Mechanical Tensions Regulate Gene Expression in the Xenopus laevis Axial Tissues
Abstract
1. Introduction
2. Results
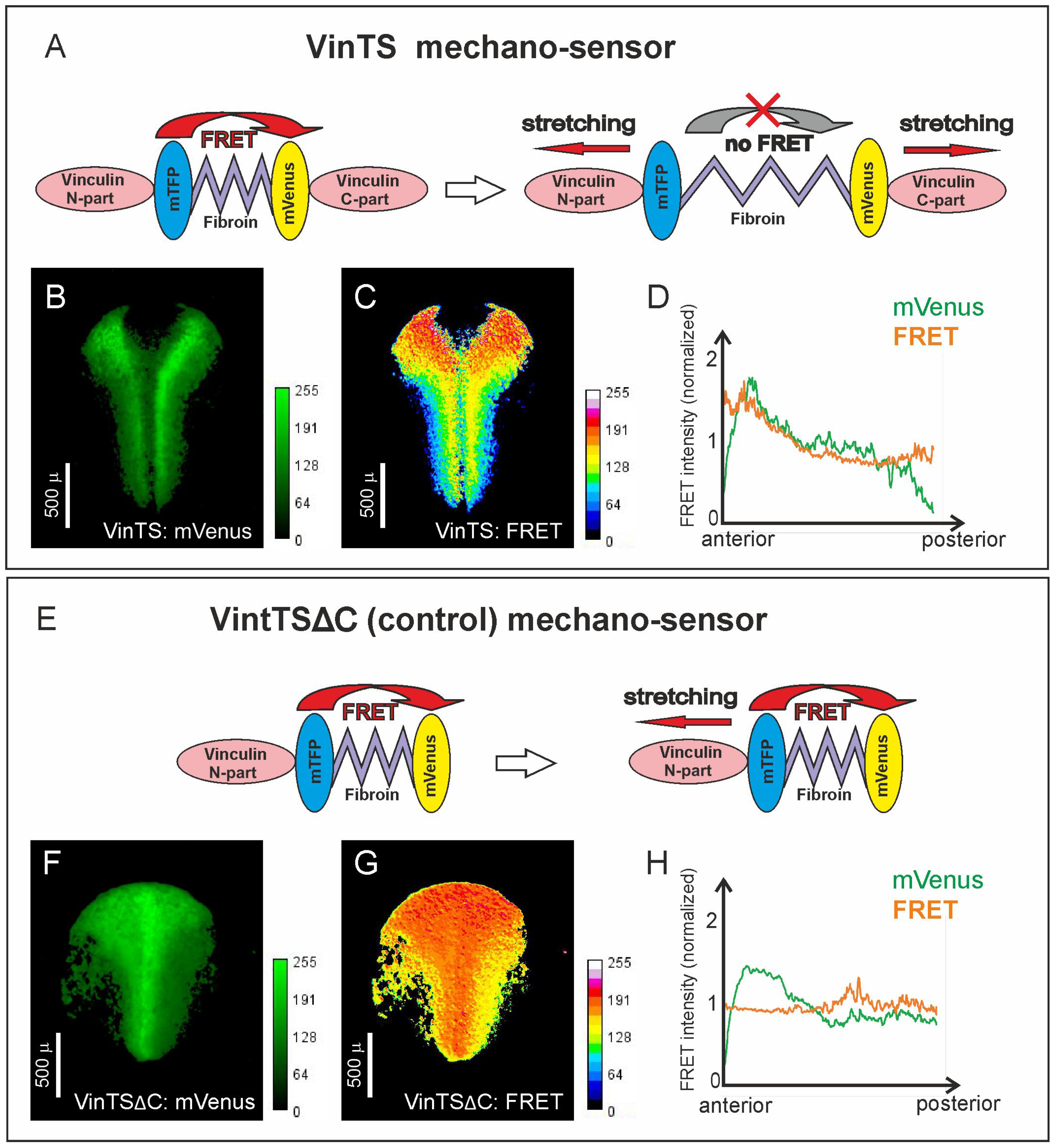
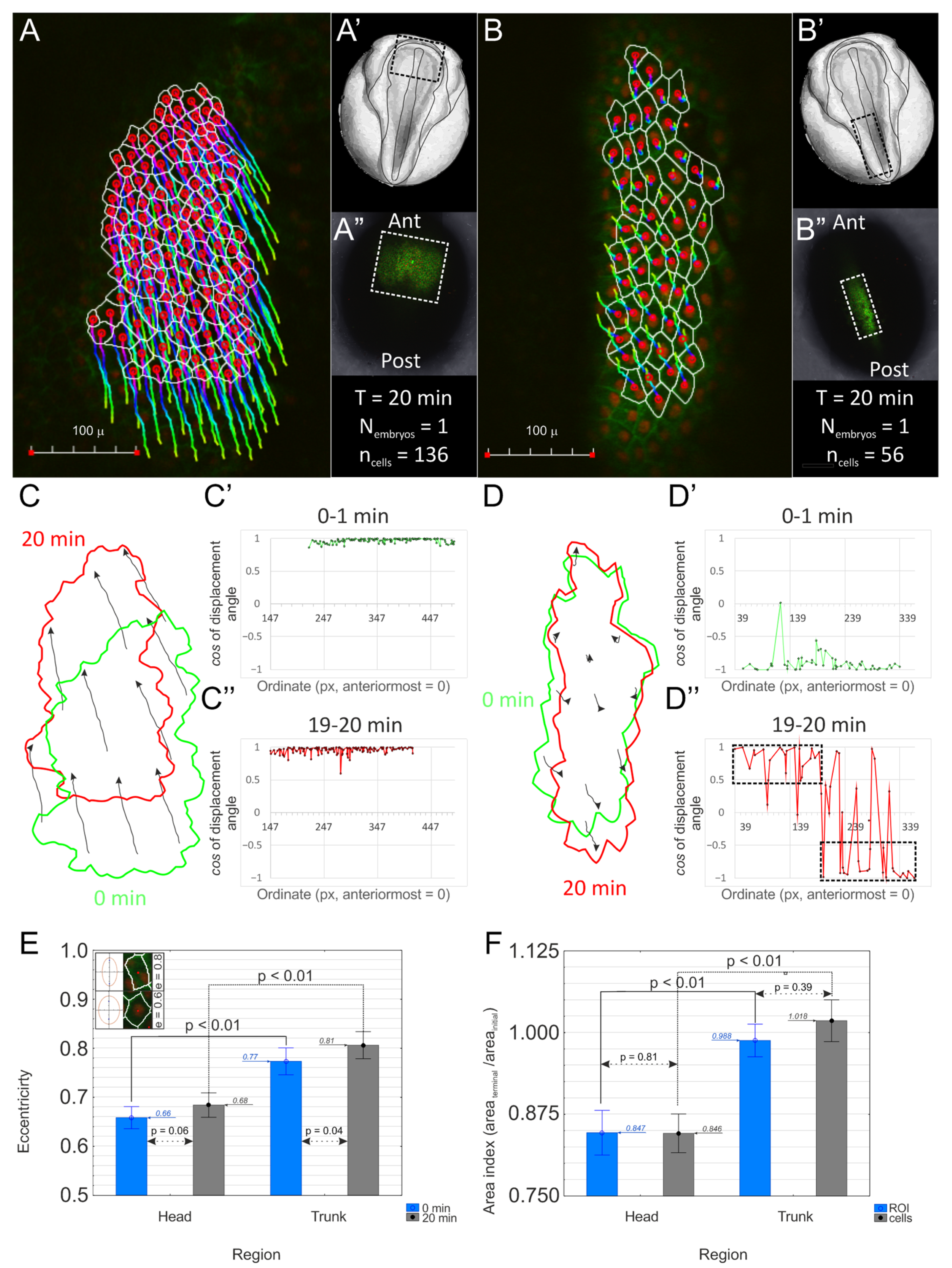
2.1. The Head and Trunk Parts of the Neuroectoderm Experience Different Mechanical Tensions
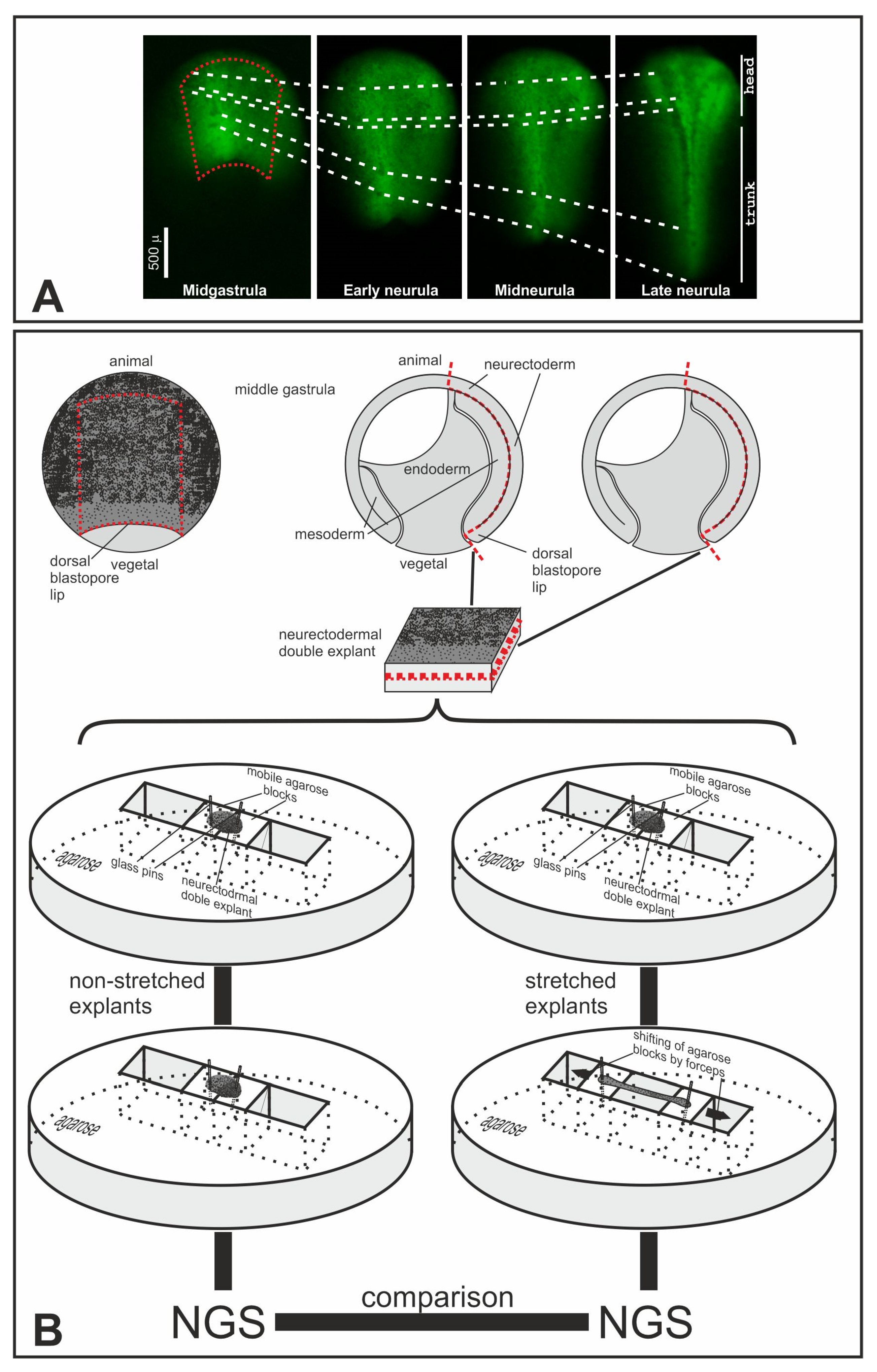
2.2. Modeling Stretching of the Trunk Neuroectoderm in Explants of the Dorsal Gastrula Ectoderm
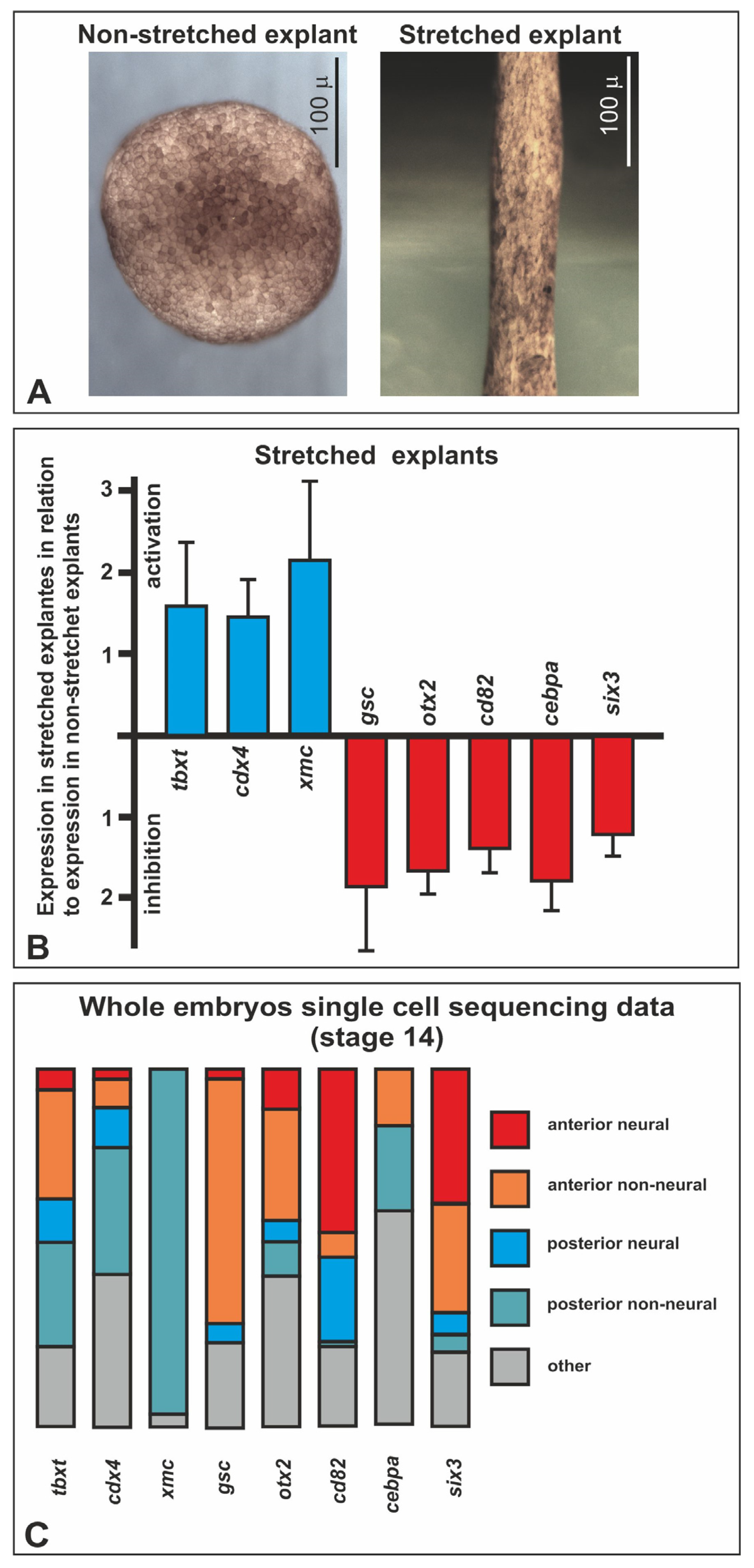
2.3. Differential Gene Expression in Stretched and Nonstretched Explants
3. Discussion
3.1. Stretched-Activated Genes
3.2. Stretched-Inhibited Genes
3.3. Possible Mediators of the Mechanotransduction
4. Materials and Methods
4.1. VinTS Cloning, RNA Microinjection, Image Acquisition, and Processing
4.2. FRET Validation
4.3. Cell Morphometry
4.4. Cutting and Stretching Explants
4.5. High-Throughput Sequencing
4.6. Xenopus Single-Cell Transcriptomic Data Analysis
4.7. QRT-PCR
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roux, W. Gesammelte Abhandlungen uË ber Entwicklungsmechanik; W. Engelmann: Leipzig, Germany, 1895; Volume II. [Google Scholar]
- Wolff, Y. Das Gesetz der Transformation der Knochen; Verlag von August Hirschwald: Berlin, Germany, 1892. [Google Scholar]
- Belintsev, B.N.; Beloussov, L.V.; Zaraisky, A.G. Model of pattern formation in epithelial morphogenesis. J. Theor. Biol. 1987, 129, 369–394. [Google Scholar] [CrossRef] [PubMed]
- Delile, J.; Herrmann, M.; Peyrieras, N.; Doursat, R. A cell-based computational model of early embryogenesis coupling mechanical behaviour and gene regulation. Nat. Commun. 2017, 8, 13929. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.D.; Oster, G.F.; Harris, A.K. A mechanical model for mesenchymal morphogenesis. J. Math. Biol. 1983, 17, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Eroshkin, F.M.; Zaraisky, A.G. Mechano-sensitive regulation of gene expression during the embryonic development. Genesis 2017, 55, e23026. [Google Scholar] [CrossRef]
- Brunet, T.; Bouclet, A.; Ahmadi, P.; Mitrossilis, D.; Driquez, B.; Brunet, A.C.; Henry, L.; Serman, F.; Bealle, G.; Menager, C.; et al. Evolutionary conservation of early mesoderm specification by mechanotransduction in Bilateria. Nat. Commun. 2013, 4, 2821. [Google Scholar] [CrossRef] [PubMed]
