The Protective Effect of the Supplementation with an Extract from Aronia melanocarpa L. Berries against Cadmium-Induced Changes of Chosen Biomarkers of Neurotoxicity in the Brain—A Study in a Rat Model of Current Lifetime Human Exposure to This Toxic Heavy Metal
Abstract
:1. Introduction
2. Results
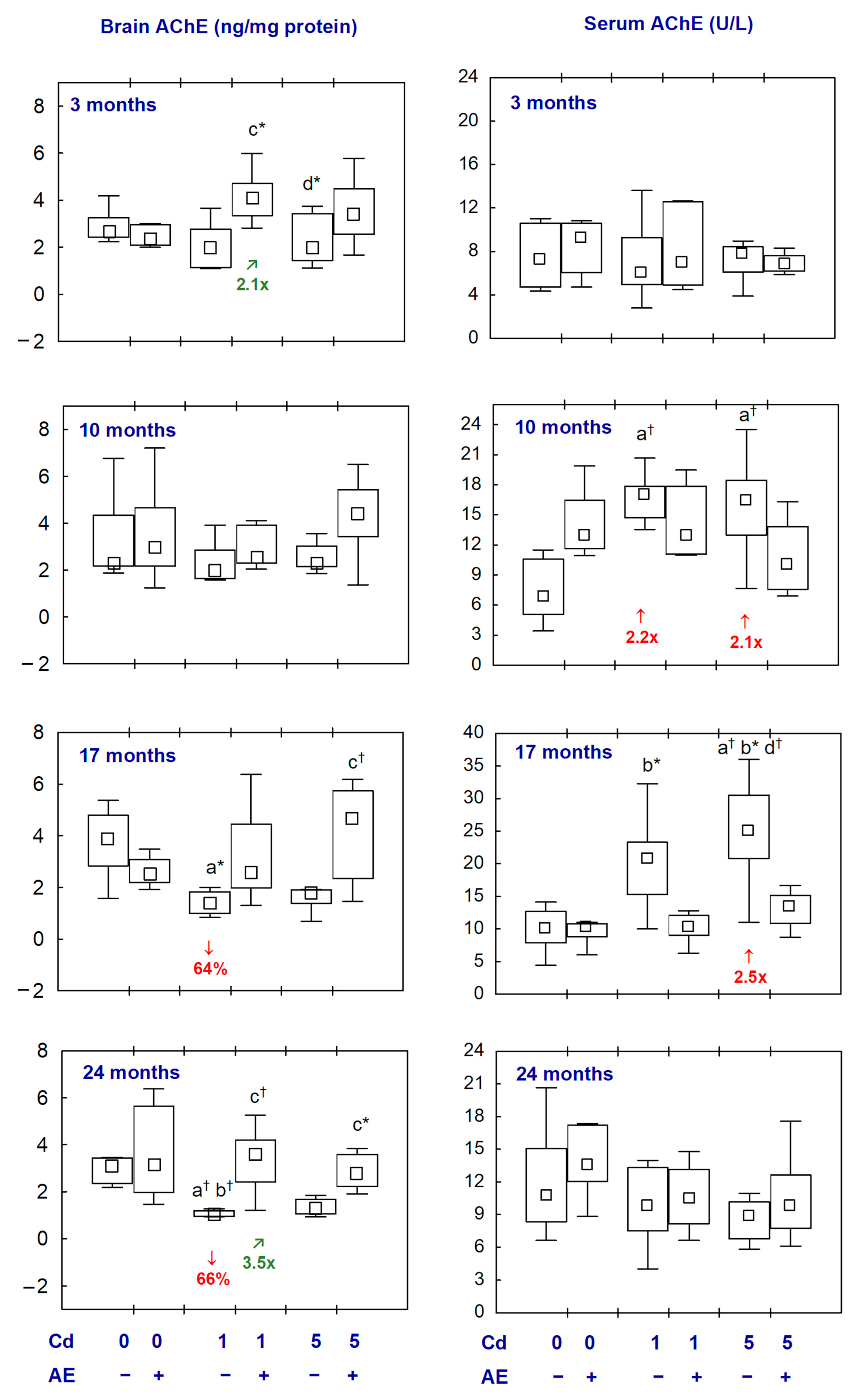
2.1. AChE in the Aliquots of the Brain Homogenates and the Serum
2.2. Na+/K+-ATPase and Ca2+/Mg2+-ATPase in the Aliquots of the Brain Homogenates, as Well as Ca and Mg in the Brain
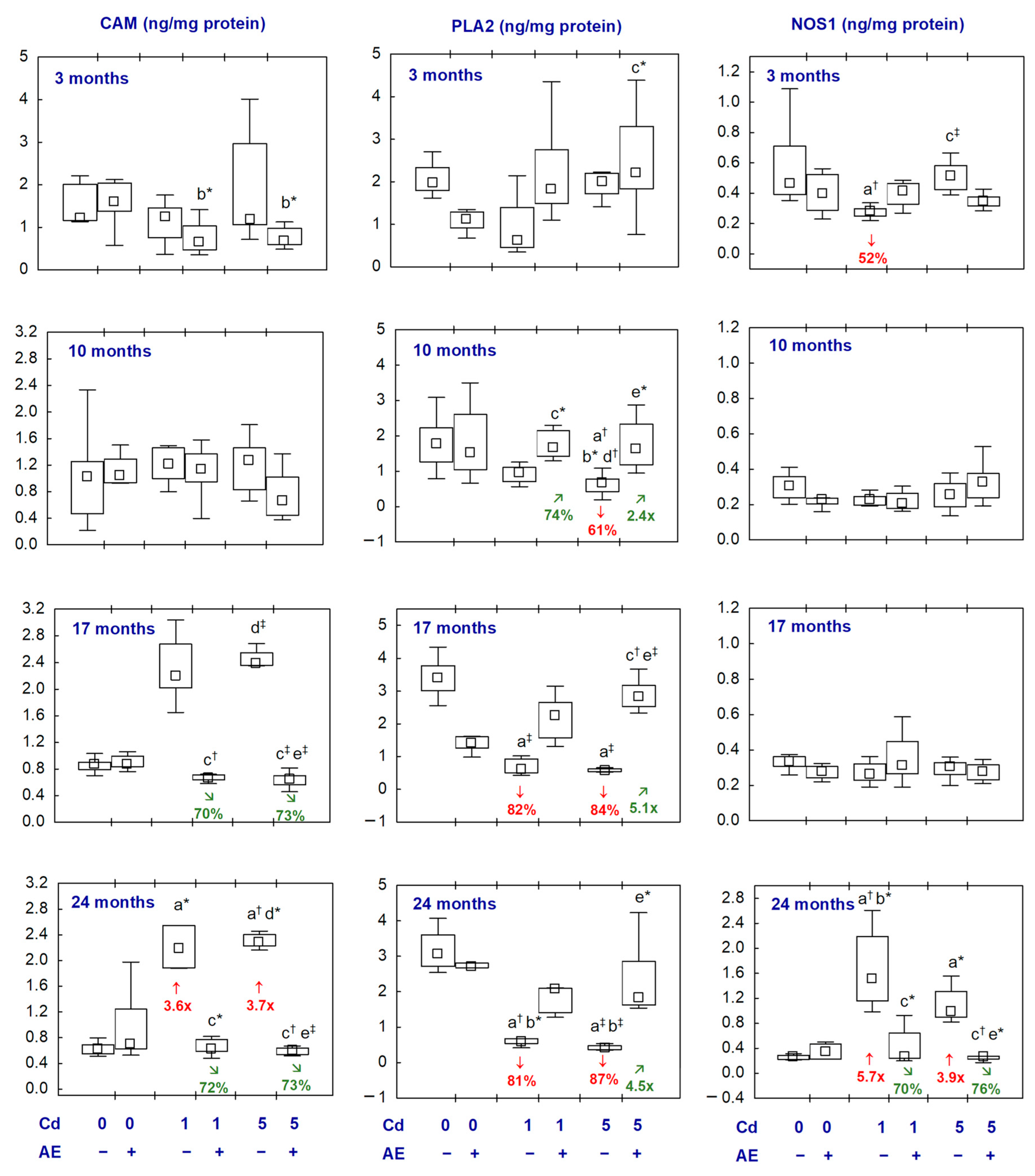
2.3. CAM, PLA2, and NOS1 in the Aliquots of the Brain Homogenates
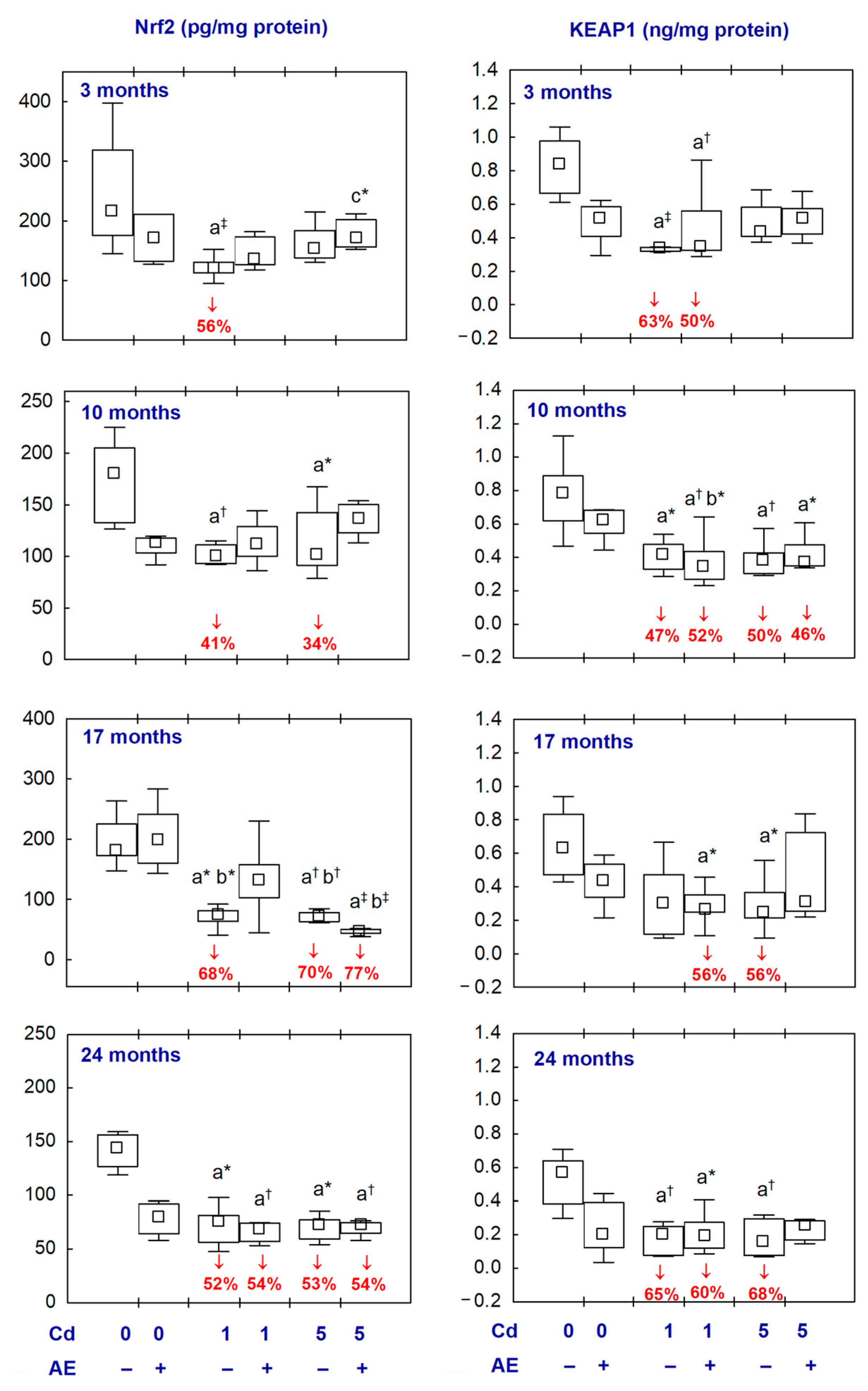
2.4. Nrf2 and KEAP1 in the Aliquots of the Brain Homogenates
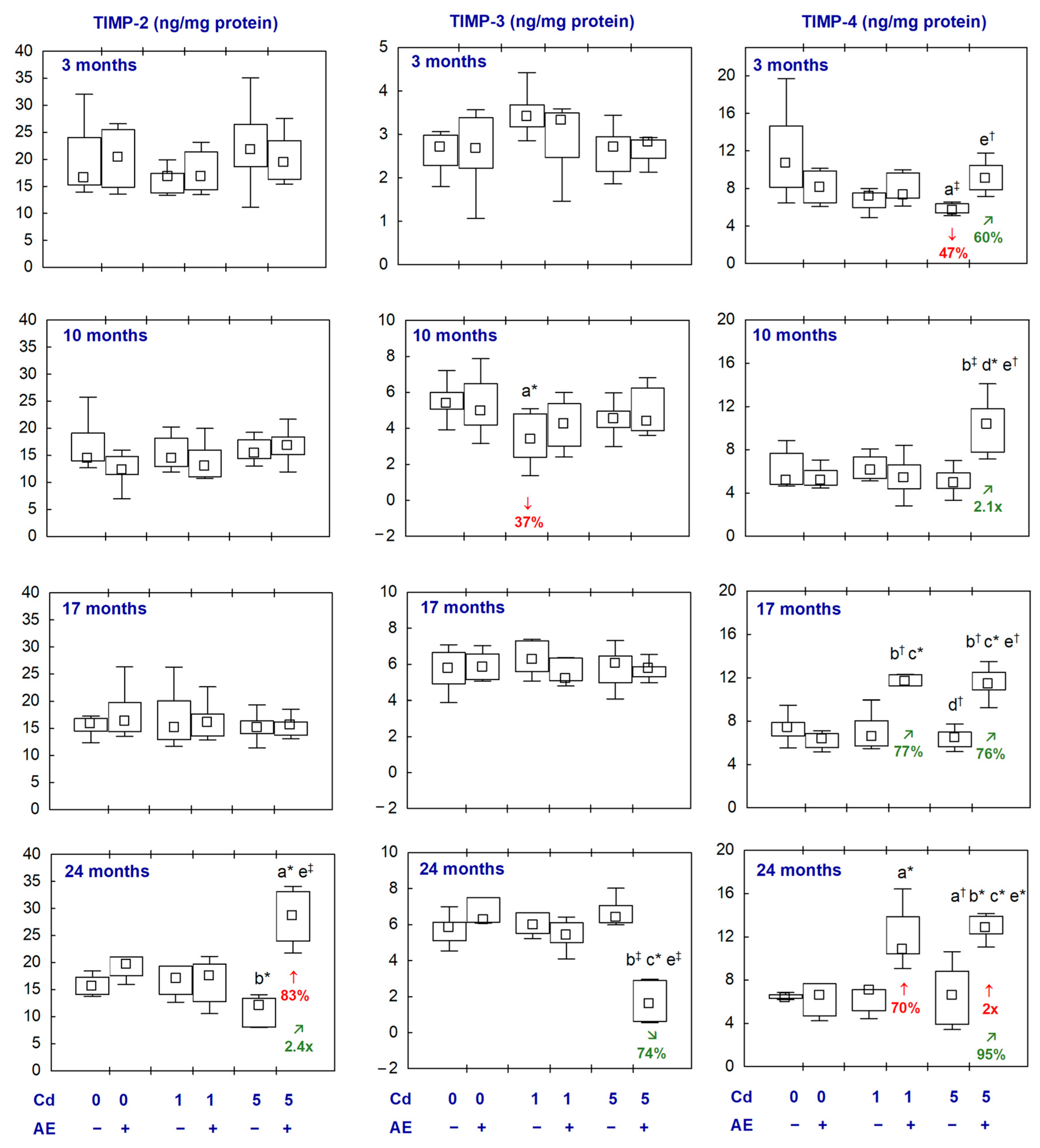
2.5. MMPs and TIMPs in the Aliquots of the Brain Homogenates
2.6. Relationships between the Determined Markers of Neurotoxicity and Cd Concentration in the Brain, Blood, and Urine
2.7. Relationships between the Determined Markers of Neurotoxicity and TOS, TAS, and OSI in the Aliquots of the Brain Homogenates
2.8. Relationships between the Determined Markers of Neurotoxicity and Indices of Oxidative Modifications of Proteins, Lipids, and DNA in the Aliquots of the Brain Homogenates
2.9. Mutual Relationships between the Determined Markers of Neurotoxicity
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cd Diet and the Extract from A. melanocarpa L. Berries
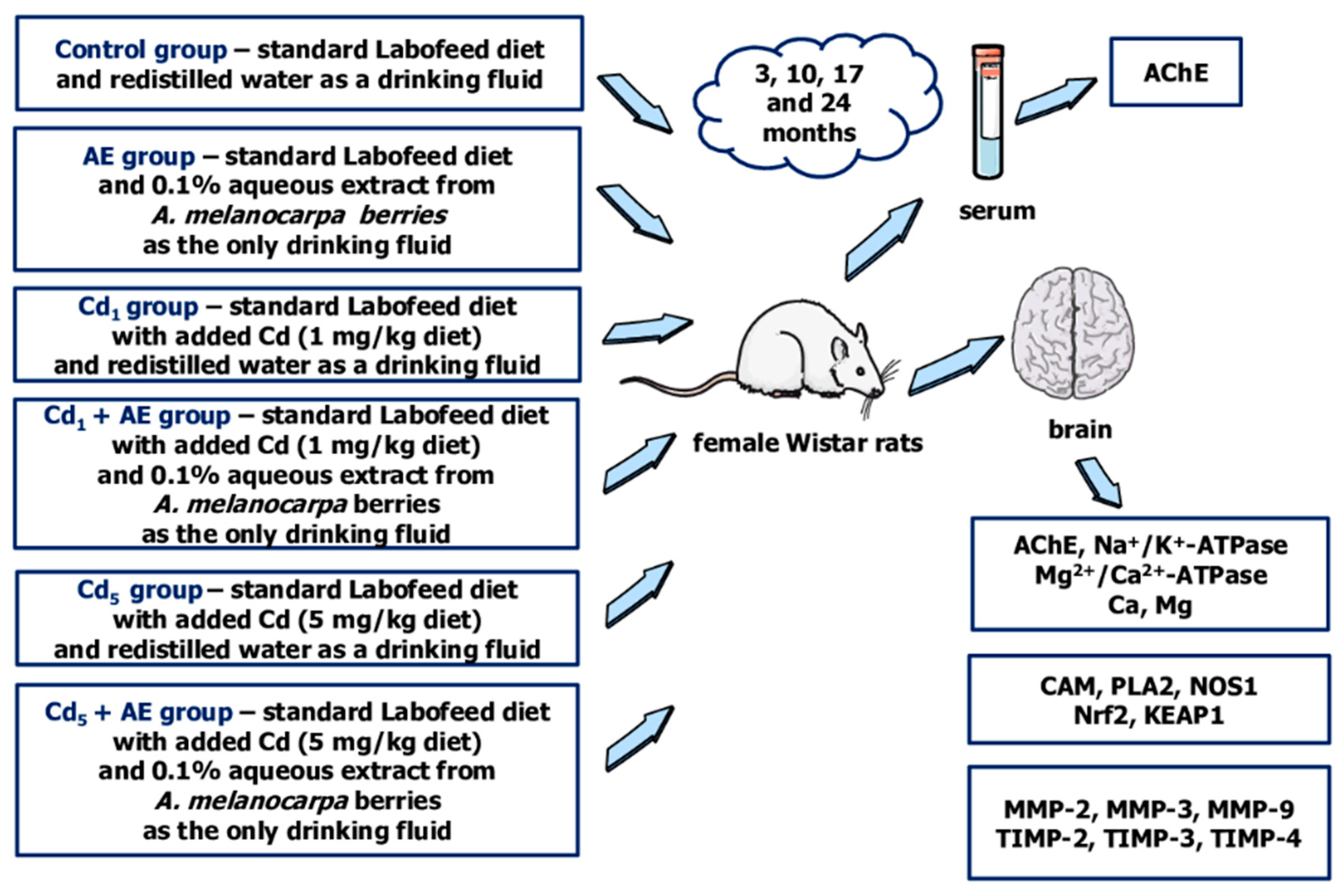
4.3. Animals and Design of the Study
4.4. Determination of Biomarkers of Neurotoxicity in the Brain
4.5. Determination of Ca and Mg in the Brain
4.6. Determination of AChE in the Serum
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Franco, G.A.; Interdonato, L.; Cordaro, M.; Cuzzocrea, S.; Di Paola, R. Bioactive compounds of the Mediterranean diet as nutritional support to fight neurodegenerative disease. Int. J. Mol. Sci. 2023, 24, 7318. [Google Scholar] [CrossRef] [PubMed]
- Grabska-Kobyłecka, I.; Szpakowski, P.; Król, A.; Książek-Winiarek, D.; Kobyłecki, A.; Głęabiński, A.; Nowak, D. Polyphenols and their impact on the prevention of neurodegenerative diseases and development. Nutrients 2023, 15, 3454. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shlomo, Y.; Darweesh, S.; Llibre-Guerra, J.; Marras, C.; San Luciano, M.; Tanner, C. The epidemiology of Parkinson’s disease. Lancet 2024, 403, 283–292. [Google Scholar] [CrossRef]
- Huat, T.J.; Camats-Perna, J.; Newcombe, E.A.; Valmas, N.; Kitazawa, M.; Medeiros, R. Metal toxicity links to Alzheimer’s disease and neuroinflammation. J. Mol. Biol. 2019, 431, 1843–1868. [Google Scholar] [CrossRef]
- Gasull, M.; Camargo, J.; Pumarega, J.; Henríquez-Hernández, L.A.; Campi, L.; Zumbado, M.; Contreras-Llanes, M.; Oliveras, L.; González-Marín, P.; Luzardo, O.P.; et al. Blood concentrations of metals, essential trace elements, rare earth elements and other chemicals in the general adult population of Barcelona: Distribution and associated sociodemographic factors. Sci. Total Environ. 2024, 909, 168502. [Google Scholar] [CrossRef] [PubMed]
- Rooney, J.P.K.; Michalke, B.; Geoghegan, G.; Heverin, M.; Bose-O’Reilly, S.; Hardiman, O.; Rakete, S. Urine concentrations of selected trace metals in a cohort of Irish adults. Environ. Sci. Pollut. Res. Int. 2022, 29, 75356–75364. [Google Scholar] [CrossRef]
- Satarug, S.; Vesey, D.A.; Gobe, G.C.; Phelps, K.R. Estimation of health risks associated with dietary cadmium exposure. Arch. Toxicol. 2023, 97, 329–358. [Google Scholar] [CrossRef]
