Expression, Functional Polymorphism, and Diagnostic Values of MIAT rs2331291 and H19 rs217727 Long Non-Coding RNAs in Cerebral Ischemic Stroke Egyptian Patients
Abstract
:1. Introduction
2. Results
2.1. Demographic and Clinical Features of CIS Cases and Healthy Controls
2.2. Serum Expression Levels of lncRNA-MIAT and H19 Levels in CIS Patients and Healthy Subjects
2.3. Different Methylation Patterns of H19 Gene Promoter in CIS Patients
2.4. Correlations between Serum lncRNA-MIAT and H19 Levels
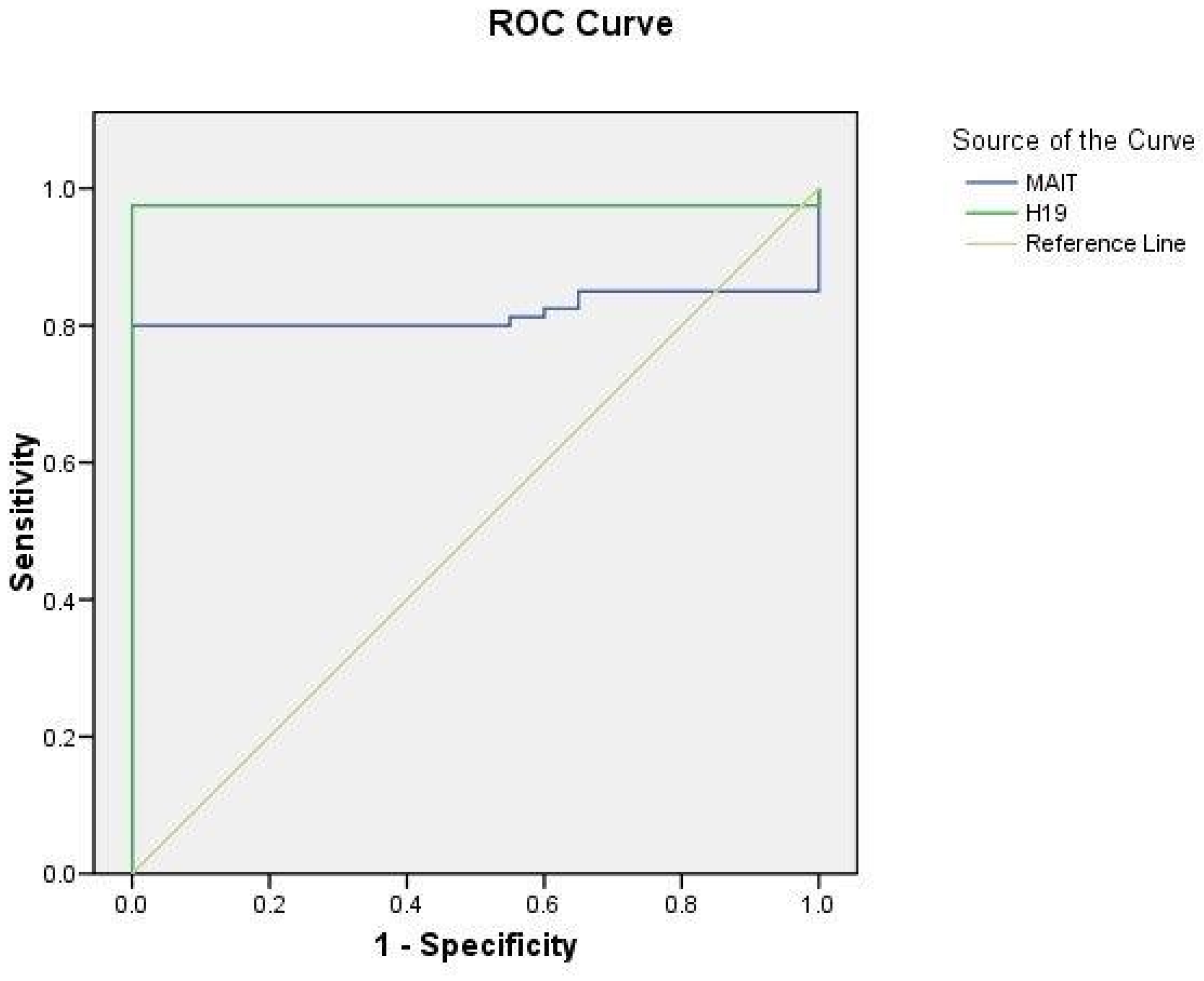
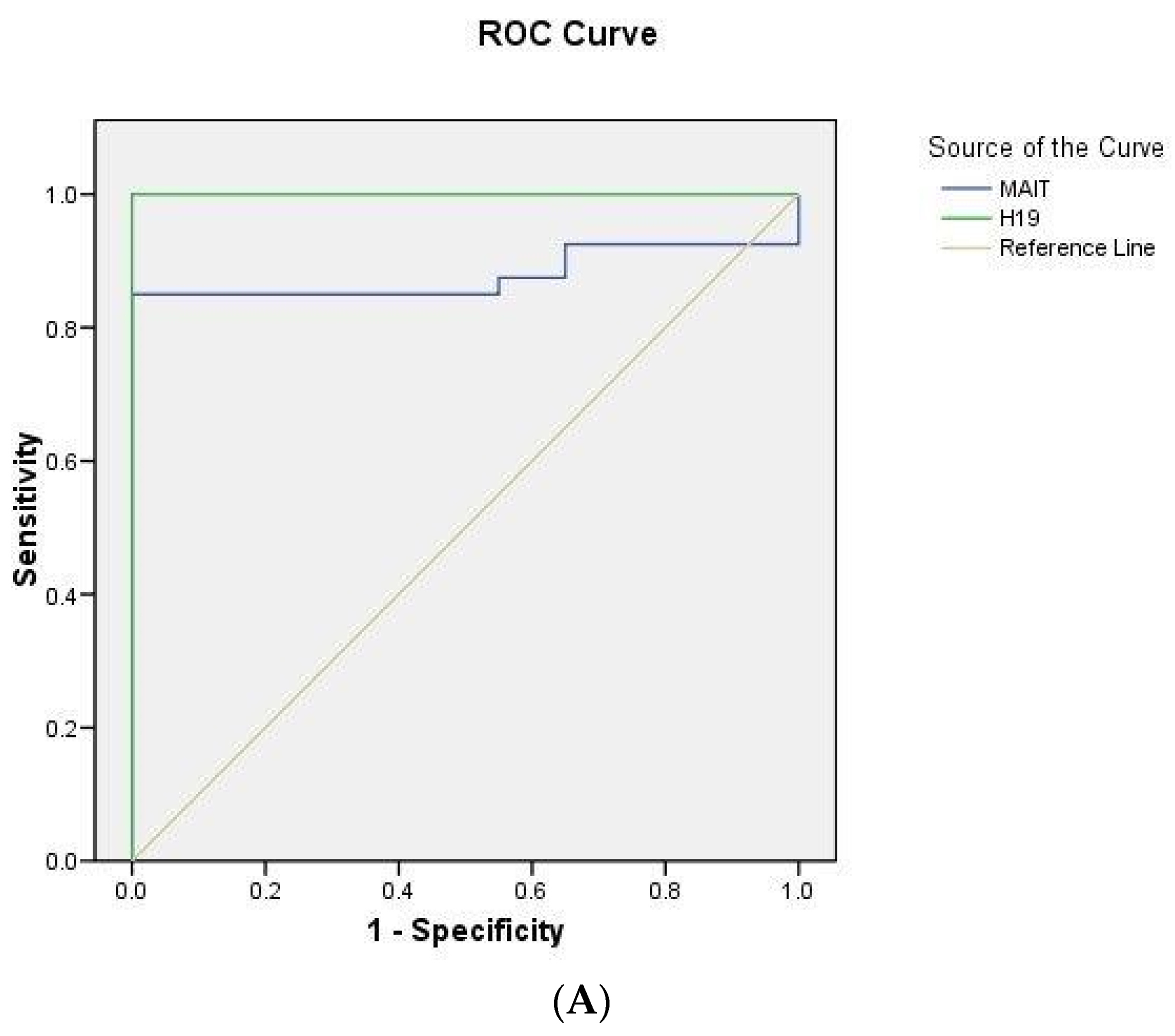
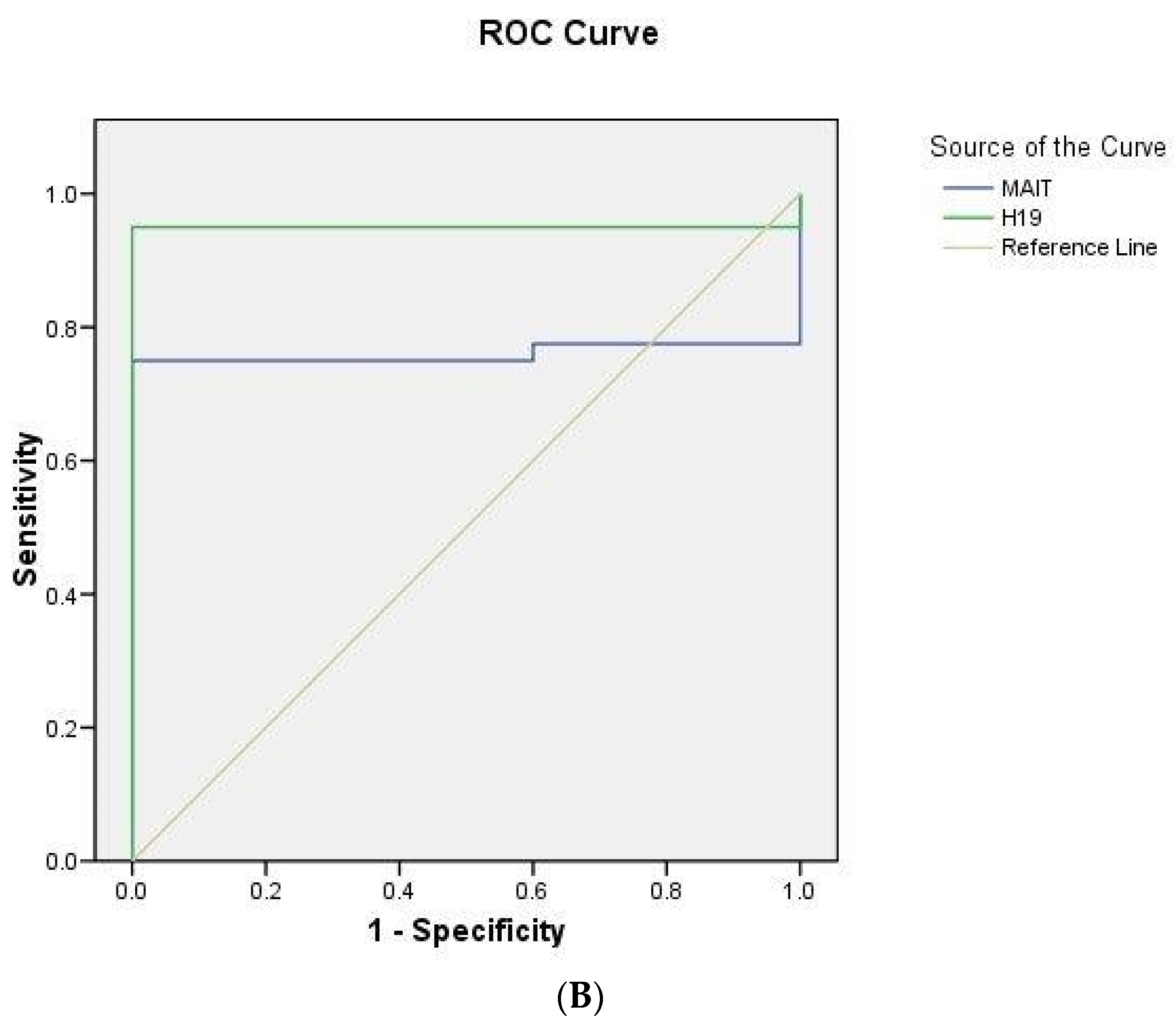
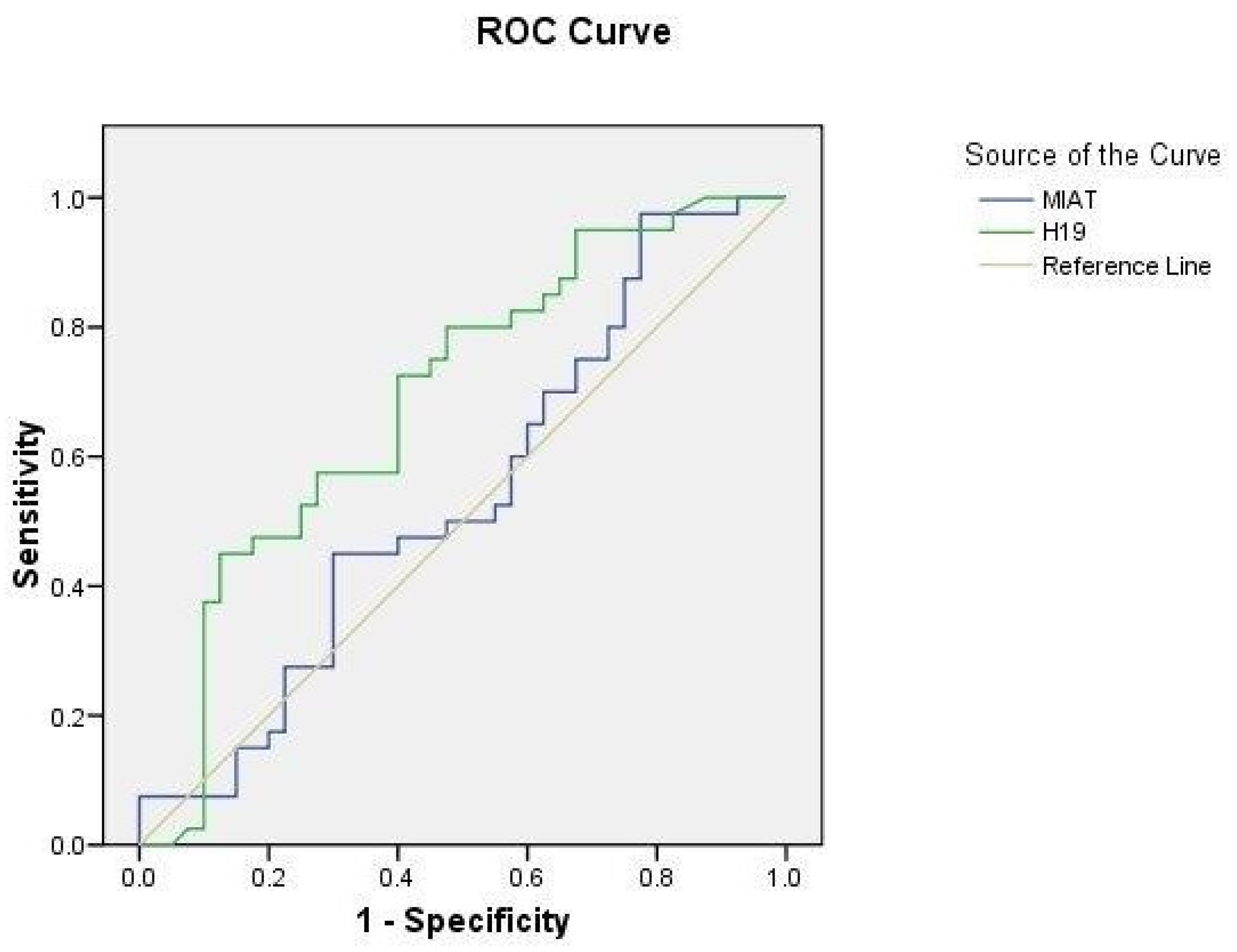
2.5. ROC Curve Analysis
3. Discussion
4. Subjects and Methods
4.1. Subjects
4.2. Blood Sampling and Laboratory Assays
4.3. DNA Extraction and Genotyping
4.4. Serum MIAT and H19 Assays by RT-qPCR
- MIAT: forward 5′-ATCACGCGTCCAGAGTCAGGGAAAAAGACC-3′
- MIAT: reverse 5′-ATCCTCGAGTTGAATTCTACCATTTTCT TACATC-3′
- H19: forward 5′-GTCGCTATCTCTAGGTGAAG-3′
- H19: reverse 5′-GTGGAGGCTTTGAATCTCTC-3′
- GAPDH: forward 5′-CCCTTCATTGACCTCAACTA-3′
- GAPDH: reverse 5′-TGGAAGATGGTGATGGGATT-3′
4.5. H19-rs217727 Promoter Methylation
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Słomka, A.; Świtońska, M.; Sinkiewicz, W.; Żekanowska, E. Haemostatic Factors Do Not Account for Worse Outcomes from Ischaemic Stroke in Patients with Higher C-Reactive Protein Concentrations. Ann. Clin. Biochem. 2017, 54, 378–385. [Google Scholar] [CrossRef]
- Gao, Y.; Xu, W.; Li, W.; Chen, Z.; Li, Q.; Liu, Z.; Liu, H.; Dai, L. Epidemiology and Prevalence of Pulmonary Sequestration in Chinese Population, 2010–2019. BMC Pulm. Med. 2023, 23, 8. [Google Scholar] [CrossRef]
- Yousufuddin, M.; Young, N. Aging and Ischemic Stroke. Aging 2019, 11, 2542. [Google Scholar] [CrossRef]
- Gao, W.; Zhu, M.; Wang, H.; Zhao, S.; Zhao, D.; Yang, Y.; Wang, Z.-M.M.; Wang, F.; Yang, Z.-J.J.; Lu, X.; et al. Association of Polymorphisms in Long Non-Coding RNA H19 with Coronary Artery Disease Risk in a Chinese Population. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2015, 772, 15–22. [Google Scholar] [CrossRef]
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, Regional, and National Prevalence of Overweight and Obesity in Children and Adults during 1980–2013: A Systematic Analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef]
- Feigin, V.L.; Krishnamurthi, R.V.; Parmar, P.; Norrving, B.; Mensah, G.A.; Bennett, D.A.; Barker-Collo, S.; Moran, A.E.; Sacco, R.L.; Truelsen, T.; et al. Update on the Global Burden of Ischemic and Hemorrhagic Stroke in 1990–2013: The GBD 2013 Study. Neuroepidemiology 2015, 45, 161–176. [Google Scholar] [CrossRef]
- Aref, H.; Zakaria, M.; Shokri, H.; Roushdy, T.; El Basiouny, A.; El Nahas, N. Changing the Landscape of Stroke in Egypt. Cerebrovasc. Dis. Extra 2021, 11, 155–159. [Google Scholar] [CrossRef]
- Vijayan, M.; Reddy, P.H. Peripheral Biomarkers of Stroke: Focus on Circulatory MicroRNAs. Biochim. Biophys. Acta 2016, 1862, 1984. [Google Scholar] [CrossRef]
- Zerna, C.; Hegedus, J.; Hill, M.D. Evolving Treatments for Acute Ischemic Stroke. Circ. Res. 2016, 118, 1425–1442. [Google Scholar] [CrossRef] [PubMed]
