Glaucoma Animal Models beyond Chronic IOP Increase
Abstract
1. Introduction
1.1. Prevalence of Glaucoma
1.2. Pathophysiology of Glaucoma
1.3. Glaucoma Treatment Options
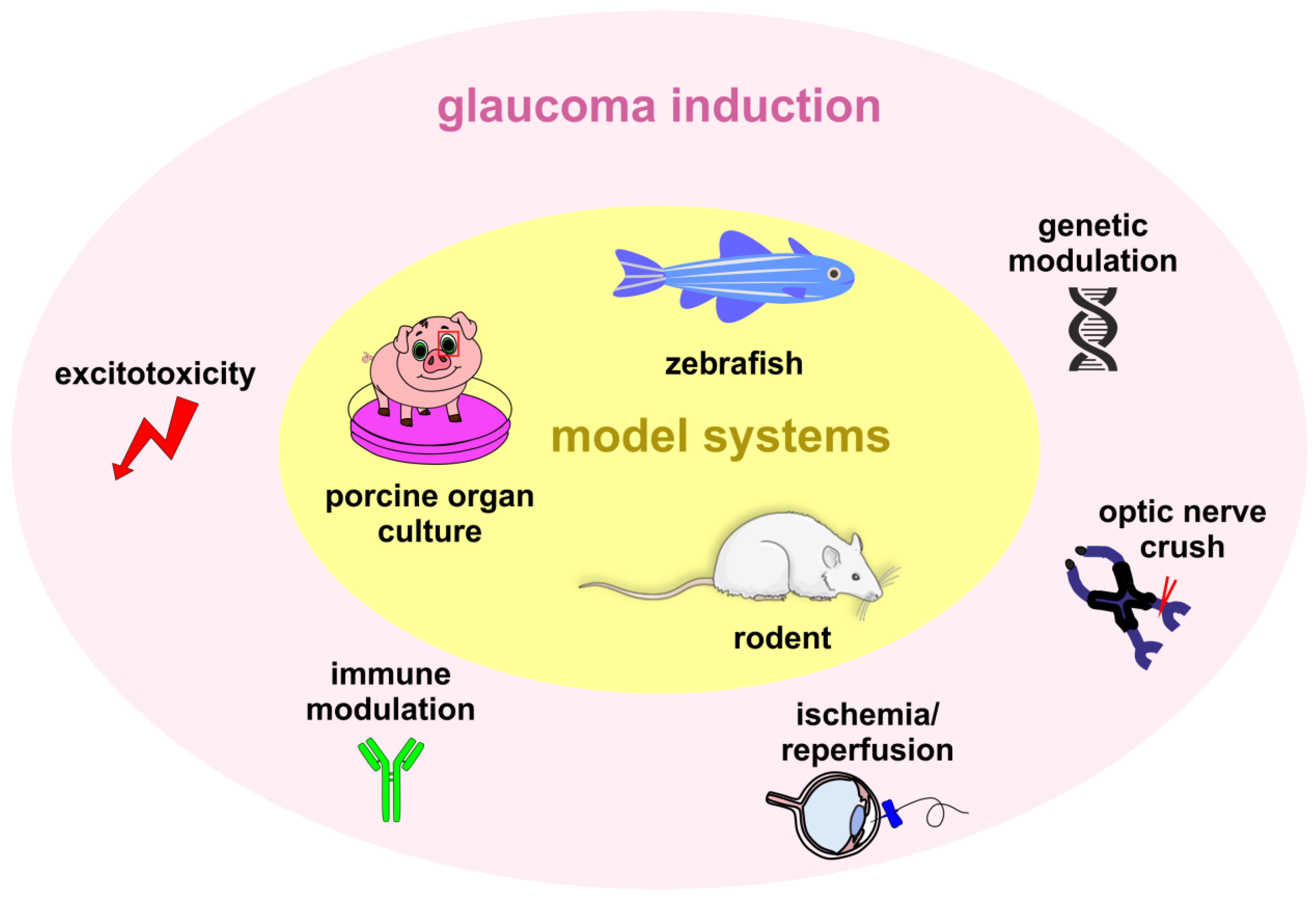
1.4. Glaucoma Animal Models
2. Genetic Modulation
2.1. Genetically Modified Normal-Tension Glaucoma Zebrafish Model
2.2. Genetic Modified Rodent Normal-Tension Glaucoma Models
3. Excitotoxicity
3.1. Excitotoxicity-Based In Vivo Glaucoma Models
3.2. Excitotoxicity-Based Organ Culture Models
4. Immune-Based Glaucoma Models
4.1. Contribution of the Immune System in Glaucoma
4.2. The Experimental Autoimmune Glaucoma Model
4.3. Combination of the EAG Model with IOP-Dependent and -Independent Glaucoma Models
5. Ischemia Models of Glaucoma
6. Optic Nerve Crush or Transection Models
7. Marmosets with Naturally Occurring NTG
8. Conclusions
9. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Finger, R.P.; Bertram, B.; Wolfram, C.; Holz, F.G. Blindness and visual impairment in Germany: A slight fall in prevalence. Dtsch. Arztebl. Int. 2012, 109, 484–489. [Google Scholar] [CrossRef]
- Azuara-Blanco, A.; Bgnasco, L.; Bagnis, A.; Barbosa Breda, J.; Bonzano, C.; Brezhnev, A.; Bron, A.; Cutolo, C.A.; Cvenkel, B.; Gandolfi, S.; et al. Terminology and Guidelines for Glaucoma; European Glaucoma Society: Edinburgh, UK, 2020. [Google Scholar]
- Casson, R.J.; Chidlow, G.; Wood, J.P.; Crowston, J.G.; Goldberg, I. Definition of glaucoma: Clinical and experimental concepts. Clin. Exp. Ophthalmol. 2012, 40, 341–349. [Google Scholar] [CrossRef]
- Leung, D.Y.L.; Tham, C.C. Normal-tension glaucoma: Current concepts and approaches-A review. Clin. Exp. Ophthalmol. 2022, 50, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Trivli, A.; Koliarakis, I.; Terzidou, C.; Goulielmos, G.N.; Siganos, C.S.; Spandidos, D.A.; Dalianis, G.; Detorakis, E.T. Normal-tension glaucoma: Pathogenesis and genetics. Exp. Ther. Med. 2019, 17, 563–574. [Google Scholar] [CrossRef]
- Vishwaraj, C.R.; Kavitha, S.; Venkatesh, R.; Shukla, A.G.; Chandran, P.; Tripathi, S. Neuroprotection in glaucoma. Indian J. Ophthalmol. 2022, 70, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Mehran, N.A.; Sinha, S.; Razeghinejad, R. New glaucoma medications: Latanoprostene bunod, netarsudil, and fixed combination netarsudil-latanoprost. Eye 2020, 34, 72–88. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, M.F.; Gandolfi, S.; Gugleta, K.; Normando, E.M.; Oddone, F. How latanoprost changed glaucoma management. Acta Ophthalmol. 2023. [Google Scholar] [CrossRef]
- Gupta, D.; Chen, P.P. Glaucoma. Am. Fam. Physician 2016, 93, 668–674. [Google Scholar] [PubMed]
- Gazzard, G.; Konstantakopoulou, E.; Garway-Heath, D.; Adeleke, M.; Vickerstaff, V.; Ambler, G.; Hunter, R.; Bunce, C.; Nathwani, N.; Barton, K.; et al. Laser in Glaucoma and Ocular Hypertension (LiGHT) Trial: Six-Year Results of Primary Selective Laser Trabeculoplasty versus Eye Drops for the Treatment of Glaucoma and Ocular Hypertension. Ophthalmology 2023, 130, 139–151. [Google Scholar] [CrossRef]
- Gallo Afflitto, G.; Swaminathan, S.S. Minimally Invasive Glaucoma Surgery. Int. Ophthalmol. Clin. 2023, 63, 33–60. [Google Scholar] [CrossRef]
- Rosdahl, J.A.; Gupta, D. Prospective Studies of Minimally Invasive Glaucoma Surgeries: Systematic Review and Quality Assessment. Clin. Ophthalmol. 2020, 14, 231–243. [Google Scholar] [CrossRef] [PubMed]
- European Glaucoma Society. European Glaucoma Society—A guide on surgical innovation for glaucoma. Br. J. Ophthalmol. 2023, 107, 1–114. [Google Scholar] [CrossRef] [PubMed]
- Wagner, I.V.; Stewart, M.W.; Dorairaj, S.K. Updates on the Diagnosis and Management of Glaucoma. Mayo Clin. Proc. Innov. Qual. Outcomes 2022, 6, 618–635. [Google Scholar] [CrossRef] [PubMed]
- Heijl, A.; Leske, M.C.; Bengtsson, B.; Hyman, L.; Bengtsson, B.; Hussein, M.; Early Manifest Glaucoma Trial Group. Reduction of intraocular pressure and glaucoma progression: Results from the Early Manifest Glaucoma Trial. Arch. Ophthalmol. 2002, 120, 1268–1279. [Google Scholar] [CrossRef]
- Bell, K.; Und Hohenstein-Blaul, N.V.T.; Teister, J.; Grus, F. Modulation of the Immune System for the Treatment of Glaucoma. Curr. Neuropharmacol. 2018, 16, 942–958. [Google Scholar] [CrossRef] [PubMed]
- Grus, F.H.; Joachim, S.C.; Hoffmann, E.M.; Pfeiffer, N. Complex autoantibody repertoires in patients with glaucoma. Mol. Vis. 2004, 10, 132–137. [Google Scholar] [PubMed]
- Mac Nair, C.E.; Nickells, R.W. Neuroinflammation in Glaucoma and Optic Nerve Damage. Prog. Mol. Biol. Transl. Sci. 2015, 134, 343–363. [Google Scholar] [CrossRef]
- Karlstetter, M.; Scholz, R.; Rutar, M.; Wong, W.T.; Provis, J.M.; Langmann, T. Retinal microglia: Just bystander or target for therapy? Prog. Retin. Eye Res. 2015, 45, 30–57. [Google Scholar] [CrossRef]
- Grozdanic, S.D.; Sakaguchi, D.S.; Kwon, Y.H.; Kardon, R.H.; Sonea, I.M. Functional characterization of retina and optic nerve after acute ocular ischemia in rats. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2597–2605. [Google Scholar] [CrossRef]
- Guan, L.; Li, C.; Zhang, Y.; Gong, J.; Wang, G.; Tian, P.; Shen, N. Puerarin ameliorates retinal ganglion cell damage induced by retinal ischemia/reperfusion through inhibiting the activation of TLR4/NLRP3 inflammasome. Life Sci. 2020, 256, 117935. [Google Scholar] [CrossRef]