- Desprat, N.; Supatto, W.; Pouille, P.A.; Beaurepaire, E.; Farge, E. Tissue deformation modulates twist expression to determine anterior midgut differentiation in Drosophila embryos. Dev. Cell 2008, 15, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Farge, E. Mechanical induction of Twist in the Drosophila foregut/stomodeal primordium. Curr. Biol. 2003, 13, 1365–1377. [Google Scholar] [CrossRef]
- Eyckmans, J.; Boudou, T.; Yu, X.; Chen, C.S. A hitchhiker’s guide to mechanobiology. Dev. Cell 2011, 21, 35–47. [Google Scholar] [CrossRef]
- Janmey, P.A.; Wells, R.G.; Assoian, R.K.; McCulloch, C.A. From tissue mechanics to transcription factors. Differentiation 2013, 86, 112–120. [Google Scholar] [CrossRef]
- Parada, C.; Banavar, S.P.; Khalilian, P.; Rigaud, S.; Michaut, A.; Liu, Y.; Joshy, D.M.; Campas, O.; Gros, J. Mechanical feedback defines organizing centers to drive digit emergence. Dev. Cell 2022, 57, 854–866.e6. [Google Scholar] [CrossRef]
- Sanketi, B.D.; Zuela-Sopilniak, N.; Bundschuh, E.; Gopal, S.; Hu, S.; Long, J.; Lammerding, J.; Hopyan, S.; Kurpios, N.A. Pitx2 patterns an accelerator-brake mechanical feedback through latent TGFbeta to rotate the gut. Science 2022, 377, eabl3921. [Google Scholar] [CrossRef] [PubMed]
- Sokol, S.Y. Mechanotransduction During Vertebrate Neurulation. Curr. Top. Dev. Biol. 2016, 117, 359–376. [Google Scholar] [PubMed]
- Beloussov, L.V. Mechanically based generative laws of morphogenesis. Phys. Biol. 2008, 5, 015009. [Google Scholar] [CrossRef] [PubMed]
- Beloussov, L.V.; Dorfman, J.G.; Cherdantzev, V.G. Mechanical stresses and morphological patterns in amphibian embryos. J. Embryol. Exp. Morphol. 1975, 34, 559–574. [Google Scholar] [CrossRef] [PubMed]
- Grashoff, C.; Hoffman, B.D.; Brenner, M.D.; Zhou, R.; Parsons, M.; Yang, M.T.; McLean, M.A.; Sligar, S.G.; Chen, C.S.; Ha, T.; et al. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature 2010, 466, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.; Tsuboi, T.; Ishinabe, N.; Kitaguchi, T.; Michiue, T. Wide and high resolution tension measurement using FRET in embryo. Sci. Rep. 2016, 6, 28535. [Google Scholar] [CrossRef] [PubMed]