- Dewey, H.M.; Thrift, A.G.; Mihalopoulos, C.; Carter, R.; Macdonell, R.A.L.; McNeil, J.J.; Donnan, G.A. Cost of Stroke in Australia from a Societal Perspective: Results from the North East Melbourne Stroke Incidence Study (NEMESIS). Stroke 2001, 32, 2409–2416. [Google Scholar] [CrossRef] [PubMed]
- Béjot, Y.; Garnier, P. Cerebral Ischemia. In Hormesis in Health and Disease; CRC Press: Boca Raton, FL, USA, 2022; pp. 185–200. [Google Scholar] [CrossRef]
- Lakhan, S.E.; Kirchgessner, A.; Hofer, M. Inflammatory Mechanisms in Ischemic Stroke: Therapeutic Approaches. J. Transl. Med. 2009, 7, 97. [Google Scholar] [CrossRef]
- Yemisci, M.; Caban, S.; Gursoy-Ozdemir, Y.; Lule, S.; Novoa-Carballal, R.; Riguera, R.; Fernandez-Megia, E.; Andrieux, K.; Couvreur, P.; Capan, Y.; et al. Systemically Administered Brain-Targeted Nanoparticles Transport Peptides across the Blood–Brain Barrier and Provide Neuroprotection. J. Cereb. Blood Flow Metab. 2015, 35, 469. [Google Scholar] [CrossRef] [PubMed]
- Jover-Mengual, T.; Hwang, J.Y.; Byun, H.R.; Court-Vazquez, B.L.; Centeno, J.M.; Burguete, M.C.; Zukin, R.S. The Role of NF-ΚB Triggered Inflammation in Cerebral Ischemia. Front. Cell. Neurosci. 2021, 15, 633610. [Google Scholar] [CrossRef] [PubMed]
- Şekerdağ, E.; Solaroğlu, I.; Gürsoy-Özdemir, Y. Cell Death Mechanisms in Stroke and Novel Molecular and Cellular Treatment Options. Curr. Neuropharmacol. 2018, 16, 1396. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zeng, L.; Lu, X.; Chen, K.; Yu, M.; Wang, B.; Zhao, M. Early Brain Injury and Neuroprotective Treatment after Aneurysmal Subarachnoid Hemorrhage: A Literature Review. Brain Sci. 2023, 13, 1083. [Google Scholar] [CrossRef]
- Manzanero, S.; Santro, T.; Arumugam, T.V. Neuronal Oxidative Stress in Acute Ischemic Stroke: Sources and Contribution to Cell Injury. Neurochem. Int. 2013, 62, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.L.; Ju, H.Q.; Lu, Y.X.; Chen, L.Z.; Zeng, Z.L.; Zhang, D.S.; Luo, H.Y.; Wang, F.; Qiu, M.Z.; Wang, D.S.; et al. Long Non-Coding RNA XIST Regulates Gastric Cancer Progression by Acting as a Molecular Sponge of MiR-101 to Modulate EZH2 Expression. J. Exp. Clin. Cancer Res. 2016, 35, 142. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Morris-Blanco, K.C.; Lopez, M.S.; Yang, T.; Zhao, H.; Vemuganti, R.; Luo, Y. Impact of MicroRNAs on Ischemic Stroke: From Pre- to Post-Disease. Prog. Neurobiol. 2018, 163–164, 59–78. [Google Scholar] [CrossRef]
- Amouyel, P. From Genes to Stroke Subtypes. Lancet Neurol. 2012, 11, 931–933. [Google Scholar] [CrossRef]
- Kakkar, P.; Kakkar, T.; Patankar, T.; Saha, S. Current Approaches and Advances in the Imaging of Stroke. Dis. Model. Mech. 2021, 14, dmm048785. [Google Scholar] [CrossRef]
- Chen, W.; Wu, J.; Wei, R.; Wu, S.; Xia, C.; Wang, D.; Liu, D.; Zheng, L.; Zou, T.; Li, R.; et al. Improving the Diagnosis of Acute Ischemic Stroke on Non-Contrast CT Using Deep Learning: A Multicenter Study. Insights Imaging 2022, 13, 184. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, A.; Satagopam, V.; Schneider, R. Long Non-Coding RNAs: Mechanisms, Experimental, and Computational Approaches in Identification, Characterization, and Their Biomarker Potential in Cancer. Front. Genet. 2021, 12, 649619. [Google Scholar] [CrossRef] [PubMed]