- Sasaki, N.; Carpenter, D.O. Associations between metal exposures and cognitive function in American older adults. Int. J. Environ. Res. Public Health 2022, 19, 2327. [Google Scholar] [CrossRef]
- Ruczaj, A.; Brzóska, M.M. Environmental exposure of the general population to cadmium as a risk factor of the damage to the nervous system: A critical review of current data. J. Appl. Toxicol. 2023, 43, 66–88. [Google Scholar] [CrossRef]
- Abdel-Aleem, G.A.; Khaleel, E.F. Rutin hydrate ameliorates cadmium chloride-induced spatial memory loss and neural apoptosis in rats by enhancing levels of acetylcholine, inhibiting JNK and ERK1/2 activation and activating mTOR signalling. Arch. Physiol. Biochem. 2018, 124, 367–377. [Google Scholar] [CrossRef]
- Adefegha, S.A.; Oboh, G.; Omojokun, O.S.; Adefegha, O.M. Alterations of Na+/K+-ATPase, cholinergic and antioxidant enzymes activity by protocatechuic acid in cadmium-induced neurotoxicity and oxidative stress in Wistar rats. Biomed. Pharmacother. 2016, 83, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Adefegha, S.A.; Omojokun, O.S.; Oboh, G.; Fasakin, O.; Ogunsuyi, O. Modulatory effects of ferulic acid on cadmium-induced brain damage. J. Evid. Based Complement. Altern. Med. 2016, 21, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Akinyemi, A.J.; Okonkwo, P.K.; Faboya, O.A.; Onikanni, S.A.; Fadaka, A.; Olayide, I.; Akinyemi, E.O.; Oboh, G. Curcumin improves episodic memory in cadmium induced memory impairment through inhibition of acetylcholinesterase and adenosine deaminase activities in a rat model. Metab. Brain Dis. 2017, 32, 87–95. [Google Scholar] [CrossRef]
- Al-Brakati, A.; Albarakati, A.J.A.; Lokman, M.S.; Theyab, A.; Algahtani, M.; Menshawi, S.; AlAmri, O.D.; Al Omairi, N.E.; Essawy, E.A.; Kassab, R.B.; et al. Possible role of kaempferol in reversing oxidative damage, inflammation, and apoptosis-mediated cortical injury following cadmium exposure. Neurotoxicol. Res. 2020, 39, 198–209. [Google Scholar] [CrossRef]
- Ali, T.; Khan, A.; Alam, S.I.; Ahmad, S.; Ikram, M.; Park, J.S.; Lee, H.J.; Kim, M.O. Cadmium, an environmental contaminant, exacerbates Alzheimer’s pathology in the aged mice’s brain. Front. Aging Neurosci. 2021, 13, 650930. [Google Scholar] [CrossRef]
- Bolat, İ.; Yıldırım, S.; Ceylan, N.; Sağlam, Y.S.; Örnek, F.K.; Bolat, M.; Kiliçoğlu, M. Investigation of neurotoxicity oxidative stress and oxidative DNA damage in cadmium induced brain injury in rats. Vet. Sci. Pract. 2023, 18, 19–24. [Google Scholar] [CrossRef]
- El-Sherbiny, E.M.; Abdel-Gawad, E.I.; Osman, H.F. Impact of nano silver composite structure on cadmium neurotoxicity in albino rats. Appl. Biol. Chem. 2022, 65, 70. [Google Scholar] [CrossRef]
- Hao, M.L.; Pan, N.; Zhang, Q.H.; Wang, X.H. Therapeutic efficacy of chlorogenic acid on cadmium-induced oxidative neuropathy in a murine model. Exp. Ther. Med. 2015, 9, 1887–1894. [Google Scholar] [CrossRef]
- Ojo, O.A.; Rotimi, D.E.; Ojo, A.B.; Ogunlakin, A.D.; Ajiboye, B.O. Gallic acid abates cadmium chloride toxicity via alteration of neurotransmitters and modulation of inflammatory markers in Wistar rats. Sci. Rep. 2023, 13, 1577. [Google Scholar] [CrossRef]
- Ruczaj, A.; Brzóska, M.M.; Rogalska, J. The protective impact of Aronia melanocarpa L. berries extract against prooxidative cadmium action in the brain—A study in an in vivo model of current environmental human exposure to this harmful element. Nutrients 2024, 16, 502. [Google Scholar] [CrossRef]
