Regulatory Peptide Pro-Gly-Pro Accelerates Neuroregeneration of Primary Neuroglial Culture after Mechanical Injury in Scratch Test
Abstract
1. Introduction
2. Results
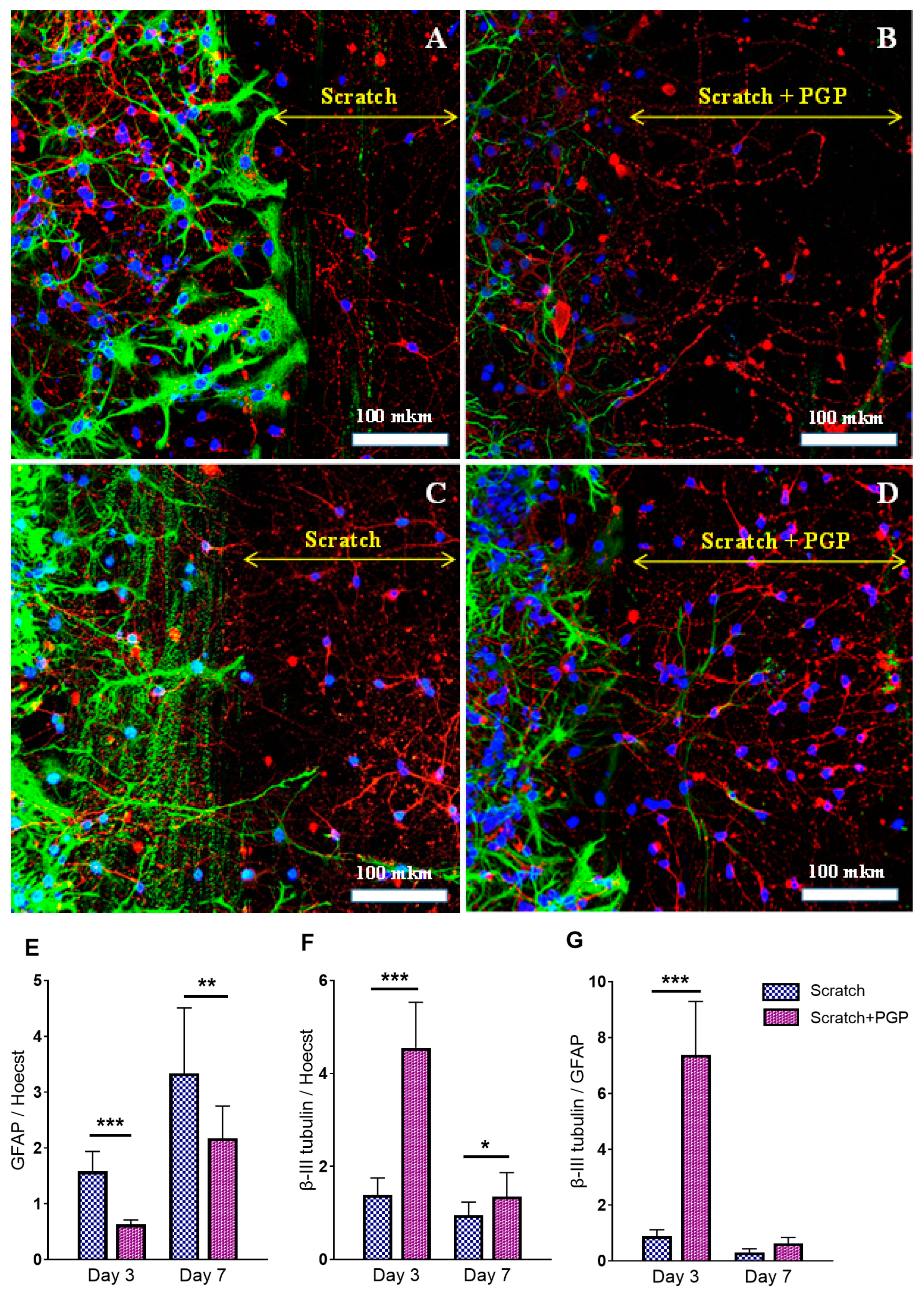
2.1. The Effect of PGP Treatment on the Survival and Regeneration Potential of Neuro-Glial Culture Cells after Mechanical Trauma
2.2. Effect of PGP on the BDNF and NSE Levels in Neuroglial Cell Culture after Mechanical Injury
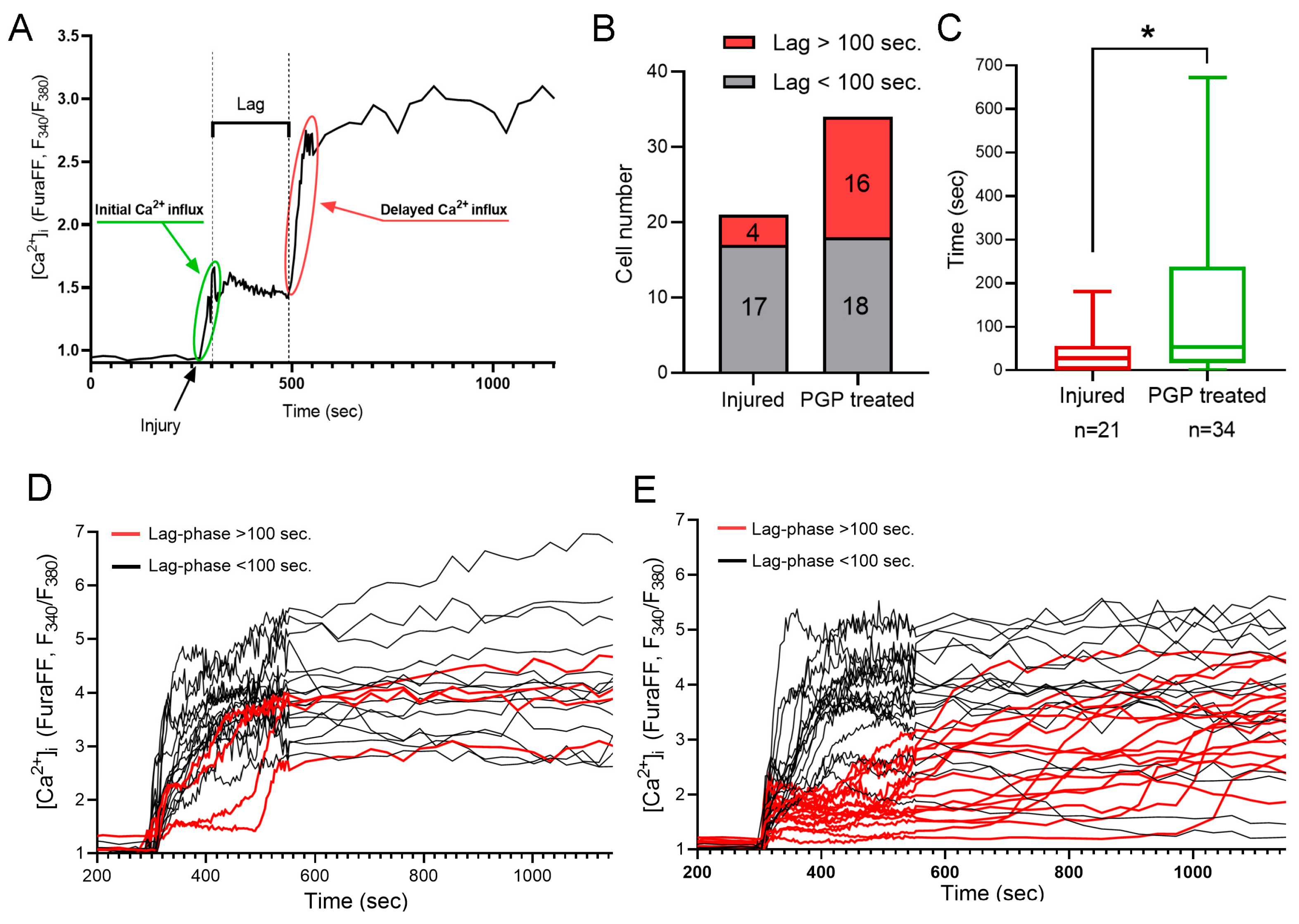
2.3. The Effect of Mechanical Injury on the Parameters of Acute Changes of [Ca2+]i and ΔΨm
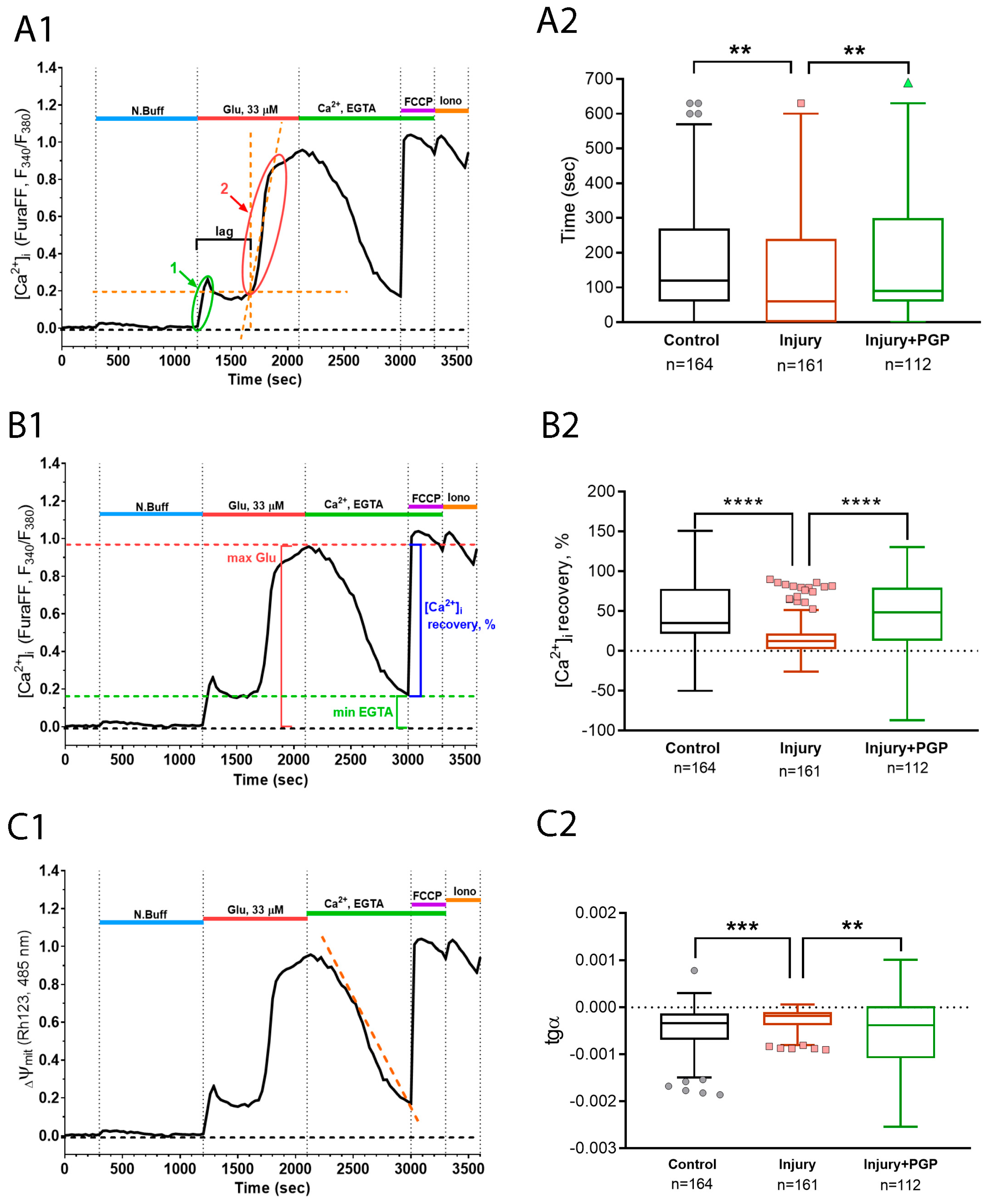
2.4. The Effect of Mechanical Injury on the Delayed Changes of [Ca2+]i and ΔΨm
3. Discussion
4. Materials and Methods
4.1. Primary Rat Cortical Cultures
4.2. Scratch Test and Migration Rate
4.3. Evaluation of Neuronal Viability
4.4. Immunofluorescence Staining of Neuroglial Culture on Markers GFAP and Beta-3 Tubulin
4.5. Determination of Neuromarkers BDNF and NSE
4.6. Application of Mechanical Injury during Calcium Imaging
4.7. Measurements of Changes of Cytosolic Free Ca2+ Concentration ([Ca2+]i) and Mitochondrial Potential (ΔΨm)
4.8. Reagents
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andriessen, T.M.J.C.; Jacobs, B.; Vos, P.E. Clinical Characteristics and Pathophysiological Mechanisms of Focal and Diffuse Traumatic Brain Injury. J. Cell. Mol. Med. 2010, 14, 2381–2392. [Google Scholar] [CrossRef] [PubMed]
- Thapa, K.; Khan, H.; Singh, T.G.; Kaur, A. Traumatic Brain Injury: Mechanistic Insight on Pathophysiology and Potential Therapeutic Targets. J. Mol. Neurosci. 2021, 71, 1725–1742. [Google Scholar] [CrossRef]
- Hiebert, J.B.; Shen, Q.; Thimmesch, A.R.; Pierce, J.D. Traumatic Brain Injury and Mitochondrial Dysfunction. Am. J. Med. Sci. 2015, 350, 132–138. [Google Scholar] [CrossRef]
- Khatri, N.; Sumadhura, B.; Kumar, S.; Kaundal, R.K.; Sharma, S.; Datusalia, A.K. The Complexity of Secondary Cascade Consequent to Traumatic Brain Injury: Pathobiology and Potential Treatments. Curr. Neuropharmacol. 2021, 19, 1984. [Google Scholar] [CrossRef]