- Schaukowitch, K.; Kim, T.K. Emerging Epigenetic Mechanisms of Long Non-Coding RNAs. Neuroscience 2014, 264, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Kazimierczyk, M.; Wrzesinski, J. Long Non-Coding RNA Epigenetics. Int. J. Mol. Sci. 2021, 22, 6166. [Google Scholar] [CrossRef] [PubMed]
- Iyer, M.K.; Niknafs, Y.S.; Malik, R.; Singhal, U.; Sahu, A.; Hosono, Y.; Barrette, T.R.; Prensner, J.R.; Evans, J.R.; Zhao, S.; et al. The Landscape of Long Noncoding RNAs in the Human Transcriptome. Nat. Genet. 2015, 47, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Shan, G. The Physiological Function of Long-Noncoding RNAs. Non-Coding RNA Res. 2020, 5, 178. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Yang, X. Pathophysiology of Long Non-Coding RNAs in Ischemic Stroke. Front. Mol. Neurosci. 2018, 11, 96. [Google Scholar] [CrossRef]
- Zhu, W.; Tian, L.; Yue, X.; Liu, J.; Fu, Y.; Yan, Y. LncRNA Expression Profiling of Ischemic Stroke during the Transition from the Acute to Subacute Stage. Front. Neurol. 2019, 10, 36. [Google Scholar] [CrossRef]
- Gao, N.; Li, Y.; Li, J.; Gao, Z.; Yang, Z.; Li, Y.; Liu, H.; Fan, T. Long Non-Coding RNAs: The Regulatory Mechanisms, Research Strategies, and Future Directions in Cancers. Front. Oncol. 2020, 10, 598817. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, W.; Zhu, W.; Dong, J.; Cheng, Y.; Yin, Z.; Shen, F. Mechanisms and Functions of Long Non-Coding RNAs at Multiple Regulatory Levels. Int. J. Mol. Sci. 2019, 20, 5573. [Google Scholar] [CrossRef]
- Li, L.; Chang, H.Y. Physiological Roles of Long Noncoding RNAs: Insights from Knockout Mice. Trends Cell Biol. 2014, 24, 594. [Google Scholar] [CrossRef] [PubMed]
- Graf, J.; Kretz, M. From Structure to Function: Route to Understanding LncRNA Mechanism. BioEssays 2020, 42, 2000027. [Google Scholar] [CrossRef] [PubMed]
- Zampetaki, A.; Albrecht, A.; Steinhofel, K. Long Non-Coding RNA Structure and Function: Is There a Link? Front. Physiol. 2018, 9, 1201. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Fullwood, M.J. Roles, Functions, and Mechanisms of Long Non-Coding RNAs in Cancer. Genomics. Proteom. Bioinform. 2016, 14, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Chang, C.P. Long Non-Coding RNA and Chromatin Remodeling. RNA Biol. 2015, 12, 1094. [Google Scholar] [CrossRef]
- Saxena, A.; Carninci, P. Long Non-Coding RNA Modifies Chromatin: Epigenetic Silencing by Long Non-Coding RNAs. Bioessays 2011, 33, 830. [Google Scholar] [CrossRef]
- Lorenzen, J.M.; Martino, F.; Thum, T. Epigenetic Modifications in Cardiovascular Disease. Basic Res. Cardiol. 2012, 107, 245. [Google Scholar] [CrossRef]
- Ma, B.; Wang, S.; Wu, W.; Shan, P.; Chen, Y.; Meng, J.; Xing, L.; Yun, J.; Hao, L.; Wang, X.; et al. Mechanisms of CircRNA/LncRNA-MiRNA Interactions and Applications in Disease and Drug Research. Biomed. Pharmacother. 2023, 162, 114672. [Google Scholar] [CrossRef]
- Ronco, C.; Haapio, M.; House, A.A.; Anavekar, N.; Bellomo, R. Cardiorenal Syndrome. J. Am. Coll. Cardiol. 2008, 52, 1527–1539. [Google Scholar] [CrossRef]