- Hardin, J.; Keller, R. The behaviour and function of bottle cells during gastrulation of Xenopus laevis. Development 1988, 103, 211–230. [Google Scholar] [CrossRef]
- Martin, A.C.; Gelbart, M.; Fernandez-Gonzalez, R.; Kaschube, M.; Wieschaus, E.F. Integration of contractile forces during tissue invagination. J. Cell Biol. 2010, 188, 735–749. [Google Scholar] [CrossRef]
- Elul, T.; Keller, R. Monopolar protrusive activity: A new morphogenic cell behavior in the neural plate dependent on vertical interactions with the mesoderm in Xenopus. Dev. Biol. 2000, 224, 3–19. [Google Scholar] [CrossRef]
- Keller, R. Shaping the vertebrate body plan by polarized embryonic cell movements. Science 2002, 298, 1950–1954. [Google Scholar] [CrossRef]
- Belousov, L.V.; Luchinskaia, N.N.; Zaraiskii, A.G. Tensotaxis-a collective movement of embryonic cells up along the gradients of mechanical tensions. Ontogenez 1999, 30, 220–228. [Google Scholar]
- Briggs, J.A.; Weinreb, C.; Wagner, D.E.; Megason, S.; Peshkin, L.; Kirschner, M.W.; Klein, A.M. The dynamics of gene expression in vertebrate embryogenesis at single-cell resolution. Science 2018, 360, eaar5780. [Google Scholar] [CrossRef]
- Butler, M.T.; Wallingford, J.B. Spatial and temporal analysis of PCP protein dynamics during neural tube closure. Elife 2018, 7, e36456. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Shen, C.; Yang, L.; Ni, C.; Huang, J.; Lin, K.; Cao, Z.; Xu, S.; Cui, W.; Wang, X.; et al. Mechanical stretching boosts expansion and regeneration of intestinal organoids through fueling stem cell self-renewal. Cell Regen. 2022, 11, 39. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, N.; Rungger, D. Antisense inhibition of Xbrachyury impairs mesoderm formation in Xenopus embryos. Dev. Growth Differ. 2002, 44, 147–159. [Google Scholar] [CrossRef]
- Itoh, K.; Sokol, S.Y. Graded amounts of Xenopus dishevelled specify discrete anteroposterior cell fates in prospective ectoderm. Mech. Dev. 1997, 61, 113–125. [Google Scholar] [CrossRef]
- Vignal, E.; de Santa Barbara, P.; Guemar, L.; Donnay, J.M.; Fort, P.; Faure, S. Expression of RhoB in the developing Xenopus laevis embryo. Gene Expr. Patterns 2007, 7, 282–288. [Google Scholar] [CrossRef]
