Therapeutic and Immunologic Effects of Short-Chain Fatty Acids in Inflammatory Bowel Disease: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Question and Eligibility Criteria
2.1.1. Inclusion Criteria
- Population: adults diagnosed with ulcerative colitis or Crohn’s disease.
- Intervention: the administration of prebiotics, probiotics, or dietary treatments aimed at restoring butyrate-producing bacteria.
- Comparator: comparison with patients who did not receive such interventions.
- Outcome: the evidence of anti-inflammatory effects associated with short-chain fatty acids.
- Publication language: English or Spanish.
- Publication date: articles published between January 2000 and August 2024.
- Study design: prospective or retrospective observational studies (cross-sectional, case-control, or cohort studies), reviews, case studies and case series.
2.1.2. Exclusion Criteria
- Articles that did not directly address the impact of SCFAs or their immunomodulatory role in inflammatory bowel disease.
- Letters to the editor, personal opinions, books, book chapters, non-original reports, conference abstracts, editorials, commentaries, or articles that did not contribute to or complement the objectives of the study.
- Studies involving patients with additional serious medical conditions, known allergies to prebiotics or probiotics, pregnancy, or participation in other clinical trials that could confound the results.
- Articles for which the full text could not be obtained.
2.2. Sources of Information and Search Strategy
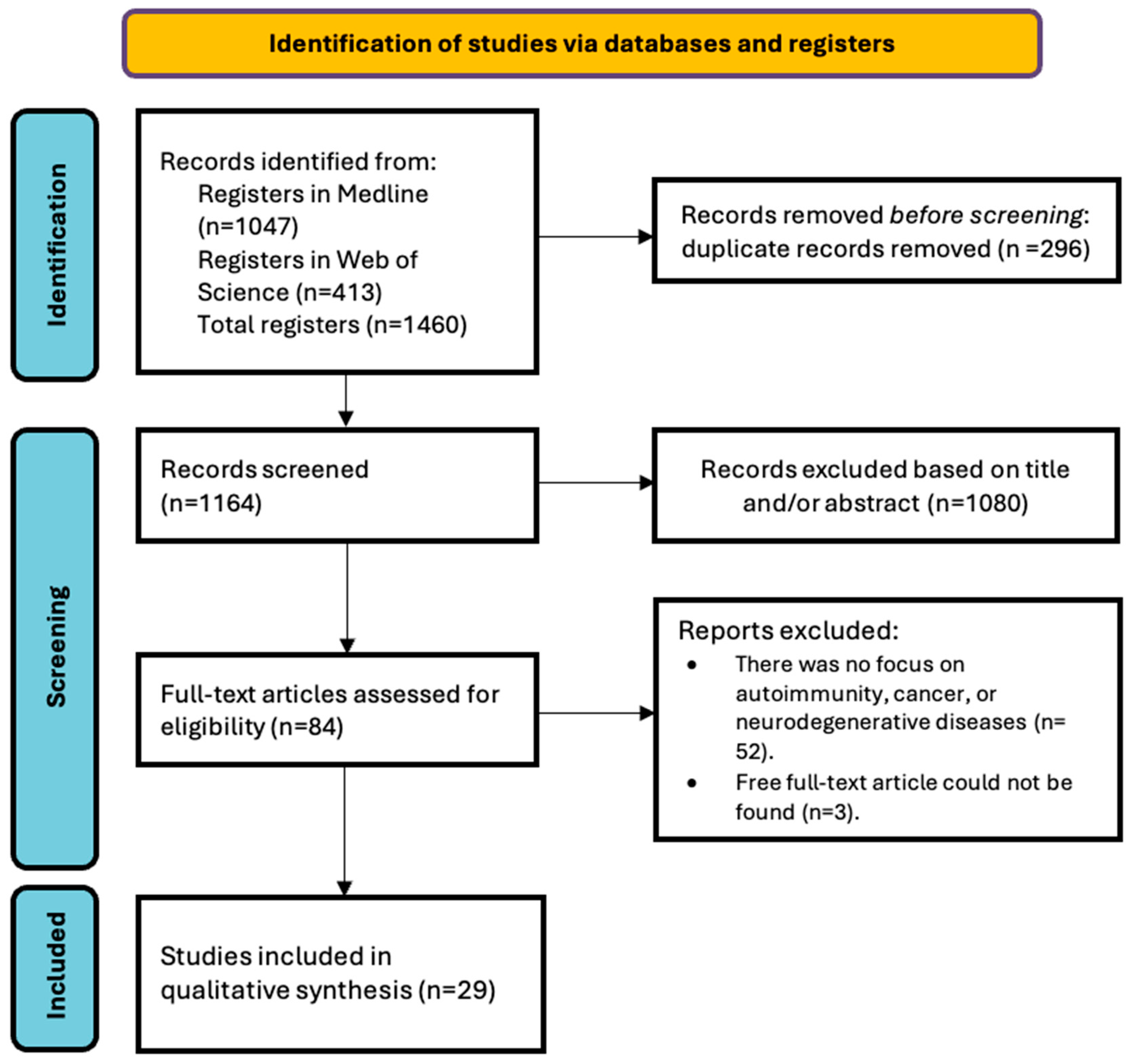
2.3. Selection of Studies
2.4. Data Extraction
2.5. Quality Assessment
3. Results
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Seyedian, S.S.; Nokhostin, F.; Malamir, M.D. A review of the diagnosis, prevention, and treatment methods of inflammatory bowel disease. J. Med. Life 2019, 12, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, G.G. The global burden of IBD: From 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.M.; Bornemann, P.H. Ulcerative colitis. Am. Fam. Physician 2013, 87, 699–705. [Google Scholar]
- Bernstein, C.N.; Wajda, A.; Svenson, L.W.; MacKenzie, A.; Koehoorn, M.; Jackson, M.; Fedorak, R.; Israel, D.; Blanchard, J.F. The epidemiology of inflammatory bowel disease in Canada: A population-based study. Am. J. Gastroenterol. 2006, 101, 1559–1568. [Google Scholar] [CrossRef]
- Cosnes, J.; Gower-Rousseau, C.; Seksik, P.; Cortot, A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology 2011, 140, 1785–1794. [Google Scholar] [CrossRef]
- Loftus, E.V., Jr. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology 2004, 126, 1504–1517. [Google Scholar] [CrossRef] [PubMed]
- Juliao-Baños, F.; Grillo-Ardila, C.F.; Alfaro, I.; Andara-Ramírez, M.T.; Avelar-Escobar, O.; Barahona-Garrido, J.; Bautista-Martínez, S.; Bosques-Padilla, F.J.; De Paula, J.A.; Ernest Suárez, K.; et al. Update of the PANCCO clinical practice guidelines for the treatment of ulcerative colitis in the adult population. Rev. Gastroenterol. Mex. 2022, 87, 342–361. [Google Scholar] [CrossRef]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)- Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar]