- Werner, C.; Engelhard, K. Pathophysiology of Traumatic Brain Injury. Br. J. Anaesth. 2007, 99, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Bakaeva, Z.; Goncharov, M.; Krasilnikova, I.; Zgodova, A.; Frolov, D.; Grebenik, E.; Timashev, P.; Pinelis, V.; Surin, A. Acute and Delayed Effects of Mechanical Injury on Calcium Homeostasis and Mitochondrial Potential of Primary Neuroglial Cell Culture: Potential Causal Contributions to Post-Traumatic Syndrome. Int. J. Mol. Sci. 2022, 23, 3858. [Google Scholar] [CrossRef]
- Hinzman, J.M.; DiNapoli, V.A.; Mahoney, E.J.; Gerhardt, G.A.; Hartings, J.A. Spreading Depolarizations Mediate Excitotoxicity in the Development of Acute Cortical Lesions. Exp. Neurol. 2015, 267, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Hartings, J.A.; Shuttleworth, C.W.; Kirov, S.A.; Ayata, C.; Hinzman, J.M.; Foreman, B.; Andrew, R.D.; Boutelle, M.G.; Brennan, K.; Carlson, A.P.; et al. The Continuum of Spreading Depolarizations in Acute Cortical Lesion Development: Examining Leão’s Legacy. J. Cereb. Blood Flow Metab. 2017, 37, 1571–1594. [Google Scholar] [CrossRef]
- Ladak, A.A.; Enam, S.A.; Ibrahim, M.T. A Review of the Molecular Mechanisms of Traumatic Brain Injury. World Neurosurg. 2019, 131, 126–132. [Google Scholar] [CrossRef]
- Arundine, M.; Tymianski, M. Molecular Mechanisms of Glutamate-Dependent Neurodegeneration in Ischemia and Traumatic Brain Injury. Cell. Mol. Life Sci. 2004, 61, 657–668. [Google Scholar] [CrossRef]
- Guerriero, R.M.; Giza, C.C.; Rotenberg, A. Glutamate and GABA Imbalance Following Traumatic Brain Injury. Curr. Neurol. Neurosci. Rep. 2015, 15, 27. [Google Scholar] [CrossRef] [PubMed]
- Bullock, R.; Zauner, A.; Woodward, J.J.; Myseros, J.; Choi, S.C.; Ward, J.D.; Marmarou, A.; Young, H.F. Factors Affecting Excitatory Amino Acid Release Following Severe Human Head Injury. J. Neurosurg. 1998, 89, 507–518. [Google Scholar] [CrossRef]
- Brittain, M.K.; Brustovetsky, T.; Sheets, P.L.; Brittain, J.M.; Khanna, R.; Cummins, T.R.; Brustovetsky, N. Delayed Calcium Dysregulation in Neurons Requires Both the NMDA Receptor and the Reverse Na+/Ca2+ Exchanger. Neurobiol. Dis. 2012, 46, 109–117. [Google Scholar] [CrossRef]
- Weber, J.T. Altered Calcium Signaling Following Traumatic Brain Injury. Front. Pharmacol. 2012, 3, 60. [Google Scholar] [CrossRef]
- Berridge, M.J.; Bootman, M.D.; Roderick, H.L. Calcium Signalling: Dynamics, Homeostasis and Remodelling. Nat. Rev. Mol. Cell Biol. 2003, 4, 517–529. [Google Scholar] [CrossRef]
