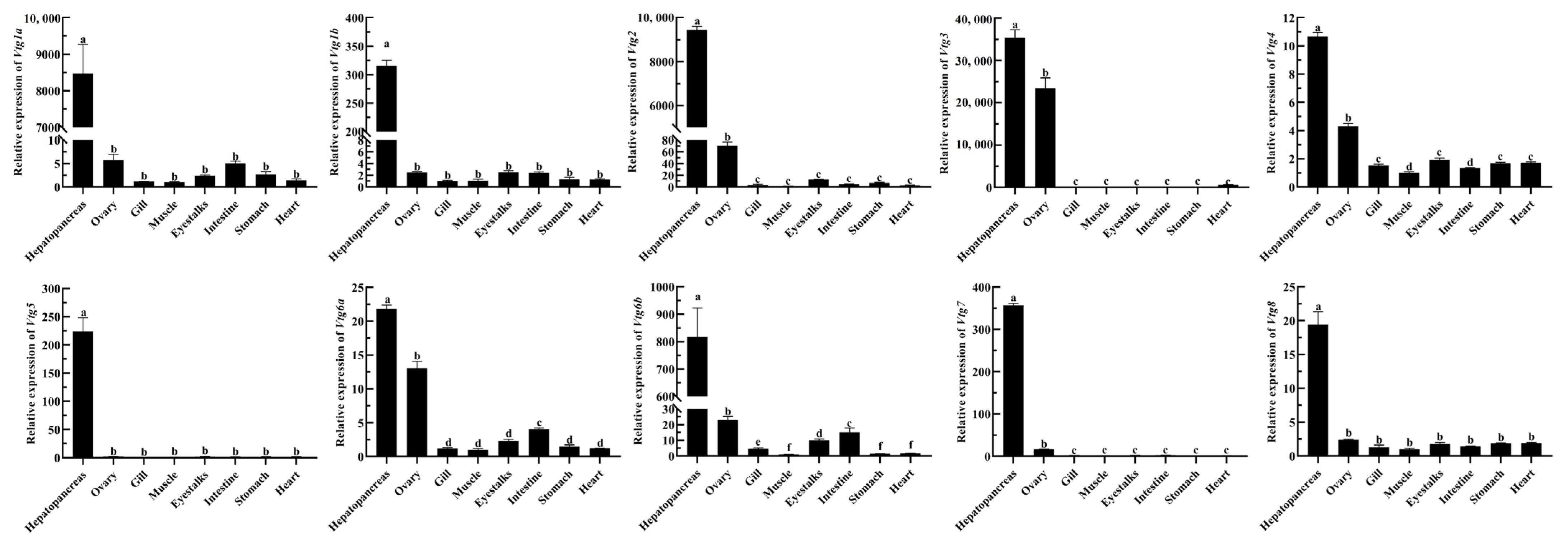
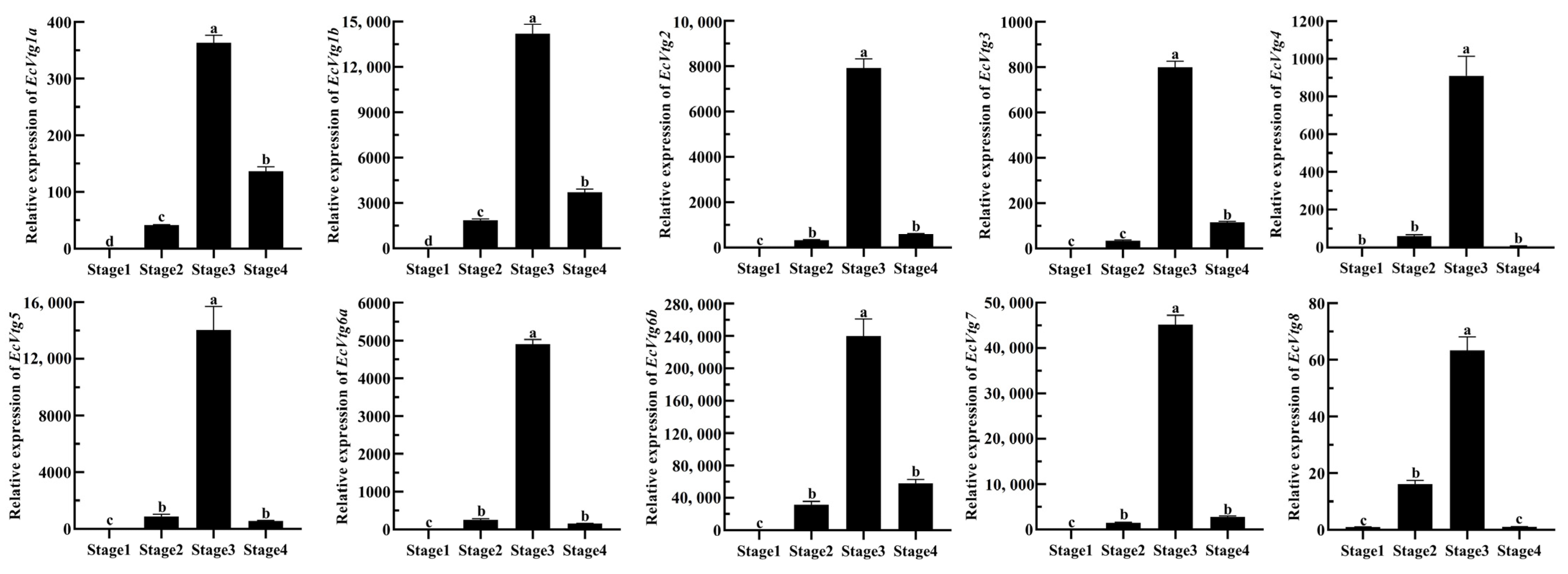
To explore the possible functions of EcVtgs, their expression profiles in different tissues and different developmental stages were characterized using qRT-PCR and RNA-seq data, respectively. As shown in Figure 4, the expression patterns of EcVtgs in different tissues were different and tissue specific. All EcVtg genes showed preferential expression in hepatopancreatic tissues, especially EcVtg1a, EcVtg1b, EcVtg2, EcVtg3, EcVtg5, EcVtg6b, and EcVtg7, which exhibited much higher expression than that in other tissues (p < 0.05), suggesting that these gene members tend to play roles in vitellogenesis. Meanwhile, six members (EcVtg2, EcVtg3, EcVtg4, EcVtg6a, EcVtg6b, and EcVtg7) were expressed at significantly higher levels in the ovaries than in other tissues (p < 0.05).
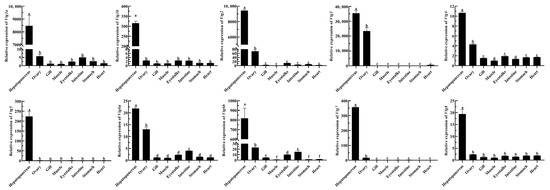
Figure 4.
Relative abundance of EcVtg mRNA transcript in eight tissues. All experiments were performed independently at least three times. Error bars represent the standard deviation of three replicates. Different lowercase letters indicated significant differences in the different tissues (p < 0.05). The β-actin serves as an internal reference gene.