- Yadav, M.K.; Kumari, I.; Singh, B.; Sharma, K.K.; Tiwari, S.K. Probiotics, prebiotics and synbiotics: Safe options for next-generation therapeutics. Appl. Microbiol. Biotechnol. 2022, 106, 505–521. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Joanna Briggs Institute. Critical Appraisal Tools. Available online: https://jbi.global/critical-appraisal-tools (accessed on 28 February 2024).
- Wilson, B.; Eyice, Ö.; Koumoutsos, I.; Lomer, M.C.; Irving, P.M.; Lindsay, J.O.; Whelan, K. Prebiotic Galactooligosaccharide Supplementation in Adults with Ulcerative Colitis: Exploring the Impact on Peripheral Blood Gene Expression, Gut Microbiota, and Clinical Symptoms. Nutrients 2021, 13, 3598. [Google Scholar] [CrossRef]
- Wei, Y.; Gong, J.; Zhu, W.; Tian, H.; Ding, C.; Gu, L.; Li, N.; Li, J. Pectin enhances the effect of fecal microbiota transplantation in ulcerative colitis by delaying the loss of diversity of gut flora. BMC Microbiol. 2016, 16, 255. [Google Scholar] [CrossRef]
- Facchin, S.; Vitulo, N.; Calgaro, M.; Buda, A.; Romualdi, C.; Pohl, D.; Perini, B.; Lorenzon, G.; Marinelli, C.; D’Incà, R.; et al. Microbiota changes induced by microencapsulated sodium butyrate in patients with inflammatory bowel disease. Neurogastroenterol. Motil. 2020, 32, e13914. [Google Scholar] [CrossRef]
- Pietrzak, A.; Banasiuk, M.; Szczepanik, M.; Borys-Iwanicka, A.; Pytrus, T.; Walkowiak, J.; Banaszkiewicz, A. Sodium Butyrate Effectiveness in Children and Adolescents with Newly Diagnosed Inflammatory Bowel Diseases-Randomized Placebo-Controlled Multicenter Trial. Nutrients 2022, 14, 3283. [Google Scholar] [CrossRef]
- Chu, N.D.; Crothers, J.W.; Nguyen, L.T.T.; Kearney, S.M.; Smith, M.B.; Kassam, Z.; Collins, C.; Xavier, R.; Moses, P.L.; Alm, E.J. Dynamic Colonization of Microbes and Their Functions after Fecal Microbiota Transplantation for Inflammatory Bowel Disease. mBio 2021, 12, e0097521. [Google Scholar] [CrossRef]
- Svolos, V.; Hansen, R.; Nichols, B.; Quince, C.; Ijaz, U.Z.; Papadopoulou, R.T.; Edwards, C.A.; Watson, D.; Alghamdi, A.; Brejnrod, A.; et al. Treatment of Active Crohn’s Disease with an Ordinary Food-based Diet That Replicates Exclusive Enteral Nutrition. Gastroenterology 2019, 156, 1354–1367.e6. [Google Scholar] [CrossRef]
- Luceri, C.; Femia, A.P.; Fazi, M.; Di Martino, C.; Zolfanelli, F.; Dolara, P.; Tonelli, F. Effect of butyrate enemas on gene expression profiles and endoscopic/histopathological scores of diverted colorectal mucosa: A randomized trial. Dig. Liver Dis. 2016, 48, 27–33. [Google Scholar] [CrossRef]
- Haskey, N.; Estaki, M.; Ye, J.; Shim, R.K.; Singh, S.; Dieleman, L.A.; Jacobson, K.; Gibson, D.L. A Mediterranean Diet Pattern Improves Intestinal Inflammation Concomitant with Reshaping of the Bacteriome in Ulcerative Colitis: A Randomised Controlled Trial. J. Crohns. Colitis. 2023, 17, 1569–1578. [Google Scholar] [CrossRef]
- Hamilton, A.L.; Kamm, M.A.; De Cruz, P.; Wright, E.K.; Feng, H.; Wagner, J.; Sung, J.J.Y.; Kirkwood, C.D.; Inouye, M.; Teo, S.M. Luminal microbiota related to Crohn’s disease recurrence after surgery. Gut. Microbes. 2020, 11, 1713–1728. [Google Scholar] [CrossRef]