- Carafoli, E.; Santella, L.; Branca, D.; Brini, M. Generation, Control, and Processing of Cellular Calcium Signals. Crit. Rev. Biochem. Mol. Biol. 2001, 36, 107–260. [Google Scholar] [CrossRef]
- Brustovetsky, T.; Bolshakov, A.; Brustovetsky, N. Calpain Activation and Na+/Ca2+ Exchanger Degradation Occur Downstream of Calcium Deregulation in Hippocampal Neurons Exposed to Excitotoxic Glutamate. J. Neurosci. Res. 2010, 88, 1317–1328. [Google Scholar] [CrossRef]
- Bernardi, P. The Mitochondrial Permeability Transition Pore: A Mystery Solved? Front. Physiol. 2013, 4, 95. [Google Scholar] [CrossRef]
- Vergun, O.; Keelan, J.; Khodorov, B.I.; Duchen, M.R. Glutamate-Induced Mitochondrial Depolarisation and Perturbation of Calcium Homeostasis in Cultured Rat Hippocampal Neurones. J. Physiol. 1999, 519, 451–466. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.; Lizama, B.N.; Chu, C.T. Excitotoxicity, Calcium and Mitochondria: A Triad in Synaptic Neurodegeneration. Transl. Neurodegener. 2022, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Fujikawa, D.G. The Role of Excitotoxic Programmed Necrosis in Acute Brain Injury. Comput. Struct. Biotechnol. J. 2015, 13, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Pearl Chung, F.K. Traumatic Brain Injury (TBI): Overview of Diagnosis and Treatment. J. Neurol. Neurophysiol. 2013, 5, 182–192. [Google Scholar] [CrossRef]
- Jarrahi, A.; Braun, M.; Ahluwalia, M.; Gupta, R.V.; Wilson, M.; Munie, S.; Ahluwalia, P.; Vender, J.R.; Vale, F.L.; Dhandapani, K.M.; et al. Revisiting Traumatic Brain Injury: From Molecular Mechanisms to Therapeutic Interventions. Biomedicines. 2020, 8, 389. [Google Scholar] [CrossRef]
- O’Reilly, P.J.; Hardison, M.T.; Jackson, P.L.; Xu, X.; Snelgrove, R.J.; Gaggar, A.; Galin, F.S.; Blalock, J.E. Neutrophils Contain Prolyl Endopeptidase and Generate the Chemotactic Peptide, PGP, from Collagen. J. Neuroimmunol. 2009, 217, 51–54. [Google Scholar] [CrossRef]
- Natunen, T.A.; Gynther, M.; Rostalski, H.; Jaako, K.; Jalkanen, A.J. Extracellular Prolyl Oligopeptidase Derived from Activated Microglia Is a Potential Neuroprotection Target. Basic Clin. Pharmacol. Toxicol. 2019, 124, 40–49. [Google Scholar] [CrossRef]