- Tezel, G.; Yang, X.; Luo, C.; Kain, A.D.; Powell, D.W.; Kuehn, M.H.; Kaplan, H.J. Oxidative stress and the regulation of complement activation in human glaucoma. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5071–5082. [Google Scholar] [CrossRef] [PubMed]
- Shu, D.Y.; Chaudhary, S.; Cho, K.S.; Lennikov, A.; Miller, W.P.; Thorn, D.C.; Yang, M.; McKay, T.B. Role of Oxidative Stress in Ocular Diseases: A Balancing Act. Metabolites 2023, 13, 187. [Google Scholar] [CrossRef] [PubMed]
- Grus, F.H.; Joachim, S.C.; Bruns, K.; Lackner, K.J.; Pfeiffer, N.; Wax, M.B. Serum autoantibodies to alpha-fodrin are present in glaucoma patients from Germany and the United States. Investig. Ophthalmol. Vis. Sci. 2006, 47, 968–976. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Agarwal, P.; Iezhitsa, I. Exploring the current use of animal models in glaucoma drug discovery: Where are we in 2023? Expert Opin. Drug Discov. 2023, 18, 1287–1300. [Google Scholar] [CrossRef]
- Loo, Y.; Chan, A.S.Y.; Khor, C.C.; Aung, T.; Wang, Z. Rodent genetically modified models of glaucoma. Mol. Aspects Med. 2023, 95, 101229. [Google Scholar] [CrossRef]
- Kim, Y.; Yang, J.; Kim, J.Y.; Lee, J.M.; Son, W.C.; Moon, B.G. HL3501, a Novel Selective A3 Adenosine Receptor Antagonist, Lowers Intraocular Pressure (IOP) in Animal Glaucoma Models. Transl. Vis. Sci. Technol. 2022, 11, 30. [Google Scholar] [CrossRef] [PubMed]
- Ghosn, C.; Rajagopalan, L.; Ugarte, S.; Mistry, S.; Orilla, W.; Goodkin, M.L.; Robinson, M.R.; Engles, M.; Dibas, M. Intraocular Pressure-Lowering Efficacy of a Sustained-Release Bimatoprost Implant in Dog Eyes Pretreated with Selective Laser Trabeculoplasty. J. Ocul. Pharmacol. Ther. 2022, 38, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.; Stowell, C.; Reynaud, J.; Gardiner, S.K.; Yang, H.; Williams, G.; Williams, I.; Marsh-Armstrong, N.; Burgoyne, C.F. Optic Nerve Head Myelin-Related Protein, GFAP, and Iba1 Alterations in Non-Human Primates With Early to Moderate Experimental Glaucoma. Investig. Ophthalmol. Vis. Sci. 2022, 63, 9. [Google Scholar] [CrossRef]
- Snider, E.J.; Hardie, B.A.; Li, Y.; Gao, K.; Splaine, F.; Kim, R.K.; Vannatta, R.T.; Read, A.T.; Ethier, C.R. A Porcine Organ-Culture Glaucoma Model Mimicking Trabecular Meshwork Damage Using Oxidative Stress. Investig. Ophthalmol. Vis. Sci. 2021, 62, 18. [Google Scholar] [CrossRef]
- Hong, Y.; Luo, Y. Zebrafish Model in Ophthalmology to Study Disease Mechanism and Drug Discovery. Pharmaceuticals 2021, 14, 716. [Google Scholar] [CrossRef]
- Ramirez, J.M.; Salobrar-Garcia, E.; de Hoz, R.; Salazar, J.J.; Matamoros, J.A.; Sanchez-Puebla, L.; Lopez-Cuenca, I.; Fernandez-Albarral, J.A.; Ramirez, A.I. Laser-Induced Ocular Hypertension in a Mouse Model of Glaucoma. Methods Mol. Biol. 2023, 2708, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Mead, B. Microbead-Induced Ocular Hypertension in a Rodent Model of Glaucoma. Methods Mol. Biol. 2023, 2708, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Pang, I.H.; Clark, A.F. Inducible rodent models of glaucoma. Prog. Retin. Eye Res. 2020, 75, 100799. [Google Scholar] [CrossRef] [PubMed]
- McDowell, C.M.; Kizhatil, K.; Elliott, M.H.; Overby, D.R.; van Batenburg-Sherwood, J.; Millar, J.C.; Kuehn, M.H.; Zode, G.; Acott, T.S.; Anderson, M.G.; et al. Consensus Recommendation for Mouse Models of Ocular Hypertension to Study Aqueous Humor Outflow and Its Mechanisms. Investig. Ophthalmol. Vis. Sci. 2022, 63, 12. [Google Scholar] [CrossRef]
- Biswas, S.; Wan, K.H. Review of rodent hypertensive glaucoma models. Acta Ophthalmol. 2019, 97, e331–e340. [Google Scholar] [CrossRef] [PubMed]
- Gould, D.B.; John, S.W. Anterior segment dysgenesis and the developmental glaucomas are complex traits. Hum. Mol. Genet. 2002, 11, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- McMahon, C.; Semina, E.V.; Link, B.A. Using zebrafish to study the complex genetics of glaucoma. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2004, 138, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Bibliowicz, J.; Tittle, R.K.; Gross, J.M. Toward a better understanding of human eye disease insights from the zebrafish, Danio rerio. Prog. Mol. Biol. Transl. Sci. 2011, 100, 287–330. [Google Scholar] [CrossRef]
- Ito, Y.A.; Goping, I.S.; Berry, F.; Walter, M.A. Dysfunction of the stress-responsive FOXC1 transcription factor contributes to the earlier-onset glaucoma observed in Axenfeld-Rieger syndrome patients. Cell Death Dis. 2014, 5, e1069. [Google Scholar] [CrossRef]
- Ferre-Fernandez, J.J.; Sorokina, E.A.; Thompson, S.; Collery, R.F.; Nordquist, E.; Lincoln, J.; Semina, E.V. Disruption of foxc1 genes in zebrafish results in dosage-dependent phenotypes overlapping Axenfeld-Rieger syndrome. Hum. Mol. Genet. 2020, 29, 2723–2735. [Google Scholar] [CrossRef]
- French, C.R. Mechanistic Insights into Axenfeld-Rieger Syndrome from Zebrafish foxc1 and pitx2 Mutants. Int. J. Mol. Sci. 2021, 22, 10001. [Google Scholar] [CrossRef] [PubMed]
- Meer, E.; Qin, V.L.; Gudiseva, H.V.; McGeehan, B.; Salowe, R.; Pistilli, M.; He, J.; Daniel, E.; Ying, G.S.; Chavali, V.R.M.; et al. LMX1B Locus Associated with Low-Risk Baseline Glaucomatous Features in the POAAGG Study. Genes 2021, 12, 1252. [Google Scholar] [CrossRef]
- Nishimura, D.Y.; Swiderski, R.E.; Alward, W.L.; Searby, C.C.; Patil, S.R.; Bennet, S.R.; Kanis, A.B.; Gastier, J.M.; Stone, E.M.; Sheffield, V.C. The forkhead transcription factor gene FKHL7 is responsible for glaucoma phenotypes which map to 6p25. Nat. Genet. 1998, 19, 140–147. [Google Scholar] [CrossRef] [PubMed]