- Langhorst, J.; Koch, A.K.; Voiss, P.; Dobos, G.J.; Rueffer, A. Distinct patterns of shortchain fatty acids during flare in patients with ulcerative colitis under treatment with mesalamine or a herbal combination of myrrh, chamomile flowers, and coffee charcoal: Secondary analysis of a randomized controlled trial. Eur. J. Gastroenterol. Hepatol. 2020, 32, 175–180. [Google Scholar] [CrossRef]
- Fritsch, J.; Garces, L.; Quintero, M.A.; Pignac-Kobinger, J.; Santander, A.M.; Fernández, I.; Ban, Y.J.; Kwon, D.; Phillips, M.C.; Knight, K.; et al. Low-Fat, High-Fiber Diet Reduces Markers of Inflammation and Dysbiosis and Improves Quality of Life in Patients with Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2021, 19, 1189–1199.e30. [Google Scholar] [CrossRef]
- Ma, H.Q.; Yu, T.T.; Zhao, X.J.; Zhang, Y.; Zhang, H.J. Fecal microbial dysbiosis in Chinese patients with inflammatory bowel disease. World J. Gastroenterol. 2018, 24, 1464–1477. [Google Scholar] [CrossRef]
- Andoh, A.; Kuzuoka, H.; Tsujikawa, T.; Nakamura, S.; Hirai, F.; Suzuki, Y.; Matsui, T.; Fujiyama, Y.; Matsumoto, T. Multicenter analysis of fecal microbiota profiles in Japanese patients with Crohn’s disease. J. Gastroenterol. 2012, 47, 1298–1307. [Google Scholar] [CrossRef]
- O’Brien, C.L.; Allison, G.E.; Pavli, P. The more the merrier: Faecalibacterium prausnitzii in Crohn’s disease. J. Gastroenterol. Hepatol. 2013, 28, 757–759. [Google Scholar] [CrossRef]
- Fujimoto, T.; Imaeda, H.; Takahashi, K.; Kasumi, E.; Bamba, S.; Fujiyama, Y.; Andoh, A. Decreased abundance of Faecalibacterium prausnitzii in the gut microbiota of Crohn’s disease. J. Gastroenterol. Hepatol. 2013, 28, 613–619. [Google Scholar] [CrossRef]
- Valcheva, R.; Koleva, P.; Martínez, I.; Walter, J.; Gänzle, M.G.; Dieleman, L.A. Inulin-type fructans improve active ulcerative colitis associated with microbiota changes and increased short-chain fatty acids levels. Gut. Microbes. 2019, 10, 334–357. [Google Scholar] [CrossRef]
- Danilova, N.A.; Abdulkhakov, S.R.; Grigoryeva, T.V.; Markelova, M.I.; Vasilyev, I.Y.; Boulygina, E.A.; Ardatskaya, M.D.; Pavlenko, A.V.; Tyakht, A.V.; Odintsova, A.K.; et al. Markers of dysbiosis in patients with ulcerative colitis and Crohn’s disease. Ter. Arkhiv 2019, 91, 17–24. [Google Scholar] [CrossRef]
- Ferrer-Picón, E.; Dotti, I.; Corraliza, A.M.; Mayorgas, A.; Esteller, M.; Perales, J.C.; Ricart, E.; Masamunt, M.C.; Carrasco, A.; Tristán, E.; et al. Intestinal Inflammation Modulates the Epithelial Response to Butyrate in Patients with Inflammatory Bowel Disease. Inflamm. Bowel. Dis. 2020, 26, 43–55. [Google Scholar] [CrossRef]
- Wang, W.; Chen, L.; Zhou, R.; Wang, X.; Song, L.; Huang, S.; Wang, G.; Xia, B. Increased proportions of Bifidobacterium and the Lactobacillus group and loss of butyrateproducing bacteria in inflammatory bowel disease. J. Clin. Microbiol. 2014, 52, 398–406. [Google Scholar] [CrossRef]
- Lopez-Siles, M.; Enrich-Capó, N.; Aldeguer, X.; Sabat-Mir, M.; Duncan, S.H.; Garcia-Gil, L.J.; Martinez-Medina, M. Alterations in the Abundance and Co-occurrence of Akkermansia muciniphila and Faecalibacterium prausnitzii in the Colonic Mucosa of Inflammatory Bowel Disease Subjects. Front. Cell Infect. Microbiol. 2018, 8, 281. [Google Scholar] [CrossRef]
- Lopez-Siles, M.; Martinez-Medina, M.; Abellà, C.; Busquets, D.; Sabat-Mir, M.; Duncan, S.H.; Aldeguer, X.; Flint, H.J.; Garcia-Gil, L.J. Mucosa-associated Faecalibacterium prausnitzii phylotype richness is reduced in patients with inflammatory bowel disease. Appl. Environ. Microbiol. 2015, 81, 7582–7592. [Google Scholar] [CrossRef]