- Yoon, J.H.; Abdelmohsen, K.; Gorospe, M. Post-Transcriptional Gene Regulation by Long Noncoding RNA. J. Mol. Biol. 2013, 425, 3723. [Google Scholar] [CrossRef]
- Katara, P. Single Nucleotide Polymorphism and Its Dynamics for Pharmacogenomics. Interdiscip. Sci. Comput. Life Sci. 2014, 6, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Alwi, Z. Bin The Use of SNPs in Pharmacogenomics Studies. Malays. J. Med. Sci. 2005, 12, 4–12. [Google Scholar] [PubMed]
- Gray, I.C.; Campbell, D.A.; Spurr, N.K. Single Nucleotide Polymorphisms as Tools in Human Genetics. Hum. Mol. Genet. 2000, 9, 2403–2408. [Google Scholar] [CrossRef]
- Liao, J.; He, Q.; Li, M.; Chen, Y.; Liu, Y.; Wang, J. LncRNA MIAT: Myocardial Infarction Associated and More. Gene 2016, 578, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.S.; Zhang, N.C.; Li, K.; Sun, H.X.; Dai, X.M.; Liu, G.L. Upregulation of Long Non-Coding RNA Myocardial Infarction-Associated Transcription Is Correlated with Coronary Artery Stenosis and Elevated Inflammation in Patients with Coronary Atherosclerotic Heart Disease. Kaohsiung J. Med. Sci. 2021, 37, 1038–1047. [Google Scholar] [CrossRef]
- Vausort, M.; Wagner, D.R.; Devaux, Y. Long Noncoding RNAs in Patients with Acute Myocardial Infarction. Circ. Res. 2014, 115, 668–677. [Google Scholar] [CrossRef]
- Borghetti, G.; Von Lewinski, D.; Eaton, D.M.; Sourij, H.; Houser, S.R.; Wallner, M. Diabetic Cardiomyopathy: Current and Future Therapies. Beyond Glycemic Control. Front. Physiol. 2018, 9, 414750. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, W.; Jin, M.; Chen, J.; Xu, W.; Kong, X. LncRNA MIAT Functions as a Competing Endogenous RNA to Upregulate DAPK2 by Sponging MiR-22-3p in Diabetic Cardiomyopathy. Cell Death Dis. 2017, 8, e2929. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, S.; Xiong, Y. Long Noncoding RNA MIAT Promotes Non-Small Cell Lung Cancer Progression by Sponging MiR-149-5p and Regulating FOXM1 Expression. Cancer Cell Int. 2020, 20, 348. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Zheng, F.S.; Yang, W.; Lu, J. Bin The Long Non-Coding RNA MIAT Regulates Zinc Finger E-Box Binding Homeobox 1 Expression by Sponging MiR-150 and Promoteing Cell Invasion in Non-Small-Cell Lung Cancer. Gene 2017, 633, 61–65. [Google Scholar] [CrossRef]
- Zhu, M.; Li, N.; Luo, P.; Jing, W.; Wen, X.; Liang, C.; Tu, J. Peripheral Blood Leukocyte Expression of LncRNA MIAT and Its Diagnostic and Prognostic Value in Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2018, 27, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Shan, K.; Qun-Wang, X.; Zhou, R.M.; Yang, H.; Liu, C.; Li, Y.J.; Yao, J.; Li, X.M.; Shen, Y.; et al. Long Non-Coding RNA-MIAT Promotes Neurovascular Remodeling in the Eye and Brain. Oncotarget 2016, 7, 49688–49698. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xiong, X.; Zhang, L.; Shen, J. Neurovascular Unit: A Critical Role in Ischemic Stroke. CNS Neurosci. Ther. 2021, 27, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Ishii, N.; Ozaki, K.; Sato, H.; Mizuno, H.; Saito, S.; Takahashi, A.; Miyamoto, Y.; Ikegawa, S.; Kamatani, N.; Hori, M.; et al. Identification of a Novel Non-Coding RNA, MIAT, That Confers Risk of Myocardial Infarction. J. Hum. Genet. 2006, 51, 1087–1099. [Google Scholar] [CrossRef] [PubMed]