- McMahon, C.; Gestri, G.; Wilson, S.W.; Link, B.A. Lmx1b is essential for survival of periocular mesenchymal cells and influences Fgf-mediated retinal patterning in zebrafish. Dev. Biol. 2009, 332, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Sarfarazi, M.; Rezaie, T. Optineurin in primary open angle glaucoma. Ophthalmol. Clin. N. Am. 2003, 16, 529–541. [Google Scholar] [CrossRef]
- Rezaie, T.; Child, A.; Hitchings, R.; Brice, G.; Miller, L.; Coca-Prados, M.; Heon, E.; Krupin, T.; Ritch, R.; Kreutzer, D.; et al. Adult-onset primary open-angle glaucoma caused by mutations in optineurin. Science 2002, 295, 1077–1079. [Google Scholar] [CrossRef]
- Ying, H.; Yue, B.Y. Optineurin: The autophagy connection. Exp. Eye Res. 2016, 144, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Minegishi, Y.; Nakayama, M.; Iejima, D.; Kawase, K.; Iwata, T. Significance of optineurin mutations in glaucoma and other diseases. Prog. Retin. Eye Res. 2016, 55, 149–181. [Google Scholar] [CrossRef]
- Tseng, H.C.; Riday, T.T.; McKee, C.; Braine, C.E.; Bomze, H.; Barak, I.; Marean-Reardon, C.; John, S.W.; Philpot, B.D.; Ehlers, M.D. Visual impairment in an optineurin mouse model of primary open-angle glaucoma. Neurobiol. Aging 2015, 36, 2201–2212. [Google Scholar] [CrossRef]
- Bond, L.M.; Peden, A.A.; Kendrick-Jones, J.; Sellers, J.R.; Buss, F. Myosin VI and its binding partner optineurin are involved in secretory vesicle fusion at the plasma membrane. Mol. Biol. Cell 2011, 22, 54–65. [Google Scholar] [CrossRef]
- Wild, P.; Farhan, H.; McEwan, D.G.; Wagner, S.; Rogov, V.V.; Brady, N.R.; Richter, B.; Korac, J.; Waidmann, O.; Choudhary, C.; et al. Phosphorylation of the autophagy receptor optineurin restricts Salmonella growth. Science 2011, 333, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Chi, Z.L.; Akahori, M.; Obazawa, M.; Minami, M.; Noda, T.; Nakaya, N.; Tomarev, S.; Kawase, K.; Yamamoto, T.; Noda, S.; et al. Overexpression of optineurin E50K disrupts Rab8 interaction and leads to a progressive retinal degeneration in mice. Hum. Mol. Genet. 2010, 19, 2606–2615. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Xiao, Z.; Yuan, H.; Xue, F.; Zhu, Y.; Zhou, X.; Yang, B.; Sun, J.; Meng, B.; Sun, X.; et al. Transgenic mice with overexpression of mutated human optineurin (E50K) in the retina. Mol. Biol. Rep. 2012, 39, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Jiang, B.O.; Lei, D.; Zhou, X.; Yuan, H. Expression profiling of microRNAs in optineurin (E50K) mutant transgenic mice. Biomed. Rep. 2016, 4, 193–196. [Google Scholar] [CrossRef]
- Aung, T.; Ebenezer, N.D.; Brice, G.; Child, A.H.; Prescott, Q.; Lehmann, O.J.; Hitchings, R.A.; Bhattacharya, S.S. Prevalence of optineurin sequence variants in adult primary open angle glaucoma: Implications for diagnostic testing. J. Med. Genet. 2003, 40, e101. [Google Scholar] [CrossRef]
- Saccuzzo, E.G.; Youngblood, H.A.; Lieberman, R.L. Myocilin misfolding and glaucoma: A 20-year update. Prog. Retin. Eye Res. 2023, 95, 101188. [Google Scholar] [CrossRef]
- Aldred, M.A.; Baumber, L.; Hill, A.; Schwalbe, E.C.; Goh, K.; Karwatowski, W.; Trembath, R.C. Low prevalence of MYOC mutations in UK primary open-angle glaucoma patients limits the utility of genetic testing. Hum. Genet. 2004, 115, 428–431. [Google Scholar] [CrossRef]
- Menaa, F.; Braghini, C.A.; Vasconcellos, J.P.; Menaa, B.; Costa, V.P.; Figueiredo, E.S.; Melo, M.B. Keeping an eye on myocilin: A complex molecule associated with primary open-angle glaucoma susceptibility. Molecules 2011, 16, 5402–5421. [Google Scholar] [CrossRef]
- Zode, G.S.; Kuehn, M.H.; Nishimura, D.Y.; Searby, C.C.; Mohan, K.; Grozdanic, S.D.; Bugge, K.; Anderson, M.G.; Clark, A.F.; Stone, E.M.; et al. Reduction of ER stress via a chemical chaperone prevents disease phenotypes in a mouse model of primary open angle glaucoma. J. Clin. Investig. 2011, 121, 3542–3553. [Google Scholar] [CrossRef]
- Naskar, R.; Vorwerk, C.K.; Dreyer, E.B. Concurrent downregulation of a glutamate transporter and receptor in glaucoma. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1940–1944. [Google Scholar]
- Casson, R.J. Possible role of excitotoxicity in the pathogenesis of glaucoma. Clin. Exp. Ophthalmol. 2006, 34, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Baba, A.; Matsuda, T.; Romano, C. Susceptibilities to and mechanisms of excitotoxic cell death of adult mouse inner retinal neurons in dissociated culture. Investig. Ophthalmol. Vis. Sci. 2004, 45, 4576–4582. [Google Scholar] [CrossRef]
- Dong, X.X.; Wang, Y.; Qin, Z.H. Molecular mechanisms of excitotoxicity and their relevance to pathogenesis of neurodegenerative diseases. Acta Pharmacol. Sin. 2009, 30, 379–387. [Google Scholar] [CrossRef]
- Lebrun-Julien, F.; Duplan, L.; Pernet, V.; Osswald, I.; Sapieha, P.; Bourgeois, P.; Dickson, K.; Bowie, D.; Barker, P.A.; Di Polo, A. Excitotoxic death of retinal neurons in vivo occurs via a non-cell-autonomous mechanism. J. Neurosci. 2009, 29, 5536–5545. [Google Scholar] [CrossRef]
- Sery, O.; Sultana, N.; Kashem, M.A.; Pow, D.V.; Balcar, V.J. GLAST But Not Least—Distribution, Function, Genetics and Epigenetics of L-Glutamate Transport in Brain—Focus on GLAST/EAAT1. Neurochem. Res. 2015, 40, 2461–2472. [Google Scholar] [CrossRef] [PubMed]