- Brotherton, C.S.; Martin, C.A.; Long, M.D.; Kappelman, M.D.; Sandler, R.S. Avoidance of Fiber Is Associated with Greater Risk of Crohn’s Disease Flare in a 6-Month Period. Clin. Gastroenterol. Hepatol. 2016, 14, 1130–1136. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Ahuja, V.; Paul, J. Fluctuations in butyrate-producing bacteria in ulcerative colitis patients of North India. World J. Gastroenterol. 2013, 19, 3404–3414. [Google Scholar] [CrossRef]
- Machiels, K.; Joossens, M.; Sabino, J.; De Preter, V.; Arijs, I.; Eeckhaut, V.; Ballet, V.; Claes, K.; Van Immerseel, F.; Verbeke, K.; et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut 2014, 63, 1275–1283. [Google Scholar] [CrossRef]
- Takahashi, K.; Nishida, A.; Fujimoto, T.; Fujii, M.; Shioya, M.; Imaeda, H.; Inatomi, O.; Bamba, S.; Sugimoto, M.; Andoh, A. Reduced Abundance of Butyrate-Producing Bacteria Species in the Fecal Microbial Community in Crohn’s Disease. Digestion 2016, 93, 59–65, Erratum in Digestion 2016, 93, 174. [Google Scholar] [CrossRef]
- Zhuang, X.; Li, T.; Li, M.; Huang, S.; Qiu, Y.; Feng, R.; Zhang, S.; Chen, M.; Xiong, L.; Zeng, Z. Systematic Review and Meta-analysis: Short-Chain Fatty Acid Characterization in Patients with Inflammatory Bowel Disease. Inflamm. Bowel. Dis. 2019, 25, 1751–1763. [Google Scholar] [CrossRef]
- Xu, H.M.; Zhao, H.L.; Guo, G.J.; Xu, J.; Zhou, Y.L.; Huang, H.L.; Nie, Y.Q. Characterization of short-chain fatty acids in patients with ulcerative colitis: A meta-analysis. BMC Gastroenterol. 2022, 22, 117. [Google Scholar] [CrossRef]
- Cao, Y.; Shen, J.; Ran, Z.H. Association between Faecalibacterium prausnitzii Reduction and Inflammatory Bowel Disease: A Meta-Analysis and Systematic Review of the Literature. Gastroenterol. Res. Pract. 2014, 2014, 872725. [Google Scholar] [CrossRef]
- Zhao, H.; Xu, H.; Chen, S.; He, J.; Zhou, Y.; Nie, Y. Systematic review and meta-analysis of the role of Faecalibacterium prausnitzii alteration in inflammatory bowel disease. J. Gastroenterol. Hepatol. 2021, 36, 320–328. [Google Scholar] [CrossRef]
- del Toro Añel, A.Y.; Pérez Tabío, Y.; Gorguet Pí, M.M.; Díaz del Toro, C.E. Estrés académico en estudiantes de Medicina durante la pandemia de covid-19. MEDISAN 2023, 27, e4398. [Google Scholar]
- Burisch, J.; Pedersen, N.; Čuković-Čavka, S.; Brinar, M.; Kaimakliotis, I.; Duricova, D.; Shonová, O.; Vind, I.; Avnstrøm, S.; Thorsgaard, N.; et al. East-West gradient in the incidence of inflammatory bowel disease in Europe: The ECCO-EpiCom inception cohort. Gut 2014, 63, 588–597. [Google Scholar] [CrossRef] [PubMed]
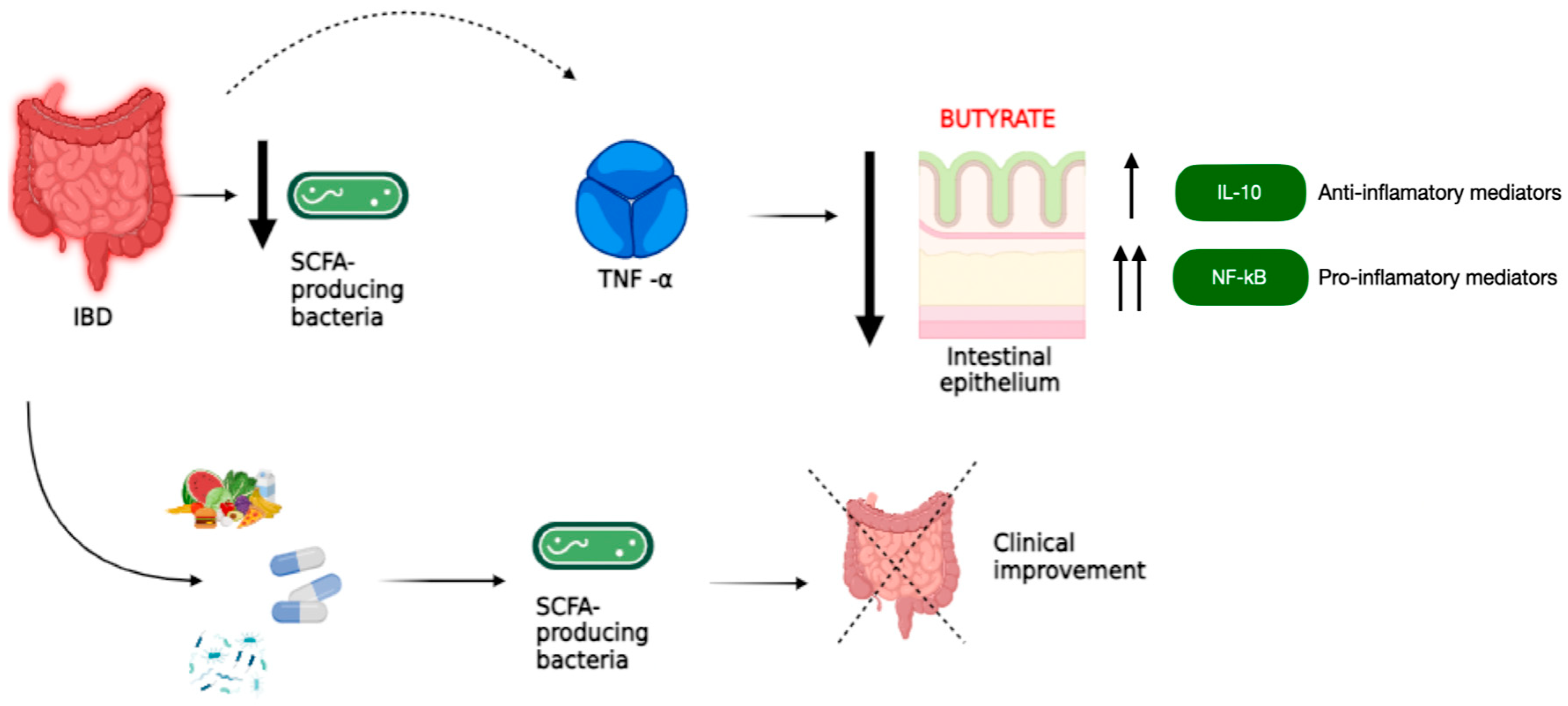
| Reference | Country | n | Objectives | Conclusions |
|---|---|---|---|---|
| Wilson B, 2021 [12] | UK | 18 (UC) | To analyze the impact of prebiotic GOS on the inflammation, disease parameters, and microbiota of UC. | Prebiotics produce an increase in bifidobacteria in vitro and in vivo, which regulate pH by producing acetate and lactate, which favors butyrate production. There are low concentrations of bifidobacteria in intestinal inflammation. Both clinical scores and inflammation were not reduced with the use of these prebiotics. Bifidobacterium increased only in patients with lower disease activity. |