- Sadek, K.M.; Lebda, M.A.; Abouzed, T.K.; Nasr, S.M.; Shoukry, M. Neuro- and nephrotoxicity of subchronic cadmium chloride exposure and the potential chemoprotective effects of selenium nanoparticles. Metab. Brain Dis. 2017, 32, 1659–1673. [Google Scholar] [CrossRef]
- Tang, K.K.; Liu, X.Y.; Wang, Z.Y.; Qu, K.C.; Fan, R.F. Trehalose alleviates cadmium-induced brain damage by ameliorating oxidative stress, autophagy inhibition, and apoptosis. Metallomics 2019, 11, 2043–2051. [Google Scholar] [CrossRef]
- Xue, H.; Cao, H.; Xing, C.; Feng, J.; Zhang, L.; Zhang, C.; Hu, G.; Yang, F. Selenium triggers Nrf2-AMPK crosstalk to alleviate cadmium-induced autophagy in rabbit cerebrum. Toxicology 2021, 459, 152855. [Google Scholar] [CrossRef] [PubMed]
- Yıldız, M.O.; Çelik, H.; Caglayan, C.; Genç, A.; Doğan, T.; Satıcı, E. Neuroprotective effects of carvacrol against cadmium-induced neurotoxicity in rats: Role of oxidative stress, inflammation and apoptosis. Metab. Brain Dis. 2022, 37, 1259–1269. [Google Scholar] [CrossRef]
- Zhang, F.; Xing, S.; Li, Z. Antagonistic effects of lycopene on cadmium-induced hippocampal dysfunctions in autophagy, calcium homeostasis and redox. Oncotarget 2017, 8, 44720–44731. [Google Scholar] [CrossRef]
- Srivastava, A.; Kumari, A.; Jagdale, P.; Ayanur, A.; Pant, A.B.; Khanna, V.K. Potential of quercetin to protect cadmium induced cognitive deficits in rats by modulating NMDA-R mediated downstream signaling and PI3K/AKT-Nrf2/ARE signaling pathways in hippocampus. Neuromol. Med. 2023, 25, 426–440. [Google Scholar] [CrossRef]
- Anwar, M.M. Oxidative stress—A direct bridge to central nervous system homeostatic dysfunction and Alzheimer’s disease. Cell Biochem. Funct. 2022, 40, 17–27. [Google Scholar] [CrossRef]
- Gholamzadeh, R.; Ramezani, F.; Tehrani, P.M.; Aboutaleb, N. Apelin-13 attenuates injury following ischemic stroke by targeting matrix metalloproteinases (MMP), endothelin-B receptor, occludin/claudin-5 and oxidative stress. J. Chem. Neuroanat. 2021, 118, 102015. [Google Scholar] [CrossRef]
- Ataizi, Z.S.; Ertilav, K.; Nazıroğlu, M. Mitochondrial oxidative stress-induced brain and hippocampus apoptosis decrease through modulation of caspase activity, Ca2+ influx and inflammatory cytokine molecular pathways in the docetaxel-treated mice by melatonin and selenium treatments. Metab. Brain Dis. 2019, 34, 1077–1089. [Google Scholar] [CrossRef]
- Baev, A.Y.; Vinokurov, A.Y.; Novikova, I.N.; Dremin, V.V.; Potapova, E.V.; Abramov, A.Y. Interaction of mitochondrial calcium and ROS in neurodegeneration. Cells 2022, 17, 706. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- Zhang, X.; Lee, W.; Bian, J.S. Recent advances in the study of Na+/K+-ATPase in neurodegenerative diseases. Cells 2022, 11, 4075. [Google Scholar] [CrossRef]
- Brzóska, M.M.; Gałażyn-Sidorczuk, M.; Jurczuk, M.; Tomczyk, M. Protective effect of Aronia melanocarpa polyphenols on cadmium accumulation in the body: A study in a rat model of human exposure to this metal. Curr. Drug Targets 2015, 16, 1470–1487. [Google Scholar] [CrossRef] [PubMed]
- Brzóska, M.M.; Rogalska, J.; Gałażyn-Sidorczuk, M.; Jurczuk, M.; Roszczenko, A.; Tomczyk, M. Protective effect of Aronia melanocarpa polyphenols against cadmium-induced disorders in bone metabolism: A study in a rat model of lifetime human exposure to this heavy metal. Chem. Biol. Interact. 2015, 229, 132–146. [Google Scholar] [CrossRef]