- Sarthy, V.P.; Pignataro, L.; Pannicke, T.; Weick, M.; Reichenbach, A.; Harada, T.; Tanaka, K.; Marc, R. Glutamate transport by retinal Muller cells in glutamate/aspartate transporter-knockout mice. Glia 2005, 49, 184–196. [Google Scholar] [CrossRef]
- Danbolt, N.C. Glutamate uptake. Prog. Neurobiol. 2001, 65, 1–105. [Google Scholar] [CrossRef]
- Harada, T.; Harada, C.; Nakamura, K.; Quah, H.M.; Okumura, A.; Namekata, K.; Saeki, T.; Aihara, M.; Yoshida, H.; Mitani, A.; et al. The potential role of glutamate transporters in the pathogenesis of normal tension glaucoma. J. Clin. Investig. 2007, 117, 1763–1770. [Google Scholar] [CrossRef] [PubMed]
- Rauen, T. Diversity of glutamate transporter expression and function in the mammalian retina. Amino Acids 2000, 19, 53–62. [Google Scholar] [CrossRef]
- Sreekumar, P.G.; Ferrington, D.A.; Kannan, R. Glutathione Metabolism and the Novel Role of Mitochondrial GSH in Retinal Degeneration. Antioxidants 2021, 10, 661. [Google Scholar] [CrossRef]
- Fernandes, K.A.; Harder, J.M.; Williams, P.A.; Rausch, R.L.; Kiernan, A.E.; Nair, K.S.; Anderson, M.G.; John, S.W.; Howell, G.R.; Libby, R.T. Using genetic mouse models to gain insight into glaucoma: Past results and future possibilities. Exp. Eye Res. 2015, 141, 42–56. [Google Scholar] [CrossRef]
- Namekata, K.; Harada, C.; Guo, X.; Kikushima, K.; Kimura, A.; Fuse, N.; Mitamura, Y.; Kohyama, K.; Matsumoto, Y.; Tanaka, K.; et al. Interleukin-1 attenuates normal tension glaucoma-like retinal degeneration in EAAC1-deficient mice. Neurosci. Lett. 2009, 465, 160–164. [Google Scholar] [CrossRef]
- Namekata, K.; Kimura, A.; Kawamura, K.; Guo, X.; Harada, C.; Tanaka, K.; Harada, T. Dock3 attenuates neural cell death due to NMDA neurotoxicity and oxidative stress in a mouse model of normal tension glaucoma. Cell Death Differ. 2013, 20, 1250–1256. [Google Scholar] [CrossRef] [PubMed]
- Kimura, A.; Guo, X.; Noro, T.; Harada, C.; Tanaka, K.; Namekata, K.; Harada, T. Valproic acid prevents retinal degeneration in a murine model of normal tension glaucoma. Neurosci. Lett. 2015, 588, 108–113. [Google Scholar] [CrossRef]
- Alix, J.J.; Domingues, A.M. White matter synapses: Form, function, and dysfunction. Neurology 2011, 76, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.C.; Yu, Y.C.; Sung, Y.; Han, J.M. Glutamine reliance in cell metabolism. Exp. Mol. Med. 2020, 52, 1496–1516. [Google Scholar] [CrossRef]
- Newsholme, P.; Procopio, J.; Lima, M.M.; Pithon-Curi, T.C.; Curi, R. Glutamine and glutamate—Their central role in cell metabolism and function. Cell Biochem. Funct. 2003, 21, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Riedel, G.; Platt, B.; Micheau, J. Glutamate receptor function in learning and memory. Behav. Brain Res. 2003, 140, 1–47. [Google Scholar] [CrossRef]
- Hynd, M.R.; Scott, H.L.; Dodd, P.R. Glutamate-mediated excitotoxicity and neurodegeneration in Alzheimer’s disease. Neurochem. Int. 2004, 45, 583–595. [Google Scholar] [CrossRef]
- Mi, Z.; Abrahamson, E.E.; Ryu, A.Y.; Malek-Ahmadi, M.; Kofler, J.K.; Fish, K.N.; Sweet, R.A.; Villemagne, V.L.; Schneider, J.A.; Mufson, E.J.; et al. Vesicular Glutamate Transporter Changes in the Cortical Default Mode Network During the Clinical and Pathological Progression of Alzheimer’s Disease. J. Alzheimer’s Dis. 2023, 94, 227–246. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, C.; Zhang, H.; Yan, W. A mini-review of the role of vesicular glutamate transporters in Parkinson’s disease. Front. Mol. Neurosci. 2023, 16, 1118078. [Google Scholar] [CrossRef]
- Meredith, G.E.; Totterdell, S.; Beales, M.; Meshul, C.K. Impaired glutamate homeostasis and programmed cell death in a chronic MPTP mouse model of Parkinson’s disease. Exp. Neurol. 2009, 219, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Iribarne, M.; Hyde, D.R. Different inflammation responses modulate Muller glia proliferation in the acute or chronically damaged zebrafish retina. Front. Cell Dev. Biol. 2022, 10, 892271. [Google Scholar] [CrossRef] [PubMed]
- Lahne, M.; Brecker, M.; Jones, S.E.; Hyde, D.R. The Regenerating Adult Zebrafish Retina Recapitulates Developmental Fate Specification Programs. Front. Cell Dev. Biol. 2020, 8, 617923. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.W.; Wang, H.T.; Wang, N.; Sheng, W.W.; Jin, M.; Lu, Y.; Bai, Y.J.; Zou, S.Q.; Pang, Y.L.; Xu, H.; et al. Establishment of an adult zebrafish model of retinal neurodegeneration induced by NMDA. Int. J. Ophthalmol. 2019, 12, 1250–1261. [Google Scholar] [CrossRef]
- Kuehn, S.; Rodust, C.; Stute, G.; Grotegut, P.; Meissner, W.; Reinehr, S.; Dick, H.B.; Joachim, S.C. Concentration-Dependent Inner Retina Layer Damage and Optic Nerve Degeneration in a NMDA Model. J. Mol. Neurosci. 2017, 63, 283–299. [Google Scholar] [CrossRef]
- Maekawa, S.; Sato, K.; Fujita, K.; Daigaku, R.; Tawarayama, H.; Murayama, N.; Moritoh, S.; Yabana, T.; Shiga, Y.; Omodaka, K.; et al. The neuroprotective effect of hesperidin in NMDA-induced retinal injury acts by suppressing oxidative stress and excessive calpain activation. Sci. Rep. 2017, 7, 6885. [Google Scholar] [CrossRef]
- Honda, S.; Namekata, K.; Kimura, A.; Guo, X.; Harada, C.; Murakami, A.; Matsuda, A.; Harada, T. Survival of Alpha and Intrinsically Photosensitive Retinal Ganglion Cells in NMDA-Induced Neurotoxicity and a Mouse Model of Normal Tension Glaucoma. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3696–3707. [Google Scholar] [CrossRef]