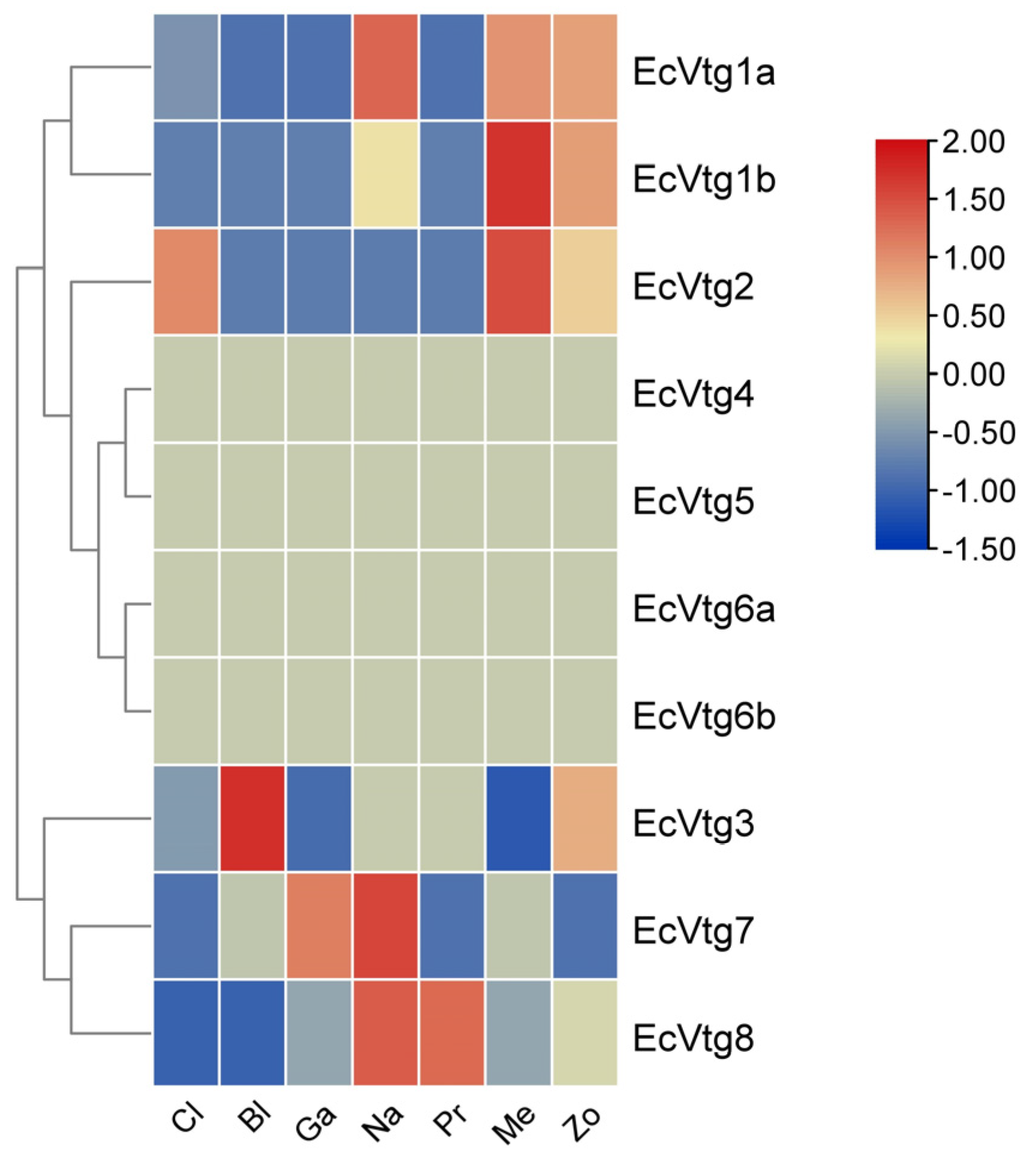
As shown in Figure 5, except for EcVtg4, EcVtg5, EcVtg6a, and EcVtg6b, six EcVtgs were expressed at various levels based on the transcriptome databases of six different embryonic development stages and the first stage of zoea. EcVtg1a, EcVtg1b, and EcVtg2 were expressed at high levels during the metazoea stage, which is the final stage of embryonic development in E. carinicauda. EcVtg7 and EcVtg8 were expressed most highly in the nauplius stage, and the highest expression of EcVtg3 was in the nauplius stage.
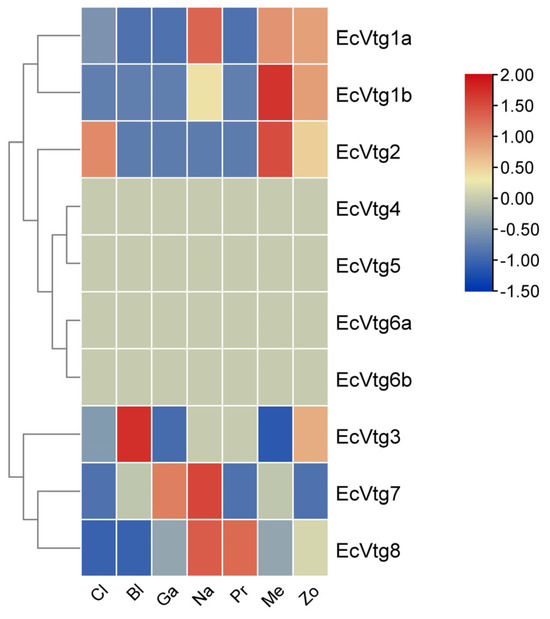
Figure 5.
Heat map of differential expression of EcVtg genes during embryonic development. Cl, cleavage; Bl, blastula; Ga, gastula; Na, nauplius; Pr, protozoea; Me, metazoea; Zo, the first stage of zoea.
2.6. EcVtg Genes Related to Ovarian Development of E. carinicauda
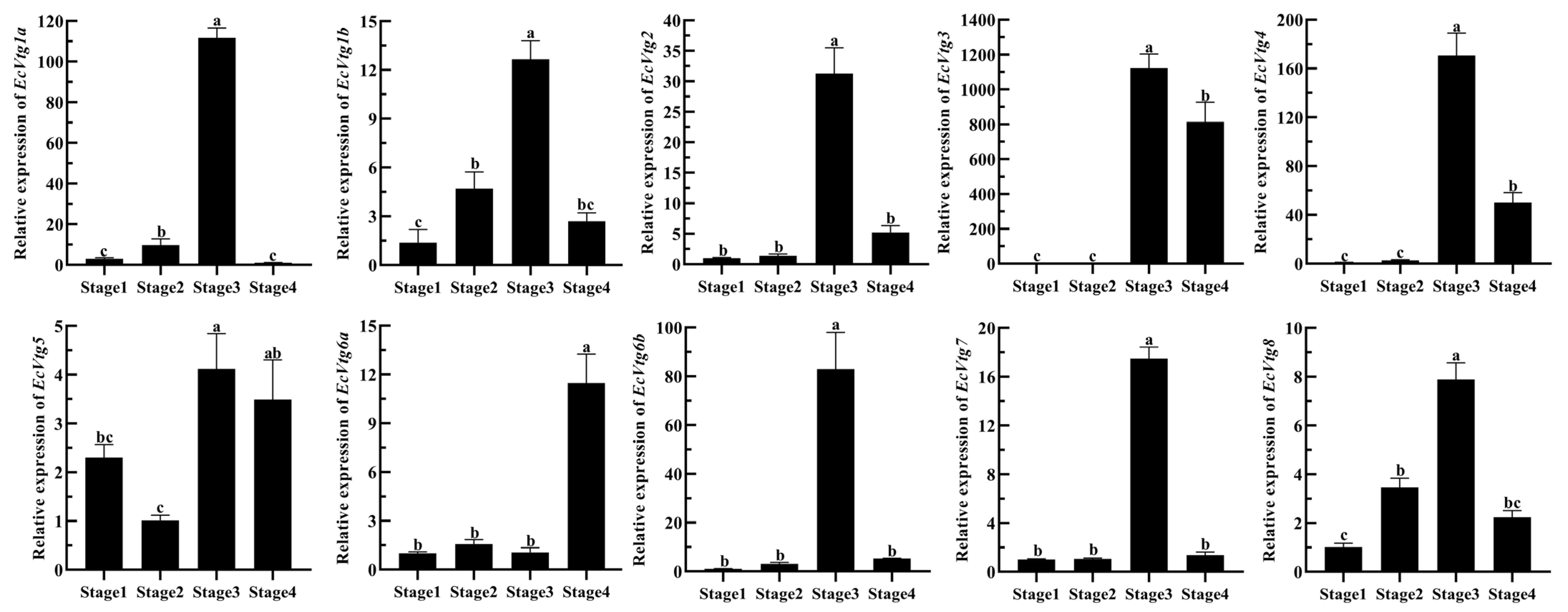
To confirm the role of EcVtg genes in the ovarian development of E. carinicauda, the expression profiles of EcVtgs at four typical stages were examined using qRT-PCR. As shown in Figure 6, all EcVtgs exhibited high expression levels in the hepatopancreas during the major-growth stage, which represents the primary growth stage. However, the expression patterns of EcVtgs differed across the other three stages of ovarian development. EcVtg1a, EcVtg1b, and EcVtg3 displayed significantly higher expression in the mature stage compared to the minor-growth stage (p < 0.05). Conversely, EcVtg8 exhibited significantly higher expression in the minor-growth stage than in the mature stage (p < 0.05). There were no significant differences between the minor-growth stage and mature stage in the expression of EcVtg2, EcVtg4, EcVtg5, EcVtg6a, EcVtg6b, and EcVtg7.
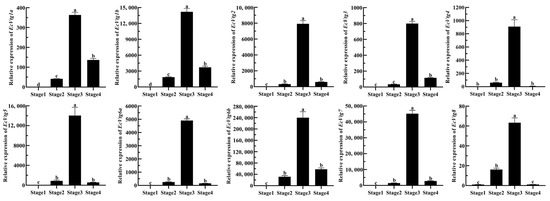
Figure 6.