- Pukhlyakova, E.; Aman, A.J.; Elsayad, K.; Technau, U. beta-Catenin-dependent mechanotransduction dates back to the common ancestor of Cnidaria and Bilateria. Proc. Natl. Acad. Sci. USA 2018, 115, 6231–6236. [Google Scholar] [CrossRef] [PubMed]
- Hudson, C.; Yasuo, H. Neuromesodermal Lineage Contribution to CNS Development in Invertebrate and Vertebrate Chordates. Genes 2021, 12, 592. [Google Scholar] [CrossRef]
- Sambasivan, R.; Steventon, B. Neuromesodermal Progenitors: A Basis for Robust Axial Patterning in Development and Evolution. Front. Cell Dev. Biol. 2021, 8, 607516. [Google Scholar] [CrossRef]
- Beck, C.W.; Slack, J.M. Analysis of the developing Xenopus tail bud reveals separate phases of gene expression during determination and outgrowth. Mech. Dev. 1998, 72, 41–52. [Google Scholar] [CrossRef]
- Faas, L.; Isaacs, H.V. Overlapping functions of Cdx1, Cdx2, and Cdx4 in the development of the amphibian Xenopus tropicalis. Dev. Dyn. 2009, 238, 835–852. [Google Scholar] [CrossRef]
- Marletaz, F.; Maeso, I.; Faas, L.; Isaacs, H.V.; Holland, P.W. Cdx ParaHox genes acquired distinct developmental roles after gene duplication in vertebrate evolution. BMC Biol. 2015, 13, 56. [Google Scholar] [CrossRef]
- Frazzetto, G.; Klingbeil, P.; Bouwmeester, T. Xenopus marginal coil (Xmc), a novel FGF inducible cytosolic coiled-coil protein regulating gastrulation movements. Mech. Dev. 2002, 113, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Haremaki, T.; Weinstein, D.C. Xmc mediates Xctr1-independent morphogenesis in Xenopus laevis. Dev. Dyn. 2009, 238, 2382–2387. [Google Scholar] [CrossRef]
- Yao, J.; Kessler, D.S. Goosecoid promotes head organizer activity by direct repression of Xwnt8 in Spemann’s organizer. Development 2001, 128, 2975–2987. [Google Scholar] [CrossRef]
- Sander, V.; Reversade, B.; De Robertis, E.M. The opposing homeobox genes Goosecoid and Vent1/2 self-regulate Xenopus patterning. Embo J. 2007, 26, 2955–2965. [Google Scholar] [CrossRef]
- Niehrs, C.; Keller, R.; Cho, K.W.; De Robertis, E.M. The homeobox gene goosecoid controls cell migration in Xenopus embryos. Cell 1993, 72, 491–503. [Google Scholar] [CrossRef]
- Andreazzoli, M.; Pannese, M.; Boncinelli, E. Activating and repressing signals in head development: The role of Xotx1 and Xotx2. Development 1997, 124, 1733–1743. [Google Scholar] [CrossRef] [PubMed]