- Brzóska, M.M.; Gałażyn-Sidorczuk, M.; Kozłowska, M.; Smereczański, N.M. The body status of manganese and activity of this element-dependent mitochondrial superoxide dismutase in a rat model of human exposure to cadmium and co-administration of Aronia melanocarpa L. extract. Nutrients 2022, 14, 4773. [Google Scholar] [CrossRef]
- Mężyńska, M.; Brzóska, M.M.; Rogalska, J.; Galicka, A. Extract from Aronia melanocarpa L. berries protects against cadmium-induced lipid peroxidation and oxidative damage to proteins and DNA in the liver: A study in a rat model of environmental human exposure to this xenobiotic. Nutrients 2019, 11, 758. [Google Scholar] [CrossRef]
- Smereczański, N.M.; Brzóska, M.M.; Rogalska, J. Protective effect of the extract from Aronia melanocarpa L. berries against cadmium-induced oxidative stress in the kidney: A study in an in vivo experimental model. Acta Pol. Pharm. 2023, 80, 627–648. [Google Scholar]
- Borowska, S.; Brzóska, M.M.; Gałażyn-Sidorczuk, M.; Rogalska, J. Effect of an extract from Aronia melanocarpa L. berries on the body status of zinc and copper under chronic exposure to cadmium: An in vivo experimental study. Nutrients 2017, 9, 1374. [Google Scholar] [CrossRef]
- Sidor, A.; Gramza-Michałowska, A. Black chokeberry Aronia melanocarpa L.—A qualitative composition, phenolic profile and antioxidant potential. Molecules 2019, 24, 3710. [Google Scholar] [CrossRef]
- Ahles, S.; Stevens, Y.R.; Joris, P.J.; Vauzour, D.; Adam, J.; de Groot, E.; Plat, J. The effect of long-term Aronia melanocarpa extract supplementation on cognitive performance, mood, and vascular function: A randomized controlled trial in healthy, middle-aged individuals. Nutrients 2020, 12, 2475. [Google Scholar] [CrossRef]
- Ahles, S.; Joris, P.J.; Plat, J. Short-term Aronia melanocarpa extract supplementation improves cognitive performance: A randomized, double-blind, placebo-controlled cross-over study in healthy young adults. Eur. J. Nutr. 2024, 63, 1545–1553. [Google Scholar] [CrossRef]
- Wen, H.; Cui, H.; Tian, H.; Zhang, X.; Ma, L.; Ramassamy, C.; Li, J. Isolation of neuroprotective anthocyanins from black chokeberry (Aronia melanocarpa) against amyloid-β-induced cognitive impairment. Foods 2020, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- Gawdi, R.; Shumway, K.R.; Emmady, P.D. Physiology, Blood Brain Barrier; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Branca, J.J.V.; Fiorillo, C.; Carrino, D.; Paternostro, F.; Taddei, N.; Gulisano, M.; Pacini, A.; Becatti, M. Cadmium-induced oxidative stress: Focus on the central nervous system. Antioxidants 2020, 9, 492. [Google Scholar] [CrossRef]
- Raghunath, A.; Sundarraj, K.; Nagarajan, R.; Arfuso, F.; Bian, J.; Kumar, A.P.; Sethi, G.; Perumal, E. Antioxidant response elements: Discovery, classes, regulation and potential applications. Redox Biol. 2018, 17, 297–314. [Google Scholar] [CrossRef]
- Tewari, D.; Sah, A.N.; Bawari, S.; Nabavi, S.F.; Dehpour, A.R.; Shirooie, S.; Braidy, N.; Fiebich, B.L.; Vacca, R.A.; Nabavi, S.M. Role of nitric oxide in neurodegeneration: Function, regulation, and inhibition. Curr. Neuropharmacol. 2021, 19, 114–126. [Google Scholar] [CrossRef]
- Chachlaki, K.; Prevot, V. Nitric oxide signalling in the brain and its control of bodily functions. Br. J. Pharmacol. 2020, 177, 5437–5458. [Google Scholar] [CrossRef]
