Investigating the Effects of Sex Hormones on Macrophage Polarization
Abstract
1. Introduction
2. Results
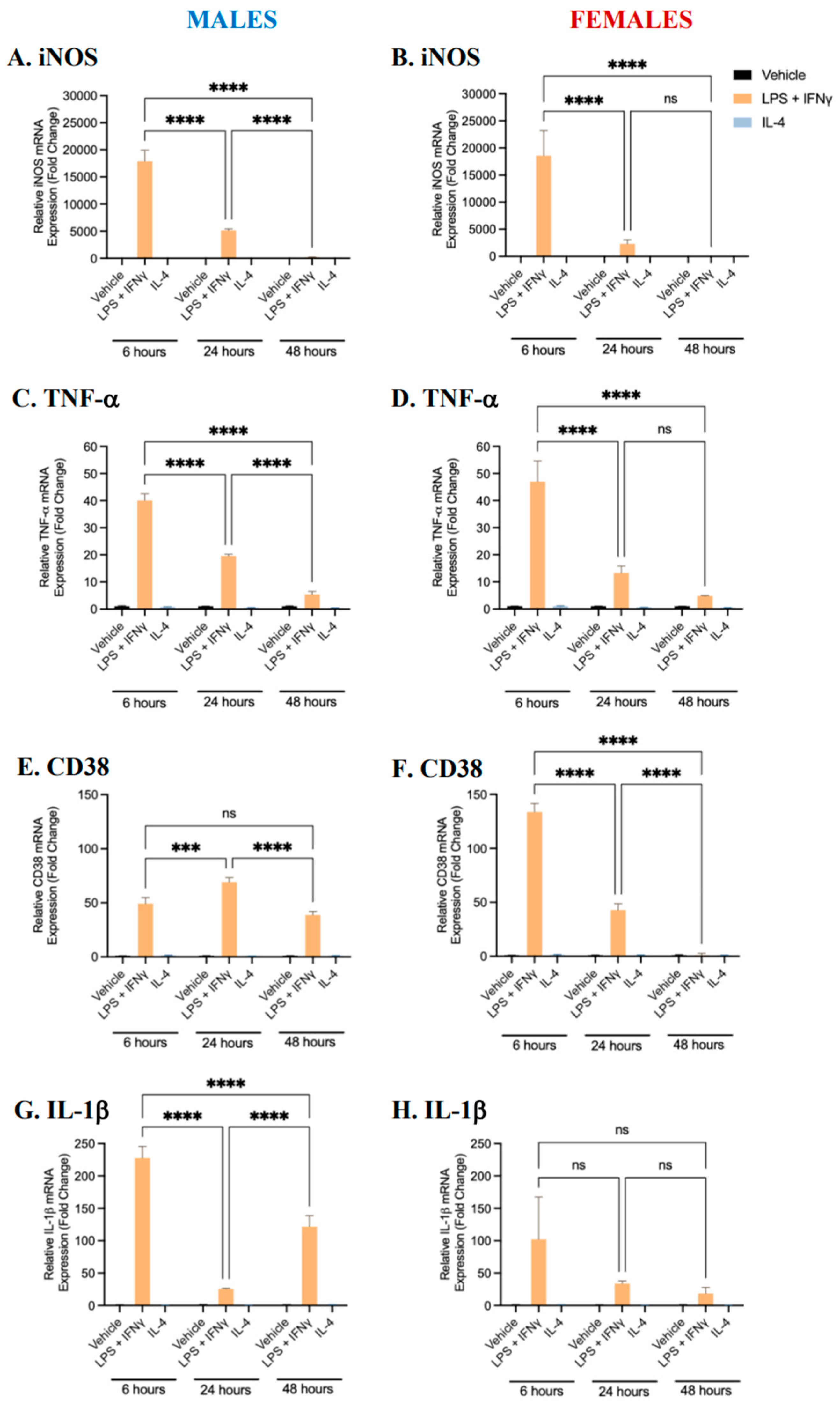
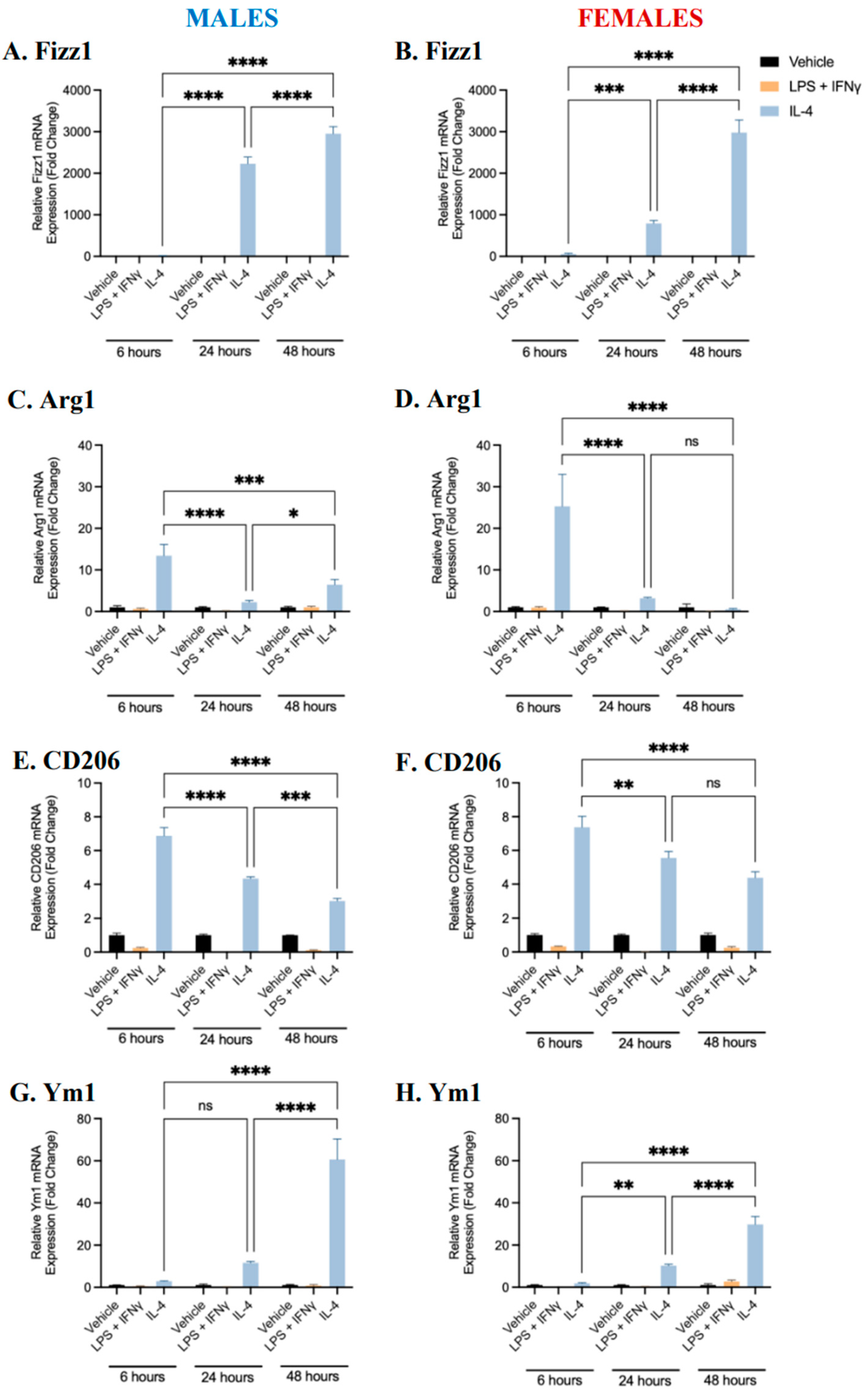
2.1. Characterization of BMDM Polarization
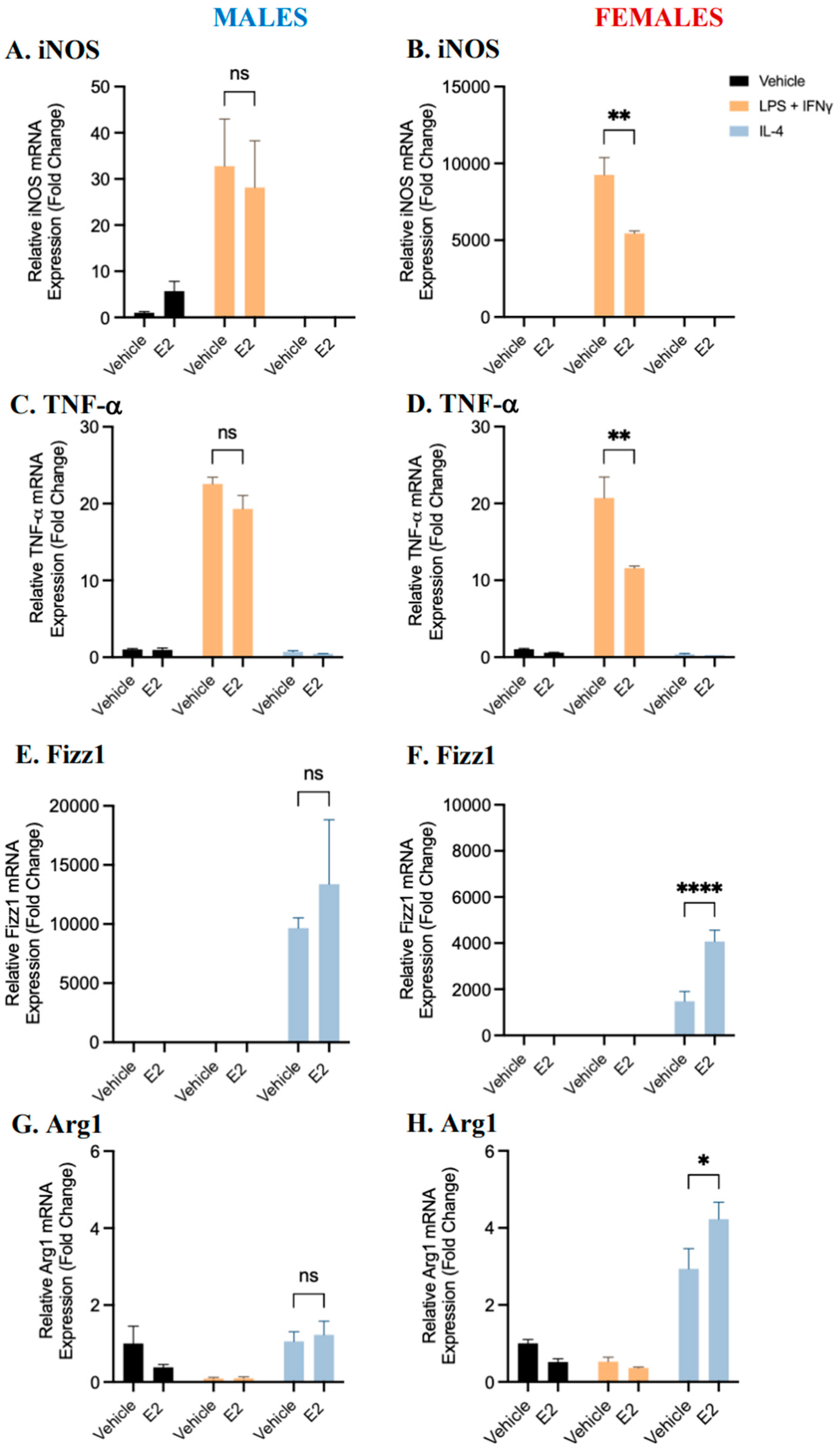
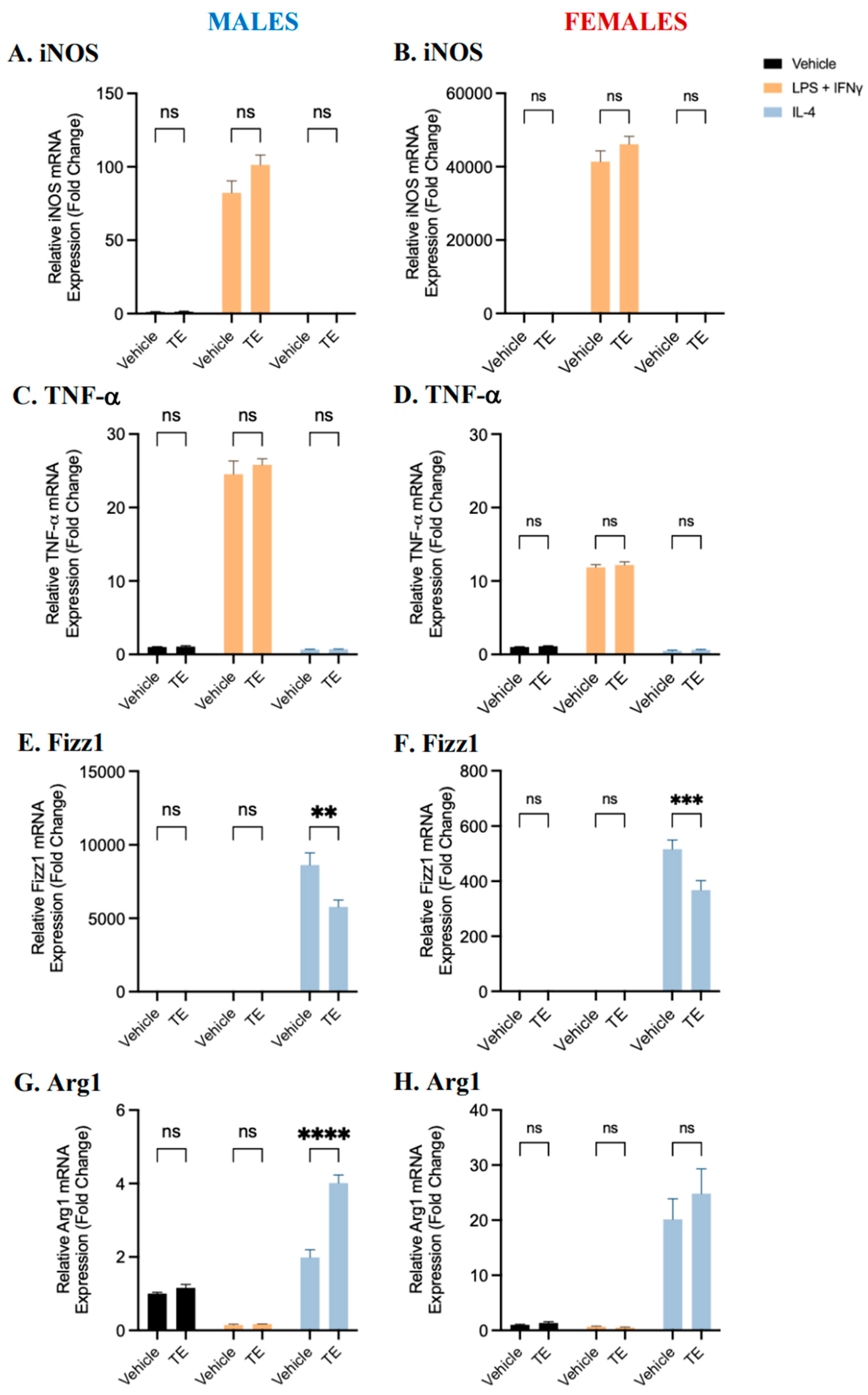
2.2. BMDM Polarization Is Affected by 17β-Estradiol and Testosterone Supplementation
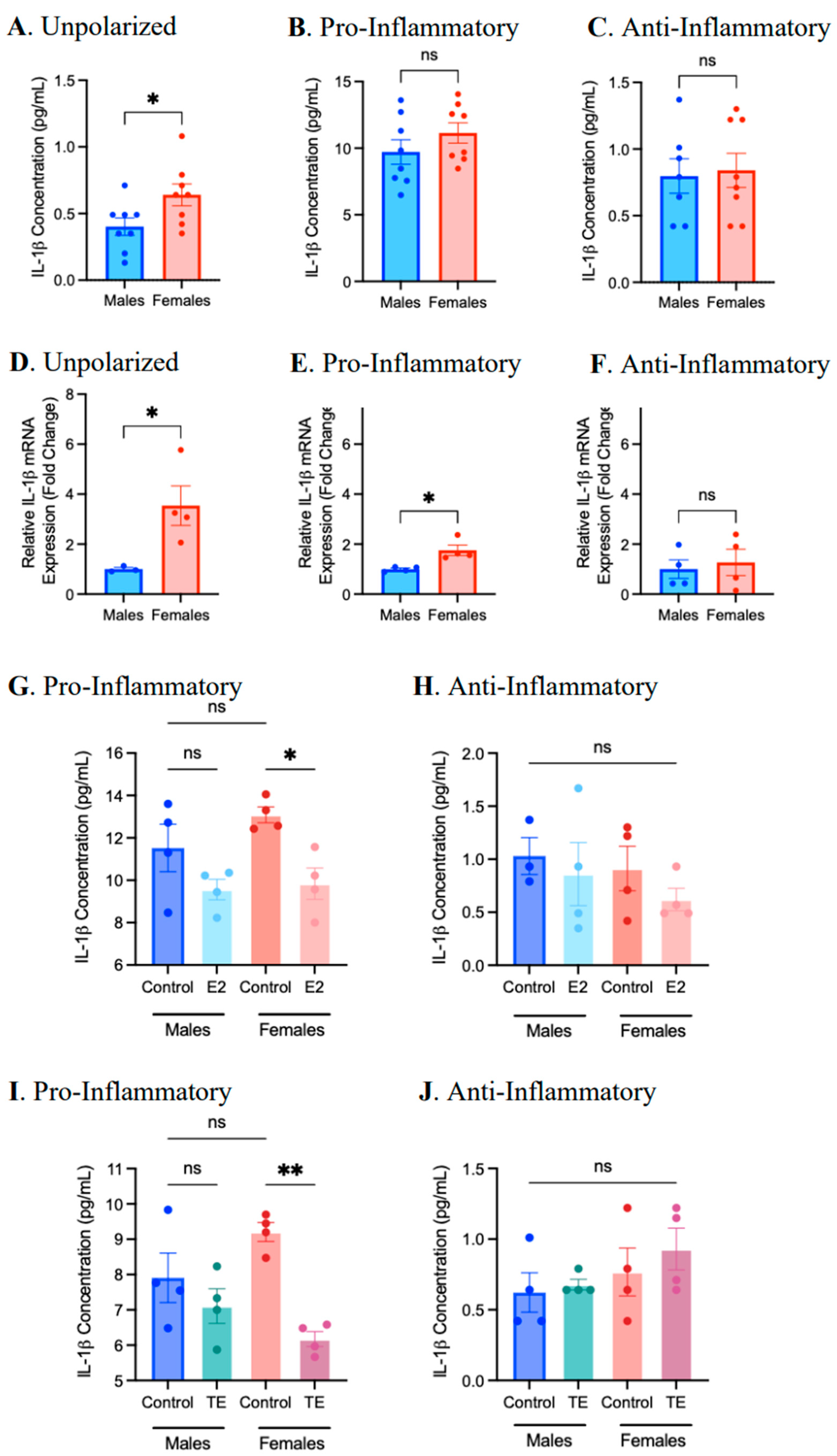
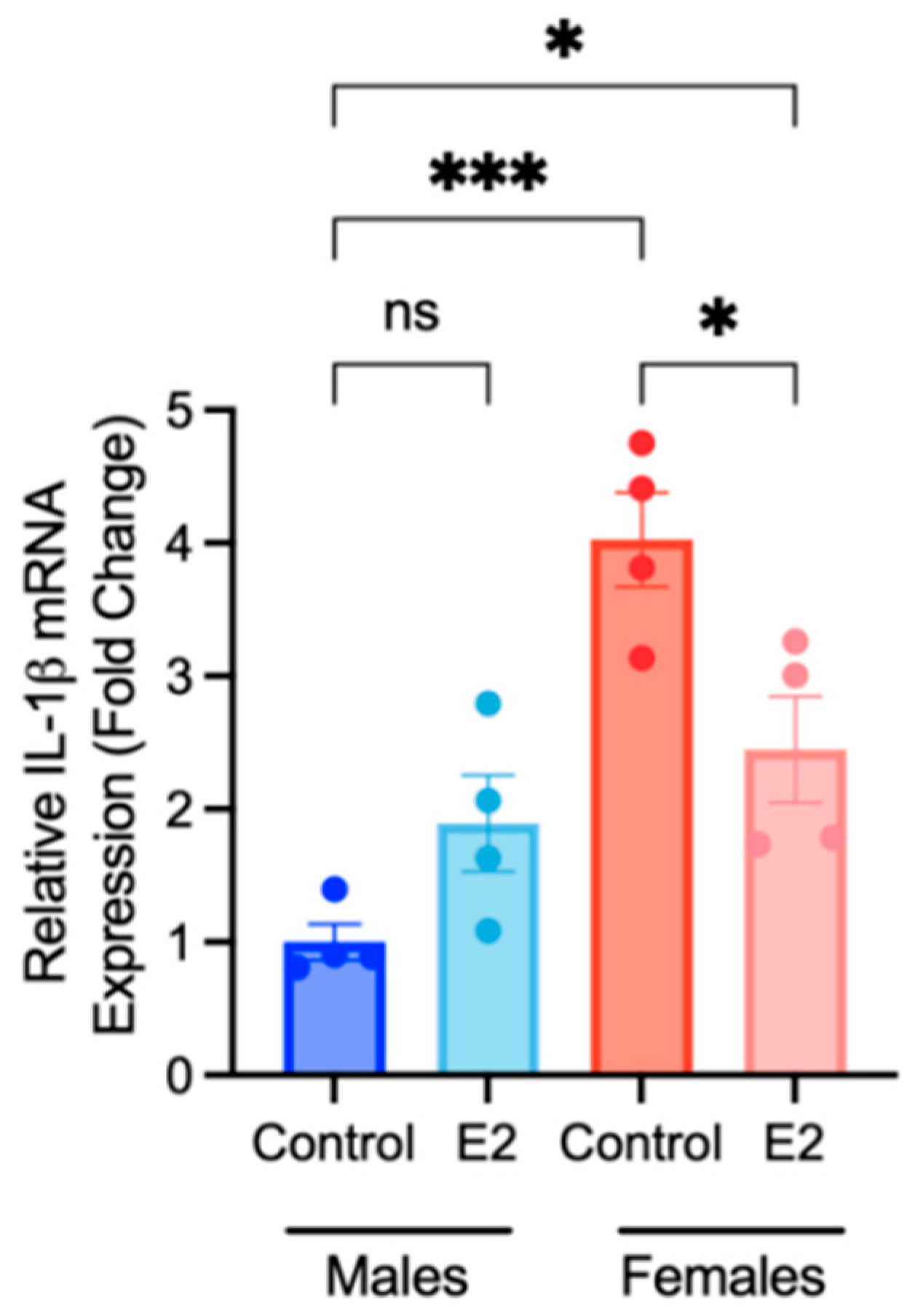
2.3. Sex Differences and the Effects of Sex Hormone Supplementation on Inflammatory Response
2.4. Sex Differences and the Effects of Sex Hormone Supplementation on the Migratory Response
3. Discussion
4. Materials and Methods
4.1. Bone Marrow-Derived Macrophage Isolation and Polarization
4.2. Hormone Pre-Treatment
4.3. Analysis of Gene Expression
4.4. Cytokine/Chemokine Detection
4.5. Macrophage Migration Assay
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Norris, C.M.; Yip, C.Y.Y.; Nerenberg, K.A.; Clavel, M.A.; Pacheco, C.; Foulds, H.J.A.; Hardy, M.; Gonsalves, C.A.; Jaffer, S.; Parry, M.; et al. State of the Science in Women’s Cardiovascular Disease: A Canadian Perspective on the Influence of Sex and Gender. J. Am. Heart Assoc. 2020, 9, e015634. [Google Scholar] [CrossRef] [PubMed]
- Mehta, L.S.; Beckie, T.M.; DeVon, H.A.; Grines, C.L.; Krumholz, H.M.; Johnson, M.N.; Lindley, K.J.; Vaccarino, V.; Wang, T.Y.; Watson, K.E.; et al. Acute Myocardial Infarction in Women: A Scientific Statement from the American Heart Association. Circulation 2016, 133, 916–947. [Google Scholar] [CrossRef] [PubMed]