- Pannese, M.; Polo, C.; Andreazzoli, M.; Vignali, R.; Kablar, B.; Barsacchi, G.; Boncinelli, E. The Xenopus homologue of Otx2 is a maternal homeobox gene that demarcates and specifies anterior body regions. Development 1995, 121, 707–720. [Google Scholar] [CrossRef]
- Carron, C.; Bourdelas, A.; Li, H.Y.; Boucaut, J.C.; Shi, D.L. Antagonistic interaction between IGF and Wnt/JNK signaling in convergent extension in Xenopus embryo. Mech. Dev. 2005, 122, 1234–1247. [Google Scholar] [CrossRef]
- Ghanbari, H.; Seo, H.C.; Fjose, A.; Brandli, A.W. Molecular cloning and embryonic expression of Xenopus Six homeobox genes. Mech. Dev. 2001, 101, 271–277. [Google Scholar] [CrossRef]
- Zhou, X.; Hollemann, T.; Pieler, T.; Gruss, P. Cloning and expression of xSix3, the Xenopus homologue of murine Six3. Mech. Dev. 2000, 91, 327–330. [Google Scholar] [CrossRef]
- Steinmetz, P.R.; Urbach, R.; Posnien, N.; Eriksson, J.; Kostyuchenko, R.P.; Brena, C.; Guy, K.; Akam, M.; Bucher, G.; Arendt, D. Six3 demarcates the anterior-most developing brain region in bilaterian animals. Evodevo 2010, 1, 14. [Google Scholar] [CrossRef]
- Carlin, D.; Sepich, D.; Grover, V.K.; Cooper, M.K.; Solnica-Krezel, L.; Inbal, A. Six3 cooperates with Hedgehog signaling to specify ventral telencephalon by promoting early expression of Foxg1a and repressing Wnt signaling. Development 2012, 139, 2614–2624. [Google Scholar] [CrossRef]
- Gestri, G.; Carl, M.; Appolloni, I.; Wilson, S.W.; Barsacchi, G.; Andreazzoli, M. Six3 functions in anterior neural plate specification by promoting cell proliferation and inhibiting Bmp4 expression. Development 2005, 132, 2401–2413. [Google Scholar] [CrossRef]
- Lagutin, O.V.; Zhu, C.C.; Kobayashi, D.; Topczewski, J.; Shimamura, K.; Puelles, L.; Russell, H.R.; McKinnon, P.J.; Solnica-Krezel, L.; Oliver, G. Six3 repression of Wnt signaling in the anterior neuroectoderm is essential for vertebrate forebrain development. Genes. Dev. 2003, 17, 368–379. [Google Scholar] [CrossRef]
- Liu, W.; Lagutin, O.; Swindell, E.; Jamrich, M.; Oliver, G. Neuroretina specification in mouse embryos requires Six3-mediated suppression of Wnt8b in the anterior neural plate. J. Clin. Investig. 2010, 120, 3568–3577. [Google Scholar] [CrossRef]