- Sampath, C.; Raju, A.V.; Freeman, M.L.; Srinivasan, S.; Gangula, P.R. Nrf2 attenuates hyperglycemia-induced nNOS impairment in adult mouse primary enteric neuronal crest cells and normalizes stomach function. Am. J. Physiol. Gastrointest. Liver Physiol. 2022, 322, 368–382. [Google Scholar] [CrossRef]
- Khan, M.H.; Parvez, S. Hesperidin ameliorates heavy metal induced toxicity mediated by oxidative stress in brain of Wistar rats. J. Trace Elem. Med. Biol. 2015, 31, 53–60. [Google Scholar] [CrossRef]
- da Costa, P.; Gonçalves, J.F.; Baldissarelli, J.; Mann, T.R.; Abdalla, F.H.; Fiorenza, A.M.; da Rosa, M.M.; Carvalho, F.B.; Gutierres, J.M.; de Andrade, C.M.; et al. Curcumin attenuates memory deficits and the impairment of cholinergic and purinergic signaling in rats chronically exposed to cadmium. Environ. Toxicol. Chem. 2017, 32, 70–83. [Google Scholar] [CrossRef]
- Petrova, E.; Pashkunova-Martic, I.; Schaier, M.; Gluhcheva, Y.; Pavlova, E.; Helbich, T.H.; Keppler, B.; Ivanova, J. Effects of subacute cadmium exposure and subsequent deferiprone treatment on cadmium accumulation and on the homeostasis of essential elements in the mouse brain. J. Trace Elem. Med. Biol. 2022, 74, 127062. [Google Scholar] [CrossRef]
- Shrivastava, A.N.; Triller, A.; Melki, R. Cell biology and dynamics of neuronal Na+/K+-ATPase in health and diseases. Neuropharmacology 2020, 169, 107461. [Google Scholar] [CrossRef]
- Chkadua, G.; Nozadze, E.; Tsakadze, L.; Shioshvili, L.; Arutinova, N.; Leladze, M.; Dzneladze, S.; Javakhishvili, M. Effect of H2O2 on Na,K-ATPase. J. Bioenerg. Biomembr. 2022, 54, 241–249. [Google Scholar] [CrossRef]
- Sivaprakasam, C.; Vijayakumar, R.; Arul, M.; Nachiappan, V. Alteration of mitochondrial phospholipid due to the PLA2 activation in rat brains under cadmium toxicity. Toxicol. Res. 2016, 5, 1680–1687. [Google Scholar] [CrossRef]
- Qin, W.; Li, J.; Zhu, R.; Gao, S.; Fan, J.; Xia, M.; Zhao, R.C.; Zhang, J. Melatonin protects blood-brain barrier integrity and permeability by inhibiting matrix metalloproteinase-9 via the NOTCH3/NF-κB pathway. Aging 2019, 11, 11391–11415. [Google Scholar] [CrossRef]
- Rivera, S. Metalloproteinases in nervous system function and pathology: Introduction. Cell. Mol. Life Sci. 2019, 76, 3051–3053. [Google Scholar] [CrossRef]
- Lopez-Navarro, E.R.; Gutierrez, J. Metalloproteinases and their inhibitors in neurological disease. Naunyn. Schmiedebergs Arch. Pharmacol. 2022, 395, 27–38. [Google Scholar] [CrossRef]
- Fomenko, O.; Shiyntum, H.; Shaulska, O.; Shevtsova, A.; Ushakova, G. Effects of cadmium on the activity of matrix metalloproteinases and metallothionein level in the rat brain. Neurophysiology 2017, 49, 154–157. [Google Scholar] [CrossRef]
- Ren, Y.; Frank, T.; Meyer, G.; Lei, J.; Grebenc, J.R.; Slaughter, R.; Gao, Y.G.; Kinghorn, A.D. Potential benefits of black chokeberry (Aronia melanocarpa) fruits and their constituents in improving human health. Molecules 2022, 27, 7823. [Google Scholar] [CrossRef]
- Alonso-Garrido, M.; Frangiamone, M.; Font, G.; Cimbalo, A.; Manyes, L. In vitro blood brain barrier exposure to mycotoxins and carotenoids pumpkin extract alters mitochondrial gene expression and oxidative stress. Food Chem. Toxicol. 2021, 153, 112261. [Google Scholar] [CrossRef]
- Al-Sowayan, N.S.; Abdullah Almarzougi, R. Vitamin E reduces oxidative stress in brains of male albino male rats undergoing immobilization. Saudi J. Biol. Sci. 2024, 31, 103900. [Google Scholar] [CrossRef]