Relative abundances of EcVtg mRNA transcripts in hepatopancreas during the ovarian development. Stage 1, as the proliferative stage, the ovary is small and completely transparent when anatomically observed, and its morphology and color cannot be distinguished with in vitro observation. Stage 2, as the minor-growth stage, the ovary is enlarged, translucent with small black spots over its outer membrane, and positioned above the heart. Stage 3, as the major-growth stage, ovarian volume continues to increase, and its length has reached the 1/2 cephalothorax length, and the ovary is faintly yellow with small black dots over its ovarian membrane. Stage 4, as the mature stage, the ovary covers almost the entire stomach, hepatopancreas, and heart. All experiments were performed independently at least three times. Error bars represent the standard deviation of three replicates. Different lowercase letters indicated significant differences in the different stages (p < 0.05). The β-actin serves as the internal reference gene.
The mRNA expression of EcVtgs of the ovary during four ovarian-development stages are shown in Figure 7. The expression patterns of EcVtg genes were similar expect for EcVtg5 and EcVtg6a, and their expression was highest in the major-growth stage, whereas EcVtg6a was highest in the mature stage (p < 0.05), and there was no difference in EcVtg5 between the major-growth stage and the mature stage. Specifically, the mRNA expression of EcVtg3 in the major-growth stage and the mature stage were thousands of times that of it in the proliferative stage and the minor-growth stage.
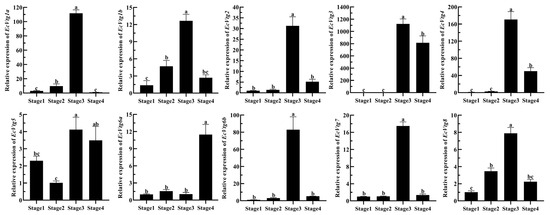
Figure 7.
Relative abundances of EcVtg mRNA transcripts in the ovary during ovarian development. All experiments were performed independently at least three times. Error bars represent the standard deviation of three replicates. Different lowercase letters indicated significant differences in the different stages (p < 0.05). The β-actin serves as the internal reference gene.
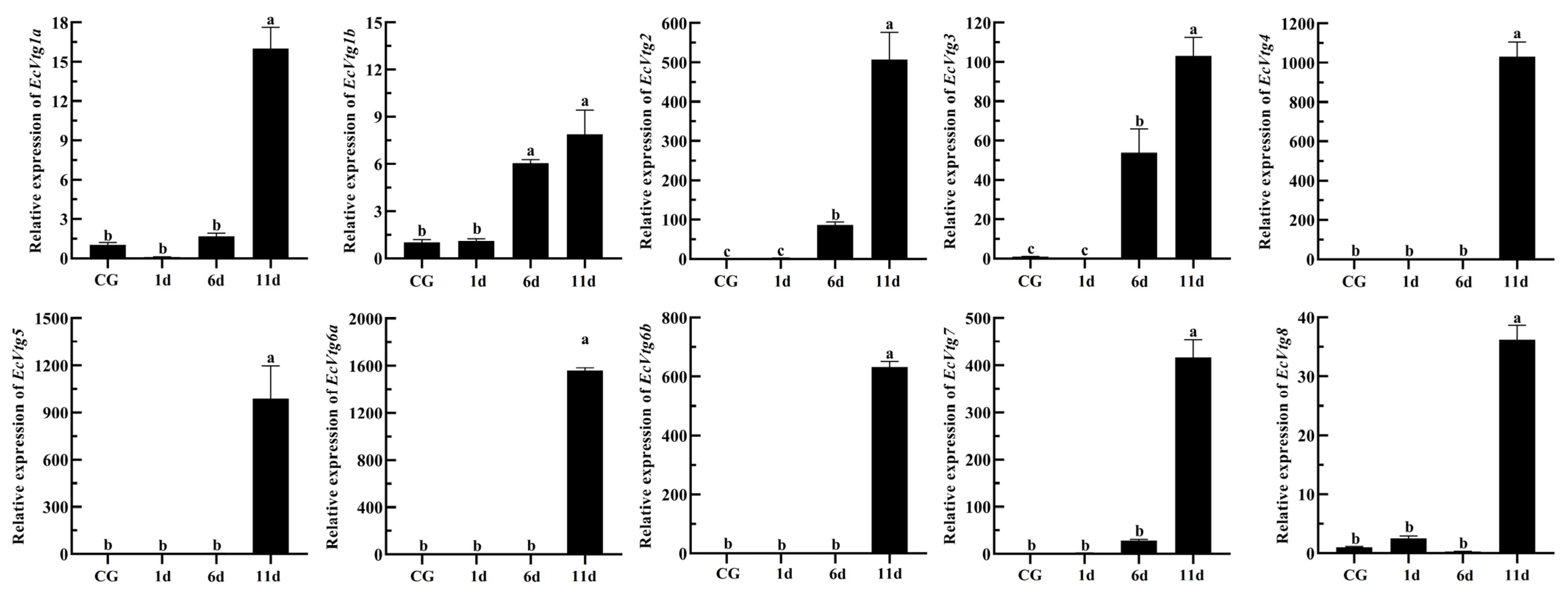
2.7. Expression of the EcVtgs after Eyestalk Ablation
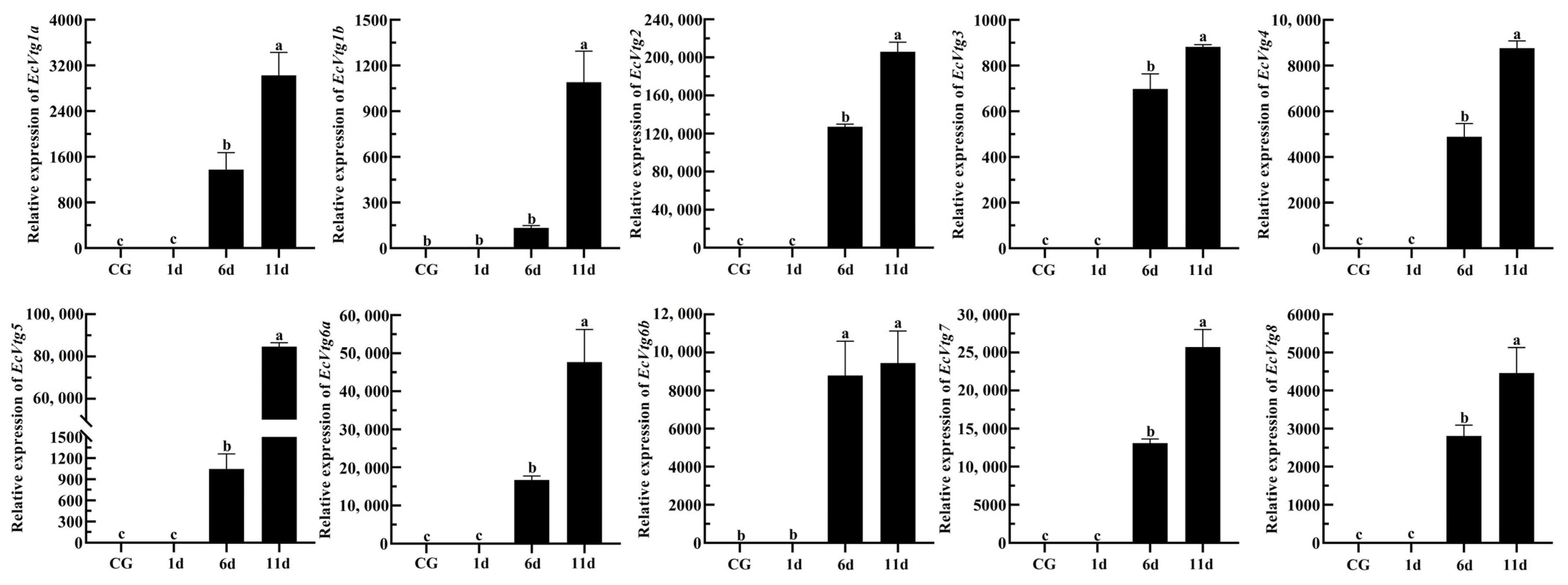
Eyestalk ablation is an effective method of promoting ovarian development in crustaceans. The expression levels of EcVtgs in the hepatopancreas and ovaries after eyestalk removal were monitored using qPCR (Figure 8 and Figure 9). When compared with that of the control group with intact eyestalks, the expression levels of the EcVtgs in the hepatopancreas were significantly upregulated 6 and 11 days after eyestalk ablation. In contrast to those in the hepatopancreas, the expression levels of the EcVtgs in the ovaries were significantly upregulated 11 days after eyestalk ablation, and only the expression levels of EcVtg1a, EcVtg2, and EcVtg3 in the ovaries in the day 11 group were higher than those in the control group, whereas the other EcVtgs exhibited no significant change in expression at 6 days after eyestalk ablation.