| Wei Y, 2016 [13] | China | 20 (IBD) | To analyze the effects of pectin on FMT in UC patients. | Gut microbiota can ferment pectin into short-chain fatty acids. This maintains the composition and balance of the gut microbiota after FMT and preserves the diversity of the gut flora after FMT. |
| Facchin S, 2020 [14] | Italia | 49 (IBD) | To analyze the effect of sodium butyrate microcapsules on the intestinal microbiota of patients with IBD and the regulation of intestinal bacterial formation, especially butyrogenic degenerogens and SCFA, by butyrate. | Oral supplementation with butyrate increases the production of intestinal SCFA-producing bacteria with anti-inflammatory activity, which will produce more endogenous butyrate for intestinal comfort. This treatment has been particularly beneficial in UC patients. |
| Pietrzak A, 2022 [15] | Polonia | 79 (IBD) | To evaluate the effectiveness of sodium butyrate as an adjunct treatment in children and adolescents newly diagnosed with IBD. | Supplementation with sodium butyrate as an adjunctive therapy for 12 weeks did not show efficacy in children and adolescents newly diagnosed with IBD. |
| Chu ND, 2021 [16] | USA | 8 (UC) | To analyze the dynamics of microbial colonization and persistence in UC patients treated with FMT, focusing on the transfer of butyrate production genes from a healthy donor to a patient. | There is an association between the loss of butyrate-producing microbes and IBD. There are difficulties in increasing the genetic capacity for butyrate production through fecal transplants. |
| Svolos V, 2018 [17] | UK | 28 (UC) | To evaluate the effects on the gut microbiome, inflammation, and clinical response of an individualized food-based diet with a composition similar to EEN. | CD-TREAT mimics EEN changes in the microbiome and reduces intestinal inflammation. It is well tolerated and effective in patients with CD. |
| Luceri C, 2015 [18] | Italia | 20 (IBD) | To study the effect of sodium butyrate enemas in preventing inflammation and mucosal atrophy in IBD patients after ileus/colostomy. | Butyrate enemas prevent colon/rectum atrophy and improve the recovery of tissue integrity. |
| Haskey N, 2023 [19] | Canada | 38 (UC) | To investigate the differential effects of the Mediterranean dietary pattern (MDP) versus the Canadian dietary pattern (CHD) on the gut microbiome and inflammation in patients with inactive UC. | The MDP group had higher SCFA levels compared to the CDH group. MDP induces changes in the gut microbiome that are associated with clinical improvement in patients with inactive UC and may be used as an adjunctive therapy in patients with UC. |
| Hamilton AL, 2020 [20] | Australia | 130 (IBD) | Identify the types of bacteria associated with the sustained remission of UC through fecal microbiota transplantation (FMT). | Butyrate and other SCFAs have been shown to induce an anti-inflammatory response in animal models and clinical trials. The efficacy of FMT in restoring butyrate-producing bacteria and ButCoA levels suggests an important role for butyrate in the long-term remission of UC and establishes butyrate or butyrate-producing bacteria as a potential treatment strategy. |