- Andreeva, L.A.; Myasoedov, N.F.; Lyapina, L.A.; Grigor’eva, M.E.; Obergan, T.Y.; Shubina, T.A. Effect of the PRO-GLY-PRO Peptide on Hemostasis and Lipid Metabolism in Rats with Hypercholesterolemia. Dokl. Biol. Sci. 2013, 453, 333. [Google Scholar] [CrossRef] [PubMed]
- Medvedeva, E.V.; Dmitrieva, V.G.; Povarova, O.V.; Limborska, S.A.; Skvortsova, V.I.; Myasoedov, N.F.; Dergunova, L.V. Effect of Semax and Its C-Terminal Fragment Pro-Gly-Pro on the Expression of VEGF Family Genes and Their Receptors in Experimental Focal Ischemia of the Rat Brain. J. Mol. Neurosci. 2013, 49, 328–333. [Google Scholar] [CrossRef]
- Stavchansky, V.V.; Kurichenkova, E.O.; Dmitrieva, V.G.; Myasoedov, N.F.; Limborska, S.A.; Dergunova, L.V. Cplx2 Gene Expression Following Semax or PGP Administration under Conditions of Two Experimental Models of Rat-Brain Ischemia. Mol. Genet. Microbiol. Virol. 2016, 31, 214–219. [Google Scholar] [CrossRef]
- V’unova, T.V.; Andreeva, L.A.; Shevchenko, K.V.; Shevchenko, V.P.; Bobrov, M.Y.; Bezuglov, V.V.; Myasoedov, N.F. Binding of Tripeptide Pro-Gly-Pro Labeled at the C-Terminal Proline Residue to Plasma Membranes of the Rat Forebrain. Dokl. Biol. Sci. 2008, 419, 95. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Wang, Q.; Wu, S.; Yang, L.; Ren, P.; Yang, Y.; Gao, J.; Li, L. Neonatal Hypoxic Ischemic Encephalopathy-Related Biomarkers in Serum and Cerebrospinal Fluid. Clin. Chim. Acta 2015, 450, 282–297. [Google Scholar] [CrossRef]
- Thelin, E.P.; Zeiler, F.A.; Ercole, A.; Mondello, S.; Büki, A.; Bellander, B.M.; Helmy, A.; Menon, D.K.; Nelson, D.W. Serial Sampling of Serum Protein Biomarkers for Monitoring Human Traumatic Brain Injury Dynamics: A Systematic Review. Front. Neurol. 2017, 8, 300. [Google Scholar] [CrossRef] [PubMed]
- Obrenovitch, T.P.; Urenjak, J. Is High Extracellular Glutamate the Key to Excitotoxicity in Traumatic Brain Injury? J. Neurotrauma 1997, 14, 677–698. [Google Scholar] [CrossRef]
- Bienkowski, R.S.; Baum, B.J.; Crystal, R.G. Fibroblasts degrade newly synthesised collagen within the cell before secretion. Nature 1978, 276, 413–416. [Google Scholar] [CrossRef]
- Ashmarin, I.P.; Karazeeva, E.P.; Lyapina, L.A.; Samonina, G.E. The simplest proline-containing peptides PG, GP, PGP, and GPGG: Regulatory activity and possible sources of biosynthesis. Biochem. Biokhimiia 1998, 63, 119–124. [Google Scholar]