- Matouk, I.J.; DeGroot, N.; Mezan, S.; Ayesh, S.; Abu-Lail, R.; Hochberg, A.; Galun, E. The H19 Non-Coding RNA Is Essential for Human Tumor Growth. PLoS ONE 2007, 2, e845. [Google Scholar] [CrossRef] [PubMed]
- Yoshimizu, T.; Miroglio, A.; Ripoche, M.A.M.-A.; Gabory, A.; Vernucci, M.; Riccio, A.; Colnot, S.; Godard, C.; Terris, B.; Jammes, H.; et al. The H19 Locus Acts in Vivo as a Tumor Suppressor. Proc. Natl. Acad. Sci. USA 2008, 105, 12417–12422. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Ma, X.; Zhao, H.; Fan, J.; Liu, T.; Luo, Y.; Guo, Y. Long Non-Coding RNA H19 Promotes Leukocyte Inflammation in Ischemic Stroke by Targeting the MiR-29b/C1QTNF6 Axis. CNS Neurosci. Ther. 2022, 28, 953–963. [Google Scholar] [CrossRef]
- Hernández-Valero, M.A.; Rother, J.; Gorlov, I.; Frazier, M.; Gorlova, O. Interplay between Polymorphisms and Methylation in the H19/IGF2 Gene Region May Contribute to Obesity in Mexican-American Children. J. Dev. Orig. Health Dis. 2013, 4, 499–506. [Google Scholar] [CrossRef]
- Tragante, V.; Barnes, M.R.; Ganesh, S.K.; Lanktree, M.B.; Guo, W.; Franceschini, N.; Smith, E.N.; Johnson, T.; Holmes, M.V.; Padmanabhan, S.; et al. Gene-Centric Meta-Analysis in 87,736 Individuals of European Ancestry Identifies Multiple Blood-Pressure-Related Loci. Am. J. Hum. Genet. 2014, 94, 349. [Google Scholar] [CrossRef]
- Rezaei, M.; Mokhtari, M.J.; Bayat, M.; Safari, A.; Dianatpuor, M.; Tabrizi, R.; Asadabadi, T.; Borhani-Haghighi, A. Long Non-Coding RNA H19 Expression and Functional Polymorphism Rs217727 Are Linked to Increased Ischemic Stroke Risk. BMC Neurol. 2021, 21, 54. [Google Scholar] [CrossRef]
- Wang, B.; Suen, C.W.; Ma, H.; Wang, Y.; Kong, L.; Qin, D.; Lee, Y.W.W.; Li, G. The Roles of H19 in Regulating Inflammation and Aging. Front. Immunol. 2020, 11, 579687. [Google Scholar] [CrossRef] [PubMed]
- Jinno, Y.; Ikeda, Y.; Yun, K.; Maw, M.; Masuzaki, H.; Fukuda, H.; Inuzuka, K.; Fujishita, A.; Ohtani, Y.; Okimoto, T.; et al. Establishment of Functional Imprinting of the H19 Gene in Human Developing Placentae. Nat. Genet. 1995, 10, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.H.; Bai, X.D.; Zhang, H.J.; Li, Y.M.; Hu, L.; Liu, L.Y.; Mao, J.P.; Yang, X.Y.; Dila, N.M. Gene Polymorphisms Affect the Effectiveness of Atorvastatin in Treating Ischemic Stroke Patients. Cell. Physiol. Biochem. 2016, 39, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Huang, L.; Li, Z.; Leng, K.; Xu, Y.; Jiang, X.; Cui, Y. Long Non-Coding RNA MIAT in Development and Disease: A New Player in an Old Game. J. Biomed. Sci. 2018, 25, 23. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.J.; Kim, O.J.; Kim, S.Y.; Oh, S.H.; Oh, D.; Kim, O.J.; Shin, B.S.; Kim, N.K. Association of the MiR-146a, MiR-149, MiR-196a2, and MiR-499 Polymorphisms with Ischemic Stroke and Silent Brain Infarction Risk. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 420–430. [Google Scholar] [CrossRef]
- MedlinePlus Genetics Genomic Research Center. March 2022. Available online: https://medlineplus.gov/genetics/understanding/genomicresearch/snp/ (accessed on 18 November 2023).