- Shrestha, P.; Jaganathan, A.; Huilgol, D.; Ballon, C.; Hwangbo, Y.; Mills, A.A. Chd5 regulates the transcription factor Six3 to promote neuronal differentiation. Stem Cells 2023, 41, 242–251. [Google Scholar] [CrossRef]
- Agricola, Z.N.; Jagpal, A.K.; Allbee, A.W.; Prewitt, A.R.; Shifley, E.T.; Rankin, S.A.; Zorn, A.M.; Kenny, A.P. Identification of genes expressed in the migrating primitive myeloid lineage of Xenopus laevis. Dev. Dyn. 2016, 245, 47–55. [Google Scholar] [CrossRef]
- Xu, Q.; Tata, J.R. Characterization and developmental expression of Xenopus C/EBP gene. Mech. Dev. 1992, 38, 69–81. [Google Scholar] [CrossRef]
- Park, E.C.; Hayata, T.; Cho, K.W.; Han, J.K. Xenopus cDNA microarray identification of genes with endodermal organ expression. Dev. Dyn. 2007, 236, 1633–1649. [Google Scholar] [CrossRef]
- Alexander, M.S.; Rozkalne, A.; Colletta, A.; Spinazzola, J.M.; Johnson, S.; Rahimov, F.; Meng, H.; Lawlor, M.W.; Estrella, E.; Kunkel, L.M.; et al. CD82 Is a Marker for Prospective Isolation of Human Muscle Satellite Cells and Is Linked to Muscular Dystrophies. Cell Stem Cell 2016, 19, 800–807. [Google Scholar] [CrossRef]
- Neumann, E.; Schwarz, M.C.; Hasseli, R.; Hulser, M.L.; Classen, S.; Sauerbier, M.; Rehart, S.; Mueller-Ladner, U. Tetraspanin CD82 affects migration, attachment and invasion of rheumatoid arthritis synovial fibroblasts. Ann. Rheum. Dis. 2018, 77, 1619–1626. [Google Scholar] [CrossRef]
- Zeng, T.D.; Zheng, B.; Zheng, W.; Chen, C. CD82/KAI1 inhibits invasion and metastasis of esophageal squamous cell carcinoma via TGF-beta1. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 5928–5937. [Google Scholar]
- Bergsma, A.; Ganguly, S.S.; Dick, D.; Williams, B.O.; Miranti, C.K. Global deletion of tetraspanin CD82 attenuates bone growth and enhances bone marrow adipogenesis. Bone 2018, 113, 105–113. [Google Scholar] [CrossRef]
- Pilon, N.; Oh, K.; Sylvestre, J.R.; Bouchard, N.; Savory, J.; Lohnes, D. Cdx4 is a direct target of the canonical Wnt pathway. Dev. Biol. 2006, 289, 55–63. [Google Scholar] [CrossRef]
- Ro, H.; Dawid, I.B. Modulation of Tcf3 repressor complex composition regulates cdx4 expression in zebrafish. Embo J. 2011, 30, 2894–2907. [Google Scholar] [CrossRef]