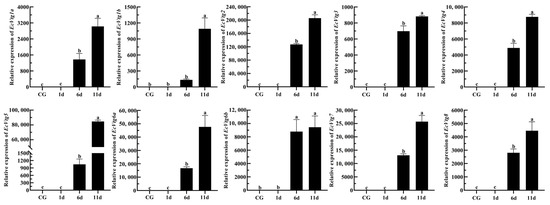
Figure 8.
Expression level of EcVtgs after eyestalk ablation in the hepatopancreas. CG represents the control group with an intact eyestalk; 1d, 6d, and 11d represent the days after eyestalk ablation. All experiments were performed independently at least three times. Error bars represent the standard deviation of three replicates. Different lowercase letters indicate significant differences in the different stages (p < 0.05). The β-actin serves as the internal reference gene.
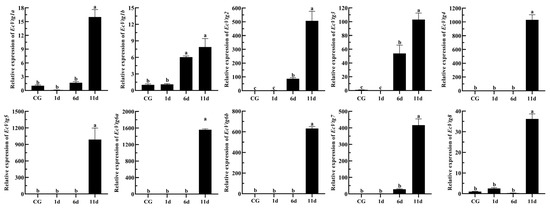
Figure 9.
Expression level of EcVtgs after eyestalk ablation in ovary. CG represent the control group the control group with intact eyestalk, 1d, 6d and 11d represent the days after eyestalk ablation. All experiments were performed independently at least three times. Error bars represent the standard deviation of three replicates. Different lowercase letters indicate significant differences in the different stages (p < 0.05). The β-actin serves as the internal reference gene.
3. Discussion
Vtg is a precursor of yolk proteins in egg-laying vertebrates and invertebrates and has been found to have multiple forms in many fish species, which play important roles in vitellogenesis and embryonic development [24]. Zebrafish have at least seven distinct and functional Vtg genes [25], whilst three Vtg genes have been found in Larimichthys crocea [24], and two Vtg genes have been identified in Opsariichthys bidens, suggesting that different species have a variety of Vtg genes. Similar to various oviparous vertebrates, multiple Vtg genes are present in single-decapod crustacean species. As Dendrobranchiata species, Fenneropenaeus merguiensis [26] and Litopenaeus vannamei [27] have three Vtg genes and Penaeus japonicus has two Vtg genes [28]. Furthermore, as Pleocyamata species, four Vtg genes were identified in the Procambarus clarkii species [29], and seven Vtg genes were identified from M. rosenbergii [22] and Macrobrachium nipponense. We identified 10 different Vtgs in E. carinicauda using homology comparison, which were much more abundant than those in other crustaceans. These results also suggest that the number of Vtgs in Pleocyemata species is higher than that in Dendrobranchiata species. Liang et al. found a Vtg (AFM82474.1) in E. carinicauda which shares 99.10% identity tandemly with EcVtg1a. In this study, we comprehensively analyzed the 10 EcVtg genes, including their chromosome location, collinear relationships, gene structure, conserved motifs, and expression patterns.
In most oviparous animals, Vtg typically comprises three conserved domains: the N-terminal domain, a domain of unknown function (1943), and the von Willebrand factor type D domain [30]. SMART analysis identified these three conserved domains in all EcVtgs. Initially recognized for its involvement in vitellogenesis and embryo development, Vtg is increasingly acknowledged as a multifunctional protein contributing to non-reproductive functions, including innate immunity and antioxidation [31,32]. The N-terminal domain represents a conserved region acknowledged as the primary phosphorylation site and protein-modification region, crucial for Vtg cleavage, recognition by Vtg receptors, and the transportation of lipids and proteins [33]. Vtg is an antibacterial agent with a wide spectrum of functions in insects [34], which also increase the lifespan of honeybees by enhancing their oxidative-stress tolerance and shielding them from oxidative damage [35]. Additionally, Vtg plays a critical role in protecting Eriocheir sinensis from bacterial infections by inhibiting bacterial proliferation and regulating the expression of antimicrobial peptides [36]. As the DUF1943 and VWD domains in Vtg always interact with and remove pathogenic microorganisms, such as viruses or bacteria, it is proposed that Vtg is primarily a pattern recognition molecule and plays an important role in immunity. This indicated that the possibility of multifunctional function of EcVtgs occur in E. carinicauda.
The EcVtg genes identified in the E. carinicauda genome displayed minor variation in their sequence structures. Regarding protein length, the range of variation observed in the amino acid sequence of EcVtgs was from 2421 aa to 2581 aa. Concerning gene structure, the variation range of the Vtg gene exon spanned from 15 to 17. Furthermore, variations within the conserved motifs of Vtg genes ranged from 26 to 30. Typically, a 15-exon Vtg gene has been reported in shrimp species, including M. ensis [37], P. monodon [38], Homarus americanus [39], and P. japonica [15]. Across these reports, the sizes and positions of exons and introns within the Vtg gene remained consistent, indicating a high level of conservation among decapod Vtg genes. However, in the case of E. carinicauda, EcVtg3 and EcVtg7 contained 16 exons, and EcVtg5 contained 17 exons. Similarly, Vtgs observed in M. rosenbergii also exhibit structures containing either 15 or 16 exons. The inquiry of whether similar Vtg gene organizations occur in other decapods necessitates further investigation. The different gene structures of EcVtgs suggest that their functions are diverse.
EcVtg1a and EcVtg1b share 91.51% of identity tandemly located on chromosome 23, and EcVtg6a and EcVtg6b share 97.92% of identity tandemly located on chromosome 10, while EcVtg3–6 were present in a tandem cluster on chromosome 10. Gene-duplication analysis revealed that the EcVtgs family have expanded with duplication events in evolution. The Ka/Ks ratio of all repetitive EcVtg gene pairs were significantly less than 1.0 (ranged from 0.1981 to 0.4303). The ratio is of the number of nonsynonymous substitutions per nonsynonymous site (Ka) to the number of synonymous substitutions per synonymous site (Ks). The Ka is much less than Ks (i.e., Ka/Ks << 1) which means that selection keeps the protein as it is (purifying selection) [40]. This suggests that repetitive EcVtg genes in E. carinicauda were predominantly subjected to purifying selection during evolution.
Vtg is synthesized and secreted from the liver of oviparous vertebrates [41] and from fat bodies in insects [42], and is then taken up for oocyte development and other functions. In crustaceans, Vtg is produced mainly in the hepatopancreas and ovaries, deposited in the hemolymph, and then accumulated in growing oocytes. Various Vtg synthesis sites have been identified in different crustacean species. Several studies have suggested that the hepatopancreas is the principal site of Vtg synthesis in Pleocyemata [43,44], and only a small amount of Vtg is synthesized in the ovaries. In Dendrobranchiata, Vtg synthesis occurs in both the hepatopancreas and ovaries [45]. As shown in Table 3, the hepatopancreas and ovaries were found to be the principal site of Vtg synthesis in all Penaeidae shrimp species, including L. vannamei, P. japonicus, and Penaeus monodon [28,46,47]; the hepatopancreas was the principal site of Vtg synthesis in most Pleocyemata species. However, other studies have demonstrated that different Vtg isoforms are differentially expressed in the hepatopancreas and ovary, contradicting the past understanding of the Vtg synthesis site. Vtg2 is primarily expressed in the hepatopancreas, whereas Vtg1 and Vtg3 are primarily expressed in the ovaries of F. merguiensis [26]. Vtg1 is expressed primarily in the hepatopancreas and ovaries, but only Vtg2 is expressed in the hepatopancreas of M. ensis [14]. Recently, some studies have reported the dual expression (in the hepatopancreas and ovaries) of Vtgs in species such as M. nipponensis [48], P. clarkii [29], and Scylla paramamosain [49].

Table 3.
The Vtg synthesis site in different crustaceans.
In the present study, all EcVtgs were highly expressed in the hepatopancreas, whereas EcVtg3, EcVtg4, and EcVtg6a were simultaneously expressed in both the ovaries and hepatopancreas. This indicates that, whilst the ovaries are a synthesis site of Vtg, the hepatopancreas is the main site of Vtg synthesis in E. carinicauda. Liang et al. also found that Vtg (AFM82474.1), which includes Vtg1a, is mainly expressed in the hepatopancreas [19]. Therefore, exogenous Vtg is the major source of Vtg, which is synthesized in the hepatopancreas, transported via the hemolymph, and absorbed by oocytes or follicle cells via receptor-mediated endocytosis. The expression pattern indicated that all EcVtgs play an important role during the ovarian development of E. carinicauda; meanwhile, EcVtg1a, EcVtg2 and EcVtg3 play major roles in exogenous vitellogenesis, and EcVtg3 plays a major role in endogenous vitellogenesis.