| Langhorst J, 2020 [21] | Germany | 38 (UC) | To evaluate the effect of butyrate and other SCFAs in patients with UC with flares treated with mesalamine or a herbal preparation. | A decrease in SCFAs and butyrate has been observed in UC flare patients treated with mesalamine. Patients treated with the herbal preparation did not show this decrease in SCFAs. |
| Fritsch J, 2020 [22] | USA | 17 (UC) | To study the effects of a low-fat, high-fiber diet (LFD) compared to a Standard American Diet (SAD). | Although the LFD diet had higher amounts of fiber than the iSAD, the only fatty acid that increased significantly was acetate. This has a beneficial effect on the intestinal barrier and inflammatory responses by binding to G protein-coupled receptors. LFD increases Bacteroides, which contain the main producers of acetate. |
| Ma HQ, 2018 [23] | China | 15 (CD) 14 (UC) | To study alterations in the intestinal microbiota of Chinese patients with IBD. | Both Butyricicoccus and Lachnobacterium are SCFA-producing bacteria that act as an energy source for colonic epithelial cells. Changes in the gut microbiota, such as a decrease in these SCFA-producing bacteria, are associated with IBD patients. |
| Andoh A, 2012 [24] | Japan | 161 (CD) | To study fecal microbiota profiles in patients with CD. | A decrease in the Clostridium class, including the genus Fecalibacterium (butyrate-producing bacteria), was found in the bacterial composition of CD patients. |
| O’Brien CL, 2013 [25] | Australia | 47 (IBD) | To study the levels of Fecalibacterium Prausnitzzi and its relationship with CD. | Reduced levels of Fecalibacterium prausnitzzi have been observed in patients with CD, which is a cause and not a consequence of the disease. Fecalibacterium prausnitzzi and other fermenting organisms provide SCFAs, including butyrate, to the colonic epithelium, which uses it as an energy source. Butyrate plays an important role in reducing oxidative stress and as an anti-inflammatory agent. The more Fecalibacterium prausnitzzi, the better the gut ecosystem. The study found that inflammatory parameters were worse in patients with low Fecalibacterium prausnitzzii. |
| Fujimoto T, 2013 [26] | Japan | 47 (IBD) | To study the relationship between dysbiosis in CD patients and the abundance of Fecalibacterium prausnitzii and Bilophila wadsworthia bacteria in Japanese patients. | The abundance of Fecalibacterium prausnitzii was significantly decreased in CD patients compared to healthy subjects. The decrease in Fecalibacterium prausnitzii results in a reduction of the anti-inflammatory activities regulated by these butyrate-producing bacteria. |
| Valcheva R, 2019 [27] | Canada | 25 (UC) | To analyze whether insulin-type fructans produce benefits in UC and whether these are related to changes in bacterial composition. | B-fructan supplementation significantly increased SCFA production in the high-dose group but not in the low-dose group. B-fructans altered the composition of SCFAs. High-dose treatment with insulin-like fructans induces a modification of the gut microbiota, thereby improving UC and showing promise as a treatment for the disease. |