- Dvoriantchikova, G.; Fleishaker, M.; Ivanov, D. Molecular mechanisms of NMDA excitotoxicity in the retina. Sci. Rep. 2023, 13, 18471. [Google Scholar] [CrossRef]
- Inokuchi, Y.; Imai, S.; Nakajima, Y.; Shimazawa, M.; Aihara, M.; Araie, M.; Hara, H. Edaravone, a free radical scavenger, protects against retinal damage in vitro and in vivo. J. Pharmacol. Exp. Ther. 2009, 329, 687–698. [Google Scholar] [CrossRef]
- Maekawa, S.; Sato, K.; Kokubun, T.; Himori, N.; Yabana, T.; Ohno-Oishi, M.; Shi, G.; Omodaka, K.; Nakazawa, T. A Plant-Derived Antioxidant Supplement Prevents the Loss of Retinal Ganglion Cells in the Retinas of NMDA-Injured Mice. Clin. Ophthalmol. 2022, 16, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, Y.; Inokuchi, Y.; Nishi, M.; Shimazawa, M.; Otsubo, K.; Hara, H. Coenzyme Q10 protects retinal cells against oxidative stress in vitro and in vivo. Brain Res. 2008, 1226, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Abd Ghapor, A.A.; Abdul Nasir, N.A.; Iezhitsa, I.; Agarwal, R.; Razali, N. Neuroprotection by trans-resveratrol in rats with N-methyl-D-aspartate (NMDA)-induced retinal injury: Insights into the role of adenosine A1 receptors. Neurosci. Res. 2023, 193, 1–12. [Google Scholar] [CrossRef]
- Lambuk, L.; Jafri, A.J.; Arfuzir, N.N.; Iezhitsa, I.; Agarwal, R.; Rozali, K.N.; Agarwal, P.; Bakar, N.S.; Kutty, M.K.; Yusof, A.P.; et al. Neuroprotective Effect of Magnesium Acetyltaurate Against NMDA-Induced Excitotoxicity in Rat Retina. Neurotox. Res. 2017, 31, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Fazel, M.F.; Abu, I.F.; Mohamad, M.H.N.; Agarwal, R.; Iezhitsa, I.; Bakar, N.S.; Juliana, N.; Mellor, I.R.; Franzyk, H. Philanthotoxin-343 attenuates retinal and optic nerve injury, and protects visual function in rats with N-methyl-D-aspartate-induced excitotoxicity. PLoS ONE 2020, 15, e0236450. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Bengtson, C.P.; Buchthal, B.; Hagenston, A.M.; Bading, H. Coupling of NMDA receptors and TRPM4 guides discovery of unconventional neuroprotectants. Science 2020, 370, eaay3302. [Google Scholar] [CrossRef]
- Rettinger, C.L.; Wang, H.C. Quantitative Assessment of Retina Explant Viability in a Porcine Ex Vivo Neuroretina Model. J. Ocul. Pharmacol. Ther. 2018, 34, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Kuehn, S.; Hurst, J.; Rensinghoff, F.; Tsai, T.; Grauthoff, S.; Satgunarajah, Y.; Dick, H.B.; Schnichels, S.; Joachim, S.C. Degenerative effects of cobalt-chloride treatment on neurons and microglia in a porcine retina organ culture model. Exp. Eye Res. 2017, 155, 107–120. [Google Scholar] [CrossRef]
- Hurst, J.; Kuehn, S.; Jashari, A.; Tsai, T.; Bartz-Schmidt, K.U.; Schnichels, S.; Joachim, S.C. A novel porcine ex vivo retina culture model for oxidative stress induced by H2O2. Altern. Lab. Anim. 2017, 45, 11–25. [Google Scholar] [CrossRef]
- Kuehn, S.; Hurst, J.; Jashari, A.; Ahrens, K.; Tsai, T.; Wunderlich, I.M.; Dick, H.B.; Joachim, S.C.; Schnichels, S. The novel induction of retinal ganglion cell apoptosis in porcine organ culture by NMDA—An opportunity for the replacement of animals in experiments. Altern. Lab. Anim. 2016, 44, 557–568. [Google Scholar] [CrossRef]
- Schnichels, S.; Paquet-Durand, F.; Loscher, M.; Tsai, T.; Hurst, J.; Joachim, S.C.; Klettner, A. Retina in a dish: Cell cultures, retinal explants and animal models for common diseases of the retina. Prog. Retin. Eye Res. 2020, 81, 100880. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.; Mueller-Buehl, A.M.; Satgunarajah, Y.; Kuehn, S.; Dick, H.B.; Joachim, S.C. Protective effect of the extremolytes ectoine and hydroxyectoine in a porcine organ culture. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020, 258, 2185–2203. [Google Scholar] [CrossRef] [PubMed]
- Bell, K.; Wilding, C.; Funke, S.; Perumal, N.; Beck, S.; Wolters, D.; Holz-Muller, J.; Pfeiffer, N.; Grus, F.H. Neuroprotective effects of antibodies on retinal ganglion cells in an adolescent retina organ culture. J. Neurochem. 2016, 139, 256–269. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Chen, Y.S.; Thakur, S.S.; Rupenthal, I.D. Ultrasound-mediated nanoparticle delivery across ex vivo bovine retina after intravitreal injection. Eur. J. Pharm. Biopharm. 2017, 119, 125–136. [Google Scholar] [CrossRef]
- Peynshaert, K.; Devoldere, J.; Forster, V.; Picaud, S.; Vanhove, C.; De Smedt, S.C.; Remaut, K. Toward smart design of retinal drug carriers: A novel bovine retinal explant model to study the barrier role of the vitreoretinal interface. Drug Deliv. 2017, 24, 1384–1394. [Google Scholar] [CrossRef]
- Auler, N.; Tonner, H.; Pfeiffer, N.; Grus, F.H. Antibody and Protein Profiles in Glaucoma: Screening of Biomarkers and Identification of Signaling Pathways. Biology 2021, 10, 1296. [Google Scholar] [CrossRef]
- Von Thun Und Hohenstein-Blaul, N.; Kunst, S.; Pfeiffer, N.; Grus, F.H. Biomarkers for glaucoma: From the lab to the clinic. Eye 2017, 31, 225–231. [Google Scholar] [CrossRef]
- Bell, K.; Gramlich, O.W.; Von Thun Und Hohenstein-Blaul, N.; Beck, S.; Funke, S.; Wilding, C.; Pfeiffer, N.; Grus, F.H. Does autoimmunity play a part in the pathogenesis of glaucoma? Prog. Retin. Eye Res. 2013, 36, 199–216. [Google Scholar] [CrossRef]
- Tezel, G.; Wax, M.B. Glaucoma. Chem. Immunol. Allergy 2007, 92, 221–227. [Google Scholar] [CrossRef]
- Hubens, W.H.G.; Beckers, H.J.M.; Gorgels, T.; Webers, C.A.B. Increased ratios of complement factors C3a to C3 in aqueous humor and serum mark glaucoma progression. Exp. Eye Res. 2021, 204, 108460. [Google Scholar] [CrossRef]