In most shrimp species, the synthesis of Vtg in the ovaries and hepatopancreas during ovarian development is regulated by the X-organ/sinus gland complex situated in the paired eyestalks. Unilateral eyestalk ablation in female shrimp is commonly practiced to induce ovarian maturation [64,65]. Removal of the eyestalk, observed in both Pleocyamata and Dendrobranchiata, has been shown to accelerate ovarian development and elevate the gonadosomatic index [66,67,68]. In the Dendrobranchiata shrimp species P. japonicus, mRNA expression of Vtg1 notably increased in the ovaries while remaining unchanged in the hepatopancreas following eyestalk ablation, whereas Vtg2 expression differed [28]. Bilateral ablation of the eyestalk significantly upregulated the mRNA expression of all Vtgs in the female hepatopancreas of M. rosenbergii but did not affect the expression of Vtgs, except Vtg1 in the ovaries [22]. Eyestalk ablation is commonly used to promote ovarian development and maturation in E. carinicauda [69], which can promote synchronous ovarian development and shorten the breeding cycle of seedlings. We previously found that the gonadosomatic index increased after eyestalk ablation. On the 6th and 11th days after eyestalk ablation, the gonadosomatic index increased approximately three- and nine-fold when compared with the control group, respectively [70]. In the current study, the expression levels of all EcVtgs in the hepatopancreas exhibited significant upregulation at 6 and 11 days following eyestalk ablation. However, the expression levels of most EcVtgs in the ovaries were significantly upregulated specifically at the 11-day mark post eyestalk ablation. These indicated that removal of the eyestalk firstly promotes the ovarian development of E. carinicauda from exogenous, and next from endogenous.
4. Materials and Methods
4.1. Genome-Wide Identification and Sequence Analysis of Vtg Genes in E. carinicauda
The genome sequence, gene annotation, and gene and protein sequences were obtained from our previous study (unpublished, the BioProject was PRJNA1055619). To identify the candidate Vtg genes, we used a HMM to search the protein database for E. carinicauda genome using the hmmsearch software in HMMER v3.0, and, in which, the HMM profiles of the conservative functional domain of the lipoprotein amino-terminal region (PF01347), vitellinogen, open beta-sheet (PF09172), and von Willebrand factor type D domain (PF00094) were used as queries with default parameters. Then, the CD-Search Tool (https://www.ncbi.nlm.nih.gov/Struture/bwrpsb/bwrpsb.cgi, National Library of Medicine, Bethesda, MD, USA, accessed on 20 May 2022) was used for further verification following manual screening of redundant genes. The basic physicochemical properties of the Vtg proteins, including the amino acid number, MW, and pI, and they were predicted using the ExPASy tool (http://www.expasy.ch/tools/pi_tool.html, Swiss Institute of Bioinformatics, Lausanne, Switzerland, accessed on 5 June 2022).
4.2. Phylogenetic Analysis and Molecular Evolution
The phylogenetic trees of Vtg genes were constructed using MEGA11 software. These Vtg protein sequences were then utilized to generate a maximum likelihood phylogenetic tree through MEGA11. The best model of the phylogenetic tree was calculated and constructed, employing the pairwise-deletion option with MEGA11, and then a phylogenetic tree of all Vtg proteins was constructed using the maximum-likelihood method with 1000 bootstrap replicates. Finally, the phylogenetic tree was visualized and beautified using the online tool iTOL (https://itol.embl.de/, Biobyte Solutions GmbH, Heidelberg, Germany, accessed on 20 August 2022).
KaKs_Calculator 2.0 (https://sourceforge.net/projects/kakscalculator2/, SourceForge, San Diego, CA, USA, accessed on 10 June 2022) [71] was employed to calculate the non-synonymous (Ka) and synonymous (Ks) substitution rates, along with the Ka/Ks ratio, for each duplicated Vtg gene.
4.3. Chromosome Distribution, Gene Structure, and Protein Motif Analyses of EcVtg Genes
The Multiple Expectation Maximization for Motif Elicitation (MEME) online program (https://meme-suite.org/meme/index.html, University of Nevada, Reno, NV, USA, accessed on 15 June 2022) (5.5.3) was performed to identify conserved motifs of EcVtg proteins, with the number of motifs set to 30, the maximum length set to 100, and the other parameters set to default.
To elucidate the exon–intron structure of Vtg genes within the E. carinicauda genome, data regarding exon and intron positions were extracted from the genome Generic Feature Format (GFF) file. Finally, the chromosome distribution, gene motif, and exon–intron structure of Vtgs were visualized using TBtools.
4.4. Subcellular-Localization and Protein-Structure Prediction
The subcellular localization of Vtg in E. carinicauda was predicted using the online Wolf PSORT tool (https://www.genscript.com/wolf-psort.html, GenScript Biotech Corporation, Nanjing, China, accessed on 23 June 2022). Additionally, the secondary structure of the Vtg proteins was predicted using online SOPMA software (https://npsa-prabi.ibcp.fr/cgi-bin/npsa_automat.pl?page=npsa_sopma.html, Institute for the Biology and Chemistry of Proteins, Lyon, France, accessed on 20 June 2022) with the number of conformational states set to four, the similarity threshold set to eight, and the window width set to 17. Signal peptides were predicted using online SignalP 5.0 software (https://services.healthtech.dtu.dk/services/SignalP-5.0/, Department of Health Technology, Lyngby, Denmark, accessed on 27 June 2022).
4.5. Expression Analyses of EcVtg Genes
To illustrate the expression patterns of EcVtg genes in various tissues and during different ovarian-development stages, healthy female adults of E. carinicauda (with a body length of 51.72 ± 1.47 mm and body weight of 1.78 ± 0.21 g) were obtained from the Yellow Sea near Rizhao City, Shandong Province, China. For tissue-specific expression determination, RNA isolation involved collecting muscle, stomach, eyestalk, heart, intestine, ovary, gill, and hepatopancreas tissues. From eighteen shrimps, three samples from each tissue type were collected (six shrimp pooled together).
According to the criteria outlined in Wang’s study for ovarian-development stages, females were evaluated daily by observing the size and color of their gonads. Subsequently, four females in distinct ovarian developmental stages were selected. A total of seventy-two shrimp (six individuals × four stages × three replicates) were collected to analyze the expression patterns of Vtg across four ovarian developmental stages.
For the eyestalk ablation experiments, 36 females underwent bilateral eyestalk ablation, while 12 females were designated as the control group. Hepatopancreatic and ovarian tissues were collected at 1-, 6-, and 11-days post eyestalk ablation, with three parallel samples obtained for both the experimental and control groups, respectively.
4.6. Quantitative RT-PCR Analysis
Total RNA was extracted using a TransZol Up Plus RNA Kit (Trans, Beijing, China) according to the manufacturer’s standard protocol. mRNA was reverse transcribed using HiScript III RT SuperMix for qPCR expression analysis. To investigate mRNA expression of EcVtgs, the qPCR assay was performed using the ChamQ SYBR Color qPCR Master Mix (Vazyme, Nanjing, China) with an ABI PRISM 7500 Sequence Detection System (Applied Biosystems, Carlsbad, CA, USA). The following conditions were used for PCR: one cycle at 95 °C for 30 s, then 40 cycles of 95 °C for 10 s, 60 °C for 30 s, followed by one cycle of 95 °C and 15 s, 60 °C for 1 min, and 95 °C for 15 s.
β-actin derived from E. carinicauda (GenBank accession number: JQ045354.1) served as the internal control for the expression analysis of E. carinicauda. This particular gene was selected due to its abundance and stability within cells, exhibiting minimal susceptibility to external regulatory influences [72]. The primers for RT-PCR are shown in Supplementary Table S5. Real-time PCR assays were performed in triplicate, and the analysis was based on the CT values of the PCR products. The expression levels of EcVtg were calculated utilizing the 2−ΔΔCT method. Statistical significance regarding differences in gene expression was determined using one-way ANOVA alongside Tukey’s multiple comparison tests, with a significance threshold set at p values < 0.05.
4.7. Expression-Pattern Analysis of EcVtg Genes during Embryonic Development
To investigate the expression patterns of EcVtg genes across six critical embryonic stages, encompassing cleavage, blastula, gastrula, nauplius, protozoa, and metazoea, the initial stage of the metazoea was collected following the methodology outlined in Wang’s study [73]. Total RNA extraction was conducted using the previously described method. Subsequently, a total of 21 libraries were generated, comprising three replicates from each of the seven groups. These libraries were sequenced using an Illumina HiSeq 4000 (San Diego, CA, USA) platform, generating 150 bp paired-end raw reads. The clean reads obtained from each RNA-seq library were aligned to the E. carinicauda genome utilizing STAR [74]. Differential expression analysis between the two groups was executed using the DEseq R package, employing criteria involving |log2fold change (FC)| ≥ 1 and a false discovery rate of <0.01. Heat maps illustrating the expression levels of the EcVtg genes were generated and visualized using the Heatmap Illustrator program within the TBtools.