- Crease, D.J.; Dyson, S.; Gurdon, J.B. Cooperation between the activin and Wnt pathways in the spatial control of organizer gene expression. Proc. Natl. Acad. Sci. USA 1998, 95, 4398–4403. [Google Scholar] [CrossRef]
- Sokol, S.Y.; Melton, D.A. Interaction of Wnt and activin in dorsal mesoderm induction in Xenopus. Dev. Biol. 1992, 154, 348–355. [Google Scholar] [CrossRef]
- Arnold, S.J.; Stappert, J.; Bauer, A.; Kispert, A.; Herrmann, B.G.; Kemler, R. Brachyury is a target gene of the Wnt/beta-catenin signaling pathway. Mech. Dev. 2000, 91, 249–258. [Google Scholar] [CrossRef]
- Holowacz, T.; Sokol, S. FGF is required for posterior neural patterning but not for neural induction. Dev. Biol. 1999, 205, 296–308. [Google Scholar] [CrossRef][Green Version]
- Cox, W.G.; Hemmati-Brivanlou, A. Caudalization of neural fate by tissue recombination and bFGF. Development 1995, 121, 4349–4358. [Google Scholar] [CrossRef] [PubMed]
- Deimling, S.J.; Drysdale, T.A. Fgf is required to regulate anterior-posterior patterning in the Xenopus lateral plate mesoderm. Mech. Dev. 2011, 128, 327–341. [Google Scholar] [CrossRef]
- Kengaku, M.; Okamoto, H. bFGF as a possible morphogen for the anteroposterior axis of the central nervous system in Xenopus. Development 1995, 121, 3121–3130. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, H.V.; Pownall, M.E.; Slack, J.M. eFGF regulates Xbra expression during Xenopus gastrulation. Embo J. 1994, 13, 4469–4481. [Google Scholar] [CrossRef] [PubMed]
- Keenan, I.D.; Sharrard, R.M.; Isaacs, H.V. FGF signal transduction and the regulation of Cdx gene expression. Dev. Biol. 2006, 299, 478–488. [Google Scholar] [CrossRef]
- Schulte-Merker, S.; Smith, J.C. Mesoderm formation in response to Brachyury requires FGF signalling. Curr. Biol. 1995, 5, 62–67. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Kinoshita, N.; Greco, T.M.; Federspiel, J.D.; Jean Beltran, P.M.; Ueno, N.; Cristea, I.M. Mechanical Force Induces Phosphorylation-Mediated Signaling that Underlies Tissue Response and Robustness in Xenopus Embryos. Cell Syst. 2019, 8, 226–241.e7. [Google Scholar] [CrossRef]
- Kinoshita, N.; Hashimoto, Y.; Yasue, N.; Suzuki, M.; Cristea, I.M.; Ueno, N. Mechanical Stress Regulates Epithelial Tissue Integrity and Stiffness through the FGFR/Erk2 Signaling Pathway during Embryogenesis. Cell Rep. 2020, 30, 3875–3888.e3. [Google Scholar] [CrossRef]