- Kaeslin, M.A.; Killer, H.E.; Fuhrer, C.A.; Zeleny, N.; Huber, A.R.; Neutzner, A. Changes to the Aqueous Humor Proteome during Glaucoma. PLoS ONE 2016, 11, e0165314. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Cho, K.S.; Vu, T.H.K.; Shen, C.H.; Kaur, M.; Chen, G.; Mathew, R.; McHam, M.L.; Fazelat, A.; Lashkari, K.; et al. Commensal microflora-induced T cell responses mediate progressive neurodegeneration in glaucoma. Nat. Commun. 2018, 9, 3209. [Google Scholar] [CrossRef] [PubMed]
- Gramlich, O.W.; Ding, Q.J.; Zhu, W.; Cook, A.; Anderson, M.G.; Kuehn, M.H. Adoptive transfer of immune cells from glaucomatous mice provokes retinal ganglion cell loss in recipients. Acta Neuropathol. Commun. 2015, 3, 56. [Google Scholar] [CrossRef] [PubMed]
- Wax, M.B.; Tezel, G.; Yang, J.; Peng, G.; Patil, R.V.; Agarwal, N.; Sappington, R.M.; Calkins, D.J. Induced autoimmunity to heat shock proteins elicits glaucomatous loss of retinal ganglion cell neurons via activated T-cell-derived fas-ligand. J. Neurosci. 2008, 28, 12085–12096. [Google Scholar] [CrossRef] [PubMed]
- Laspas, P.; Gramlich, O.W.; Muller, H.D.; Cuny, C.S.; Gottschling, P.F.; Pfeiffer, N.; Dick, H.B.; Joachim, S.C.; Grus, F.H. Autoreactive antibodies and loss of retinal ganglion cells in rats induced by immunization with ocular antigens. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8835–8848. [Google Scholar] [CrossRef] [PubMed]
- Joachim, S.C.; Gramlich, O.W.; Laspas, P.; Schmid, H.; Beck, S.; von Pein, H.D.; Dick, H.B.; Pfeiffer, N.; Grus, F.H. Retinal ganglion cell loss is accompanied by antibody depositions and increased levels of microglia after immunization with retinal antigens. PLoS ONE 2012, 7, e40616. [Google Scholar] [CrossRef]
- Joachim, S.C.; Mondon, C.; Gramlich, O.W.; Grus, F.H.; Dick, H.B. Apoptotic retinal ganglion cell death in an autoimmune glaucoma model is accompanied by antibody depositions. J. Mol. Neurosci. 2014, 52, 216–224. [Google Scholar] [CrossRef]
- Ehrnthaller, C.; Ignatius, A.; Gebhard, F.; Huber-Lang, M. New insights of an old defense system: Structure, function, and clinical relevance of the complement system. Mol. Med. 2011, 17, 317–329. [Google Scholar] [CrossRef]
- Reinehr, S.; Reinhard, J.; Gandej, M.; Kuehn, S.; Noristani, R.; Faissner, A.; Dick, H.B.; Joachim, S.C. Simultaneous Complement Response via Lectin Pathway in Retina and Optic Nerve in an Experimental Autoimmune Glaucoma Model. Front. Cell Neurosci. 2016, 10, 140. [Google Scholar] [CrossRef]
- Reinehr, S.; Reinhard, J.; Wiemann, S.; Hesse, K.; Voss, C.; Gandej, M.; Dick, H.B.; Faissner, A.; Joachim, S.C. Transfer of the Experimental Autoimmune Glaucoma Model from Rats to Mice-New Options to Study Glaucoma Disease. Int. J. Mol. Sci. 2019, 20, 2563. [Google Scholar] [CrossRef]
- Paolicelli, R.C.; Sierra, A.; Stevens, B.; Tremblay, M.E.; Aguzzi, A.; Ajami, B.; Amit, I.; Audinat, E.; Bechmann, I.; Bennett, M.; et al. Microglia states and nomenclature: A field at its crossroads. Neuron 2022, 110, 3458–3483. [Google Scholar] [CrossRef]
- Reinehr, S.; Guntermann, A.; Theile, J.; Benning, L.; Grotegut, P.; Kuehn, S.; Serschnitzki, B.; Dick, H.B.; Marcus, K.; Joachim, S.C.; et al. Proteomic Analysis of Retinal Tissue in an S100B Autoimmune Glaucoma Model. Biology 2021, 11, 16. [Google Scholar] [CrossRef] [PubMed]
- Reinehr, S.; Gomes, S.C.; Gassel, C.J.; Asaad, M.A.; Stute, G.; Schargus, M.; Dick, H.B.; Joachim, S.C. Intravitreal Therapy Against the Complement Factor C5 Prevents Retinal Degeneration in an Experimental Autoimmune Glaucoma Model. Front. Pharmacol. 2019, 10, 1381. [Google Scholar] [CrossRef]
- Johnson, E.C.; Jia, L.; Cepurna, W.O.; Doser, T.A.; Morrison, J.C. Global changes in optic nerve head gene expression after exposure to elevated intraocular pressure in a rat glaucoma model. Investig. Ophthalmol. Vis. Sci. 2007, 48, 3161–3177. [Google Scholar] [CrossRef]
- Reinehr, S.; Reinhard, J.; Wiemann, S.; Stute, G.; Kuehn, S.; Woestmann, J.; Dick, H.B.; Faissner, A.; Joachim, S.C. Early remodelling of the extracellular matrix proteins tenascin-C and phosphacan in retina and optic nerve of an experimental autoimmune glaucoma model. J. Cell Mol. Med. 2016, 20, 2122–2137. [Google Scholar] [CrossRef]
- Wiemann, S.; Reinhard, J.; Reinehr, S.; Cibir, Z.; Joachim, S.C.; Faissner, A. Loss of the Extracellular Matrix Molecule Tenascin-C Leads to Absence of Reactive Gliosis and Promotes Anti-inflammatory Cytokine Expression in an Autoimmune Glaucoma Mouse Model. Front. Immunol. 2020, 11, 566279. [Google Scholar] [CrossRef] [PubMed]
- Reinehr, S.; Girbig, R.M.; Schulte, K.K.; Theile, J.; Asaad, M.A.; Fuchshofer, R.; Dick, H.B.; Joachim, S.C. Enhanced glaucomatous damage accompanied by glial response in a new multifactorial mouse model. Front. Immunol. 2022, 13, 1017076. [Google Scholar] [CrossRef]
- Nennstiel, S. Basics Allgemeine Pathologie; Elsevier, Urban & Fischer: Berkeley, CA, USA, 2009. [Google Scholar]
- Fortmann, S.D.; Grant, M.B. Molecular mechanisms of retinal ischemia. Curr. Opin. Physiol. 2019, 7, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Muthaian, R.; Minhas, G.; Anand, A. Pathophysiology of stroke and stroke-induced retinal ischemia: Emerging role of stem cells. J. Cell Physiol. 2012, 227, 1269–1279. [Google Scholar] [CrossRef]