| Danilova NA, 2019 [28] | Russia | 95 (IBD) | To study the composition of the gut microbiota in patients with IBD to identify key markers of dysbiosis in the disease. | The decrease in acetate CoA transferase, the decrease in the total number of SCFAs, as well as the particulate and major SCFA in IBD patients may indicate an inhibition of the functional activity and the amount of anaerobic microflora. These signs can be considered as typical for dysbiosis in IBD and can be used for the development of future treatments. |
| Ferrer-Picón E, 2020 [29] | Spain | 137 (IBD) | To study the effects of butyrate on healthy and IBD-affected intestinal epithelium. | There is a reduction in butyrate-producing gut bacteria in patients with active IBD. The response to butyrate is not intrinsically altered in patients with IBD, but TNFα (a cytokine involved in the pathophysiology of IBD) produces a decrease in the epithelial response to this metabolite. This rejects the hypothesis of butyrate supplementation during active inflammation. The main effects of butyrate on the intestinal epithelium and the relevance of inflammation on the ability of epithelial cells to absorb, metabolize, and respond to this bacterial metabolite were determined. |
| Wang W, 2014 [30] | China | 11 (CD) 20 (UC) | To compare the composition of fecal and mucosa-associated bacteria in patients with IBD and healthy controls. | Butyrate-producing bacteria of the Clostridium IV and XIVa groups were found to be decreased in IBD patients, particularly F. prausnitzzi. Previous reports have shown that F. prausnitzii produces butyrate and that its fermented product provides energy for colonic epithelial cells and is important for the immune system and epithelial barrier integrity. Butyrate-producing bacteria should be considered as probiotics for patients in the acute phase of IBD. |
| Lopez-Siles M, 2018 [31] | Spain | 23 (UC) 37 (IBD) | To determine the variation of A. muciniphila and F. prausnitzii between healthy subjects and IBD patients. | In addition to butyrate production (which can reduce the inflammation of the intestinal mucosa and is the main energy source for colonocytes), other anti-inflammatory properties have been attributed to F. prausnitzii. IBD patients showed a decrease in this bacterium. |
| Lopez-Siles M, 2015 [32] | Spain | 64 (IBD) 23 (UC) | To study whether patients with gastrointestinal diseases, particularly IBD, have differences in F. prausnitzii populations with respect to healthy subjects. | The population of F. prausnitzii, which is a part of the butyrate-producing bacteria group, is present in both healthy subjects and those with intestinal disease. However, there is a loss of abundance and altered distribution in patients with IBD. |
| Brotherton CS, 2016 [33] | USA | 489 (UC) 1130 (CD) | To study whether dietary fiber intake is associated with exacerbations in IBD. | There are reasons to believe that fiber is beneficial for IBD patients because of the production of SCFAs, such as butyrate. Fiber intake is associated with reduced flares in CD but not in UC. |
| Kumari R, 2013 [34] | India | 26 (UC) | To study fecal samples to determine the concentration of butyrate and the butyrate-producing bacteria in patients with UC. | With reduced butyrate levels, there is a decrease in members of the clostridial group that contribute to the etiology of UC. The decrease in butyrate-producing clostridia was associated with a decrease in SCFAs in UC patients. |