5. Conclusions
The EcVtg-family genes were meticulously and systematically characterized in this study. A total of 10 EcVtg genes were identified within the genome of E. carinicauda, marking the most extensive number of Vtgs found in crustaceans so far. Phylogenetic analysis revealed that the Vtg phylogenetic tree aligned with the traditional taxonomic structure, illustrating the subdivision of the crustacean group into four distinct clusters: Astacidea, Brachyra, Penaeidae, and Palaemonidae. Molecular evolutionary analysis indicated that the evolution of EcVtg genes primarily underwent purifying selection. Furthermore, the qRT-PCR results demonstrated notably higher expression of all EcVtgs in the female hepatopancreas compared to other tissues. EcVtg1a, EcVtg2, and EcVtg3 play major roles in exogenous vitellogenesis, and EcVtg3 also plays a major role in endogenous vitellogenesis. The stimulation of ovarian development following eyestalk ablation was evidenced by the induction of EcVtgs expression in the hepatopancreas and, subsequently, in the ovaries, albeit at a later stage. This indicates that the hepatopancreas serves as the primary site for Vtg synthesis. The observed differential expression patterns among the EcVtg genes suggest a diverse array of functions during ovarian development in E. carinicauda. The differential expression patterns of various EcVtg genes in different tissues and during ovarian development indicates that their functions are diversified in E. carinicauda.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ijms25021089/s1.
Author Contributions
Conceptualization, J.W. and J.L. (Jitao Li); methodology, J.W. and J.L. (Jian Li); software, J.W. and Q.W.; validation, J.W. and S.T.; formal analysis, J.W. and Y.H.; investigation, J.W. and J.L. (Jitao Li); resources, J.W. and J.L. (Jitao Li); data curation, J.W. and Q.G.; writing—original draft preparation, J.W.; writing—review and editing, J.W. and J.L. (Jitao Li); visualization, J.W. and X.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Key Research and Development Program of China, grant number 2022YFD2400104, Shandong Natural Science Foundation, grant number ZR2023MC055, Central Public-interest Scientific Institution Basal Research Fund, grant number 2023TD50, and the earmarked fund for CARS-48.
Institutional Review Board Statement
The animal-study protocol was approved by the Institute Animal Care and Use committee.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Carducci, F.; Biscotti, M.A.; Canapa, A. Vitellogenin gene family in vertebrates: Evolution and functions. Eur. Zool. J. 2019, 86, 233–240. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, S. Immune-Relevant and Antioxidant Activities of Vitellogenin and Yolk Proteins in Fish. Nutrients 2015, 7, 8818–8829. [Google Scholar] [CrossRef]
- Dietrich, M.A.; Adamek, M.; Teitge, F.; Teich, L.; Jung-Schroers, V.; Malinowska, A.; Świderska, B.; Rakus, K.; Kodzik, N.; Chadzińska, M.; et al. Proteomic analysis of carp seminal plasma provides insights into the immune response to bacterial infection of the male reproductive system. Fish Shellfish Immunol. 2022, 127, 822–835. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, X.J.; Wu, Y.M.; Yang, L.; Li, W.; Wang, Q. Vitellogenin regulates antimicrobial responses in Chinese mitten crab, Eriocheir sinensis. Fish Shellfish Immunol. 2017, 69, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Chen, Y.-Z.; Lou, Y.-H.; Zhang, C.-X. Vitellogenin and Vitellogenin-Like Genes in the Brown Planthopper. Front. Physiol. 2019, 10, 1181. [Google Scholar] [CrossRef]
- Roth, Z.; Weil, S.; Aflalo, E.D.; Manor, R.; Sagi, A.; Khalaila, I. Identification of Receptor-Interacting Regions of Vitellogenin within Evolutionarily Conserved β-Sheet Structures by Using a Peptide Array. ChemBioChem 2013, 14, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Sadasivam, M.; Ding, J.L. Receptor-Ligand Interaction between Vitellogenin Receptor (VtgR) and Vitellogenin (Vtg), Implications on Low Density Lipoprotein Receptor and Apolipoprotein. J. Biol. Chem. 2003, 278, 2799–2806. [Google Scholar] [CrossRef]
- Sun, W.; Li, H.; Zhao, Y.; Bai, L.; Qin, Y.; Wang, Q.; Li, W. Distinct vitellogenin domains differentially regulate immunological outcomes in invertebrates. J. Biol. Chem. 2021, 296, 100060. [Google Scholar] [CrossRef]
- Wu, B.; Liu, Z.; Zhou, L.; Ji, G.; Yang, A. Molecular cloning, expression, purification and characterization of vitellogenin in scallop Patinopecten yessoensis with special emphasis on its antibacterial activity. Dev. Comp. Immunol. 2015, 49, 249–258. [Google Scholar] [CrossRef]
- Liu, X.; Qiao, X.; Yu, S.; Li, Y.; Wu, S.; Liu, J.; Wang, L.; Song, L. The DUF1943 and VWD domains endow Vitellogenin from Crassostrea gigas with the agglutination and inhibition ability to microorganism. Dev. Comp. Immunol. 2023, 143, 104679. [Google Scholar] [CrossRef]
- Yilmaz, O.; Patinote, A.; Nguyen, T.; Com, E.; Pineau, C.; Bobe, J. Genome editing reveals reproductive and developmental dependencies on specific types of vitellogenin in zebrafish (Danio rerio). Mol. Reprod. Dev. 2019, 86, 1168–1188. [Google Scholar] [CrossRef]
- Williams, V.N.; Reading, B.J.; Hiramatsu, N.; Amano, H.; Glassbrook, N.; Hara, A.; Sullivan, C.V. Multiple vitellogenins and product yolk proteins in striped bass, Morone saxatilis: Molecular characterization and processing during oocyte growth and maturation. Fish Physiol. Biochem. 2014, 40, 395–415. [Google Scholar] [CrossRef]
- Yilmaz, O.; Patinote, A.; Com, E.; Pineau, C.; Bobe, J. Knock out of specific maternal vitellogenins in zebrafish (Danio rerio) evokes vital changes in egg proteomic profiles that resemble the phenotype of poor quality eggs. BMC Genom. 2021, 22, 308. [Google Scholar] [CrossRef] [PubMed]
- Kung, S.Y.; Chan, S.M.; Hui, J.H.; Tsang, W.S.; Mak, A.; He, J.G. Vitellogenesis in the sand shrimp, metapenaeus ensis: The contribution from the hepatopancreas-specific vitellogenin gene (MeVg2). Biol. Reprod. 2004, 71, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.-M.; Kim, B.-K.; Kim, Y.-J.; Kim, H.-W. Structural Similarity and Expression Differences of Two Pj-Vg Genes from the Pandalus Shrimp Pandalopsis japonica. Fish. Aquat. Sci. 2011, 14, 22–30. [Google Scholar] [CrossRef][Green Version]
- Xu, W.; Xie, J.; Shi, H.; Li, C. Hematodinium infections in cultured ridgetail white prawns, Exopalaemon carinicauda, in eastern China. Aquaculture 2010, 300, 25–31. [Google Scholar] [CrossRef]
- Wang, J.; Ge, Q.; Li, J.; Li, J. Effects of inbreeding on growth and survival rates, and immune responses of ridgetail white prawn Exopalaemon carinicauda against the infection of Vibrio parahaemolyticus. Aquaculture 2020, 519, 734755. [Google Scholar] [CrossRef]
- Yuan, J.; Gao, Y.; Zhang, X.; Wei, J.; Liu, C.; Li, F.; Xiang, J. Genome Sequences of Marine Shrimp Exopalaemon carinicauda Holthuis Provide Insights into Genome Size Evolution of Caridea. Mar. Drugs 2017, 15, 213. [Google Scholar] [CrossRef]
- Liang, J.-P.; Zhang, W.-L.; Li, H.; Li, J.-T.; Li, J. Abundances of vitellogenin and heat shock protein 90 during ovarian and embryonic development of Exopalaemon carinicauda. Anim. Reprod. Sci. 2020, 223, 106633. [Google Scholar] [CrossRef]