- Shibata, A.; Morioka, I.; Ashi, C.; Nagasaki, S.; Tode, C.; Morikawa, S.; Miwa, A.; Enomoto, M.; Saiki, K.; Yokoyama, N.; et al. Identification of N-acetyl Proline–Glycine–Proline (acPGP) in human serum of adults and newborns by liquid chromatography–tandem mass spectrometry. Clin. Chim. Acta 2009, 402, 124–128. [Google Scholar] [CrossRef]
- Ashmarin, I.P.; Baglikova, K.E.; Edeeva, S.E.; IuA, Z.; Kozik, V.S.; Dadaian, A.K.; Dorokhova, E.M.; LIu, A.; Andreeva, L.A.; Kopylova, G.N.; et al. A comparative analysis of distribution of glyprolines administered by various routes. Bioorganicheskaia Khimiia 2008, 34, 464–470. [Google Scholar] [PubMed]
- Berger, R.P.; Pierce, M.C.; Wisniewski, S.R.; Adelson, P.D.; Clark, R.S.; Ruppel, R.A.; Kochanek, P.M. Neuron-specific enolase and S100B in cerebrospinal fluid after severe traumatic brain injury in infants and children. Pediatrics 2002, 109, e31. [Google Scholar] [CrossRef]
- Gustafsson, D.; Klang, A.; Thams, S.; Rostami, E. The Role of Bdnf in Experimental and Clinical Traumatic Brain Injury. Int. J. Mol. Sci. 2021, 22, 3582. [Google Scholar] [CrossRef]
- Werner, J.K.; Stevens, R.D. Traumatic Brain Injury: Recent Advances in Plasticity and Regeneration. Curr. Opin. Neurol. 2015, 28, 565–573. [Google Scholar] [CrossRef]
- Wang, C.S.; Kavalali, E.T.; Monteggia, L.M. BDNF Signaling in Context: From Synaptic Regulation to Psychiatric Disorders. Cell. 2022, 185, 62–76. [Google Scholar] [CrossRef]
- Failla, M.D.; Conley, Y.P.; Wagner, A.K. Brain-Derived Neurotrophic Factor (BDNF) in Traumatic Brain Injury–Related Mortality: Interrelationships between Genetics and Acute Systemic and Central Nervous System BDNF Profiles. Neurorehabil. Neural Repair 2016, 30, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Alcañiz, J.A.; Haege, S.; Mueller, W.; Pla, R.; Mackay, F.; Schulz, S.; López-Bendito, G.; Stumm, R.; Marín, O. Cxcr7 Controls Neuronal Migration by Regulating Chemokine Responsiveness. Neuron 2011, 69, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.E.S.; Goodkey, K.; Footz, T.; Voronova, A. Regulation of CNS Precursor Function by Neuronal Chemokines. Neurosci. Lett. 2020, 715, 134533. [Google Scholar] [CrossRef]