- Falk, E.; Nakano, M.; Bentzon, J.F.; Finn, A.; Virmani, R. Update on acute coronary syndromes: The pathologists’ view. Eur. Heart J. 2013, 34, 719–728. [Google Scholar] [CrossRef]
- Lerner, D.J.; Kannel, W.B.; Boston, M. Patterns of coronary heart disease morbidity and mortality in the sexes: A 26-year follow-up of the Framingham population. Am. Heart J. 1986, 111, 383–390. [Google Scholar] [CrossRef]
- Mosca, L.; Barrett-Connor, E.; Kass Wenger, N. Sex/gender differences in cardiovascular disease prevention: What a difference a decade makes. Circulation 2011, 124, 2145–2154. [Google Scholar] [CrossRef] [PubMed]
- Hayward, C.S.; Kelly, R.P.; Collins, P. The roles of gender, the menopause and hormone replacement on cardiovascular function. Cardiovasc. Res. 2000, 46, 28–49. [Google Scholar] [CrossRef]
- Ruggiero, R.J.; Likis, F.E. Estrogen: Physiology, Pharmacology, and Formulations for Replacement Therapy. J. Midwifery Women’s Health 2002, 47, 130–138. [Google Scholar] [CrossRef]
- Murphy, E. Estrogen signaling and cardiovascular disease. Circ. Res. 2011, 109, 687–696. [Google Scholar] [CrossRef]
- Brouchet, L.; Krust, A.; Dupont, S.; Chambon, P.; Bayard, F.; Arnal, F. Estradiol Accelerates Reendothelialization in Mouse Carotid Artery Through Estrogen Receptor-but Not Estrogen Receptor. Circulation 2001, 103, 423–428. [Google Scholar] [CrossRef]