- Uengwetwanit, T.; Pootakham, W.; Nookaew, I.; Sonthirod, C.; Angthong, P.; Sittikankaew, K.; Rungrassamee, W.; Arayamethakorn, S.; Wongsurawat, T.; Jenjaroenpun, P.; et al. A chromosome-level assembly of the black tiger shrimp (Penaeus monodon) genome facilitates the identification of growth-associated genes. Mol. Ecol. Resour. 2021, 21, 1620–1640. [Google Scholar] [CrossRef]
- Cui, Z.; Liu, Y.; Yuan, J.; Zhang, X.; Ventura, T.; Ma, K.Y.; Sun, S.; Song, C.; Zhan, D.; Yang, Y.; et al. The Chinese mitten crab genome provides insights into adaptive plasticity and developmental regulation. Nat. Commun. 2021, 12, 2395. [Google Scholar] [CrossRef]
- Jiang, K.; Fang, X.; Li, Y.-L.; Qiu, G.-F. Genome-wide identification, phylogeny, expression and eyestalk neuroendocrine regulation of vitellogenin gene family in the freshwater giant prawn Macrobrachium rosenbergii. Gen. Comp. Endocrinol. 2023, 340, 114306. [Google Scholar] [CrossRef]
- Innan, H.; Kondrashov, F. The evolution of gene duplications: Classifying and distinguishing between models. Nat. Rev. Genet. 2010, 11, 97–108. [Google Scholar] [CrossRef]
- Gao, X.-M.; Zhou, Y.; Zhang, D.-D.; Hou, C.-C.; Zhu, J.-Q. Multiple vitellogenin genes (vtgs) in large yellow croaker (Larimichthys crocea): Molecular characterization and expression pattern analysis during ovarian development. Fish Physiol. Biochem. 2019, 45, 829–848. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yan, T.; Tan, J.T.T.; Gong, Z. A zebrafish vitellogenin gene (vg3) encodes a novel vitellogenin without a phosvitin domain and may represent a primitive vertebrate vitellogenin gene. Gene 2000, 256, 303–310. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, W.; Wang, C.; Shi, L.; Wang, G.; Sun, C.; Chan, S.F. The presence of multiple copies of the vitellogenin gene in Fenneropenaeus merguiensis (De Man, 1888) (Decapoda: Dendrobranchiata: Penaeidae): Evidence for gene expansion and functional diversification in shrimps. J. Crustac. Biol. 2021, 41, 100. [Google Scholar] [CrossRef]
- Wang, W.; Li, B.; Zhou, T.; Wang, C.; Kyei, A.B.; Shi, L.; Chan, S. Investigation of Gene Sequence Divergence, Expression Dynamics, and Endocrine Regulation of the Vitellogenin Gene Family in the Whiteleg Shrimp Litopenaeus vannamei. Front. Endocrinol. 2020, 11, 577745. [Google Scholar] [CrossRef] [PubMed]
- El-Desoky, M.S.; Jogatani, T.; Yamane, F.; Izumikawa, K.; Kakinuma, M.; Sakamoto, T.; Tsutsui, N. Identification of an additional vitellogenin gene showing hepatopancreas-specific expression in the kuruma prawn Marsupenaeus japonicus. Fish. Sci. 2023, 89, 613–623. [Google Scholar] [CrossRef]
- Manfrin, C.; Tom, M.; Avian, M.; Battistella, S.; Pallavicini, A.; Giulianini, P.G. Characterization and Gene Expression of Vitellogenesis-Related Transcripts in the Hepatopancreas and Ovary of the Red Swamp Crayfish, Procambarus clarkii (Girard, 1852), during Reproductive Cycle. Diversity 2021, 13, 445. [Google Scholar] [CrossRef]
- Leipart, V.; Montserrat-Canals, M.; Cunha, E.S.; Luecke, H.; Herrero-Galán, E.; Halskau, Ø.; Amdam, G.V. Structure prediction of honey bee vitellogenin: A multi-domain protein important for insect immunity. FEBS Open Bio 2022, 12, 51–70. [Google Scholar] [CrossRef]
- Mengal, K.; Kor, G.; Siino, V.; Buřič, M.; Kozák, P.; Levander, F.; Niksirat, H. Quantification of proteomic profile changes in the hemolymph of crayfish during in vitro coagulation. Dev. Comp. Immunol. 2023, 147, 104760. [Google Scholar] [CrossRef]
- Zhang, J.-J.; Xi, G.-S.; Zhao, J. Vitellogenin regulates estrogen-related receptor expression by crosstalk with the JH and IIS-TOR signaling pathway in Polyrhachis vicina Roger (Hymenoptera, Formicidae). Gen. Comp. Endocrinol. 2021, 310, 113836. [Google Scholar] [CrossRef]
- Huo, Y.; Yu, Y.; Chen, L.; Li, Q.; Zhang, M.; Song, Z.; Chen, X.; Fang, R.; Zhang, L. Insect tissue-specific vitellogenin facilitates transmission of plant virus. PLoS Pathog. 2018, 14, e1006909. [Google Scholar] [CrossRef] [PubMed]
- Kodrík, D.; Ibrahim, E.; Gautam, U.K.; Čapková Frydrychová, R.; Bednářová, A.; Krištůfek, V.; Jedlička, P. Changes in vitellogenin expression caused by nematodal and fungal infections in insects. J. Exp. Biol. 2019, 222, jeb202853. [Google Scholar] [CrossRef] [PubMed]
- Havukainen, H.; Münch, D.; Baumann, A.; Zhong, S.; Halskau, Ø.; Krogsgaard, M.; Amdam, G.V. Vitellogenin Recognizes Cell Damage through Membrane Binding and Shields Living Cells from Reactive Oxygen Species. J. Biol. Chem. 2013, 288, 28369–28381. [Google Scholar] [CrossRef]
- Sun, W.; Li, L.; Li, H.; Zhou, K.; Li, W.; Wang, Q. Vitellogenin receptor expression in ovaries controls innate immunity in the Chinese mitten crab (Eriocheir sinensis) by regulating vitellogenin accumulation in the hemolymph. Fish Shellfish Immunol. 2020, 107, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Tsang, W.-S.; Quackenbush, L.S.; Chow, B.K.C.; Tiu, S.H.K.; He, J.-G.; Chan, S.-M. Organization of the shrimp vitellogenin gene: Evidence of multiple genes and tissue specific expression by the ovary and hepatopancreas. Gene 2003, 303, 99–109. [Google Scholar] [CrossRef]
- Tiu, S.H.K.; Hui, J.H.L.; Mak, A.S.C.; He, J.-G.; Chan, S.-M. Equal contribution of hepatopancreas and ovary to the production of vitellogenin (PmVg1) transcripts in the tiger shrimp, Penaeus monodon. Aquaculture 2006, 254, 666–674. [Google Scholar] [CrossRef]
- Tiu, S.H.K.; Hui, H.-L.; Tsukimura, B.; Tobe, S.S.; He, J.-G.; Chan, S.-M. Cloning and expression study of the lobster (Homarus americanus) vitellogenin: Conservation in gene structure among decapods. Gen. Comp. Endocrinol. 2009, 160, 36–46. [Google Scholar] [CrossRef]
- Hurst, L.D. The Ka/Ks ratio: Diagnosing the form of sequence evolution. Trends Genet. 2002, 18, 486–487. [Google Scholar] [CrossRef]
- Ghosh, P.; Das, D.; Juin, S.K.; Hajra, S.; Kachari, A.; Das, D.N.; Nath, P.; Maitra, S. Identification and partial characterization of Olyra longicaudata (McClelland, 1842) vitellogenins: Seasonal variation in plasma, relative to estradiol-17β and ovarian growth. Aquac. Rep. 2016, 3, 120–130. [Google Scholar] [CrossRef]
- Tufail, M.; Takeda, M. Molecular characteristics of insect vitellogenins. J. Insect Physiol. 2008, 54, 1447–1458. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Chen, L.; Zhou, Z.; Li, E.; Zhao, X.; Guo, H. The site of vitellogenin synthesis in Chinese mitten-handed crab Eriocheir sinensis. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2006, 143, 453–458. [Google Scholar] [CrossRef]
- Kang, B.J.; Nanri, T.; Lee, J.M.; Saito, H.; Han, C.H.; Hatakeyama, M.; Saigusa, M. Vitellogenesis in both sexes of gonochoristic mud shrimp, Upogebia major (Crustacea): Analyses of vitellogenin gene expression and vitellogenin processing. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2008, 149, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Subramoniam, T. Mechanisms and control of vitellogenesis in crustaceans. Fish. Sci. 2011, 77, 1–21. [Google Scholar] [CrossRef]