- Shi, Y.; Han, L.; Zhang, X.; Xie, L.; Pan, P.; Chen, F. Selenium alleviates cerebral ischemia/reperfusion injury by regulating oxidative stress, mitochondrial fusion and ferroptosis. Neurochem. Res. 2022, 47, 2992–3002. [Google Scholar] [CrossRef]
- Fabre, G.; Bayach, I.; Berka, K.; Paloncýová, M.; Starok, M.; Rossi, C.; Duroux, J.L.; Otyepka, M.; Trouillas, P. Synergism of antioxidant action of vitamins E, C and quercetin is related to formation of molecular associations in biomembranes. Chem. Commun. 2015, 51, 7713–7716. [Google Scholar] [CrossRef]
- Zhang, L.; Guan, Q.; Jiang, J.; Khan, M.S. Tannin complexation with metal ions and its implication on human health, environment and industry: An overview. Int. J. Biol. Macromol. 2023, 253, 127485. [Google Scholar] [CrossRef]
- Kumar, G.; Paliwal, P.; Mukherjee, S.; Patnaik, N.; Krishnamurthy, S.; Patnaik, R. Pharmacokinetics and brain penetration study of chlorogenic acid in rats. Xenobiotica 2019, 49, 339–345. [Google Scholar] [CrossRef]
- Toro-Román, V.; Robles-Gil, M.C.; Munoz, D.; Bartolomé, I.; Grijota, F.J.; Maynar-Marino, M. Sex differences in cadmium and lead concentrations in different biological matrices in athletes. Relationship with iron status. Environ. Toxicol. Pharmacol. 2023, 99, 104107. [Google Scholar] [CrossRef]
- Del Bo’, C.; Bernardi, S.; Marino, M.; Porrini, M.; Tucci, M.; Guglielmetti, S.; Cherubini, A.; Carrieri, B.; Kirkup, B.; Kroon, P.; et al. Systematic review on polyphenol intake and health outcomes: Is there sufficient evidence to define a health-promoting polyphenol-rich dietary pattern? Nutrients 2019, 11, 1355. [Google Scholar] [CrossRef]
- Ellman, G.; Courtney, K.; Andres, V.J.; Feather-Stone, R. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]



| Parameter | Cd in the Brain 1 | Cd in the Blood | Cd in the Urine | |||
|---|---|---|---|---|---|---|
| Without AE 2 | With AE 3 | Without AE | With AE | Without AE | With AE | |
| AChE | −0.309 † 4 | 0.279 † | −0.397 ‡ | NS | −0.250 * | NS |
| Na+/K+-ATPase | 0.280 † | NS | NS | NS | NS | NS |
| Ca2+/Mg2+-ATPase | NS | NS | NS | NS | NS | NS |
| Ca | NS | NS | 0.387 ‡ | NS | 0.263 * | NS |
| Mg | 0.260 * | NS | NS | NS | NS | NS |
| CAM | 0.296 ‡ | −0.308 † | 0.472 ‡ | −0.464 ‡ | 0.409 ‡ | −0.469 ‡ |
| PLA2 | NS | NS | NS | NS | NS | NS |
| NOS1 | NS | 0.263 * | NS | NS | NS | NS |
| Nrf2 | −0.432 ‡ | NS | −0.567 ‡ | NS | −0.452 ‡ | NS |
| KEAP1 | −0.501 ‡ | NS | −0.561 ‡ | NS | −0.490 ‡ | NS |
| MMP-2 | NS | NS | NS | 0.264 * | NS | 0.243 * |
| MMP-3 | NS | NS | NS | NS | NS | NS |
| MMP-9 | 0.451 ‡ | −0.228 * | 0.572 ‡ | −0.360 ‡ | 0.601 ‡ | −0.344 ‡ |
| TIMP-2 | NS | NS | NS | NS | NS | NS |
| TIMP-3 | NS | −0.372 ‡ | NS | NS | NS | −0.220 * |
| TIMP-4 | −0.318 † | 0.270 † | −0.246 * | 0.553 ‡ | −0.302 † | 0.468 ‡ |
| Parameter | TOS 1 | TAS | OSI | |||
|---|---|---|---|---|---|---|
| Without AE 2 | With AE 3 | Without AE | With AE | Without AE | With AE | |
| AChE | −0.276 * 4 | NS | 0.391 ‡ | NS | −0.536 ‡ | NS |
| Na+/K+-ATPase | NS | NS | −0.427 ‡ | NS | 0.279 † | NS |
| Ca2+/Mg2+-ATPase | NS | NS | NS | 0.244 * | NS | −0.214 * |
| Ca | NS | −0.275 † | −0.732 ‡ | −0.437 ‡ | 0.528 ‡ | NS |
| Mg | NS | 0.264 * | 0.259 * | 0.206 * | NS | NS |
| CAM | 0.311 † | NS | NS | NS | 0.472 † | NS |
| PLA2 | NS | NS | 0.566 ‡ | −0.245 * | −0.219 * | NS |
| NOS1 | NS | NS | NS | 0.311 † | NS | NS |
| Nrf2 | −0.479 ‡ | 0.365 ‡ | 0.687 ‡ | 0.579 ‡ | −0.700 ‡ | NS |
| KEAP1 | −0.546 ‡ | NS | 0.606 ‡ | 0.371 ‡ | −0.659 ‡ | NS |