- Sone, M.; Hayashi, T.; Tarui, H.; Agata, K.; Takeichi, M.; Nakagawa, S. The MRNA-like Noncoding RNA Gomafu Constitutes a Novel Nuclear Domain in a Subset of Neurons. J. Cell Sci. 2007, 120, 2498–2506. [Google Scholar] [CrossRef]
- Alipoor, F.J.; Asadi, M.H.; Torkzadeh-Mahani, M. MIAT LncRNA Is Overexpressed in Breast Cancer and Its Inhibition Triggers Senescence and G1 Arrest in MCF7 Cell Line. J. Cell. Biochem. 2018, 119, 6470–6481. [Google Scholar] [CrossRef]
- Sattari, A.; Siddiqui, H.; Moshiri, F.; Ngankeu, A.; Nakamura, T.; Kipps, T.J.; Croce, C.M. Upregulation of Long Noncoding RNA MIAT in Aggressive Form of Chronic Lymphocytic Leukemias. Oncotarget 2016, 7, 54174–54182. [Google Scholar] [CrossRef]
- Yan, B.; Yao, J.; Liu, J.Y.; Li, X.M.; Wang, X.Q.; Li, Y.J.; Tao, Z.F.; Song, Y.C.; Chen, Q.; Jiang, Q. LncRNA-MIAT Regulates Microvascular Dysfunction by Functioning as a Competing Endogenous RNA. Circ. Res. 2015, 116, 1143–1156. [Google Scholar] [CrossRef]
- Shen, Y.; Dong, L.F.; Zhou, R.M.; Yao, J.; Song, Y.C.; Yang, H.; Jiang, Q.; Yan, B. Role of Long Non-Coding RNA MIAT in Proliferation, Apoptosis and Migration of Lens Epithelial Cells: A Clinical and in Vitro Study. J. Cell. Mol. Med. 2016, 20, 537–548. [Google Scholar] [CrossRef]
- Li, M.H.; Leng, T.D.; Feng, X.C.; Yang, T.; Simon, R.P.; Xiong, Z.G. Modulation of Acid-Sensing Ion Channel 1a by Intracellular PH and Its Role in Ischemic Stroke. J. Biol. Chem. 2016, 291, 18370–18383. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, C.; Yao, Y.; Chen, L.; Liu, G.; Zhang, R.; Liu, Q.; Shi, F.D.; Hao, J. MTORC1 Pathway Disruption Ameliorates Brain Inflammation Following Stroke via a Shift in Microglia Phenotype from M1 Type to M2 Type. FASEB J. 2016, 30, 3388–3399. [Google Scholar] [CrossRef] [PubMed]
- Hartings, J.A.; York, J.; Carroll, C.P.; Hinzman, J.M.; Mahoney, E.; Krueger, B.; Winkler, M.K.L.; Major, S.; Horst, V.; Jahnke, P.; et al. Subarachnoid Blood Acutely Induces Spreading Depolarizations and Early Cortical Infarction. Brain 2017, 140, 2673–2690. [Google Scholar] [CrossRef] [PubMed]
- Lückl, J.; Lemale, C.L.; Kola, V.; Horst, V.; Khojasteh, U.; Oliveira-Ferreira, A.I.; Major, S.; Winkler, M.K.L.; Kang, E.J.; Schoknecht, K.; et al. The Negative Ultraslow Potential, Electrophysiological Correlate of Infarction in the Human Cortex. Brain 2018, 141, 1734–1752. [Google Scholar] [CrossRef] [PubMed]
- Mayor, D.; Tymianski, M. Neurotransmitters in the Mediation of Cerebral Ischemic Injury. Neuropharmacology 2018, 134, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Veenman, L.; Singh, S.; Ouyang, F.; Liang, J.; Huang, W.; Marek, I.; Zeng, J.; Gavish, M. 2-Cl-MGV-1 Ameliorates Apoptosis in the Thalamus and Hippocampus and Cognitive Deficits after Cortical Infarct in Rats. Stroke 2017, 48, 3366–3374. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Qiu, J.; Fan, Y.; Zhang, Q.; Cheng, B.; Wu, Y.; Bai, B. Apelin-13 Attenuates ER Stress-Mediated Neuronal Apoptosis by Activating Gαi/Gαq-CK2 Signaling in Ischemic Stroke. Exp. Neurol. 2018, 302, 136–144. [Google Scholar] [CrossRef]
- Giral, H.; Landmesser, U.; Kratzer, A. Into the Wild: GWAS Exploration of Non-Coding RNAs. Front. Cardiovasc. Med. 2018, 5, 181. [Google Scholar] [CrossRef]
- Bao, M.-H.H.; Szeto, V.; Yang, B.B.; Zhu, S.-Z.Z.; Sun, H.-S.S.; Feng, Z.-P.P. Long Non-Coding RNAs in Ischemic Stroke. Cell Death Dis. 2018, 9, 281. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, H.; Fan, Z.; Li, G.; Ma, Q.; Tao, Z.; Wang, R.; Feng, J.; Luo, Y. Long Noncoding RNA H19 Promotes Neuroinflammation in Ischemic Stroke by Driving Histone Deacetylase 1–Dependent M1 Microglial Polarization. Stroke 2017, 48, 2211–2221. [Google Scholar] [CrossRef]
- Wang, J.; Cao, B.; Han, D.; Sun, M.; Feng, J. Long Non-Coding RNA H19 Induces Cerebral Ischemia Reperfusion Injury via Activation of Autophagy. Aging Dis. 2017, 8, 71. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Cao, W.; Yang, J.J.; Shi, K.H.; Zhou, X.; Liu, L.P.; Li, J. Long Noncoding RNA H19 Controls DUSP5/ERK1/2 Axis in Cardiac Fibroblast Proliferation and Fibrosis. Cardiovasc. Pathol. 2016, 25, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Puyal, J.; Clarke, P.G.H. Targeting Autophagy to Prevent Neonatal Stroke Damage. Autophagy 2009, 5, 1060–1061. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, L.; Mao, Y.; Nan, G. Long Noncoding RNA-H19 Contributes to Atherosclerosis and Induces Ischemic Stroke via the Upregulation of Acid Phosphatase 5. Front. Neurol. 2019, 10, 32. [Google Scholar] [CrossRef] [PubMed]