- Pare, G.; Krust, A.; Karas, R.H.; Dupont, S.; Aronovitz, M.; Chambon, P.; Mendelsohn, M.E. Estrogen receptor-alpha mediates the protective effects of estrogen against vascular injury. Circ. Res. 2002, 90, 1087–1092. [Google Scholar] [CrossRef]
- Hodgin, J.B.; Maeda, N. Minireview: Estrogen and Mouse Models of Atherosclerosis. Endocrinology 2002, 143, 4495–4501. [Google Scholar] [CrossRef] [PubMed]
- Bourghardt, J.; Wilhelmson, A.S.; Alexanderson, C.; De Gendt, K.; Verhoeven, G.; Krettek, A.; Ohlsson, C.; Tivesten, A. Androgen receptor-dependent and independent atheroprotection by testosterone in male mice. Endocrinology 2010, 151, 5428–5437. [Google Scholar] [CrossRef] [PubMed]
- Nathan, L.; Shi, W.; Dinh, H.; Mukherjee, T.K.; Wang, X.; Lusis, A.J.; Chaudhuri, G. Testosterone inhibits early atherogenesis by conversion to estradiol: Critical role of aromatase. Proc. Natl. Acad. Sci. USA 2001, 98, 3589–3593. [Google Scholar] [CrossRef] [PubMed]
- Mendelsohn, M.E.; Rosano, G.M.C. Hormonal Regulation of Normal Vascular Tone in Males. Circ. Res. 2003, 93, 1142–1145. [Google Scholar] [CrossRef] [PubMed]
- Colin, S.; Chinetti-Gbaguidi, G.; Staels, B. Macrophage phenotypes in atherosclerosis. Immunol. Rev. 2014, 262, 153–166. [Google Scholar] [CrossRef]
- Campbell, L.; Emmerson, E.; Williams, H.; Saville, C.R.; Krust, A.; Chambon, P.; Mace, K.A.; Hardman, M.J. Estrogen receptor-alpha promotes alternative macrophage activation during cutaneous repair. J. Investig. Dermatol. 2014, 134, 2447–2457. [Google Scholar] [CrossRef] [PubMed]