| MMP-2 | NS | 0.257 * | 0.532 ‡ | NS | −0.281 † | NS |
| MMP-3 | NS | 0.279 † | NS | 0.212 * | NS | NS |
| MMP-9 | 0.479 ‡ | NS | NS | 0.296 † | 0.363 ‡ | NS |
| TIMP-2 | NS | NS | 0.416 ‡ | NS | NS | NS |
| TIMP-3 | NS | −0.347 ‡ | −0.720 ‡ | −0.382 ‡ | 0.250 * | NS |
| TIMP-4 | −0.223 * | NS | NS | −0.249 * | −0.226 * | 0.211 * |
| Parameter | 3-NT 1 | PC | LPO | 8-iso | γ-H2AX | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Without AE 2 | With AE 3 | Without AE | With AE | Without AE | With AE | Without AE | With AE | Without AE | With AE | |
| AChE | −0.215 * 4 | NS | −0.325 † | NS | −0.292 † | NS | −0.396 ‡ | NS | −0.246 * | NS |
| Na+/K+-ATPase | NS | NS | NS | NS | NS | 0.218 * | NS | NS | −0.273 * | NS |
| Ca2+/Mg2+-ATPase | NS | NS | NS | 0.242 * | NS | 0.339 ‡ | NS | NS | 0.238 * | NS |
| Ca | NS | −0.304 † | 0.470 ‡ | NS | NS | −0.263 * | 0.591 ‡ | NS | 0.391 ‡ | −0.263 * |
| Mg | NS | 0.210 * | NS | −0.254 * | NS | NS | NS | NS | NS | NS |
| CAM | 0.411 ‡ | 0.235 * | 0.459 ‡ | −0.222 * | 0.371 ‡ | NS | 0.647 ‡ | NS | 0.415 ‡ | NS |
| PLA2 | NS | NS | NS | NS | NS | NS | −0.324 † | NS | NS | NS |
| NOS1 | 0.256 * | NS | 0.416 ‡ | NS | 0.241 * | 0.239 * | 0.318 † | NS | 0.522 ‡ | 0.287 † |
| Nrf2 | NS | 0.613 ‡ | −0.495 ‡ | NS | −0.334 ‡ | 0.508 ‡ | −0.622 ‡ | NS | −0.284 † | 0.375 ‡ |
| KEAP1 | NS | 0.456 ‡ | −0.471 ‡ | NS | −0.317 † | 0.497 ‡ | −0.467 ‡ | NS | −0.299 † | 0.407 * |
| MMP-2 | NS | NS | −0.278 † | NS | NS | 0.324 † | −0.254 * | NS | NS | NS |
| MMP-3 | NS | NS | NS | NS | NS | 0.209 * | NS | NS | NS | NS |
| MMP-9 | 0.393 ‡ | 0.324 † | 0.485 ‡ | NS | 0.528 ‡ | NS | 0.206 * | NS | 0.246 * | NS |
| TIMP-2 | NS | NS | NS | 0.386 ‡ | NS | NS | NS | −0.221 * | NS | NS |
| TIMP-3 | −0.208 * | NS | 0.256 * | NS | NS | NS | 0.501 ‡ | 0.343 ‡ | 0.315 † | NS |
| TIMP-4 | NS | −0.360 ‡ | NS | 0.229 * | NS | NS | NS | −0.371 ‡ | 0.209 * | −0.238 * |
| Parameter | AChE | Na+/K+ -ATPase | Ca2+/Mg2+-ATPase | Ca | Mg | CAM | PLA2 | NOS1 | Nrf2 | KEAP1 | MMP-2 | MMP-3 | MMP-9 | TIMP-2 | TIMP-3 | TIMP-4 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AChE | - | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Na+/K+-ATPase | NS 1 | - | 0.503 ‡ | NS | NS | 0.272 † | −0.246 * | NS | NS | NS | 0.641 ‡ | 0.309 † | 0.517 ‡ | 0.578 ‡ | NS | 0.390 ‡ |
| Ca2+/Mg2+-ATPase | NS | −0.461 ‡ | - | NS | NS | NS | NS | 0.277 † | 0.215 * | NS | 0.318 † | NS | 0.398 ‡ | 0.552 ‡ | −0.252 * | 0.249 * |
| Ca | −0.344 † | NS | NS | - | −0.215 * | NS | NS | NS | −0.384 ‡ | −0.390 ‡ | NS | NS | NS | NS | 0.423 ‡ | 0.254 * |
| Mg | NS | 0.214 * | −0.419 ‡ | NS | - | NS | NS | NS | NS | 0.236 * | NS | NS | 0.207 * | −0.284 † | NS | −0.324 † |
| CAM | −0.457 ‡ | NS | NS | 0.350 ‡ | NS | - | NS | NS | NS | NS | 0.215 * | NS | 0.416 ‡ | NS | NS | NS |
| PLA2 | NS | NS | NS | −0.437 ‡ | 0.332 † | NS | - | NS | −0.255 * | NS | −0.292 ‡ | NS | NS | NS | 0.248 * | NS |
| NOS1 | NS | −0.438 ‡ | NS | NS | −0.267 † | 0.271 * | NS | - | 0.361 ‡ | NS | NS | NS | NS | NS | −0.240 * | NS |
| Nrf2 | 0.595 ‡ | −0.509 ‡ | NS | −0.601 ‡ | NS | −0.378 ‡ | 0.437 ‡ | NS | - | 0.469 ‡ | 0.225 * | NS | 0.329 † | NS | −0.413 ‡ | −0.231 * |
| KEAP1 | 0.554 ‡ | −0.383 ‡ | NS | NS | NS | −0.274 * | 0.329 † | NS | 0.437 ‡ | - | NS | NS | NS | NS | −0.283 † | −0.313 † |
| MMP-2 | 0.335 † | −0.533 ‡ | NS | −0.304 † | NS | NS | 0.346 ‡ | NS | 0.498 ‡ | 0.448 ‡ | - | 0.440 ‡ | 0.472 ‡ | 0.549 ‡ | NS | 0.443 ‡ |