- Raviv, S.; Parnes, S.; Segall, C.; Davis, C.; Sagi, A. Complete sequence of Litopenaeus vannamei (Crustacea: Decapoda) vitellogenin cDNA and its expression in endocrinologically induced sub-adult females. Gen. Comp. Endocrinol. 2006, 145, 39–50. [Google Scholar] [CrossRef]
- Hiransuchalert, R.; Thamniemdee, N.; Khamnamtong, B.; Yamano, K.; Klinbunga, S. Expression profiles and localization of vitellogenin mRNA and protein during ovarian development of the giant tiger shrimp Penaeus monodon. Aquaculture 2013, 412, 193–201. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, W.; Xiong, Y.; Cheng, D.; Wang, J.; Jin, S.; Gong, Y.; Wu, Y.; Qiao, H.; Fu, H. Hepatopancreas transcriptome analyses provide new insights into the molecular regulatory mechanism of fast ovary maturation in Macrobrachium nipponense. BMC Genom. 2022, 23, 625. [Google Scholar] [CrossRef]
- Jia, X.; Chen, Y.; Zou, Z.; Lin, P.; Wang, Y.; Zhang, Z. Characterization and expression profile of Vitellogenin gene from Scylla paramamosain. Gene 2013, 520, 119–130. [Google Scholar] [CrossRef]
- Yang, W.; Ohira, T.; Tsutsui, N.; Subramoniam, T.; Do, H.; Aida, K.; Wilder, M. Determination of amino acid sequence and site of mRNA expression of four vitellins in the giant freshwater prawn, Macrobrachium rosenbergii. J. Exp. Zool. 2000, 287, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, N.; Saido-Sakanaka, H.; Yang, W.-J.; Jayasankar, V.; Jasmani, S.; Okuno, A.; Ohira, T.; Okumura, T.; Aida, K.; Wilder, M.N. Molecular characterization of a cDNA encoding vitellogenin in the coonstriped shrimp, Pandalus hypsinotus and site of vitellogenin mRNA expression. J. Exp. Zool. Part A Comp. Exp. Biol. 2004, 301A, 802–814. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.-M.; Lee, S.-O.; Kim, K.S.; Baek, H.-J.; Kim, S.; Kim, I.-K.; Mykles, D.L.; Kim, H.-W. Characterization of two vitellogenin cDNAs from a Pandalus shrimp (Pandalopsis japonica): Expression in hepatopancreas is down-regulated by endosulfan exposure. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2010, 157, 102–112. [Google Scholar] [CrossRef]
- Zmora, N.; Trant, J.; Chan, S.-M.; Chung, J.S. Vitellogenin and Its Messenger RNA During Ovarian Development in the Female Blue Crab, Callinectes sapidus: Gene Expression, Synthesis, Transport, and Cleavage1. Biol. Reprod. 2007, 77, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Nagaraju, G.P.C.; Novotney, D.; Lovett, D.L.; Borst, D.W. Yolk protein expression in the green crab, Carcinus maenas. Aquaculture 2010, 298, 325–331. [Google Scholar] [CrossRef]
- Mak, A.S.C.; Choi, C.L.; Tiu, S.H.K.; Hui, J.H.L.; He, J.-G.; Tobe, S.S.; Chan, S.-M. Vitellogenesis in the red crab Charybdis feriatus: Hepatopancreas-specific expression and farnesoic acid stimulation of vitellogenin gene expression. Mol. Reprod. Dev. 2005, 70, 288–300. [Google Scholar] [CrossRef]
- Rani, K.; Subramoniam, T. Vitellogenesis in the Mud Crab Scylla serrata: An in Vivo Isotope Study. J. Crust. Biol. 1997, 17, 659. [Google Scholar] [CrossRef]
- Girish, B.P.; Swetha, C.H.; Reddy, P.S. Hepatopancreas but not ovary is the site of vitellogenin synthesis in female fresh water crab, Oziothelphusa senex senex. Biochem. Biophys. Res. Commun. 2014, 447, 323–327. [Google Scholar] [CrossRef]
- Abdu, U.; Davis, C.; Khalaila, I.; Sagi, A. The vitellogenin cDNA of Cherax quadricarinatus encodes a lipoprotein with calcium binding ability, and its expression is induced following the removal of the androgenic gland in a sexually plastic system. Gen. Comp. Endocrinol. 2002, 127, 263–272. [Google Scholar] [CrossRef]
- Tiu, S.H.K.; Hui, J.H.L.; HE, J.-G.; Tobe, S.S.; Chan, S.-M. Characterization of vitellogenin in the shrimp Metapenaeus ensis: Expression studies and hormonal regulation of MeVg1 transcription in vitro. Mol. Reprod. Dev. 2006, 73, 424–436. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, H.; Gao, X.; Cai, L. Studies on site of vitellogenin synthesis in the shrimp Fenneropenaeus chinensis. Mar. Fish. Res. 2006, 27, 7–13. [Google Scholar]
- Yi Kyung, K.; Naoaki, T.; Ichiro, K.; Takuji, O.; Toyoji, K.; Katsumi, A. Localization and Developmental Expression of mRNA for Cortical Rod Protein in Kuruma Prawn Marsupenaeus japonicus. Zool. Sci. 2005, 22, 675–680. [Google Scholar] [CrossRef]
- Khayat, M.; Lubzens, E.; Tietz, A.; Funkenstein, B. Cell-Free Synthesis of Vitellin in the Shrimp Penaeus semisulcatus (de Haan). Gen. Comp. Endocrinol. 1994, 93, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Phiriyangkul, P.; Puengyam, P.; Jakobsen, I.B.; Utarabhand, P. Dynamics of vitellogenin mRNA expression during vitellogenesis in the banana shrimp Penaeus (Fenneropenaeus) merguiensis using real-time PCR. Mol. Reprod. Dev. 2007, 74, 1198–1207. [Google Scholar] [CrossRef]
- Sittikankaew, K.; Pootakham, W.; Sonthirod, C.; Sangsrakru, D.; Yoocha, T.; Khudet, J.; Nookaew, I.; Uawisetwathana, U.; Rungrassamee, W.; Karoonuthaisiri, N. Transcriptome analyses reveal the synergistic effects of feeding and eyestalk ablation on ovarian maturation in black tiger shrimp. Sci. Rep. 2020, 10, 3239. [Google Scholar] [CrossRef]
- Amankwah, B.K.; Wang, C.; Zhou, T.; Liu, J.; Shi, L.; Wang, W.; Chan, S. Eyestalk Ablation, a Prerequisite for Crustacean Reproduction: A review. Isr. J. Aquac.-Bamidgeh 2019, 71, 1–14. [Google Scholar]
- Takuji, O. Effects of Bilateral and Unilateral Eyestalk Ablation on Vitellogenin Synthesis in Immature Female Kuruma Prawns, Marsupenaeus japonicus. Zool. Sci. 2007, 24, 233–240. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, M.; Gao, B.; Lv, J.; Li, J.; Liu, P. Integrative Proteomic and MicroRNA Analysis: Insights into Mechanisms of Eyestalk Ablation-Induced Ovarian Maturation in the Swimming Crab Portunus trituberculatus. Front. Endocrinol. 2020, 11, 00533. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, M.; Ruan, Y.; Chen, X.; Ren, C.; Yang, H.; Zhang, X.; Liu, J.; Li, H.; Zhang, L.; et al. Transcriptomic analysis reveals yolk accumulation mechanism from the hepatopancreas to ovary in the pacific white shrimp Litopenaeus vannamei. Front. Mar. Sci. 2022, 9, 948105. [Google Scholar] [CrossRef]
- Wang, C.; Chen, Y.; Chen, Y.; Zhao, Y. A preliminary study on artificial ripening of Exopalaemon carinicauda. J. Zhejiang Coll. Fish. 1995, 14, 243–246. [Google Scholar]
- Jia, S.; Li, J.; Lv, J.; Ren, X.; Wang, J.; Wang, Q.; Liu, P.; Li, J. Molecular Characterization Related to Ovary Early Development Mechanisms after Eyestalk Ablation in Exopalaemon carinicauda. Biology 2023, 12, 596. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Y.; Zhang, Z.; Zhu, J.; Yu, J. KaKs_Calculator 2.0: A Toolkit Incorporating Gamma-Series Methods and Sliding Window Strategies. Genom. Proteom. Bioinform. 2010, 8, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Zhang, P.; Li, Z.; Zhao, L.; Lai, X.; Gao, H.; Li, J.; Yan, B. Cloning, expression and stability analysis of the reference gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in Exopalaemon carinicauda. J. Fish Sci. China 2017, 24, 1003–1012. [Google Scholar] [CrossRef]
- Wang, X.E. Early embryonis development on Exopalaemon carinicauda and relation of its incubation with temperature and salinity. J. Fish. China 1989, 13, 59–64. [Google Scholar]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).