- Dou, C.; Ding, N.; Zhao, C.; Hou, T.; Kang, F.; Cao, Z.; Liu, C.; Bai, Y.; Dai, Q.; Ma, Q.; et al. Estrogen Deficiency-Mediated M2 Macrophage Osteoclastogenesis Contributes to M1/M2 Ratio Alteration in Ovariectomized Osteoporotic Mice. J. Bone Miner. Res. 2018, 33, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Shanker, G.; Sorci-Thomas, M.; Adams, M.R. Estrogen modulates the expression of tumor necrosis factor alpha mRNA in phorbol ester-stimulated human monocytic THP-1 cells. Lymphokine Cytokine Res. 1994, 13, 377–382. [Google Scholar]
- Bolego, C.; Cignarella, A.; Staels, B.; Chinetti-Gbaguidi, G. Macrophage function and polarization in cardiovascular disease: A role of estrogen signaling? Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1127–1134. [Google Scholar] [CrossRef]
- Becerra-Díaz, M.; Strickland, A.B.; Keselman, A.; Heller, N.M. Androgen and Androgen Receptor as Enhancers of M2 Macrophage Polarization in Allergic Lung Inflammation. J. Immunol. 2018, 201, 2923–2933. [Google Scholar] [CrossRef]
- Cioni, B.; Zaalberg, A.; van Beijnum, J.R.; Melis, M.H.M.; van Burgsteden, J.; Muraro, M.J.; Hooijberg, E.; Peters, D.; Hofland, I.; Lubeck, Y.; et al. Androgen receptor signalling in macrophages promotes TREM-1-mediated prostate cancer cell line migration and invasion. Nat. Commun. 2020, 11, 4498. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Werstuck, G.H. Characterizing the Role of Glycogen Synthase Kinase-3α/β in Macrophage Polarization and the Regulation of Pro-Atherogenic Pathways in Cultured Ldlr−/− Macrophages. Front. Immunol. 2021, 12, 676752. [Google Scholar] [CrossRef]