- Kardash, E.; Bandemer, J.; Raz, E. Imaging protein activity in live embryos using fluorescence resonance energy transfer biosensors. Nat. Protoc. 2011, 6, 1835–1846. [Google Scholar] [CrossRef] [PubMed]
- Dunn, K.W.; Kamocka, M.M.; McDonald, J.H. A practical guide to evaluating colocalization in biological microscopy. Am. J. Physiol. Cell Physiol. 2011, 300, C723–C742. [Google Scholar] [CrossRef] [PubMed]
- Orlov, E.E.; Nesterenko, A.M.; Korotkova, D.D.; Parshina, E.A.; Martynova, N.Y.; Zaraisky, A.G. Targeted search for scaling genes reveals matrixmetalloproteinase 3 as a scaler of the dorsal-ventral pattern in Xenopus laevis embryos. Dev. Cell 2022, 57, 95–111.e12. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eroshkin, F.M.; Fefelova, E.A.; Bredov, D.V.; Orlov, E.E.; Kolyupanova, N.M.; Mazur, A.M.; Sokolov, A.S.; Zhigalova, N.A.; Prokhortchouk, E.B.; Nesterenko, A.M.; et al. Mechanical Tensions Regulate Gene Expression in the Xenopus laevis Axial Tissues. Int. J. Mol. Sci. 2024, 25, 870. https://doi.org/10.3390/ijms25020870
Eroshkin FM, Fefelova EA, Bredov DV, Orlov EE, Kolyupanova NM, Mazur AM, Sokolov AS, Zhigalova NA, Prokhortchouk EB, Nesterenko AM, et al. Mechanical Tensions Regulate Gene Expression in the Xenopus laevis Axial Tissues. International Journal of Molecular Sciences. 2024; 25(2):870. https://doi.org/10.3390/ijms25020870
Chicago/Turabian StyleEroshkin, Fedor M., Elena A. Fefelova, Denis V. Bredov, Eugeny E. Orlov, Nataliya M. Kolyupanova, Alexander M. Mazur, Alexey S. Sokolov, Nadezhda A. Zhigalova, Egor B. Prokhortchouk, Alexey M. Nesterenko, and et al. 2024. "Mechanical Tensions Regulate Gene Expression in the Xenopus laevis Axial Tissues" International Journal of Molecular Sciences 25, no. 2: 870. https://doi.org/10.3390/ijms25020870
APA StyleEroshkin, F. M., Fefelova, E. A., Bredov, D. V., Orlov, E. E., Kolyupanova, N. M., Mazur, A. M., Sokolov, A. S., Zhigalova, N. A., Prokhortchouk, E. B., Nesterenko, A. M., & Zaraisky, A. G. (2024). Mechanical Tensions Regulate Gene Expression in the Xenopus laevis Axial Tissues. International Journal of Molecular Sciences, 25(2), 870. https://doi.org/10.3390/ijms25020870