| MMP-3 | NS | −0.352 ‡ | NS | NS | NS | NS | NS | 0.203 * | NS | NS | NS | - | 0.209 * | NS | NS | NS |
| MMP-9 | NS | NS | NS | 0.213 * | NS | 0.387 ‡ | 0.249 * | 0.241 * | NS | −0.209 * | NS | NS | - | 0.380 ‡ | NS | NS |
| TIMP-2 | NS | −0.581 ‡ | 0.347 ‡ | NS | NS | NS | 0.316 † | NS | 0.383 ‡ | 0.388 ‡ | 0.493 ‡ | NS | 0.352 ‡ | - | NS | 0.568 ‡ |
| TIMP-3 | NS | NS | NS | 0.611 ‡ | −0.294 † | NS | −0.408 ‡ | NS | −0.398 ‡ | −0.315 † | −0.207 * | NS | NS | NS | - | NS |
| TIMP-4 | NS | −0.519 ‡ | 0.399 ‡ | NS | NS | NS | NS | 0.260 * | 0.208 * | 0.249 * | NS | NS | NS | 0.359 ‡ | NS | - |
| Parameter | Kind of Commercial Kit | Precision of Measurements | |
|---|---|---|---|
| Intra-CV | Inter-CV | ||
| AChE | ELISA Kit for Acetylcholinesterase (AChE) (No. SEB447Ra) Cloud-Clone Corp. | <5.3% and <6.6% | <4.2% |
| Ca2+/Mg2+-ATPase | Rat Ca-Mg-ATPase ELISA Kit (No. MBS3809417) MyBiosource | <0.3% and <2% | <0.3% |
| Na+/K+-ATPase | Rat Na-K-ATPase ELISA Kit (No. MBS3809783) MyBiosource | <2.2% and <4% | <3% |
| CAM | ELISA Kit for Calmodulin (CAM) (No. SEA640Ra) Cloud-Clone Corp. | <4% and <5% | <6% |
| PLA2 | Rat PLA2G2A (Phospholipase A2, Group IIA) ELISA Kit (No. ER1276), FineTest | <7.1% and <8.3% | <5.2% |
| NOS1 | ELISA Kit for Nitric Oxide Synthase 1, Neuronal (NOS1) (No. SEA815Ra), Cloud-Clone Corp. | <6.6% and <5% | <2.5% |
| Nrf2 | Rat Nuclear Factor Erythroid 2-Related Factor 2 (NRF2) ELISA Kit (No. MBS012148), MyBiosource | <3.6% and <7.9% | <3.2% |
| KEAP1 | Rat Kelch like ECH Associated Protein 1 (KEAP1) ELISA kit (No. MBS7218529), MyBiosource | <3% and <8% | <3.3% |
| MMP-2 | Rat (MMP-2) ELISA Kit (No. 201-11-0136) SunRed | <4% for both kits | <5.4% |
| MMP-3 | Rat (MMP-3) ELISA Kit (No. 201-11-0317) SunRed | <3% and <4% | <4% |
| MMP-9 | Rat (MMP-9) ELISA Kit (No. 201-11-0322) SunRed | <4.6% and <3.7% | <8.8% |
| TIMP-2 | Rat (TIMP-2) ELISA Kit (No. 201-11-0324) SunRed | <2.5% and <2.3% | <2.8% |
| TIMP-3 | Rat (TIMP-3) ELISA Kit (No. 201-11-0325) SunRed | <2% and <1% | <2% |
| TIMP-4 | Rat (TIMP-4) ELISA Kit (No. 201-11-0326) SunRed | <3% and <1% | <1% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruczaj, A.; Rogalska, J.; Gałażyn-Sidorczuk, M.; Brzóska, M.M. The Protective Effect of the Supplementation with an Extract from Aronia melanocarpa L. Berries against Cadmium-Induced Changes of Chosen Biomarkers of Neurotoxicity in the Brain—A Study in a Rat Model of Current Lifetime Human Exposure to This Toxic Heavy Metal. Int. J. Mol. Sci. 2024, 25, 10887. https://doi.org/10.3390/ijms252010887
Ruczaj A, Rogalska J, Gałażyn-Sidorczuk M, Brzóska MM. The Protective Effect of the Supplementation with an Extract from Aronia melanocarpa L. Berries against Cadmium-Induced Changes of Chosen Biomarkers of Neurotoxicity in the Brain—A Study in a Rat Model of Current Lifetime Human Exposure to This Toxic Heavy Metal. International Journal of Molecular Sciences. 2024; 25(20):10887. https://doi.org/10.3390/ijms252010887
Chicago/Turabian StyleRuczaj, Agnieszka, Joanna Rogalska, Małgorzata Gałażyn-Sidorczuk, and Małgorzata M. Brzóska. 2024. "The Protective Effect of the Supplementation with an Extract from Aronia melanocarpa L. Berries against Cadmium-Induced Changes of Chosen Biomarkers of Neurotoxicity in the Brain—A Study in a Rat Model of Current Lifetime Human Exposure to This Toxic Heavy Metal" International Journal of Molecular Sciences 25, no. 20: 10887. https://doi.org/10.3390/ijms252010887