- He, L.; Jhong, J.H.; Chen, Q.; Huang, K.Y.; Strittmatter, K.; Kreuzer, J.; DeRan, M.; Wu, X.; Lee, T.Y.; Slavov, N.; et al. Global characterization of macrophage polarization mechanisms and identification of M2-type polarization inhibitors. Cell Rep. 2021, 37, 109955. [Google Scholar] [CrossRef] [PubMed]
- Tugal, D.; Liao, X.; Jain, M.K. Transcriptional control of macrophage polarization. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Ying, W.; Cheruku, P.S.; Bazer, F.W.; Safe, S.H.; Zhou, B. Investigation of Macrophage Polarization Using Bone Marrow Derived Macrophages. J. Vis. Exp. 2013, 50323. [Google Scholar] [CrossRef]
- Gong, M.; Zhuo, X.; Ma, A. STAT6 Upregulation Promotes M2 Macrophage Polarization to Suppress Atherosclerosis. Med. Sci. Monit. Basic. Res. 2017, 23, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Lin, X.; Xing, L.; Kenney, H.M.; Bell, R.; Schwarz, E.; Rahimi, H. Variable Effects of Testosterone on Male versus Female Derived Macrophages in Inflammatory Arthritis. In Proceedings of the American College of Rheumatology Convergence 2021, Virtual. 5–9 November 2021. [Google Scholar]
- Rettew, J.A.; Huet-Hudson, Y.M.; Marriott, I. Testosterone Reduces Macrophage Expression in the Mouse of Toll-Like Receptor 4, a Trigger for Inflammation and Innate Immunity. Biol. Reprod. 2008, 78, 432–437. [Google Scholar] [CrossRef]
- Mai, W.; Liao, Y. Targeting IL-1β in the Treatment of Atherosclerosis. Front. Immunol. 2020, 11, 589654. [Google Scholar] [CrossRef]