- Haddox, J.L.; Pfister, R.R.; Muccio, D.D.; Villain, M.; Sommers, C.I.; Chaddha, M.; Anantharamaiah, G.M.; Brouillette, W.J.; DeLucas, L.J. Bioactivity of peptide analogs of the neutrophil chemoattractant, N-acetyl-proline-glycine-proline. Investig. Ophthalmol. Vis. Sci. 1999, 40, 2427–2429. [Google Scholar]
- Patel, D.F.; Snelgrove, R.J. The multifaceted roles of the matrikine Pro-Gly-Pro in pulmonary health and disease. Eur. Respir. Rev. 2018, 27, 180017. [Google Scholar] [CrossRef]
- Weathington, N.M.; Van Houwelingen, A.H.; Noerager, B.D.; Jackson, P.L.; Kraneveld, A.D.; Galin, F.S.; Folkerts, G.; Nijkamp, F.P.; Blalock, J.E. A novel peptide CXCR ligand derived from extracellular matrix degradation during airway inflammation. Nat. Med. 2006, 12, 317–323. [Google Scholar] [CrossRef]
- Lacy, M.; Jones, J.; Whittemore, S.R.; Haviland, D.L.; Wetsel, R.A.; Barnum, S.R. Expression of the receptors for the C5a anaphylatoxin, interleukin-8 and FMLP by human astrocytes and microglia. J. Neuroimmunol. 1995, 61, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Müller-Ladner, U.; Jones, J.L.; Wetsel, R.A.; Gay, S.; Raine, C.S.; Barnum, S.R. Enhanced expression of chemotactic receptors in multiple sclerosis lesions. J. Neurol. Sci. 1996, 144, 135–141. [Google Scholar] [CrossRef]
- Horuk, R.; Martin, A.W.; Wang, Z.X.; Schweitzer, L.; Gerassimides, A.; Guo, H.; Lu, Z.H.; Hesselgesser, J.; Perez, H.D.; Kim, J. Expression of chemokine receptors by subsets of neurons in the central nervous system. J. Immunol. 1997, 158, 2882–2890. [Google Scholar] [CrossRef]
- Clark, D.P.Q.; Perreau, V.M.; Shultz, S.R.; Brady, R.D.; Lei, E.; Dixit, S.; Taylor, J.M.; Beart, P.M.; Boon, W.C. Inflammation in Traumatic Brain Injury: Roles for Toxic A1 Astrocytes and Microglial–Astrocytic Crosstalk. Neurochem. Res. 2019, 44, 1410–1424. [Google Scholar] [CrossRef]
- Michinaga, S.; Koyama, Y. Pathophysiological Responses and Roles of Astrocytes in Traumatic Brain Injury. Int. J. Mol. Sci. 2021, 22, 6418. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.S.; Lefkowitz, R.J.; Hausdorff, W.P. Beta-Adrenergic Receptor Sequestration. A Potential Mechanism of Receptor Resensitization. J. Biol. Chem. 1993, 268, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Samonina, G.; Lyapina, L.; Kopylova, G.; Pastorova, V.; Bakaeva, Z.; Jeliaznik, N.; Zuykova, S.; Ashmarin, I. Protection of Gastric Mucosal Integrity