- Granger, D.N.; Kvietys, P.R. Reperfusion injury and reactive oxygen species: The evolution of a concept. Redox Biol. 2015, 6, 524–551. [Google Scholar] [CrossRef]
- Palmhof, M.; Frank, V.; Rappard, P.; Kortenhorn, E.; Demuth, J.; Biert, N.; Stute, G.; Dick, H.B.; Joachim, S.C. From Ganglion Cell to Photoreceptor Layer: Timeline of Deterioration in a Rat Ischemia/Reperfusion Model. Front. Cell Neurosci. 2019, 13, 174. [Google Scholar] [CrossRef]
- Renner, M.; Stute, G.; Alzureiqi, M.; Reinhard, J.; Wiemann, S.; Schmid, H.; Faissner, A.; Dick, H.B.; Joachim, S.C. Optic Nerve Degeneration after Retinal Ischemia/Reperfusion in a Rodent Model. Front. Cell Neurosci. 2017, 11, 254. [Google Scholar] [CrossRef]
- Kim, B.J.; Braun, T.A.; Wordinger, R.J.; Clark, A.F. Progressive morphological changes and impaired retinal function associated with temporal regulation of gene expression after retinal ischemia/reperfusion injury in mice. Mol. Neurodegener. 2013, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Minhas, G.; Morishita, R.; Anand, A. Preclinical models to investigate retinal ischemia: Advances and drawbacks. Front. Neurol. 2012, 3, 75. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Yoshitomi, T.; Zorumski, C.F.; Izumi, Y. Experimentally Induced Mammalian Models of Glaucoma. Biomed Res. Int. 2015, 2015, 281214. [Google Scholar] [CrossRef] [PubMed]
- Schmid, H.; Renner, M.; Dick, H.B.; Joachim, S.C. Loss of inner retinal neurons after retinal ischemia in rats. Investig. Ophthalmol. Vis. Sci. 2014, 55, 2777–2787. [Google Scholar] [CrossRef] [PubMed]
- Osborne, N.N.; Casson, R.J.; Wood, J.P.; Chidlow, G.; Graham, M.; Melena, J. Retinal ischemia: Mechanisms of damage and potential therapeutic strategies. Prog. Retin. Eye Res. 2004, 23, 91–147. [Google Scholar] [CrossRef]
- Zhao, R.; He, T.; Xing, Y.; Luo, J. COG1410 regulates microglial states and protects retinal ganglion cells in retinal ischemia-reperfusion injury. Exp. Eye Res. 2023, 237, 109678. [Google Scholar] [CrossRef]
- Canonica, J.; Foxton, R.; Garrido, M.G.; Lin, C.M.; Uhles, S.; Shanmugam, S.; Antonetti, D.A.; Abcouwer, S.F.; Westenskow, P.D. Delineating effects of angiopoietin-2 inhibition on vascular permeability and inflammation in models of retinal neovascularization and ischemia/reperfusion. Front. Cell Neurosci. 2023, 17, 1192464. [Google Scholar] [CrossRef]
- Conti, F.; Romano, G.L.; Eandi, C.M.; Toro, M.D.; Rejdak, R.; Di Benedetto, G.; Lazzara, F.; Bernardini, R.; Drago, F.; Cantarella, G.; et al. Brimonidine is Neuroprotective in Animal Paradigm of Retinal Ganglion Cell Damage. Front. Pharmacol. 2021, 12, 705405. [Google Scholar] [CrossRef]
- Li, X.; Ye, Z.; Pei, S.; Zheng, D.; Zhu, L. Neuroprotective effect of minocycline on rat retinal ischemia-reperfusion injury. Mol. Vis. 2021, 27, 438–456. [Google Scholar]
- Sung, M.S.; Heo, H.; Eom, G.H.; Kim, S.Y.; Piao, H.; Guo, Y.; Park, S.W. HDAC2 Regulates Glial Cell Activation in Ischemic Mouse Retina. Int. J. Mol. Sci. 2019, 20, 5159. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Zhuang, J.; Hu, P.; Ye, W.; Chen, S.; Pang, Y.; Li, N.; Deng, C.; Zhang, X. Resveratrol Delays Retinal Ganglion Cell Loss and Attenuates Gliosis-Related Inflammation From Ischemia-Reperfusion Injury. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3879–3888. [Google Scholar] [CrossRef]
- Wagner, N.; Reinehr, S.; Palmhof, M.; Schuschel, D.; Tsai, T.; Sommer, E.; Frank, V.; Stute, G.; Dick, H.B.; Joachim, S.C. Microglia Activation in Retinal Ischemia Triggers Cytokine and Toll-Like Receptor Response. J. Mol. Neurosci. 2021, 71, 527–544. [Google Scholar] [CrossRef] [PubMed]
- Musayeva, A.; Unkrig, J.C.; Zhutdieva, M.B.; Manicam, C.; Ruan, Y.; Laspas, P.; Chronopoulos, P.; Gobel, M.L.; Pfeiffer, N.; Brochhausen, C.; et al. Betulinic Acid Protects from Ischemia-Reperfusion Injury in the Mouse Retina. Cells 2021, 10, 2440. [Google Scholar] [CrossRef]
- Reinhard, J.; Renner, M.; Wiemann, S.; Shakoor, D.A.; Stute, G.; Dick, H.B.; Faissner, A.; Joachim, S.C. Ischemic injury leads to extracellular matrix alterations in retina and optic nerve. Sci. Rep. 2017, 7, 43470. [Google Scholar] [CrossRef] [PubMed]
- Wiemann, S.; Yousf, A.; Joachim, S.C.; Peters, C.; Mueller-Buehl, A.M.; Wagner, N.; Reinhard, J. Knock-Out of Tenascin-C Ameliorates Ischemia-Induced Rod-Photoreceptor Degeneration and Retinal Dysfunction. Front. Neurosci. 2021, 15, 642176. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Z.; Jiang, L.; He, S.; Long, X.; Zheng, X. Combination Therapy with N-Acetylserotonin and Aflibercept Activated the Akt/Nrf2 Pathway to Inhibit Apoptosis and Oxidative Stress in Rats with Retinal Ischemia-Reperfusion Injury. Curr. Eye Res. 2023, 1–8. [Google Scholar] [CrossRef]
- Zeng, S.; Du, L.; Lu, G.; Xing, Y. CREG Protects Retinal Ganglion Cells loss and Retinal Function Impairment Against ischemia-reperfusion Injury in mice via Akt Signaling Pathway. Mol. Neurobiol. 2023, 60, 6018–6028. [Google Scholar] [CrossRef]
- Yu, A.; Wang, S.; Xing, Y.; Han, M.; Shao, K. 7,8-Dihydroxyflavone alleviates apoptosis and inflammation induced by retinal ischemia-reperfusion injury via activating TrkB/Akt/NF-kB signaling pathway. Int. J. Med. Sci. 2022, 19, 13–24. [Google Scholar] [CrossRef]