- Stöger, J.L.; Gijbels, M.J.; van der Velden, S.; Manca, M.; van der Loos, C.M.; Biessen, E.A.; Daemen, M.J.; Lutgens, E.; de Winther, M.P. Distribution of macrophage polarization markers in human atherosclerosis. Atherosclerosis 2012, 225, 461–468. [Google Scholar] [CrossRef]
- Bäck, M.; Yurdagul, A.; Tabas, I.; Öörni, K.; Kovanen, P.T. Inflammation and its resolution in atherosclerosis: Mediators and therapeutic opportunities. Nat. Rev. Cardiol. 2019, 16, 389–406. [Google Scholar] [CrossRef]
- Yang, H.; Sun, Y.; Li, Q.; Jin, F.; Dai, Y. Diverse Epigenetic Regulations of Macrophages in Atherosclerosis. Front. Cardiovasc. Med. 2022, 9, 868788. [Google Scholar] [CrossRef] [PubMed]
- Kovács, T.; Szabó-Meleg, E.; Ábrahám, I.M. Molecular Sciences Estradiol-Induced Epigenetically Mediated Mechanisms and Regulation of Gene Expression. Int. J. Mol. Sci. 2020, 21, 3177. [Google Scholar] [CrossRef] [PubMed]
- Lefèvre, N.; Corazza, F.; Valsamis, J.; Delbaere, A.; De Maertelaer, V.; Duchateau, J.; Casimir, G. The Number of X Chromosomes Influences Inflammatory Cytokine Production Following Toll-Like Receptor Stimulation. Front. Immunol. 2019, 10, 1052. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Thuren, T.; Zalewski, A.; Libby, P. Interleukin-1β inhibition and the prevention of recurrent cardiovascular events: Rationale and Design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS). Am. Heart J. 2011, 162, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.M.; Sellers, D.J.; Woodroofe, M.N.; Jones, T.H.; Channer, K.S. Effect of Testosterone on Inflammatory Markers in the Development of