by Gelatin and Simple Proline-Containing Peptides. Pathophysiology 2000, 7, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Bakaeva, Z.V.; Sangadzhieva, A.D.; Tani, S.; Myasoedov, N.F.; Andreeva, L.A.; Torshin, V.I.; Wallace, J.L.; Tanaka, T. Glyprolines Exert Protective and Repair-Promoting Effects in the Rat Stomach: Potential Role of the Cytokine GRO/CINC-1. J. Physiol. Pharmacol. 2016, 67, 253–260. [Google Scholar]
- Bakaeva, Z.V.; Ermakova, N.V.; Mankaeva, O.V.; Sveshnikov, D.S.; Severin, A.E.; Sinel’nikova, A.N.; Starshinov, Y.P.; Radysh, I.V.; Torshin, V.I.; Frolov, D.A.; et al. Collagen Hydrolysis Products Reduce the Formation of Stress-Induced Ulcers by Regulating Stress-Associated Activation of the Neuroendocrine and Immune Systems. Bull. Exp. Biol. Med. 2018, 165, 449–452. [Google Scholar] [CrossRef] [PubMed]
- Bondarenko, N.S.; Yusipovich, A.I.; Kovalenko, S.S.; Kopylova, G.N.; Umarova, B.A.; Graevskaya, E.E.; Maksimov, G.V. Application of Laser Interference Microscopy (LIM) for Investigation of the Protective Effect of Prolyl-Glycyl-Proline (Pro-Gly-Pro) on Mast Cells. Biochem. Suppl. Ser. A Membr. Cell Biol. 2013, 7, 222–227. [Google Scholar] [CrossRef]
- Storozhevykh, T.P.; Tukhbatova, G.R.; Senilova, Y.E.; Pinelis, V.G.; Andreeva, L.A.; Myasoyedov, N.F. Effects of Semax and Its Pro-Gly-Pro Fragment on Calcium Homeostasis of Neurons and Their Survival under Conditions of Glutamate Toxicity. Bull. Exp. Biol. Med. 2007, 143, 601–604. [Google Scholar] [CrossRef]
- Bakaeva, Z.V.; Surin, A.M.; Lizunova, N.V.; Zgodova, A.E.; Krasilnikova, I.A.; Fisenko, A.P.; Frolov, D.A.; Andreeva, L.A.; Myasoedov, N.F.; Pinelis, V.G. Neuroprotective Potential of Peptides HFRWPGP (ACTH6–9PGP), KKRRPGP, and PyrRP in Cultured Cortical Neurons at Glutamate Excitotoxicity. Dokl. Biochem. Biophys. 2020, 491, 62–66. [Google Scholar] [CrossRef]
- Tolosa, L.; Mir, M.; Asensio, V.J.; Olmos, G.; Lladó, J. Vascular Endothelial Growth Factor Protects Spinal Cord Motoneurons against Glutamate-Induced Excitotoxicity via Phosphatidylinositol 3-Kinase. J. Neurochem. 2008, 105, 1080–1090. [Google Scholar] [CrossRef]
- Kroemer, G.; Galluzzi, L.; Brenner, C. Mitochondrial Membrane Permeabilization in Cell Death. Physiol. Rev. 2007, 87, 99–163. [Google Scholar] [CrossRef]