| Machiels K, 2014 [35] | Belgium | 127 (UC) | To study the dysbiosis present in UC by quantifying bacterial metabolites. | SCFAs have been shown to be reduced in UC patients. The gut microbiota of UC patients shows a reduction in R. hominis and F. prausnitzii, known as butyrate-producing bacteria. |
| Takahashi K, 2016 [36] | Japan | 78 (CD) | To study changes in the fecal microbiota in patients with CD. | In patients with CD, there is a decrease in butyrate-producing bacteria such as Blautia faecis, Roseburia inulinivorans, Ruminococcus torques, Clostridium la-valense, Bacteroides uniformis, and Fecalibacterium prausnitzii. This reduction in butyrate-producing bacteria characterizes the dysbiosis characteristic of CD patients. |
| Zhuang X, 2019 [37] | China | 472 (IBD) | To characterize SCFAs in IBD patients and explore their potential role in initiating and developing IBD. | There are changes in CCFA in patients with IBD. There are inverse changes in CCFA in patients with active UC and those in remission. |
| Xu HM, 2022 [38] | China | 4453 (IBD) | To study CCFA alterations in UC patients to investigate their role in disease pathogenesis. | Patients with UC have significantly lower concentrations of total SCFAs compared to healthy patients. |
| Cao Y, 2014 [39] | China | 1180 (IBD) | To determine the relative risk of F. prausnitzzi decline in patients with and without IBD. | F. prausnitzii may protect against the development of IBD. Variation between studies and the possibility of bias preclude the certainty of this statement. Efforts are being made to use F. prausnitzii as an adjunct treatment for IBD by producing butyric acid. |
| Zhao H, 2021 [40] | China | 1669 (IBD) | To study the relationship between intestinal F. prausnitzii and IBD. | There is a negative relationship between the amount of F. prausnitzzi and IBD activity. A cut-off level of F. prausnitzzi cannot be established to diagnose or initiate treatment for IBD. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ventura, I.; Chomon-García, M.; Tomás-Aguirre, F.; Palau-Ferré, A.; Legidos-García, M.E.; Murillo-Llorente, M.T.; Pérez-Bermejo, M. Therapeutic and Immunologic Effects of Short-Chain Fatty Acids in Inflammatory Bowel Disease: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 10879. https://doi.org/10.3390/ijms252010879
Ventura I, Chomon-García M, Tomás-Aguirre F, Palau-Ferré A, Legidos-García ME, Murillo-Llorente MT, Pérez-Bermejo M. Therapeutic and Immunologic Effects of Short-Chain Fatty Acids in Inflammatory Bowel Disease: A Systematic Review. International Journal of Molecular Sciences. 2024; 25(20):10879. https://doi.org/10.3390/ijms252010879
Chicago/Turabian StyleVentura, Ignacio, Miryam Chomon-García, Francisco Tomás-Aguirre, Alma Palau-Ferré, María Ester Legidos-García, María Teresa Murillo-Llorente, and Marcelino Pérez-Bermejo. 2024. "Therapeutic and Immunologic Effects of Short-Chain Fatty Acids in Inflammatory Bowel Disease: A Systematic Review" International Journal of Molecular Sciences 25, no. 20: 10879. https://doi.org/10.3390/ijms252010879