Early Atherogenesis in the Testicular-Feminized Mouse Model. Endocr. Res. 2013, 38, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Grebe, A.; Hoss, F.; Latz, E. NLRP3 inflammasome and the IL-1 pathway in atherosclerosis. Circ. Res. 2018, 122, 1722–1740. [Google Scholar] [CrossRef]
- Jiang, C.; Xie, S.; Yang, G.; Wang, N. Spotlight on NLRP3 Inflammasome: Role in Pathogenesis and Therapies of Atherosclerosis. J. Inflamm. Res. 2021, 14, 7143–7172. [Google Scholar] [CrossRef] [PubMed]
- Barrett, T.J. Macrophages in Atherosclerosis Regression. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 20–33. [Google Scholar] [CrossRef]
- Cui, K.; Ardell, C.L.; Podolnikova, N.P.; Yakubenko, V.P. Distinct migratory properties of M1, M2, and resident macrophages are regulated by αdβ2and αmβ2integrin-mediated adhesion. Front. Immunol. 2018, 9, 2650. [Google Scholar] [CrossRef]
- Fijak, M.; Schneider, E.; Klug, J.; Bhushan, S.; Hackstein, H.; Schuler, G.; Wygrecka, M.; Gromoll, J.; Meinhardt, A. Testosterone replacement effectively inhibits the development of experimental autoimmune orchitis in rats: Evidence for a direct role of testosterone on regulatory T cell expansion. J. Immunol. 2011, 186, 5162–5172. [Google Scholar] [CrossRef]


| Gene | Forward Primer (5′ → 3′) | Reverse Primer (5′ → 3′) |
|---|---|---|
| β-actin | GGC ACC ACA CCT TCT ACA ATG | GGG GTG TTG AAG GTC TCA AAC |
| iNOS | CAG CTG GGC TGT ACA AAC CTT | CAT TGG AAG TGA AGC GGT TCG |
| TNF-α | ACC ACA GTC CAT GCC ATC AC | CAC CAC CCT GTT GCT GTA GCC |