- Hardman, D.; Mcfalls, D.; Fakas, S. Characterization of Phosphatidic Acid Phosphatase Activity in the Oleaginous Yeast Yarrowia Lipolytica and Its Role in Lipid Biosynthesis. Yeast 2017, 34, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Liu, X.; He, Z. Long Non-Coding RNA H19 and MALAT1 Gene Variants in Patients with Ischemic Stroke in a Northern Chinese Han Population. Mol. Brain 2018, 11, 58. [Google Scholar] [CrossRef] [PubMed]
- Muka, T.; Koromani, F.; Portilla, E.; O’Connor, A.; Bramer, W.M.; Troup, J.; Chowdhury, R.; Dehghan, A.; Franco, O.H. The Role of Epigenetic Modifications in Cardiovascular Disease: A Systematic Review. Int. J. Cardiol. 2016, 212, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Mahley, R.W. Apolipoprotein E: Structure and Function in Lipid Metabolism, Neurobiology, and Alzheimer’s Diseases. Neurobiol. Dis. 2014, 72, 3–12. [Google Scholar] [CrossRef]
- Van Giau, V.; Bagyinszky, E.; An, S.S.A.; Kim, S.Y. Role of Apolipoprotein E in Neurodegenerative Diseases. Neuropsychiatr. Dis. Treat. 2015, 11, 1723–1737. [Google Scholar] [CrossRef]
- Lagging, C.; Lorentzen, E.; Stanne, T.M.; Pedersen, A.; Söderholm, M.; Cole, J.W.; Jood, K.; Lemmens, R.; Phuah, C.L.; Rost, N.S.; et al. APOE Ε4 Is Associated with Younger Age at Ischemic Stroke Onset but Not with Stroke Outcome. Neurology 2019, 93, 849. [Google Scholar] [CrossRef]
- Satizabal, C.L.; Samieri, C.; Davis-Plourde, K.L.; Voetsch, B.; Aparicio, H.J.; Pase, M.P.; Romero, J.R.; Helmer, C.; Vasan, R.S.; Kase, C.S.; et al. APOE and the Association of Fatty Acids With the Risk of Stroke, Coronary Heart Disease, and Mortality. Stroke 2018, 49, 2822–2829. [Google Scholar] [CrossRef] [PubMed]
- Stanzione, R.; Cotugno, M.; Bianchi, F.; Marchitti, S.; Forte, M.; Volpe, M.; Rubattu, S. Pathogenesis of Ischemic Stroke: Role of Epigenetic Mechanisms. Genes 2020, 11, 89. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yang, L.; Zhong, T.; Mueller, M.; Men, Y.; Zhang, N.; Xie, J.; Giang, K.; Chung, H.; Sun, X.; et al. H19 LncRNA Alters DNA Methylation Genome Wide by Regulating S-Adenosylhomocysteine Hydrolase. Nat. Commun. 2015, 6, 10221. [Google Scholar] [CrossRef] [PubMed]
- Devlin, A.M.; Bottiglieri, T.; Domann, F.E.; Lentz, S.R. Tissue-Specific Changes in H19 Methylation and Expression in Mice with Hyperhomocysteinemia. J. Biol. Chem. 2005, 280, 25506–25511. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhang, H.; Sun, T.; Wang, J.; Sun, W.; Gong, H.; Yang, B.; Shi, Y.; Wei, J. The Comprehensive Effects of Hyperlipidemia and Hyperhomocysteinemia on Pathogenesis of Atherosclerosis and DNA Hypomethylation in ApoE-/- Mice. Acta Biochim. Biophys. Sin. 2012, 44, 866–875. [Google Scholar] [CrossRef]
- Senousy, M.A.; Shaker, O.G.; Sayed, N.H.; Fathy, N.; Kortam, M.A. LncRNA GAS5 and MiR-137 Polymorphisms and Expression Are Associated with Multiple Sclerosis Risk: Mechanistic Insights and Potential Clinical Impact. ACS Chem. Neurosci. 2020, 11, 1651–1660. [Google Scholar] [CrossRef]
- Shaker, O.G.; Senousy, M.A.; Elbaz, E.M. Association of Rs6983267 at 8q24, HULC Rs7763881 Polymorphisms and Serum LncRNAs CCAT2 and HULC with Colorectal Cancer in Egyptian Patients. Sci. Rep. 2017, 7, 16246. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]

| Parameter | CIS (n = 80) | Control (n = 40) | p-Value |
|---|---|---|---|
| Sex | |||
| Male | 62 (77.5%) | 31 (77.5%) | 0.99 |
| Female | 18 (22.5%) | 9 (22.5%) | |
| Age | 57.3 ±10.66 | 56.95 ± 10.71 | 0.73 |
| Total cholesterol | 211.5 ± 40.9 | 107 ± 23.3 | 0.001 * |
| TG | 146.6 ± 61.3 | 90.9 ± 18.3 | 0.001 * |
| LDL | 145.4 ± 40.9 | 73.3 ± 17.8 | 0.001 * |
| HDL | 37.9 ± 11.9 | 54.4 ± 9.3 | 0.001 * |
| Smoking | 51 (63.75%) | 25 (62.5%) | 0.89 |
| Sex | p-Value | Age (Years) | p-Value | Smoking | Non-Smoking | p-Value | |||
|---|---|---|---|---|---|---|---|---|---|
| Male (%) | Female (%) | ||||||||
| CIS (n = 80) | HTN (n = 40) | 30 (75%) | 10 (25%) | 0.592 | 58.9 ± 8.88 | 0.162 | 25 (62.5%) | 15 (37.5%) | 0.816 |
| Non- HTN (n = 40) | 32 (80%) | 8 (20%) | 55.6 ± 12 | 26 (65%) | 14 (35%) | ||||
| Hypertensive CIS (n = 40) | D.M (n = 20) | 13 (65%) | 7 (35%) | 0.27 | 63.3 ± 6.4 | 0.001 * | 10 (50%) | 10 (50%) | 0.103 |
| Non-D.M (n = 20) | 17 (85%) | 3 (15%) | 54.6 ± 8.9 | 15 (75%) | 5 (25%) | ||||
| Non- Hypertensive CIS (n = 40) | D.M (n = 18) | 14 (77.8%) | 4 (22.2%) | 1.0 | 60.2 ± 7.5 | 0.072 | 12 (67%) | 6 (33%) | 0.84 |
| Non-D.M (n = 22) | 18 (81.8%) | 4 (18.2%) | 51.9 ± 13.9 | 14 (64%) | 8 (36%) | ||||
| Genotype Allele | CIS | Control | ||||
|---|---|---|---|---|---|---|
| MIAT rs2331291 | Observed Frequency | Expected Frequency | p-Value | Observed Frequency | Expected Frequency | p-Value |
| CC | 52.5% | 45.5% | 0.005 * | 82.5% | 83.25% | 0.54 |
| CT | 30% | 43.9% | 17.5% | 16% | ||
| TT | 17.5% | 10.56% | 0.0% | 0.75% | ||
| H19 rs217727 | ||||||