- Au, N.P.B.; Ma, C.H.E. Neuroinflammation, Microglia and Implications for Retinal Ganglion Cell Survival and Axon Regeneration in Traumatic Optic Neuropathy. Front. Immunol. 2022, 13, 860070. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Z. Optic Nerve Crush Injury in Rodents to Study Retinal Ganglion Cell Neuroprotection and Regeneration. Methods Mol. Biol. 2023, 2708, 99–106. [Google Scholar] [CrossRef]
- Hilla, A.M.; Diekmann, H.; Fischer, D. Microglia Are Irrelevant for Neuronal Degeneration and Axon Regeneration after Acute Injury. J. Neurosci. 2017, 37, 6113–6124. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Cui, Q.; Li, Y.; Irwin, N.; Fischer, D.; Harvey, A.R.; Benowitz, L.I. Macrophage-derived factors stimulate optic nerve regeneration. J. Neurosci. 2003, 23, 2284–2293. [Google Scholar] [CrossRef]
- Sanchez-Migallon, M.C.; Valiente-Soriano, F.J.; Nadal-Nicolas, F.M.; Vidal-Sanz, M.; Agudo-Barriuso, M. Apoptotic Retinal Ganglion Cell Death After Optic Nerve Transection or Crush in Mice: Delayed RGC Loss With BDNF or a Caspase 3 Inhibitor. Investig. Ophthalmol. Vis. Sci. 2016, 57, 81–93. [Google Scholar] [CrossRef]
- Kalesnykas, G.; Oglesby, E.N.; Zack, D.J.; Cone, F.E.; Steinhart, M.R.; Tian, J.; Pease, M.E.; Quigley, H.A. Retinal ganglion cell morphology after optic nerve crush and experimental glaucoma. Investig. Ophthalmol. Vis. Sci. 2012, 53, 3847–3857. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; McDowell, C.M.; Zhang, Z.; Tebow, H.E.; Wordinger, R.J.; Clark, A.F. Monitoring retinal morphologic and functional changes in mice following optic nerve crush. Investig. Ophthalmol. Vis. Sci. 2014, 55, 3766–3774. [Google Scholar] [CrossRef]
- Levkovitch-Verbin, H.; Dardik, R.; Vander, S.; Nisgav, Y.; Kalev-Landoy, M.; Melamed, S. Experimental glaucoma and optic nerve transection induce simultaneous upregulation of proapoptotic and prosurvival genes. Investig. Ophthalmol. Vis. Sci. 2006, 47, 2491–2497. [Google Scholar] [CrossRef]
- Levkovitch-Verbin, H.; Waserzoog, Y.; Vander, S.; Makarovsky, D.; Ilia, P. Minocycline mechanism of neuroprotection involves the Bcl-2 gene family in optic nerve transection. Int. J. Neurosci. 2014, 124, 755–761. [Google Scholar] [CrossRef]
- Wang, X.; Lin, J.; Arzeno, A.; Choi, J.Y.; Boccio, J.; Frieden, E.; Bhargava, A.; Maynard, G.; Tsai, J.C.; Strittmatter, S.M. Intravitreal delivery of human NgR-Fc decoy protein regenerates axons after optic nerve crush and protects ganglion cells in glaucoma models. Investig. Ophthalmol. Vis. Sci. 2015, 56, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- Cameron, E.G.; Xia, X.; Galvao, J.; Ashouri, M.; Kapiloff, M.S.; Goldberg, J.L. Optic Nerve Crush in Mice to Study Retinal Ganglion Cell Survival and Regeneration. Bio-Protocol 2020, 10, e3559. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, X.; Mao, J. The Neuroprotective Effect of Activation of Sigma-1 Receptor on Neural Injury by Optic Nerve Crush. Investig. Ophthalmol. Vis. Sci. 2023, 64, 9. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.E.; Donahue, R.J.; Schlamp, C.L.; Marola, O.J.; Libby, R.T.; Nickells, R.W. BAX activation in mouse retinal ganglion cells occurs in two temporally and mechanistically distinct steps. Mol. Neurodegener. 2023, 18, 67. [Google Scholar] [CrossRef]
- Noro, T.; Namekata, K.; Kimura, A.; Azuchi, Y.; Hashimoto, N.; Moriya-Ito, K.; Komaki, Y.; Lee, C.Y.; Okahara, N.; Guo, X.; et al. Normal tension glaucoma-like degeneration of the visual system in aged marmosets. Sci. Rep. 2019, 9, 14852. [Google Scholar] [CrossRef]
- Kimura, A.; Noro, T.; Harada, T. Role of animal models in glaucoma research. Neural Regen. Res. 2020, 15, 1257–1258. [Google Scholar] [CrossRef]
- Kalesnykas, G.; Niittykoski, M.; Rantala, J.; Miettinen, R.; Salminen, A.; Kaarniranta, K.; Uusitalo, H. The expression of heat shock protein 27 in retinal ganglion and glial cells in a rat glaucoma model. Neuroscience 2007, 150, 692–704. [Google Scholar] [CrossRef] [PubMed]
| Models | Outcomes | Advantages | Challenges | References | |
|---|---|---|---|---|---|
| Genetic normal-tension glaucoma models | E50K |
|
|
| [50,53,54] |
| GLAST, EAAC1 |
|
|
| [69,73,75] | |
| Excitotoxicity-based glaucoma models | NMDA |
|
|
| [87,97,101] |
| Immune-based glaucoma models | EAGs (HSPs, S100B, ONA) |
|
|
| [115,118,121] |
| Ischemia models | Increased IOP, chronic carotid occlusion, transient obstruction of retinal/cerebral artery, vasoconstriction of retinal vessels |
|
|
| [21,138,147] |
| Optic nerve crush or transection models |
|
|
| [160,163,168] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, T.; Reinehr, S.; Deppe, L.; Strubbe, A.; Kluge, N.; Dick, H.B.; Joachim, S.C. Glaucoma Animal Models beyond Chronic IOP Increase. Int. J. Mol. Sci. 2024, 25, 906. https://doi.org/10.3390/ijms25020906
Tsai T, Reinehr S, Deppe L, Strubbe A, Kluge N, Dick HB, Joachim SC. Glaucoma Animal Models beyond Chronic IOP Increase. International Journal of Molecular Sciences. 2024; 25(2):906. https://doi.org/10.3390/ijms25020906
Chicago/Turabian StyleTsai, Teresa, Sabrina Reinehr, Leonie Deppe, Alexandra Strubbe, Nils Kluge, H. Burkhard Dick, and Stephanie C. Joachim. 2024. "Glaucoma Animal Models beyond Chronic IOP Increase" International Journal of Molecular Sciences 25, no. 2: 906. https://doi.org/10.3390/ijms25020906
APA StyleTsai, T., Reinehr, S., Deppe, L., Strubbe, A., Kluge, N., Dick, H. B., & Joachim, S. C. (2024). Glaucoma Animal Models beyond Chronic IOP Increase. International Journal of Molecular Sciences, 25(2), 906. https://doi.org/10.3390/ijms25020906