| Fizz1 | TCC AGC TGA TGG TCC CAG TGA ATA | ACA AGC ACA CCC AGT AGC AGT CAT |
| Arg1 | ACC TGG CCT TTG TTG ATC TCC CTA | AGA GAT GCT TCC AAC TGC CAG ACT |
| IL-1β | CTG CTT CCA AAC CTT TGA CC | AGC TTC TCC AGA GCC ACA AT |
| CD38 | TTG CAA GGG TTC TTG GAA AC | CGC TGC CTC ATC TAC ACT CA |
| Ym1 | AGA AGG GAG TTT CAA ACC | GTC TTG CTC ATG TGT AGT GA |
| CD206 | CAG GTG TGG GCT CAG GTA GT | TGT GGT GAG CTG AAA GGT GA |
| AR | TTG CAA GAG AGC TGC ATC AGT T | ACT GTG TGT GGA AAT AGA TGG GC |
| ERα | ACC ATT GAC AAG AAC CGG AG | CCT GAA GCA CCC ATT TCA TT |
| ERβ | TGT GTG TGA AGG CCA TGA TT | TCT TCG AAA TCA CCC AGA CC |
| GPER1 | TCA TTT CTG CCA TGC ACC CA | GTG GAC AGG GTG TCT GAT GT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enright, S.; Werstuck, G.H. Investigating the Effects of Sex Hormones on Macrophage Polarization. Int. J. Mol. Sci. 2024, 25, 951. https://doi.org/10.3390/ijms25020951
Enright S, Werstuck GH. Investigating the Effects of Sex Hormones on Macrophage Polarization. International Journal of Molecular Sciences. 2024; 25(2):951. https://doi.org/10.3390/ijms25020951
Chicago/Turabian StyleEnright, Sophie, and Geoff H. Werstuck. 2024. "Investigating the Effects of Sex Hormones on Macrophage Polarization" International Journal of Molecular Sciences 25, no. 2: 951. https://doi.org/10.3390/ijms25020951
APA StyleEnright, S., & Werstuck, G. H. (2024). Investigating the Effects of Sex Hormones on Macrophage Polarization. International Journal of Molecular Sciences, 25(2), 951. https://doi.org/10.3390/ijms25020951