| CC | 71.3% | 68% | 0.048 * | 57.5% | 58.1% | 0.8 |
| CT | 22.5% | 28.8% | 37.5% | 36.2% | ||
| TT | 6.3% | 3.12% | 5% | 5.7% | ||
| Genotype Allele | HTN (n = 40) | Non-HTN (n = 40) | ||||
|---|---|---|---|---|---|---|
| MIAT rs2331291 | Observed Frequency | Expected Frequency | p-Value | Observed Frequency | Expected Frequency | p-Value |
| CC | 35% | 28.93% | 0.120 | 70% | 66% | 0.098 |
| CT | 37.5% | 49.7% | 22.5% | 30.5% | ||
| TT | 27.5% | 21.37% | 7.5% | 3.5% | ||
| H19 rs217727 | ||||||
| CC | 70% | 66% | 0.098 | 72.5% | 70.1% | 0.273 |
| CT | 22.5% | 30.5% | 22.5% | 27.2% | ||
| TT | 7.5% | 3.5% | 5% | 2.7% | ||
| Genotype Allele | CIS (n = 80) | Control (n = 40) | p-Value |
|---|---|---|---|
| MIAT rs2331291 | |||
| CC | 42 (52.5%) | 33 (82.5%) | 0.02 * |
| CT | 24 (30%) | 7 (17.5%) | |
| TT | 14 (17.5%) | 0 (0%) | |
| C | 108 (67.5%) | 73 (91.3%) | 0.0001 * |
| T | 52 (32.5%) | 7 (8.8%) | |
| H19 rs217727 | |||
| CC | 57 (71.3%) | 23 (57.5%) | 0.22 |
| CT | 18 (22.5%) | 15 (37.5%) | |
| TT | 5 (6.3%) | 2 (5%) | |
| C | 132 (82.5%) | 61 (76.3%) | 0.25 |
| T | 28 (17.5%) | 19 (23.8) | |
| Genotype Allele | CIS | p-Value | |
|---|---|---|---|
| HTN (n = 40) | Non-HTN (n = 40) | ||
| MIAT rs2331291 | |||
| CC | 14 (35%) | 28 (70%) | 0.004 * |
| CT | 15 (37.5%) | 9 (22.5%) | |
| TT | 11 (27.5%) | 3 (7.5%) | |
| C | 43 (53.8%) | 65 (81.3%) | 0.0001 * |
| T | 37 (46.3%) | 15 (18.7%) | |
| H19 rs217727 | |||
| CC | 28 (70%) | 29 (72.5%) | 0.89 |
| CT | 9 (22.5%) | 9 (22.5%) | |
| TT | 3 (7.5%) | 2 (5%) | |
| C | 65 (81.3%) | 67 (83.8%) | 0.677 |
| T | 15 (18.8%) | 13 (16.3%) | |
| LncRNA | Fold Change | p-Value | |
|---|---|---|---|
| Downregulated | |||
| MIAT | 0.569 ± 0.94 | 0.0001 * | |
| HTN (n = 40) | Non-HTN (n = 40) | ||
| MIAT | 0.384 ± 0.55 | 0.754 ± 1.19 | 0.08 |
| Upregulated | |||
| H19 | 23.19 ± 20.87 | 0.0001 * | |
| HTN (n = 40) | Non-HTN (n = 40) | ||
| H19 | 15.99 ± 14.5 | 30.38 ± 23.79 | 0.002 * |
| Genotype Allele | CIS (n = 80) | p-Value |
|---|---|---|
| MIAT rs2331291 | ||
| CC (n = 42) | 0.77 ± 1.2 | 0.157 |
| CT (n = 24) | 0.2 ± 0.17 | |
| TT (n = 14) | 0.56 ± 0.46 | |
| H19 rs217727 | ||
| CC (n = 57) | 21.57± 19.14 | 0.793 |
| CT (n = 18) | 28.3 ± 24.9 | |
| TT (n = 5) | 23.04 ± 25.9 | |
| Genotype Allele | HTN (n = 40) | p-Value | Non-HTN (n = 40) | p-Value |
|---|---|---|---|---|
| MIAT rs2331291 | ||||
| CC | 0.4 ± 0.77 (n = 14) | 0.157 | 0.96 ± 1.37 (n = 28) | 0.564 |
| CT | 0.19 ± 0.18 (n = 15) | 0.22 ± 0.16 (n = 9) | ||
| TT | 0.61 ± 0.51 (n = 11) | 0.39 ± 0.17 (n = 3) | ||
| H19 rs217727 | ||||
| CC | 15.33 ± 11.8(n = 28) | 0.128 | 27.58 ± 22.8 (n = 29) | 0.304 |
| CT | 22 ± 21.4 (n = 9) | 34.7 ± 27.7 (n = 9) | ||
| TT | 4.11 ± 0.57 (n = 3) | 51.4 ± 0.0 (n = 2) | ||
| CIS (n = 80) | Control (n = 40) | ||
|---|---|---|---|
| Expression Level | 53.79 ± 5.45 | 45.4 ± 0.6 | |
| p-Value | 0.0001 * | ||
| Genotype Allele | |||
| CC | 53.89 ± 5.3 (n = 57) | 45.5 ± 0.63 (n = 23) | |
| CT | 53.9 ± 6 (n = 18) | 45.1 ± 0.6 (n = 15) | |
| TT | 51.9 ± 5.2 (n = 5) | 45.2 ± 0.8 (n = 2) | |
| p-Value | 0.817 | 0.261 | |
| Genotype Allele | HTN (n = 40) | Non-HTN (n = 40) | |
| CC | 53.5 ± 5.1 (n = 28) | 54.26 ± 5.5 (n = 29) | |
| CT | 53.1 ± 6 (n = 9) | 54.8 ± 6.3 (n = 9) | ------- |
| TT | 49.8 ± 3.2 (n = 3) | 54.9 ± 7.6 (n = 2) | ------- |
| p-Value | 0.598 | 0.854 | ------- |
| CIS | ||||
|---|---|---|---|---|
| HTN (n = 40) | Non-HTN (n = 40) | |||
| r | 0.284 | 0.259 | ||
| p-value | 0.07 | 0.107 | ||
| D.M (n = 20) | Non-D.M (n = 20) | D.M (n = 18) | Non-D.M (n = 22) | |
| r | 0.045 | 0.646 | 0.017 | 0.422 |
| p-value | 0.85 | 0.002 * | 0.945 | 0.064 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motawi, T.K.; Sadik, N.A.H.; Shaker, O.G.; Ghaleb, M.M.H.; Elbaz, E.M. Expression, Functional Polymorphism, and Diagnostic Values of MIAT rs2331291 and H19 rs217727 Long Non-Coding RNAs in Cerebral Ischemic Stroke Egyptian Patients. Int. J. Mol. Sci. 2024, 25, 842. https://doi.org/10.3390/ijms25020842
Motawi TK, Sadik NAH, Shaker OG, Ghaleb MMH, Elbaz EM. Expression, Functional Polymorphism, and Diagnostic Values of MIAT rs2331291 and H19 rs217727 Long Non-Coding RNAs in Cerebral Ischemic Stroke Egyptian Patients. International Journal of Molecular Sciences. 2024; 25(2):842. https://doi.org/10.3390/ijms25020842
Chicago/Turabian StyleMotawi, Tarek K., Nermin Abdel Hamid Sadik, Olfat G. Shaker, Maggy Maged Haider Ghaleb, and Eman M. Elbaz. 2024. "Expression, Functional Polymorphism, and Diagnostic Values of MIAT rs2331291 and H19 rs217727 Long Non-Coding RNAs in Cerebral Ischemic Stroke Egyptian Patients" International Journal of Molecular Sciences 25, no. 2: 842. https://doi.org/10.3390/ijms25020842