- Zhong, L.T.; Kane, D.J.; Bredesen, D.E. BCL-2 Blocks Glutamate Toxicity in Neural Cell Lines. Mol. Brain Res. 1993, 19, 353–355. [Google Scholar] [CrossRef] [PubMed]
- Martynova, K.V.; Andreeva, L.A.; Klimova, P.A.; Kirillova, I.G.; Shevchenko, V.P.; Nagaev, I.I.; Shram, I.I.; Shvets, V.I.; Miasoedov, N.F. Structural-functional study of glycine-and-proline-containing peptides (glyprolines) as potential neuroprotectors. Bioorg. Khim. 2009, 35, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Kovács, Z.; Ikezaki, K.; Samoto, K.; Inamura, T.; Fukui, M. VEGF and Flt: Expression Time Kinetics in Rat Brain Infarct. Stroke 1996, 27, 1865–1873. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakaeva, Z.; Goncharov, M.; Frolov, F.; Krasilnikova, I.; Sorokina, E.; Zgodova, A.; Smolyarchuk, E.; Zavadskiy, S.; Andreeva, L.; Myasoedov, N.; et al. Regulatory Peptide Pro-Gly-Pro Accelerates Neuroregeneration of Primary Neuroglial Culture after Mechanical Injury in Scratch Test. Int. J. Mol. Sci. 2024, 25, 10886. https://doi.org/10.3390/ijms252010886
Bakaeva Z, Goncharov M, Frolov F, Krasilnikova I, Sorokina E, Zgodova A, Smolyarchuk E, Zavadskiy S, Andreeva L, Myasoedov N, et al. Regulatory Peptide Pro-Gly-Pro Accelerates Neuroregeneration of Primary Neuroglial Culture after Mechanical Injury in Scratch Test. International Journal of Molecular Sciences. 2024; 25(20):10886. https://doi.org/10.3390/ijms252010886
Chicago/Turabian StyleBakaeva, Zanda, Mikhail Goncharov, Fyodor Frolov, Irina Krasilnikova, Elena Sorokina, Arina Zgodova, Elena Smolyarchuk, Sergey Zavadskiy, Liudmila Andreeva, Nikolai Myasoedov, and et al. 2024. "Regulatory Peptide Pro-Gly-Pro Accelerates Neuroregeneration of Primary Neuroglial Culture after Mechanical Injury in Scratch Test" International Journal of Molecular Sciences 25, no. 20: 10886. https://doi.org/10.3390/ijms252010886
APA StyleBakaeva, Z., Goncharov, M., Frolov, F., Krasilnikova, I., Sorokina, E., Zgodova, A., Smolyarchuk, E., Zavadskiy, S., Andreeva, L., Myasoedov, N., Fisenko, A., & Savostyanov, K. (2024). Regulatory Peptide Pro-Gly-Pro Accelerates Neuroregeneration of Primary Neuroglial Culture after Mechanical Injury in Scratch Test. International Journal of Molecular Sciences, 25(20), 10886. https://doi.org/10.3390/ijms252010886






